Abstract
Purpose
Risk of suicide is increased among men with prostate cancer. We investigated this association among men with low-risk cancer, usually detected by prostate specific antigen (PSA)-testing.
Patients and Methods
Relative risk (RR) of suicide was calculated by use of Poisson regression analysis within the Prostate Cancer data Base Sweden (PCBaSe) 2.0, a nationwide, population-based database, comparing 105,736 men diagnosed with prostate cancer between 1997–2009 to 528,658 matched prostate cancer-free men.
Results
During the first six months after diagnosis, there were 38 suicides among men with prostate cancer; incidence rate 0.73 per 1,000 person-years (PY) and 30 suicides in the comparison cohort; 0.11 per 1,000 PY, corresponding to a RR of suicide of 6.5 (95% CI 4.0–10). Risk was highest among men with distant metastases, incidence rate 1.25 per 1,000 PY, RR 10 (95% CI 5.1–21) but risk was also increased for men with low-risk tumors, incidence rate difference 0.45 per 1,000 PY and RR 5.2 (95% CI 2.3–12) and across categories of socioeconomic status and comorbidity. Eighteen months after diagnosis, risk of suicide had decreased to 0.27 per 1,000 PY, RR 1.0 (95% CI 0.68–1.5) for low-risk prostate cancer but remained increased among men with metastases, 0.57 per 1,000 PY, RR 1.8 (95% CI 1.1–2.9).
Conclusion
Although the increase in absolute risk of suicide was modest, our findings reflect the severe psychological stress that prostate cancer patients may experience after diagnosis. The increased risk of suicide observed in men with prostate cancer, including low-risk, calls for increased awareness.
Keywords: prostatic neoplasms, suicide, mass screening
Introduction
Cancer is often perceived as a life-threatening disease, even though many cancers are currently diagnosed in an early and curable stage(1). Men diagnosed with prostate cancer are at increased risk of suicide and also of anxiety, depression, post-traumatic stress disorder, psychiatric hospitalization and treatment(2–4). An increased risk of suicide and cardiovascular events shortly after the date of cancer diagnosis was recently reported, highlighting that a cancer diagnosis per se may evoke severe stress and trigger these events(3–8).
Prostate cancer has an extremely variable prognosis ranging from a 10-year risk of cancer death of 3% for low-risk prostate cancer, (cT1–T2, GS 2–6, serum levels of Prostate-Specific Antigen, PSA, below 10 ng/mL)(9) to 70% among men with metastatic disease.(10, 11) Since the introduction of PSA testing for early detection of prostate cancer, there has been a sharp rise in the number of men diagnosed with low-risk prostate cancer(12, 13). If these men also have an increased risk of suicide is unknown.
The aim of the present study was therefore to assess the risk of suicide in men with prostate cancer with special reference to time after diagnosis and disease risk category. We further aimed to explore the influence of co-morbidity and socioeconomic status on the risk of suicide among men newly diagnosed with prostate cancer.
Materials and methods
This is a prospective study of two cohorts within the Prostate Cancer data Base Sweden (PCBaSe) 2.0, a database that has been described in detail previously(14). In brief, the prostate cancer cohort consisted of men diagnosed with prostate cancer between January 1, 1997 and December 31, 2009 in the National Prostate Cancer Register (NPCR), which captures more than 96% of incident prostate cancer cases compared to the Swedish Cancer Register to which registration is compulsory and mandated by law. Data available in NPCR include diagnostic unit, date of diagnosis, serum level of PSA at diagnosis, tumor stage (TNM), tumor grade and primary treatment performed or decided within 6 months after date of diagnosis. Since 2000, details on the evaluation leading to the diagnosis are also recorded (symptoms of disease, health control, i.e. unorganized screening or other reasons). We used a modification of the National Comprehensive Cancer Network risk group classification (Table 1)(9).
Table 1.
Baseline characteristics of men with prostate cancer in Prostate Cancer data Base Sweden (PCBaSe) 2.0 who committed early suicide, suicide and full cohort.
| Death due to ICD10 X60–84 | Death due to ICD10 X60–84 within 6 months from prostate cancer diagnosis | All men with prostate cancer | |
|---|---|---|---|
| (n = 211) | (n = 38) | (n = 105 736) | |
| Follow-up, yr, mean (sd) | 2.7 (2.5) | 0.3 (0.2) | 4.9 (3.2) |
| Age, yr, median (IQR) | 72 (65–79.5) | 72.5 (64.5–80) | 71 (64–78) |
| Age, yr, No (%) | |||
| <65 | 51 (24) | 10 (26) | 28197 (27) |
| 65–74 | 70 (33) | 11 (29) | 38914 (37) |
| ≥75 | 90 (43) | 17 (45) | 38625 (37) |
| Year of PC diagnosis, No (%) | |||
| 1997–1999 | 45 (21) | 3 (8) | 18162 (17) |
| 2000–2002 | 71 (34) | 8 (21) | 22305 (21) |
| 2003–2005 | 53 (25) | 9 (24) | 28304 (27) |
| 2006–2009 | 42 (20) | 18 (47) | 36965 (35) |
| Mode of detection1, No (%) | |||
| Symptoms | 113 (68) | 26 (74) | 51231 (59) |
| Health control | 22 (13) | 5 (14) | 24349 (28) |
| Other reason | 15 (9) | 1 (3) | 6009 (7) |
| Missing | 16 (10) | 3 (9) | 5985 (7) |
| T stage, No (%) | |||
| T1c | 56 (27) | 15 (39) | 34551 (33) |
| T0 | 0 (0) | 0 (0) | 496 (<1) |
| T1ab | 13 (6) | 2 (5) | 6625 (6) |
| T2 | 61 (29) | 6 (16) | 33352 (32) |
| T3–4 | 75 (36) | 15 (39) | 28708 (27) |
| Missing | 6 (3) | 0 (0) | 2004 (2) |
| M stage, No (%) | |||
| M0 | 48 (23) | 5 (13) | 28886 (27) |
| M1 | 22 (10) | 6 (16) | 10350 (10) |
| MX | 140 (66) | 27 (71) | 65483 (62) |
| Missing | 1 (<1) | 0 (0) | 1017 (<1) |
| PSA ng/mL, No (%) | |||
| PSA <4 | 9 (4) | 2 (5) | 6933 (7) |
| PSA ≥4 – <10 | 62 (29) | 10 (26) | 34222 (32) |
| PSA ≥10 – <20 | 41 (19) | 10 (26) | 22414 (21) |
| PSA ≥20 – <100 | 68 (32) | 9 (24) | 26602 (25) |
| PSA ≥100 | 25 (12) | 7 (18) | 12879 (12) |
| Missing | 6 (3) | 0 (0) | 2686 (3) |
| Gleason score2, No (%) | |||
| 2–6 | 100 (47) | 16 (42) | 47189 (45) |
| 7 | 59 (28) | 9 (24) | 34850 (33) |
| 8–10 | 45 (21) | 10 (26) | 21275 (20) |
| Missing | 7 (3) | 3 (8) | 2422 (2) |
| Risk group3, No (%) | |||
| Low risk | 49 (23) | 7 (18) | 25297 (24) |
| Intermediate risk | 35 (17) | 8 (21) | 24692 (23) |
| High risk | 59 (28) | 10 (26) | 26986 (26) |
| Regionally metastatic | 27 (13) | 2 (5) | 8980 (8) |
| Distant metastases | 34 (16) | 10 (26) | 17012 (16) |
| Missing | 7 (3) | 1 (3) | 2769 (3) |
| Planned initial treatment, No (%) | |||
| Expectancy | 49 (23) | 3 (8) | 27502 (26) |
| Treatment with curative intent | 46 (22) | 5 (13) | 34826 (33) |
| Treatment with hormonal therapy | 92 (44) | 14 (37) | 40387 (38) |
| Missing | 24 (11) | 16 (42) | 3021 (3) |
| Charlson comorbidity index, No (%) | |||
| 0 | 135 (64) | 27 (71) | 68706 (65) |
| 1 | 28 (13) | 1 (3) | 19161 (18) |
| 2+ | 48 (23) | 10 (26) | 17869 (17) |
| Socioeconomic status, No (%) | |||
| High | 95 (45) | 17 (45) | 51731 (49) |
| Low | 114 (54) | 20 (53) | 52531 (50) |
| Not gainfully employed | 1 (<1) | 0 (0) | 396 (<1) |
| Unclassified/Missing | 1 (<1) | 1 (3) | 1078 (1) |
| Marital status, No (%) | |||
| Unmarried | 30 (14) | 8 (21) | 9308 (9) |
| Married | 111 (53) | 18 (47) | 71907 (68) |
| Divorced | 35 (17) | 6 (16) | 13098 (12) |
| Widower | 35 (17) | 6 (16) | 11414 (11) |
| Missing | 0 (0) | 0 (0) | 9 (<1) |
For each case, PCBaSe 2.0 includes five randomly selected men that were prostate cancer-free at the time of the case’s diagnosis, matched to the index case on year of birth (+/− 1 year) and county of residence. These men formed our comparison cohort.
By use of the individually unique personal identity number assigned to all residents in Sweden, NPCR was linked to a number of other nation-wide population-based health care registers and demographic databases.(14)
The Cause of Death Register was used to identify suicide as defined by International Classification of Diseases [ICD]-10 code recorded as “certain suicide” (ICD-10 code X60.84). The Swedish Longitudinal integration database for health insurance and labor market studies (LISA) provided information on socioeconomic status and marital status.
We classified comorbidity for the prostate cancer patients and men in the comparison cohort according to Charlson’s co-morbidity index (CCI)(15): 0=no co-morbidity, 1= mild, 2+ =severe co-morbidity based on data in the National Patient Register according to ICD-9 or ICD-10.
Follow-up started on the date of diagnosis of the index case and ended on the date of death, emigration, or December 31, 2010, whichever came first.
Psychiatric disease as indicated by medication may modify the risk of suicide and therefore, we assessed patterns of filings of drug prescriptions (antidepressants ATC code N06A, neuroleptics ATC code N05A, sedatives and ataractics ATC code N05B and sleep medications and sedatives ATC code N05C) through linkage with the Prescribed Drug Register. In a sub-analysis, we assessed the exposure (psychiatric medication use never/ever) in the pre-diagnosis period July 2005 (when the Prescribed Drug Register started) to Dec 31, 2006 and assessed the outcome suicide during the period 2007–2009.
Statistics
Age during follow-up was divided into five-year categories and the number of suicides along with person-time at risk was calculated separately for the two cohorts. In order to study the effect of time since diagnosis, the data were further divided into three categories of follow-up time (0–6 months, 6–18 months, and >18 months since date of diagnosis of the index case). Poisson regression models were used with the observed number of events as response and the logarithm of person-time at risk as offset. The covariates included in the model were age (as natural cubic splines with two internal knots), calendar time since inclusion, an indicator of cohort, and the interaction between this indicator and the time since inclusion. The relative risks of suicide in the three follow-up intervals, along with 95% confidence limits, were calculated.
Relative risks were also estimated with flexible parametric models(17), using an indicator of cohort and age at diagnosis (as natural cubic splines) as covariates. This allowed for the relative risks to be plotted as a continuous function of time since diagnosis.
A p-value less than 0.05 was considered statistically significant. Statistical analyses were performed with the Stata statistical software v.11.0 (Stata Corp 2009, College Station, TX, U.S.A.) and the R statistical software package v.2.12.2.(18)
Results
During the entire follow-up time, mean time 4.9 (SD 3.2) years for men with prostate cancer and 5.4 (SD 3.3) years for the comparison cohort (Table 1 & 2), we observed an overall increased relative risk of suicide of 1.5 (95% CI 1.3–1.8) (Table 3) based on 211 suicides among men with prostate cancer and 785 suicides in the comparison cohort. Over the entire study period, the incidence rate was 0.41/1,000 person-years (PY) among prostate cancer cases and 0.27/1,000 PY in the comparison cohort.
Table 2.
Baseline characteristics of prostate-cancer free men in Prostate Cancer data Base Sweden (PCBaSe) 2.0 who committed early suicide, suicide and full cohort.
| Death due to ICD10 X60–84 | All prostate cancer-free men | |
|---|---|---|
| (n = 785) | (n = 528 658) | |
| Follow-up, yr, mean (sd) | 4.1 (2.8) | 5.4 (3.3) |
| Age, yr, median (IQR) | 73 (65–79) | 71 (65–78) |
| Age, yr, No (%) | ||
| <65 | 188 (24) | 132087 (25) |
| 65–74 | 259 (33) | 194022 (37) |
| ≥75 | 338 (43) | 202549 (38) |
| Charlson comorbidity index, No (%) | ||
| 0 | 506 (64) | 339708 (64) |
| 1 | 152 (19) | 96214 (18) |
| 2+ | 127 (16) | 92736 (18) |
| Socioeconomic status, No (%) | ||
| High | 306 (39) | 237227 (45) |
| Low | 461 (59) | 276426 (52) |
| Not gainfully employed | 6 (<1) | 3521 (<1) |
| Unclassified/Missing | 12 (2) | 11484 (2) |
| Marital status, No (%) | ||
| Unmarried | 162 (21) | 60326 (11) |
| Married | 384 (49) | 339433 (64) |
| Divorced | 140 (18) | 70777 (13) |
| Widower | 99 (13) | 58122 (11) |
| Missing | 0 (0) | 0 (0) |
Table 3.
Relative risk of suicide after prostate cancer diagnosis according to time after diagnosis.
| 0–6 months after diagnosis
|
6–18 months after diagnosis
|
>18 months after diagnosis
|
Overall, disregarding time since diagnosis
|
|||||
|---|---|---|---|---|---|---|---|---|
| RR | (95% CI) | RR | (95% CI) | RR | (95% CI) | RR | (95% CI) | |
| Full cohort | 6.5 | (4.0–10) | 2.1 | (1.5–2.9) | 1.2 | (0.96–1.4) | 1.5 | (1.3–1.8) |
| Age, yr1 | ||||||||
| <65 | 4.7 | (1.9–11) | 2.4 | (1.3–4.7) | 0.90 | (0.60–1.4) | 1.3 | (0.99–1.8) |
| 65–74 | 6.9 | (2.8–17) | 2.0 | (1.1–3.6) | 1.1 | (0.82–1.6) | 1.5 | (1.1–1.9) |
| ≥75 | 7.8 | (3.7–16) | 2.0 | (1.2–3.4) | 1.4 | (1.0–1.8) | 1.7 | (1.4–2.2) |
| Year of PC diagnosis | ||||||||
| 1997–1999 | 3.0 | (0.90–9.7) | 2.8 | (1.5–5.2) | 1.0 | (0.72–1.5) | 1.3 | (0.97–1.8) |
| 2000–2002 | 6.4 | (2.9–14) | 2.0 | (1.1–3.9) | 1.6 | (1.2–2.1) | 1.8 | (1.4–2.3) |
| 2003–2005 | 5.8 | (2.7–12) | 2.1 | (1.2–3.7) | 1.0 | (0.70–1.4) | 1.4 | (1.0–1.8) |
| 2006–2009 | 8.8 | (4.9–16) | 1.8 | (1.0–3.1) | 0.75 | (0.40–1.4) | 1.6 | (1.2–2.3) |
| Mode of detection | ||||||||
| Symptoms | 8.9 | (5.1–15) | 1.8 | (1.2–3.0) | 1.5 | (1.2–2.0) | 2.0 | (1.6–2.4) |
| Health control | 3.8 | (1.5–10) | 1.3 | (0.61–2.8) | 0.49 | (0.26–0.91) | 0.81 | (0.53–1.2) |
| Other reason | 3.0 | (0.41–22) | 3.8 | (1.5–9.3) | 1.3 | (0.65–2.4) | 1.7 | (1.0–2.9) |
| T stage | ||||||||
| T1c | 8.2 | (4.4–15) | 1.9 | (1.1–3.3) | 0.79 | (0.53–1.2) | 1.3 | (0.98–1.7) |
| T0 | - | - | - | - | - | - | - | - |
| T1ab | 5.3 | (1.3–22) | 1.3 | (0.33–5.4) | 1.0 | (0.54–2.0) | 1.2 | (0.72–2.1) |
| T2 | 3.2 | (1.4–7.8) | 1.9 | (1.1–3.3) | 1.1 | (0.79–1.5) | 1.3 | (1.0–1.7) |
| T3–4 | 9.1 | (4.9–17) | 2.3 | (1.3–4.0) | 1.9 | (1.4–2.6) | 2.3 | (1.8–2.9) |
| M stage | ||||||||
| M0 | 3.2 | (1.3–8.3) | 1.1 | (0.52–2.4) | 0.91 | (0.65–1.3) | 1.0 | (0.77–1.4) |
| M1 | 11 | (4.4–25) | 1.6 | (0.50–4.9) | 2.6 | (1.5–4.5) | 2.8 | (1.8–4.2) |
| MX | 7.3 | (4.4–12) | 2.6 | (1.8–3.8) | 1.2 | (0.95–1.5) | 1.7 | (1.4–2.1) |
| PSA ng/mL | ||||||||
| PSA <4 | 5.3 | (1.3–22) | 3.3 | (1.4–8.2) | 0.28 | (0.07–1.1) | 0.99 | (0.51–1.9) |
| PSA ≥4 – <10 | 5.5 | (2.7–11) | 2.5 | (1.5–4.0) | 0.95 | (0.67–1.3) | 1.4 | (1.1–1.8) |
| PSA ≥10 – <20 | 8.1 | (4.0–17) | 1.2 | (0.54–2.8) | 0.95 | (0.64–1.4) | 1.3 | (0.93–1.7) |
| PSA ≥20 – <100 | 5.8 | (2.8–12) | 1.8 | (0.99–3.4) | 1.7 | (1.3–2.3) | 1.9 | (1.5–2.4) |
| PSA ≥100 | 9.4 | (4.1–21) | 1.9 | (0.78–4.7) | 1.7 | (0.98–3.0) | 2.1 | (1.4–3.2) |
| Gleason score | ||||||||
| 2–6 | 6.3 | (3.4–12) | 2.3 | (1.4–3.5) | 1.1 | (0.84–1.4) | 1.5 | (1.2–1.8) |
| 7 | 4.6 | (2.2–9.7) | 2.4 | (1.4–3.9) | 0.92 | (0.65–1.3) | 1.3 | (1.0–1.7) |
| 8–10 | 8.2 | (4.0–17) | 1.1 | (0.46–2.7) | 2.0 | (1.4–2.9) | 2.1 | (1.5–2.8) |
| Risk group | ||||||||
| Low risk | 5.2 | (2.3–12) | 2.6 | (1.5–4.5) | 1.0 | (0.68–1.5) | 1.4 | (1.1–1.9) |
| Intermediate risk | 6.0 | (2.8–13) | 1.9 | (0.98–3.5) | 0.62 | (0.38–1.0) | 1.0 | (0.73–1.4) |
| High risk | 6.5 | (3.2–13) | 1.6 | (0.86–3.1) | 1.3 | (0.93–1.8) | 1.5 | (1.2–2.0) |
| Regionally metastatic | 3.8 | (0.91–16) | 2.0 | (0.73–5.4) | 2.4 | (1.5–3.7) | 2.3 | (1.6–3.4) |
| Distant metastases | 10 | (5.1–21) | 1.8 | (0.78–4.0) | 1.8 | (1.1–2.9) | 2.2 | (1.6–3.2) |
| Planned initial treatment | ||||||||
| Expectancy | 2.0 | (0.60–6.4) | 2.3 | (1.3–4.0) | 0.96 | (0.67–1.4) | 1.2 | (0.89–1.6) |
| Trt w curative intent | 2.7 | (1.1–7.0) | 1.7 | (0.98–3.1) | 0.74 | (0.51–1.1) | 1.0 | (0.74–1.4) |
| Trt w hormonal therapy | 6.0 | (3.2–11) | 1.9 | (1.2–3.2) | 1.8 | (1.4–2.3) | 1.9 | (1.6–2.4) |
| Charlson comorbidity index2 | ||||||||
| 0 | 11 | (5.7–22) | 2.2 | (1.4–3.4) | 1.1 | (0.86–1.4) | 1.5 | (1.3–1.8) |
| 1 | 0.57 | (0.07–4.5) | 1.7 | (0.76–3.8) | 0.94 | (0.58–1.5) | 1.0 | (0.70–1.6) |
| 2+ | 6.0 | (2.4–15) | 2.3 | (1.2–4.5) | 1.8 | (1.2–2.8) | 2.2 | (1.6–3.1) |
| Socioeconomic status3 | ||||||||
| High | 8.1 | (3.7–18) | 3.1 | (1.9–5.0) | 1.1 | (0.80–1.5) | 1.6 | (1.3–2.0) |
| Low | 5.4 | (2.9–10) | 1.5 | (0.92–2.5) | 1.3 | (0.99–1.6) | 1.5 | (1.2–1.9) |
| Not gainfully employed | - | - | - | - | - | - | - | - |
| Marital status4 | ||||||||
| Unmarried | 9.0 | (3.1–26) | 2.4 | (1.0–5.5) | 0.91 | (0.53–1.6) | 1.5 | (0.98–2.1) |
| Married | 7.9 | (3.7–17) | 1.9 | (1.1–3.1) | 1.3 | (0.97–1.6) | 1.6 | (1.3–1.9) |
| Divorced | 5.5 | (1.8–17) | 2.1 | (0.99–4.6) | 1.1 | (0.70–1.8) | 1.5 | (1.0–2.2) |
| Widower | 4.6 | (1.5–14) | 3.0 | (1.4–6.2) | 1.6 | (0.97–2.7) | 2.2 | (1.5–3.2) |
Men with prostate cancer compared to prostate cancer-free men in comparison cohort of the same age group at inclusion.
Men with prostate cancer compared to prostate cancer-free men in comparison cohort of the same CCI at inclusion.
Men with prostate cancer compared to prostate cancer-free men in comparison cohort of the same SES at inclusion.
Men with prostate cancer compared to prostate cancer-free men in comparison cohort of the same marital status at inclusion.
The median age at date of diagnosis among men with prostate cancer was 72 years (IQR 65–79.5). The median age at suicide for men with prostate cancer was 75 years (67–83) and 76 (69–83) in the comparison cohort. (Table 1) There was an almost equal distribution in socioeconomic status in the cohort of men with prostate cancer and the comparison cohort and the majority was married and had no co-morbid conditions. (Table 1&2)
Risk of suicide by time since diagnosis
Within the first six month after date of diagnosis, we observed an increased relative risk of suicide of 6.5 (95% CI 4.0–10) (Table 3); with 38 suicides among men with prostate cancer; incidence rate 0.73 per 1,000 PY and 30 suicides in the comparison cohort; 0.11 per 1,000 PY (Table 4), i.e. an excess of 0.62 suicides/1,000 PY among men with prostate cancer.
Table 4.
Number of events (death due to ICD10 X60–84), person-years at risk, and number of events per 1000 py.
| Number of events | Person-years at risk | Number of events per 1000 py | |
|---|---|---|---|
| Prostate cancer-free men in comparison cohort | |||
| 0–6 months after inclusion | 30 | 263653 | 0.11 |
| 6–18 months after inclusion | 121 | 507589 | 0.24 |
| >18 months after inclusion | 634 | 2100308 | 0.30 |
| Men with prostate cancer | |||
| 0–6 months after diagnosis | 38 | 51947 | 0.73 |
| 6–18 months after diagnosis | 48 | 97546 | 0.49 |
| >18 months after diagnosis | 125 | 368461 | 0.34 |
| Early detection features: | |||
| Men with prostate cancer - T1c | |||
| 0–6 months after diagnosis | 15 | 17163 | 0.87 |
| 6–18 months after diagnosis | 14 | 33122 | 0.42 |
| >18 months after diagnosis | 27 | 125366 | 0.22 |
| Men with prostate cancer - Mode of detection1 - Health control | |||
| 0–6 months after diagnosis | 5 | 12140 | 0.41 |
| 6–18 months after diagnosis | 7 | 23445 | 0.30 |
| >18 months after diagnosis | 10 | 76840 | 0.13 |
| Men with prostate cancer - Low risk | |||
| 0–6 months after diagnosis | 7 | 12612 | 0.56 |
| 6–18 months after diagnosis | 14 | 24612 | 0.57 |
| >18 months after diagnosis | 28 | 103557 | 0.27 |
| Men with prostate cancer - Intermediate risk | |||
| 0–6 months after diagnosis | 8 | 12274 | 0.65 |
| 6–18 months after diagnosis | 10 | 23688 | 0.42 |
| >18 months after diagnosis | 17 | 95989 | 0.18 |
| Men with prostate cancer - Treatment with curative intent | |||
| 0–6 months after diagnosis | 5 | 17392 | 0.29 |
| 6–18 months after diagnosis | 13 | 34003 | 0.38 |
| >18 months after diagnosis | 28 | 144349 | 0.19 |
Presented for subcohort with PC diagnosis after January 1, 2000.
This risk decreased to RR 2.1 (95% CI 1.5–2.9) 6–18 months after date of diagnosis and was lower and not statistically significant, RR 1.2 (95% CI 0.96–1.4) beyond 18 months after diagnosis (Table 3).
Risk of suicide by risk category
There was an increasing relative risk of suicide within 6 months with higher risk category; men with low-risk tumors relative the comparison cohort had a RR of 5.2 (95% CI 2.3–12), incidence rate difference 0.45 per 1,000 PY and men with distant metastases had an RR 10 (95% CI 5.1–21), incidence rate 1.25 per 1,000 PY, (Table 3,4). Eighteen months after diagnosis, risk of suicide had decreased to 0.27 per 1,000 PY, RR 1.0 (95% CI 0.68–1.5) for low-risk prostate cancer but remained increased among men with metastases 0.57 per 1,000 person-years, RR 1.8 (95%CI 1.1–2.9). (Table 3,4)
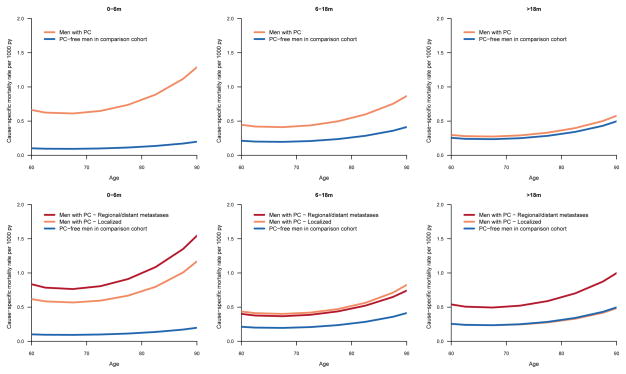
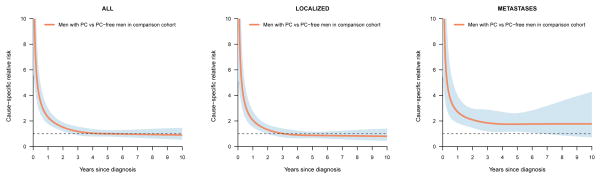
The absolute risk of suicide increased with increasing risk category, particularly among older men (Figure 1). Risk remained elevated over time for men with distant metastases whereas for men with localized disease it decreased to the same level as among controls 3 years after diagnosis (Figure 2).
Figure 1.
Cause-specific mortality of suicide for men with prostate cancer and prostate cancer-free men in the comparison cohort according to age:
Figure 2.
Relative risk of suicide in men with prostate cancer in relation to prostate cancer-free men in the comparison cohort according to time since diagnosis.
We also observed increased RR’s in the first 6 months among men diagnosed with T1c tumors (RR 8.2; 95% CI 4.4–15), in men with low or slightly elevated PSA levels, in men with intermediate risk tumors, and in asymptomatic men who were diagnosed as a result of PSA testing (Table 3).
Risk of suicide by treatment modality
The risk of suicide within 6 months was increased among all treatment strategies. This risk was somewhat lower (RR 2.0, 95% CI 0.6–6.4) for men managed expectantly as compared to those treated with curative intent (RR 2.7, 95% CI 1.1–7.0) or with hormonal therapy (RR 6.0, 95% CI 3.2–11). (Table 3)
Risk of suicide by history of filed prescriptions
Prostate cancer cases with no filed prescriptions of antidepressants, antipsychotic medication or sleeping pills, “never users”, had a lower absolute risk of suicide (0.37 per 1,000 PY) than cases on psychiatric medication, “ever users”, (0.87 per 1,000 PY) and “never user” cases also had a lower absolute increase in risk of suicide than “ever user” cases (Appendix 1). However, the relative risk was higher among never user cases (RR 2.7, 95% CI 1.6–4.7) than ever user cases (RR 1.9, 95% CI 0.94–3.7).
Appendix 1.
Risk of suicide in relation to psychiatric medication usage
| Overall, disregarding time since diagnosis |
Men with prostate cancer
|
Prostate-cancer free men in the comparison cohort |
||||||
|---|---|---|---|---|---|---|---|---|
| RR | (95% CI) | Number of events |
Person-years at risk |
Number of events per 1000 py |
Number of events |
Person-years at risk |
Number of events per 1000 py |
|
|
|
|
|
||||||
| Men with prostate cancer compared to prostate-cancer free men in the same category in respect to ever/never users | ||||||||
| Ever users | ||||||||
| Prostate-cancer free men in the comparison cohort | 1.0 | |||||||
| Men with prostate cancer | 1.9 | (0.94–3.7) | 11 | 12628 | 0.87 | 32 | 70225 | 0.46 |
| Never users | ||||||||
| Prostate-cancer free men in the comparison cohort | 1.0 | |||||||
| Men with prostate cancer | 2.7 | (1.6–4.7) | 19 | 50863 | 0.37 | 36 | 258357 | 0.14 |
Inclusion 2007–2009, follow-up until end of 2010. Ever users are those on drugs (N05A, N05B, N05C, N06A) within 18 months before inclusion.
Risk of suicide by socioeconomic status and comorbidity burden
The same relationship was seen for cases with high socioeconomic status (SES) and low comorbidity (CCI), i.e. a lower absolute increase of suicide than cases with low SES and high CCI, but a higher increase in relative risk (Table 1). The risk of suicide among men with prostate cancer was increased during the first six months among men with high (RR 8.1, 95% CI 3.7–18), and low socioeconomic status (RR 5.4, 95% CI 2.9–10) and severe comorbidity (RR 6.0, 95% CI 2.4–15) and no recorded comorbidity (RR 11, 95% CI 5.7–22), compared with men in the comparison cohort with the same socioeconomic status and comorbidity index (Table 3).
Discussion
In this population-based, nation-wide cohort study of prostate cancer in Sweden, we found an increased risk of suicide following a prostate cancer diagnosis. The relative risk was highest during the first six months after diagnosis among all prostate cancer cases. The risk increased with more advanced disease from a five-fold increase among men with low-risk disease to a ten-fold increase among men with metastatic disease.
Risk estimates were stratified on comorbidity, socioeconomic status and previous psychiatric morbidity and we found an increased risk of suicide among prostate cancer cases with and without comorbidity, with low and high socioeconomic status and with and without previous psychiatric morbidity. Although we cannot exclude confounding by unknown factors we consider it highly unlikely that an unknown confounder would have resulted in a peak of suicides during the first 6 months after diagnosis and thereafter a gradual decline.
There was an increased risk of suicide also among men with low-risk tumors most often diagnosed as a result of opportunistic screening. Given that there has been a considerable uptake of PSA-testing with ensuing increase in incidence of low-risk prostate cancer in Europe and many other countries in the Western world our findings are relevant outside of Sweden. For example, in the Nordic countries of Finland, Sweden, Iceland, and Norway, prostate cancer incidence rates are amongst the highest in Europe.(19)
Our interpretation of these findings has clear clinical relevance. First, there was an increased risk of suicide also among men with low-risk tumors following early detection. Our data thus underline the importance of an awareness of the increased suicide risk among men with newly diagnosed prostate cancer – in all risk categories. It has been estimated that more than one third of Swedish men aged 50–75 years underwent PSA-testing between 2000 and 2007(13, 20) even though no systematic PSA-screening has been, or is, in operation(21) (apart from in a screening trial in Göteborg(22)). Given that many low-risk cancers would not have been diagnosed if PSA-testing had not been performed, an increase in suicide risk is a concern. Vigilance concerning suicide risk and appropriate counseling and treatment when indicated is therefore important in the management of men with newly diagnosed prostate cancer.(23)
Secondly, although repeated PSA-screening decreased prostate cancer mortality in two large well-designed randomized screening trials(22, 24), the balance between the harms and benefits remains debated. Our current study adds some further data to be included in the trade-off calculation. Whereas the observation of a substantially increased relative risk of suicide among all prostate cancer patients warrants caution as discussed above, this potential harm of PSA-testing was low in absolute terms (approximately 1 extra suicide per 2000 person-years). Further, PSA-testing may speculatively affect suicide risk in two opposite ways; increasing the number low-risk prostate cancers with modestly increased risk of suicide, but at the same time reducing the risk of metastatic disease(22, 24, 25), which is associated with a larger increase in risk(3, 4).
Fang and colleagues recently reported that a cancer diagnosis increases risk of suicide across all tumor stages: regional, localized and metastasized prostate cancer.(3) Our findings in a data set with higher precision in tumor and patient characterization corroborate these results.
In a previous publication, based on data from the PCBaSe linkage 1.0 including 77,439 men diagnosed with prostate cancer between 1997 to 2006 with 128 suicides, we found no increased risk of suicide among men diagnosed with T1c tumors, whereas the risk was twice as high among men with locally advanced and metastatic disease compared to an age-matched background population.(4) In our present study, we compared the relative risk of men with prostate cancer with that of prostate cancer- free men matched for age and residency with information on socioeconomic status and comorbidity. Therefore, with an increased number of events (n=211) and additional data on cases and comparison men, we were able to assess the risk of suicide with increased precision also allowing for stratification for time after diagnosis.
Regarding treatment, there was an increased risk of suicide within the first 6 months for all treatment categories. This risk was lower for men managed with expectancy or active surveillance in comparison with men receiving curative or palliative treatment. This finding is line with findings from a study where men on active surveillance reported lower short-term levels of anxiety and distress as compared to data in the literature for men on other treatments.(26)
Conclusions
Men diagnosed with prostate cancer, also low-risk prostate cancer, had an increased risk of suicide, especially shortly after diagnosis. Our data underline the importance of an awareness of the risk of suicide among men with newly diagnosed prostate cancer.
Acknowledgments
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chairman), Anders Widmark, Lars Egevad, Magnus Törnblom, Stefan Carlsson, Jan Adolfsson, Anna Bill-Axelson, Jan-Erik Johanssson, Ove Andrén, Mats Lambe, Erik Holmberg, David Robinson, Bill Pettersson, Jonas Hugosson, Jan-Erik Damber, Ola Bratt, Göran Ahlgren, Karin Hellström, and Maria Nyberg.
Funding
This study was supported by funding from Swedish Research Council 825-2010-5950, the Swedish Cancer Society and the Prostate Cancer Foundation. S.C. is supported by grants from the Swedish Cancer Society, the Sweden America Foundation, the Swedish Council for Working Life and Social Research and the Swedish Society for Medical Research.
Footnotes
Conflict of interest
None of the co-authors have any conflict of interest to declare.
Ethics/human subjects statement
The study was approved by the Umeå Research Ethics committe Umeå university dnr 2010 234 31M
Author contributions
A.B-A. and P.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. P.S. created and financed the database, conceived statistical analyses together with A.B.A. and F.S., participated in drafting and writing of manuscript.
S.C. performed the literature search, contributed to the concept of the study, to the analysis and interpretation of data and important intellectual revisions, drafted and coordinated the revisions and finalized the manuscript.
J.A. and K.F. contributed to the data analysis and interpretation of data and in the manuscript writing.
F.S. and M.L. carried out the statistical analyses, interpreted the results and participated in writing and revising the manuscript.
All authors approved the final version of the manuscript to be published.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of where and when the study has been presented in part elsewhere: Oct 4, 2012, annual meeting of Swedish Urology Association
References
- 1.Hugosson J, Aus G, Lilja H, Lodding P, Pihl CG, Pileblad EA. Prostate specific antigen based biennial screening is sufficient to detect almost all prostate cancers while still curable. J Urol. 2003;169(5):1720–3. doi: 10.1097/01.ju.0000061183.43229.2e. [DOI] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Garmo H, Nyberg U, Lambe M, Bratt O, Stattin P, et al. Psychiatric treatment in men with prostate cancer--results from a Nation-wide, population-based cohort study from PCBaSe Sweden. Eur J Cancer. 2011;47(14):2195–201. doi: 10.1016/j.ejca.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Fang F, Keating NL, Mucci LA, Adami HO, Stampfer MJ, Valdimarsdottir U, et al. Immediate risk of suicide and cardiovascular death after a prostate cancer diagnosis: cohort study in the United States. J Natl Cancer Inst. 2010;102(5):307–14. doi: 10.1093/jnci/djp537. [DOI] [PubMed] [Google Scholar]
- 4.Bill-Axelson A, Garmo H, Lambe M, Bratt O, Adolfsson J, Nyberg U, et al. Suicide risk in men with prostate-specific antigen-detected early prostate cancer: a nationwide population-based cohort study from PCBaSe Sweden. Eur Urol. 2010;57(3):390–5. doi: 10.1016/j.eururo.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 5.Fall K, Fang F, Mucci LA, Ye W, Andren O, Johansson JE, et al. Immediate risk for cardiovascular events and suicide following a prostate cancer diagnosis: prospective cohort study. PLoS Med. 2009;6(12):e1000197. doi: 10.1371/journal.pmed.1000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang F, Fall K, Mittleman MA, Sparen P, Ye W, Adami HO, et al. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med. 2012;366(14):1310–8. doi: 10.1056/NEJMoa1110307. [DOI] [PubMed] [Google Scholar]
- 7.Llorente MD, Burke M, Gregory GR, Bosworth HB, Grambow SC, Horner RD, et al. Prostate cancer: a significant risk factor for late-life suicide. Am J Geriatr Psychiatry. 2005;13(3):195–201. doi: 10.1176/appi.ajgp.13.3.195. [DOI] [PubMed] [Google Scholar]
- 8.Yousaf U, Christensen ML, Engholm G, Storm HH. Suicides among Danish cancer patients 1971–1999. Br J Cancer. 2005;92(6):995–1000. doi: 10.1038/sj.bjc.6602424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohler J, Bahnson RR, Boston B, Busby JE, D’Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8(2):162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 10.Stattin P, Holmberg E, Johansson JE, Holmberg L, Adolfsson J, Hugosson J. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst. 2010;102(13):950–8. doi: 10.1093/jnci/djq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rider JR, Sandin F, Andren O, Wiklund P, Hugosson J, Stattin P. Long-term Outcomes Among Noncuratively Treated Men According to Prostate Cancer Risk Category in a Nationwide, Population-based Study. Eur Urol. 2012 doi: 10.1016/j.eururo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178(3 Pt 2):S14–9. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bratt O, Berglund A, Adolfsson J, Johansson JE, Tornblom M, Stattin P. Prostate cancer diagnosed after prostate-specific antigen testing of men without clinical signs of the disease: a population-based study from the National Prostate Cancer Register of Sweden. Scand J Urol Nephrol. 2010;44(6):384–90. doi: 10.3109/00365599.2010.498793. [DOI] [PubMed] [Google Scholar]
- 14.Van Hemelrijck M, Wigertz A, Sandin F, Garmo H, Hellstrom K, Fransson P, et al. Cohort Profile: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base Sweden 2.0. Int J Epidemiol. 2012 doi: 10.1093/ije/dys068. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Farmaceutiska Specialiteter i Sverige
- 17.Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata Journal. 2009;9:265–90. [Google Scholar]
- 18.R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: R Development Core Team; 2011. [Google Scholar]
- 19.Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46(17):3040–52. doi: 10.1016/j.ejca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson H, Holmstrom B, Duffy SW, Stattin P. Uptake of prostate-specific antigen testing for early prostate cancer detection in Sweden. Int J Cancer. 2010 doi: 10.1002/ijc.25846. [DOI] [PubMed] [Google Scholar]
- 21.Stattin P, Garmo H, Steineck G, Bill-Axelson A. Re: Immediate risk of suicide and cardiovascular death after a prostate cancer diagnosis: cohort study in the United States. J Natl Cancer Inst. 2010;102(18):1447–8. doi: 10.1093/jnci/djq307. author reply 1448. [DOI] [PubMed] [Google Scholar]
- 22.Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, et al. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11(8):725–32. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anguiano L, Mayer DK, Piven ML, Rosenstein D. A Literature Review of Suicide in Cancer Patients. Cancer Nurs. 2011 doi: 10.1097/NCC.0b013e31822fc76c. [DOI] [PubMed] [Google Scholar]
- 24.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aus G, Bergdahl S, Lodding P, Lilja H, Hugosson J. Prostate cancer screening decreases the absolute risk of being diagnosed with advanced prostate cancer--results from a prospective, population-based randomized controlled trial. Eur Urol. 2007;51(3):659–64. doi: 10.1016/j.eururo.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 26.van den Bergh RC, Essink-Bot ML, Roobol MJ, Wolters T, Schroder FH, Bangma CH, et al. Anxiety and distress during active surveillance for early prostate cancer. Cancer. 2009;115(17):3868–78. doi: 10.1002/cncr.24446. [DOI] [PubMed] [Google Scholar]