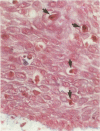
Abstract
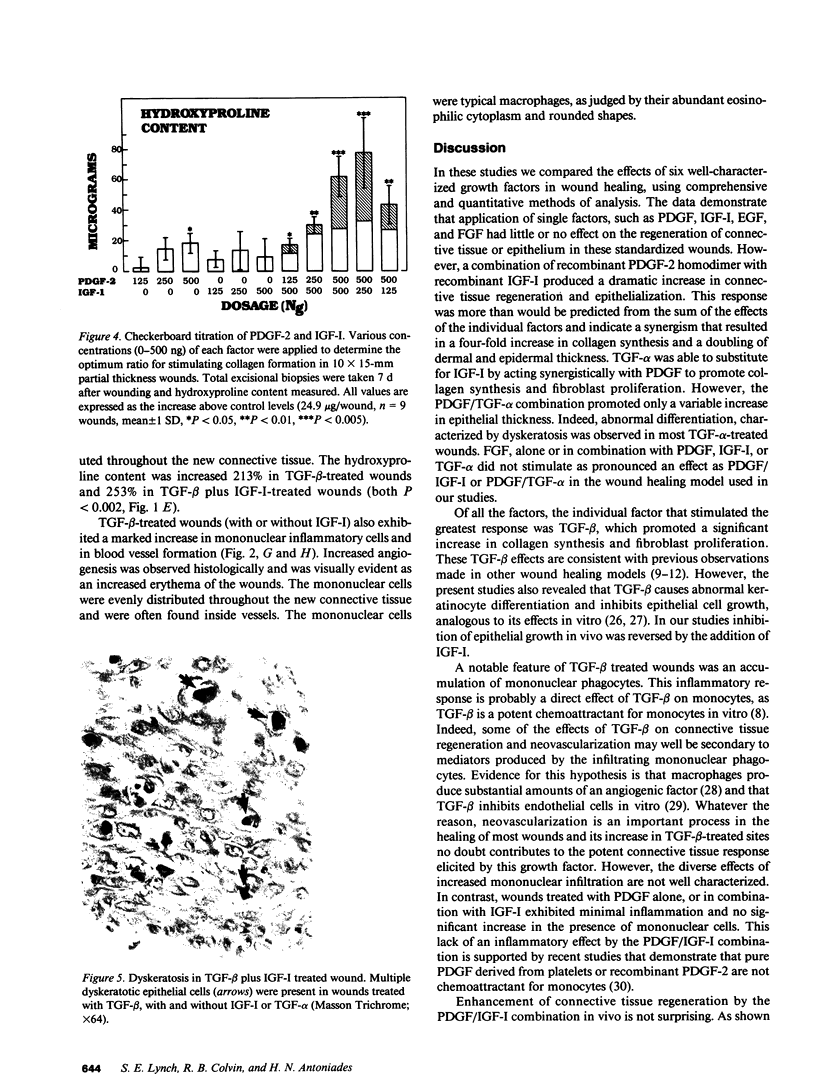
Several growth factors are potential mediators of wound healing, although their actual roles, interactions, and therapeutic use are not established. Six well-characterized human growth factors were chosen for detailed investigation by topical application to standardized skin wounds in swine: epidermal growth factor (EGF), transforming growth factors alpha and beta (TGF-alpha and TGF-beta), fibroblast growth factor (FGF), insulin-like growth factor-I (IGF-I), and platelet-derived growth factor (PDGF). When applied singly in doses up to 1,500 ng, only TGF-beta produced a marked tissue response, as demonstrated by an increase in the new connective tissue volume, the collagen content and maturity, and increased angiogenesis. However, TGF-beta enhanced inflammation and caused abnormal epithelial differentiation and decreased epithelial volume, the last reversed by addition of IGF-I. Recombinant PDGF-2 homodimer, if given in combination with recombinant IGF-I, caused a similar increase in the new connective tissue volume and collagen content and maturity, but without increased inflammation. In addition, this combination stimulated increased amounts of epithelium with normal differentiation. The synergy of PDGF-2 and IGF-I was optimal at a ratio of 2:1 by weight. Of the six individual factors and nine combinations tested, the combinations of PDGF-2 and IGF-I or PDGF-2 and TGF-alpha were the most potent stimulators of healing in the absence of increased inflammation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Sporn M. B. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986 Apr;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightwell J. R., Riddle S. L., Eiferman R. A., Valenzuela P., Barr P. J., Merryweather J. P., Schultz G. S. Biosynthetic human EGF accelerates healing of Neodecadron-treated primate corneas. Invest Ophthalmol Vis Sci. 1985 Jan;26(1):105–110. [PubMed] [Google Scholar]
- Brown G. L., Curtsinger L., 3rd, Brightwell J. R., Ackerman D. M., Tobin G. R., Polk H. C., Jr, George-Nascimento C., Valenzuela P., Schultz G. S. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986 May 1;163(5):1319–1324. doi: 10.1084/jem.163.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley A., Davidson J. M., Kamerath C. D., Wolt T. B., Woodward S. C. Sustained release of epidermal growth factor accelerates wound repair. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7340–7344. doi: 10.1073/pnas.82.21.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Sipes N. J., Bascom C. C., Graves-Deal R., Pennington C. Y., Weissman B. E., Moses H. L. Growth modulation of mouse keratinocytes by transforming growth factors. Cancer Res. 1988 Mar 15;48(6):1596–1602. [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Schweigerer L., Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987 May;8(2):95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Fibroblast growth factor. Mol Cell Endocrinol. 1986 Aug;46(3):187–204. doi: 10.1016/0303-7207(86)90001-8. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Grotendorst G. R., Antoniades H. N., Schwartz C. J., Valente A. J. Platelet-derived growth factor is not chemotactic for human peripheral blood monocytes. Exp Cell Res. 1989 Feb;180(2):497–503. doi: 10.1016/0014-4827(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Höckel M., Sasse J., Wissler J. H. Purified monocyte-derived angiogenic substance (angiotropin) stimulates migration, phenotypic changes, and "tube formation" but not proliferation of capillary endothelial cells in vitro. J Cell Physiol. 1987 Oct;133(1):1–13. doi: 10.1002/jcp.1041330102. [DOI] [PubMed] [Google Scholar]
- Lawrence W. T., Norton J. A., Sporn M. B., Gorschboth C., Grotendorst G. R. The reversal of an Adriamycin induced healing impairment with chemoattractants and growth factors. Ann Surg. 1986 Feb;203(2):142–147. doi: 10.1097/00000658-198602000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Van Wyk J. J., O'Keefe E. J., Pledger W. J. Epidermal growth factor (EGF) is required only during the traverse of early G1 in PDGF stimulated density-arrested BALB/c-3T3 cells. Exp Cell Res. 1983 Aug;147(1):202–208. doi: 10.1016/0014-4827(83)90285-9. [DOI] [PubMed] [Google Scholar]
- Lynch S. E., Nixon J. C., Colvin R. B., Antoniades H. N. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7696–7700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Müller G., Behrens J., Nussbaumer U., Böhlen P., Birchmeier W. Inhibitory action of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5600–5604. doi: 10.1073/pnas.84.16.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niall M., Ryan G. B., O'Brien B. M. The effect of epidermal growth factor on wound healing in mice. J Surg Res. 1982 Aug;33(2):164–169. doi: 10.1016/0022-4804(82)90024-5. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Marquardt H., Todaro G. J., Gallis B., Casnellie J. E., Bornstein P., Krebs E. G. Transforming growth factor and epidermal growth factor stimulate the phosphorylation of a synthetic, tyrosine-containing peptide in a similar manner. J Biol Chem. 1982 Dec 25;257(24):14628–14631. [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Platelet-derived growth factor. Annu Rev Med. 1987;38:71–79. doi: 10.1146/annurev.me.38.020187.000443. [DOI] [PubMed] [Google Scholar]
- Schultz G. S., White M., Mitchell R., Brown G., Lynch J., Twardzik D. R., Todaro G. J. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987 Jan 16;235(4786):350–352. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Pittelkow M. R., Wille J. J., Jr, Scott R. E., Moses H. L. Reversible inhibition of normal human prokeratinocyte proliferation by type beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986 Apr;46(4 Pt 2):2068–2071. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Shull J. H., Smith J. M., Ward J. M., Sodek J. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science. 1983 Mar 18;219(4590):1329–1331. doi: 10.1126/science.6572416. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer B. R., Summer G. K. Improved method for hydroxyproline analysis in tissue hydrolyzates. Anal Biochem. 1971 Feb;39(2):487–491. doi: 10.1016/0003-2697(71)90438-6. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]