ABSTRACT
In China, both inactivated hepatitis A (HA) vaccine and live attenuated HA vaccine are available. We conducted a trial to evaluate 5-year immune persistence induced by one dose of inactivated or live attenuated HA vaccines in children. Subjects with no HA vaccination history had randomly received one dose of inactivated or live attenuated HA vaccine at 18–60 months of age. Anti-HAV antibody concentrations were measured before vaccination and at the first, second, and fifth year after vaccination. Suspected cases of hepatitis A were monitored during the study period. A total of 332 subjects were enrolled and 182 provided evaluable serum samples at all planned time points. seropositive rate at 5 y was 85.9% in the inactivated HA vaccine group and 90.7% in the live attenuated HA vaccine group. GMCs were 76.3% mIU/ml (95% CI: 61.7 – 94.4) and 66.8mIU/ml (95% CI: 57.8 – 77.3), respectively. No significant difference in antibody persistence between 2 groups was found. No clinical hepatitis A case was reported. A single dose of an inactivated or live attenuated HA vaccine at 18–60 months of age resulted in high HAV seropositive rate and anti-HAV antibody concentrations that lasted for at least 5 y.
KEYWORDS: children, inactivated hepatitis A vaccine, live attenuated hepatitis A vaccine, persistence
Introduction
Hepatitis A (HA) is a global public health issue. However, hepatitis A virus (HAV) infection and disease could be prevented by early immunization with inactivated or live-attenuated hepatitis A vaccines. In 2008, hepatitis A immunization was recommended for national routine childhood immunization program in China. Due to changing socioeconomic conditions and increasing HA vaccine use, the annual national incidence rate of hepatitis A in China was reported to decline from 52.6/100 000 in 1990 to 5.9/100 000 in 2007,1 and then showed another 72.1% decline from 2007 to 2013.2
Live attenuated HA vaccine is given as a single dose to children older than 18 months, and inactivated HA vaccine is given in a 2-dose schedule with an interval of 6–12 months to children aged over 1 y. Previously published immunogenicity studies indicated that one dose of inactivated HA vaccines induced enough immune response and provided protection for children and adults against infection.3 A single dose of the vaccine is known to control outbreaks of infection and to protect individuals living in regions at risk of repeated outbreaks. In Argentina, a single dose schedule of inactivated HA vaccine for children 12 months of age was added to the national schedule since 2005. By 2010, with vaccination coverage of 95%, the incidence of HAV disease was 10.2 cases per 100,000, representing a decrease of 88% compared with the average incidence between 1998 and 2001.4 Therefore, WHO position paper on hepatitis A vaccines in 2012 suggested that national immunization programs may consider inclusion of single-dose inactivated hepatitis A vaccines in immunization schedules.5 This option seems to be comparable in terms of effectiveness, and is less expensive and easier to implement than the classical 2-dose schedule. Nevertheless, a request is made for further experience and evidence of long-term protection achieved by the single-dose use.
One study in Nanchang, China had shown that 1-dose inactivated HA vaccine in young adults could maintain high immunogenicity for at least 3 y with seropositive rate of 93% and GMC of 93.87 mIU/mL.6 To provide more evidence for the development of HA vaccine immunization strategy, this study was designed to determine the 5-year persistence of antibodies to HAV in Chinese children after one dose of Chinese domestic live attenuated vaccine (Biovac™) or inactivated vaccine (Healive®).
Results
Participants
A total of 332 subjects participated in the study and received one dose of inactivated or live attenuated HA vaccines in October 2008. Of these, 63 subjects were excluded due to protocol deviation (2 aged under 18 months old, 9 with non-compliance to the blood sampling schedule, 38 with prior hepatitis A vaccination and 14 with anti-HAV positivity before vaccination).
The long-term follow-up ended in December 2013. A total of 256, 225, and 220 subjects from both groups returned for the follow-up visits at year 1, 2 and 5, respectively. The reasons for the dropout were lost to follow-up or moving away. According to protocol compliance, 182 subjects were included in the modified PP data set. The reasons for exclusion of subjects from the PP data set at year 5 were: non-compliance to the blood sampling schedule (n = 64) and laboratory test failure due to insufficient serum sample (n = 2) (Fig. 1).
Figure 1.
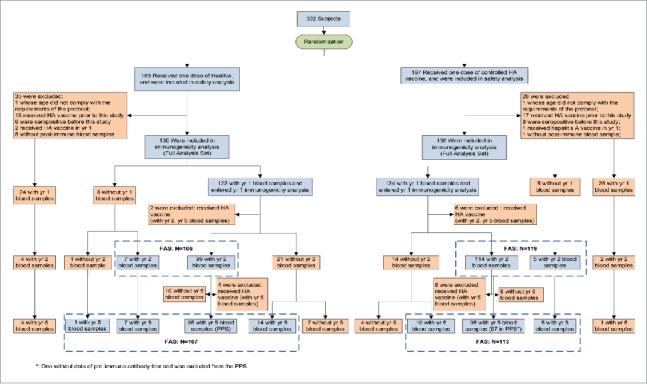
Flow chart of different analysis data sets reflecting the follow-up of the subjects in the trial.
The demographic characteristics were similar in both groups at baseline and 5 y (Table 1).
Table 1.
Characteristics of study participants at baseline and 5 year.
| At baseline |
At 5 year |
|||||
|---|---|---|---|---|---|---|
| Inactivated HA | Attenuated HA | P value | Inactivated HA | Attenuated HA | P value | |
| N | 85 | 97 | 85 | 97 | ||
| Age, month | ||||||
| Mean ± SD | 34.7 ± 4.4 | 35.6 ± 5.4 | 0.468 | 96.3 ± 8.5 | 97.4 ± 8.4 | 0.392 |
| Height, cm | ||||||
| Mean ± SD | 95.4 ± 6.6 | 96.5 ± 8.6 | 0.344 | 130.1 ± 6.7 | 131.6 ± 8.1 | 0.189 |
| Weight, kg | ||||||
| Mean ± SD | 14.9 ± 2.9 | 15.6 ± 3.0 | 0.072 | 29.1 ± 6.4 | 31.2 ± 7.8 | 0.050 |
Antibody persistence
Seropositive rates in both groups remained high within 5 y after the vaccination, with 95.3% (95% CI: 87.7 – 99.0) in Group A and 91.8% (95% CI: 83.9 – 96.4) in Group B at year 1 (P > 0.05), 90.6% (95% CI: 81.8 – 95.9) and 90.7% (95% CI: 82.7 – 95.7) at year 2 (P > 0.05), 85.9% (95% CI: 76.2–92.5) and 90.7% (95% CI: 82.7 – 95.7) at year 5 (P > 0.05), respectively. From 1 to 5 y after the vaccination, GMCs in Group A changed slightly from 92.1 mIU/ml (95% CI: 74.1 – 114.4) to 76.3 mIU/ml (95% CI: 61.7 – 94.4); while GMC in Group B increased moderately from 62.7mIU/ml (95% CI: 52.6 – 74.6) to 66.8mIU/ml (95% CI: 57.8 – 77.3). The difference in GMCs between both groups showed no statistical significance at year 2 and 5 (P > 0.05), except at year 1 (P = 0.007) (Table 2, Fig. 2).
Table 2.
Five-year antibody persistence of 1 dose inactivated or attenuated hepatitis A vaccine.
| Time-point | Parameter | Inactivated HA N = 85 | Attenuated HA N = 97 | P value |
|---|---|---|---|---|
| Baseline | GMC, (95% CI) | 2.6 (2.5∼2.7) | 2.5 (2.5∼2.6) | 0.468 |
| 1 yr | GMC, (95% CI) | 92.1 (74.1∼114.4) | 62.7 (52.6∼74.6) | 0.007 |
| Seropositive %, (95% CI) | 95.3 (87.7∼99.0) | 91.8 (83.9∼96.4) | 0.337 | |
| 2 yr | GMC, (95% CI) | 86.0 (68.9∼107.3) | 69.1 (58.8∼81.2) | 0.115 |
| Seropositive %, (95% CI) | 90.6 (81.8∼95.9) | 90.7 (82.7∼95.7) | 0.975 | |
| 5 yr | GMC, (95% CI) | 76.3(61.7∼94.4) | 66.8 (57.8∼77.3) | 0.307 |
| Seropositive %, (95% CI) | 85.9 (76.2∼92.5) | 90.7 (82.7∼95.7) | 0.308 |
Figure 2.

Five-year change of immune response in inactivated or attenuated hepatitis A vaccine group.
Immune memory induced by boosting dose
Among all subjects receiving at least one dose of HA vaccine, 33 subjects (12 in Group A and 21 Group B) received an additional injection of HA vaccine between year 1 and year 5 out of personal reasons. From year 1 to 5, seropositive rates of Group A increased from 83.3% (10/12) to 100.0% (12/12) and that of Group B increased from 52.4% (11/21) to 95.2% (20/21); GMCs increased significantly from 45.8 mIU/ml (95% CI: 23.5 – 89.5) to 911.4 mIU/ml (95% CI: 449.5 – 1847.7) in Group A and from 39.0 mIU/ml (95% CI: 21.8 – 69.9) to 955.1 mIU/ml (95% CI: 507.7 – 1796.8) in Group B.
Clinical investigation of disease status
No obvious clinical infections were reported in any of the subjects, and no hepatitis A infections were reported in their communities or schools.7
Safety
No statistical difference of incidence of adverse events was observed between the 2 groups (P = 0.209), with 9.7%(16/165) in inactivated HA group and 6.0%(10/167) in attenuated HA group. 73.1%(19/26) of the AEs were mild, and fever was the most common reported AE with an incidence of 7.3% and 6.0% respectively. Other symptoms included allergic reaction, headache, abdominal pain and diarrhea.
Discussion
Healive® developed by Sinovac and introduced in 2002, is the first Chinese domestic inactivated hepatitis A vaccine. Over 10 y since its licensure, approximately 20 million Chinese received more than 35 million doses of Healive® and the vaccine has outstanding safety profile. Post-marketing clinical studies demonstrated that the vaccine induced equal or similar immunogenicity with internationally used inactivated hepatitis A vaccines and were interchangeable for the course of HAV immunization in children.8 Healive® also provided lasting protection in healthy individuals with 2-dose schedule and was effective in curbing outbreaks of hepatitis A with one single dose.9
China is one of the world's few countries, in which both inactivated HA vaccine and live attenuated hepatitis A vaccine are used. Due to the benefits of low cost, a single dose of injection, high protection, and increased duration of immunity through natural boosting of subclinical infection,10 27 of the 31 provinces of China enroll the live vaccine into their routine childhood immunization program. The major disadvantages are substantial horizontal transmission due to vaccine virus shedding and the theoretically possible reverse mutation of the live vaccine strains.11,12 In addition, the live vaccines do not provide post-exposure protection because of slow immune response to the vaccines and cannot be used for emergency immunization during control of outbreaks.9,10 Hence, for safety concern, the remaining 4 rich municipalities/provinces (Beijing, Tianjin, Shanghai, Jiangsu) purchased the more expensive, inactivated HAV vaccines for the routine childhood immunization program. Barriers to the introduction of universal inactivated HA vaccine immunization of children include higher vaccine prices and 2-dose schedule with availability of simpler one-dose attenuated HA vaccine schedule.
In recent years, several clinical trials compared the immunogenicity of Healive® and live attenuated hepatitis A vaccines after one single vaccination, which suggested that both vaccines were similarly immunogenic in children. In a double-blind, randomized trial which compared them in children in terms of early antibody response, seroconversion rates of anti-HAV antibody were 25% and 35% at 7 day for a live attenuated HA vaccine and Healive®, respectively; 90% and 93%, respectively, at 14 day; 98% and 100%, respectively, at 28 day, with no significant difference(p > 0.05).13 And, GMC in recipients of live attenuated and inactivated HA vaccines were 6.3 and 7.9 mIU/ml, respectively, at 7 day; 42.6 and 73.3 mIU/ml, respectively, at 14 day; 46.8 and 71.3 mIU/ml, respectively, at 28 day. Similarly, both vaccines induced equal immunogenicity in young adults.14 Another study compared the one-year antibody persistency between Healive® and 3 kinds of live attenuated vaccines in children.15 At 12 month, no significant differences were observed in seroconversion rates among the 4 groups(range: 92.8% – 96.6%; P> 0.05); however, the GMC of Healive® was lower than that in the attenuated vaccine B and C groups (112.7 vs. 203.3, 212.8mIU/ml, p < 0.05) and similar to the attenuated vaccine A group (112.7 vs. 135.8 mIU/ml, P > 0.05). And all the above studies showed similar safety profile between Healive® and live attenuated HA vaccine.
The present study first reported that Healive® and the live attenuated HA vaccine showed similar 5-year antibody persistence in healthy children after one single dose. There was no significant difference in seroconversion rates and GMC between the 2 groups at 1, 2, and 5 y except GMC at 1 y. Compared to the historical clinical data of Healive® with a 2-dose schedule, although both the seroconversion rate and GMC of a one-dose regimen was lower than that following 2 doses vaccinations, the 85.9% of seroconversion rate and 76.3 mIU/mL were still acceptable.9 However, one study in Indonesia revealed that 1-dose schedule performed much better in terms of health economics, with an incremental cost-effectiveness ratio of $4,933 per quality adjusted life years(QALY), compared with $14,568 per QALY for 2-dose schedule.16
Studies assessing immune persistence achieved by a single dose of inactivated hepatitis A vaccine are limited.3 The major objective of those studies was to assess the response to a booster dose several years after the first dose, mostly among healthy adult travelers. There is an on-going study among children in Argentina, which was designed to determine the 5-year antibody persistence in children at 12–23 months of age following one dose of an inactivated HA vaccine. The interim results indicated that seropositive rate (anti-HAV ≥ 10 mIU/mL) was 99.7% and 93%, and GMC was 170.2 and 97.96 mIU/mL at 3 year, 4 y respectively.17,18 The evidence found on the longest time interval suggests that protective anti-HAV antibody levels after a single dose of inactivated hepatitis A vaccine could persist for almost 11 y and increase or reappear after booster vaccination. Furthermore, one study modeling the persistence post 2-dose Healive® showed that the seroconversion rate was 45.3% at 25 years(to be published), which means the SR at 25 y for one-dose schedule would not be satisfactory, however, whether a booster dose is needed for seronegative adults like travelers still need more evidences.
During the study there were 21 subjects whose anti-HAV antibody level increased abnormally between 2 consecutive measurements. An abnormal increase was defined as at least 2-fold increase in antibody concentration when the concentration was ≥ 100 mIU/ml or at least 4-fold increase when the concentration was <100 mIU/ml at the reference time point. To our knowledge, the increase was most likely due to natural exposure or undocumented additional hepatitis A vaccination. To account for the booster effect, additional immunogenicity analysis was performed on data from modified PP cohort, in which the 21 subjects were excluded, and the results did not change significantly in terms of the 5-year antibody persistence.
There were a few limitations to our study. Firstly, because most hepatitis A infections in children are asymptomatic, it was difficult to screen the infected and/or subclinical persons during the follow-up period. To minimize the impact, the study was designed to be conducted in Tianjin city with low epidemic, in which the incidence of hepatitis A was below 0.5 cases per one hundred thousand since 2008 and the immunization coverage of HA vaccine in children was approximately 70% – 90% in 20077. Furthermore, subjects whose anti-HAV antibody levels increased abnormally between 2 consecutive follow-up measurements were excluded out of the modified PP cohort.19 Nevertheless, GMC of the live attenuated vaccine group in the modified PP cohort at year 2 increased abnormally, with the increase rate of 23% compared with GMC at year 1. Secondly, hepatitis A immunization was included into Expanded Program on Immunization (EPI) in China since 2008, so the inclusion criteria of subjects was expanded to children with no more than 5 y old and no HAV vaccination history. Due to small sample size, the data for immune persistency could not be analyzed according to age stratification.
In conclusion, a single dose of a domestic inactivated HA vaccine at 18–60 months of age resulted in high HAV seropositive rate that lasted for at least 5 y. And anti-HAV antibody concentrations in the vaccinees during 5 y of follow-up remained far higher than the seropositive threshold of 20 mIU/mL. Further long-term evaluation is required to confirm the protective effectiveness of a single vaccination of the vaccine.
Methods
Study design and subjects
Beginning in 2008, this randomized, double-blind, controlled clinical trial was conducted in Tianjin municipality, China. Healthy children aged 18 – 60 months, with pre-vaccination anti-HAV antibody titers <20 mIU/ml, were randomized into 2 groups at 1:1 ratio, to receive either one dose of inactivated HA vaccine (Containing 250 unit hepatitis A antigen, Healive®, Lot number: 20080309) manufactured by Sinovac Biotech Co. LTD., Beijing, China (Group A) or one dose of live attenuated HA vaccine (containing 6.50lgCCID50 hepatitis A antigen, Biovac™, Lot number: 20080302c) manufactured by Zhejiang Pukang Biotechnology Co. LTD., Zhejiang, China (Group B). These 2 groups were followed for a period of 5 years, with visits at year 1, 2, 5. The trial was registered with the ClinicalTrial.gov number NCT02002065.
Immunogenicity assessment
Blood samples were collected at pre-vaccination and post-vaccination at year 1, 2, 5. Anti-HAV antibody concentrations were measured using commercial microparticle enzyme immunoassay (MEIA, Abbott's AXSYM® HAVAB 2.0 quantification kit) at the Sinovac Biologicals' laboratory (cut-off value:≥ 5mIU/ml). The undetectable titer (< 5 mIU/ml) was recorded as 2.5 mIU/ml, and >20,000 mIU/ml was recorded as 20,000 mIU/ml. Seroconversion was defined as an antibody titer ≥ 20 mIU/ml.
Disease monitoring and safety assessment
At each follow-up time point, subjects were asked about their history of jaundice or symptoms of hepatitis related illness. Other hepatitis vaccinations during the follow-up period were also recorded. Any related serious adverse events(SAEs) were recorded based on retrospective reporting by subjects.
Statistical analyses
The primary analyses of immunogenicity for the long-term follow-up were performed on per-protocol(PP) data set, while the analyses for safety was performed on safety data set. PP data set was defined as all enrolled subjects who received the vaccine correctly, provided evaluable serum samples at all particular time points, and had no major protocol deviations. Subjects were excluded from the immunogenicity analyses if they had received any additional hepatitis vaccine other than the study vaccine or had hepatitis infection during the study period.
Anti-HAV (≥ 20mIU/ml) seropositive rates and geometric mean concentrations (GMCs) were tabulated with 95% confidence interval(95% CI). GMC values were calculated by taking anti-log of the mean of the log-titer transformations. We used Pearson's chi-square test or Fisher's exact test to compare seropositive rates when relevant. We used non-parametric test or t test to compare GMCs when relevant.
All statistical analyses were performed using SPSS version 13.0. A 2-tailed p-value <0.05 was considered significant.
Ethical Statements
All necessary approvals for the long-term follow-up of the study were obtained from the independent ethics committee of Tianjin Center for Disease Control and Prevention(CDC) and the study was conducted according to Good Clinical Practices and the Declaration of Helsinki. Before enrollment, written informed consent was obtained from each subject's parents or guardian. Considering the long time interval between the last 2 follow-up visits, additional approval by the local ethics committee was obtained before 5-year visit. Parents or guardians of all subjects provided written consent before participating in the last visit.
Disclosure of potential conflicts of interest
Yuansheng Hu, Yufei Song, Gang Zeng, Lifei Song, Jiangting Chen are employed by Sinovac. All other authors: no conflicts.
Acknowledgments
We would like to express our thanks to the staffs of Jixian county Center for Disease Control and Prevention for their efforts in the implementation on the study site.
Funding
This project was funded by Sinovac Biotech Co., LTD.
Trial registration: ClinicalTrial.gov identifier: NCT02002065
References
- [1].Cui F, Hadler SC, Zheng H, Wang F, Zhenhua W, Yuansheng H, Gong X, Chen Y, Liang X. Hepatitis A surveillance and vaccine use in China from 1990 through 2007. J Epidemiol 2009; 19:189-95; PMID: 19561383; https://doi.org/ 10.2188/jea.JE20080087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang FZ, Zheng H, Liu JH, Sun XJ, Miao N, Shen LP, Zhang GM, Cui FQ. [The coverage of hepatitis A vaccine among 2–29 year olds and the reporting incidence of hepatitis A in China, 2014]. Zhonghua Liu Xing Bing Xue Za Zhi 2016; 37:1099-104; PMID: 27539340 [DOI] [PubMed] [Google Scholar]
- [3].Ott JJ, Wiersma ST. Single-dose administration of inactivated hepatitis A vaccination in the context of hepatitis A vaccine recommendations. Int J Infect Dis 2013; 17:e939-44; PMID: 23791857; https://doi.org/ 10.1016/j.ijid.2013.04.012 [DOI] [PubMed] [Google Scholar]
- [4].Vacchino MN. Incidence of Hepatitis A in Argentina after vaccination. J Viral Hepat 2008; 15(Suppl 2):47-50; PMID: 18837834; https://doi.org/ 10.1111/j.1365-2893.2008.01029.x [DOI] [PubMed] [Google Scholar]
- [5].WHO position paper on hepatitis A vaccines - June 2012. Wkly Epidemiol Rec 2012; 87:261-76; PMID: 22905367 [PubMed] [Google Scholar]
- [6].Liu XE, Chen HY, Liao Z, Zhou Y, Wen H, Peng S, Liu Y, Li R, Li J, Zhuang H. Comparison of Immunogenicity Between Inactivated and Live Attenuated Hepatitis A Vaccines Among Young Adults: A 3-Year Follow-up Study. J Infect Dis 2015; 212:1232-6; PMID: 25969561; https://doi.org/ 10.1093/infdis/jiv213 [DOI] [PubMed] [Google Scholar]
- [7].Zhang ZL, Zhu XJ, Shan AL, Gao ZG, Zhang Y, Ding YX, Liu H, Wu WS, Liu Y, He HY, et al.. Effectiveness of 10-year vaccination (2001–2010) on Hepatitis A in Tianjin, China. Hum Vaccin Immunother 2014; 10:1008-12; PMID: 24503599; https://doi.org/ 10.4161/hv.27775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang ZL, Zhu XJ, Wang X, Liang M, Sun J, Liu Y, Gao ZG, Wu JY, Dong XJ, Liu RK, et al.. Interchangeability and tolerability of two inactivated hepatitis A vaccines in Chinese children. Vaccine 2012; 30:4028-33; PMID: 22537990; https://doi.org/ 10.1016/j.vaccine.2012.04.038 [DOI] [PubMed] [Google Scholar]
- [9].Wu JY, Liu Y, Chen JT, Xia M, Zhang XM. Review of 10 years of marketing experience with Chinese domestic inactivated hepatitis A vaccine Healive(R). Hum Vaccin Immunother 2012; 8:1836-44; PMID: 23032165; https://doi.org/ 10.4161/hv.21909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rao S, Mao JS, Motlekar S, Fangcheng Z, Kadhe G. A Review of Immunogenicity and Tolerability of Live Attenuated Hepatitis A Vaccine in Children. Hum Vaccin Immunother 2016; 12(12):3160-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cui F, Liang X, Wang F, Zheng H, Hutin YJ, Yang W. Development, production, and postmarketing surveillance of hepatitis A vaccines in China. J Epidemiol 2014; 24:169-77; PMID: 24681843; https://doi.org/ 10.2188/jea.JE20130022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu ZY, Wang XY. Live attenuated hepatitis A vaccines developed in China. Hum Vaccin Immunother 2014; 10:659-66; PMID: 24280971; https://doi.org/ 10.4161/hv.27124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zheng H, Chen Y, Wang F, Gong X, Wu Z, Miao N, Zhang X, Li H, Chen C, Hou X, et al.. Comparing live attenuated and inactivated hepatitis A vaccines: an immunogenicity study after one single dose. Vaccine 2011; 29:9098-103; PMID: 21875638; https://doi.org/ 10.1016/j.vaccine.2011.08.078 [DOI] [PubMed] [Google Scholar]
- [14].Liao Z, Liu X, Wang X, et al.. Immunogenicity and safety of inactivated and live attenuated hepatitis A vaccines in Chinese young adults. Chin J Viral Dis 2012; 4:310-4; https://doi.org/ 10.16505/j.2095-0136.2012.04.004 [DOI] [Google Scholar]
- [15].Liu XE, Wushouer F, Gou A, Kuerban M, Li X, Sun Y, Zhang J, Liu Y, Li J, Zhuang H. Comparison of immunogenicity between inactivated and live attenuated hepatitis A vaccines: a single-blind, randomized, parallel-group clinical trial among children in Xinjiang Uighur Autonomous Region, China. Hum Vaccin Immunother 2013; 9:1460-5; PMID: 23571173; https://doi.org/ 10.4161/hv.24366 [DOI] [PubMed] [Google Scholar]
- [16].Suwantika AA, Beutels P, Postma MJ. Cost-effectiveness of hepatitis A vaccination in Indonesia. Hum Vaccin Immunother 2014; 10:2342-9; PMID: 25424941; https://doi.org/ 10.4161/hv.29353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Espul C, Benedetti L, Cuello H, Houillon G, Rasuli A. Persistence of immunity from 1 year of age after one or two doses of hepatitis A vaccine given to children in Argentina. Hepat Med 2012; 4:53-60; PMID: 24367232; https://doi.org/ 10.2147/HMER.S33847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vizzotti C, Gonzalez J, Rearte A, Uruena A, Perez Carrega M, Calli R, Gentile A, Uboldi A, Ramonet M, Cañero-Velasco M, et al.. Single-Dose Universal Hepatitis A Immunization in Argentina: Low Viral Circulation and High Persistence of Protective Antibodies Up to 4 Years. J Pediatric Infect Dis Soc 2015; 4:e62-7; PMID: 26582885; https://doi.org/ 10.1093/jpids/piu068 [DOI] [PubMed] [Google Scholar]
- [19].Hens N, Habteab Ghebretinsae A, Hardt K, Van Damme P, Van Herck K. Model based estimates of long-term persistence of inactivated hepatitis A vaccine-induced antibodies in adults. Vaccine 2014; 32:1507-13; PMID: 24508042; https://doi.org/ 10.1016/j.vaccine.2013.10.088 [DOI] [PubMed] [Google Scholar]


