ABSTRACT
We report on the design of a phase I, non-randomized, open-label study of idiotypic DNA vaccination in patients with B-cell non-Hodgkin's lymphoma (ISRCTN31090206). The study uses DNA fusion gene vaccination encoding patient-specific single chain variable fragment, or idiotype, linked to an immunostimulatory sequence. Two types of immunostimulatory sequence are being explored: potato virus X coat protein and human chemokine MIP3α. Linear polyethylenimine with low molecular weight (8 kDa) is used as a synthetic vehicle for vaccine delivery. Humoral and T-cellular immune responses to vaccination will be measured by ELISA and ELISPOT, respectively. The primary study endpoints are safety, tolerability and immunogenicity of DNA-PEI vaccination.
KEYWORDS: cancer vaccine, DNA vaccine, idiotype, lymphoma, polyethylenimine
Introduction
Despite recent advances in chemotherapy, B-cell non-Hodgkin's lymphoma (NHL) remains an incurable disease with a slow progression and recurrent relapses. In 2014, the incidence of lymphoma in Belarus was 707 cases,1 of which approximately 80% were mature NHL, including diffuse large B-cell lymphoma (22 %), which has a median survival of 7–10 y from diagnosis, small lymphocytic lymphoma (16.7%) and follicular lymphoma (11.6%).2 Immunotherapy, including vaccination, could be used for the stabilization of first remission.
Immunoglobulin (Ig) on the surface of lymphoma cells is unique for each malignant clone. The Ig heavy and light chain variable regions form the idiotype (Id), which serves as a tumor-specific antigen and can be targeted by vaccination. Anti-Id vaccination was first used as a therapy in 1992 when Ron Levy and Larry Kwak from Stanford University immunized 9 patients with hybridoma-produced, tumor-specific Id and showed both immune and clinical responses to vaccination.3 Subsequently, several phase I and II clinical trials4-7 and one phase III trial8 have used hybridoma-produced or recombinant Id protein for vaccination and have demonstrated both immunogenicity and clinical benefit, while toxicity was low. Such clinical trials require the preparation of patient-specific vaccines and a significant drawback to the hybridoma approach is that of the complexity, long duration and high cost of custom made Id protein vaccines. Alternative methods of recombinant protein production include the expression of Id in insect cells using baculovirus,9 mouse lymphoma cell lines5 and plants.10,11
In recent decades, DNA vaccines have attracted considerable attention because of their simple preparation and flexibility. A DNA vaccine is a plasmid vector that encodes the antigen of interest under the control of a mammalian promoter.12,13 Clinical trials using DNA vaccination are rapidly increasing in number and diversity;14-16 currently, 1024 trials are registered on СlinicalTrials.gov, of which 229 target cancer. DNA vaccines have several significant advantages facilitating their clinical use: first, they can be easily produced in bacteria; second, DNA injection is safe and well tolerated with minimal side effects; third, bacterial DNA is able to stimulate the innate immune system through hypomethylated CpG sequences, thus providing an adjuvant effect;13 and finally, DNA vaccines offer a precise but flexible strategy for vaccine design, for example, immuno-enhancing sequences can be easily added through genetic manipulation to produce fusion genes that can increase the immunogenicity of tumor antigens. Several immune-enhancing sequences have been used previously, including fragment C of tetanus toxin (FrC),17 human chemokine genes,18 potato virus X coat protein (PVXCP)19 and E.coli heat labile toxin.20
The first trial of Id DNA vaccination was performed by Timmerman et al. in 2002,21 in which 2 plasmids, one encoding Id and the second the immunostimulatory gene GM-CSF, were used. Vaccine formulation was not wholly successful with only one of 12 patients developing an anti-idiotypic immune response. A second trial was performed in patients with myeloma in which the variable regions were assembled as a single chain variable fragment (scFv) fused to FrC (scFv-FrC). Intramuscular vaccination of 25 patients resulted in the generation of anti-FrC (72% of patients) and anti-Id (38% of patients) immune responses.13 These data have provided proof-of-principle for Id DNA vaccination, but indicate that immunogenicity requires further enhancement.
Polyethylenimine (PEI) is a cationic polymer commonly used as a transfection reagent in vitro22 and synthetic vehicle for transgene and DNA vaccine delivery in vivo.23-25 It has been shown that T-cell immune responses can be increased by 20-fold with DNA-PEI complexes compared with responses elicited by intramuscular injections with naked DNA.24 Enhancement of plasmid DNA immunogenicity with linear PEI has also been demonstrated in mouse models.26 In this study, we attempt to evaluate Id DNA vaccination, delivered as a DNA/PEI complex, in patients with NHL.
Objectives and study design
Most idiotypic vaccination clinical studies have been conducted in patients with follicular lymphoma, but positive benefit has also been shown for other types of lymphoma.27 Vaccination has clinical utility for lymphoma with slow tumor progression, where remission is expected to be maintined and partial or complete immune reconstitution after chemotherapy is likely to be achieved by 6 months to enable the development of immune responses following vaccination.
The study opened in April 2014 and remains on-going. This study will include a maximum of 30 patients with stage 3 or 4 of the disease with the following diagnoses and in whom partial or complete clinical remission has been achieved: follicular lymphoma; small lymphocytic lymphoma/chronic lymphocytic leukemia; mantle cell lymphoma; nodal marginal zone B cell lymphoma; MALT lymphoma; lymphoplasmacytic lymphoma; diffuse large B-cell lymphoma. Patient eligibility criteria are shown in Table 1. During vaccination, patients should avoid taking non-steroidal anti-inflammatory drugs or steroids; if a patient is forced to take them for more than 1 week for medical reasons, vaccination will cease and the patient will be excluded from further participation in the study.
Table 1.
Eligibility criteria.
| Inclusion criteria |
Exclusion criteria |
| 1. Surface immunoglobulin G or M isotype expression on tumor cells | 1. Pregnancy and lactation. |
| 2. Presence of tumor tissue biopsy before any treatment | 2. The presence of multiple primary cancer. |
| 3. The physical status scale ESOG 0 – 2. | 3. History of autoimmune diseases (except Hashimoto's thyroiditis); |
| 4. Life expectancy at least 24 months. | 4. Severe diseases, including proceeding with symptomatic, untreated inflammatory and infectious processes. |
| 5. The age of patients from 18 to 75 years. | 5. Social, economic or geographic circumstances, which can impede proper compliance with treatment protocols and follow-up. |
| 6. Adequate renal, hepatic, and bone marrow function | 6. Polysensitisation. |
| 7. Signed written informed consent. | 7. Positive tests for human immunodeficiency virus (HIV), hepatitis B or C. |
| 8. The patient's ability to carry out the instructions of the doctor-researcher and comply with the treatment plan | |
| Vaccination begins (continues) if: | Disposal criteria |
| 1. Tumor idiotype identified and cloned. | 1. Individual intolerance of the vaccine |
| 2. No progression requiring chemotherapy or radiotherapy. | 2. The desire of the patient to stop the study. |
| 3. Peripheral blood test: leukocytes count >1.5 × 109/L, platelets >75.0 × 109/L., hemoglobin >80 g/L. | 3. Nonresponders to standard chemotherapy (progression or reduction in tumor volume less than 25% of the original) |
| 4. More than 2 month after the last cytostatic or immunosuppressive chemotherapy (4 month after Rituximab). | 4. Serious adverse events occurring in patients during the study. |
| 5. Patient violation of conditions of the study. | |
| 6. Pregnancy. | |
| 7. Identification of a second cancer. |
Tumor tissue is obtained by lymph node biopsy (lymphomas) or bone marrow aspiration (CLL). Surgically excised lymph node (approx. 1 cm3) is delivered fresh to the laboratory in a sterile container containing RPMI medium plus 15% FBS. Tissue is placed in a small petri dish filled with the same medium and milled with a scalpel or surgical scissors. The cell suspension is collected, filtered through a nylon cell strainer and washed once with sterile PBS. A cell count is performed in the Gorjaev's chamber with 3% acetic acid and methylene blue. In case of bone marrow biopsy, mononuclear cells (MNCs) are isolated by centrifugation on density gradient (Histopaque-1077, Sigma).
Lymphoma cell counts and expression of surface Ig is determined by flow cytometry. Cell suspension aliquots containing approx. 250 000 cells are stained with monoclonal antibodies in 4 tubes: 1. Isotype control; 2. CD45-FITC, CD20-PE, CD3-PC5, CD19-PE-Cy7; 3. IgG-PE-Cy5, IgM-FITC, CD19-PE-Cy7; and 4. kappa-FITC, lambda-PE, CD19-PE-Cy7. Immunoglobulin expression is estimated on lymphocytes as gated using SSC/FSC and CD19+. Samples containing more than 80% of malignant lymphoma cells with a clear monoclonal population of CD19+ cells expressing one type of heavy and light chain are used for vaccine production. Aliquots of 5–8×109 cells are lysed for RNA and DNA extraction, the remaining cells are immediately cryopreserved in liquid nitrogen for further immunological testing.
RNA is isolated using TRIreagent (Sigma) according to the manufacturer's instructions, dissolved in sterile water and evaluated by spectrophotometry before immediate storage at −80°C. One μg of total RNA is used for cDNA synthesis with SuperScriptIII reverse transcriptase (Invitrogen) and Oligo-dT. Variable region genes of heavy and light Ig chains identified by flow cytometry are amplified as described previously.28 In brief, semi-nested PCR using high-fidelity DNA-polymerase with a set of forward primers and a distal reverse primer is used. First step PCR products are separated by polyacrylamide gel electrophoresis and products of the expected size are extracted and the DNA eluted and sequenced. In a second step, PCR restriction sites, start, stop codons and 6-His tags are added to the VH and VL fragments by 5′ extension of primers. Overlap extension PCR (OE-PCR) is used to fuse VH and VL fragments as scFv. Following extraction, scFv fragments are cloned into the pTZ57R vector and used to transform E.coli XL10Gold. Transformation is performed by the calcium-cold method and bacteria are seeded on Petri dishes with S-Gal/IPTG for LacZ-selection. At least 10 white colonies are selected for plasmid isolation and sequencing. scFvs with the correct sequence are subcloned into the pING vector together with the costimulatory gene, either PVXCP or MIP-3A (Fig. 1). Alternatively, the scFv fragment is cloned directly into the vector by OE-PCR and homology recombination in E.coli.29 The assembled plasmids are verified by restriction mapping and DNA sequencing. Extraction of plasmids is performed by MaxiPrep Kit (Life Technologies). Plasmid DNA is eluted using sterile DPBS buffer. Quality control measures include agarose electrophoresis for plasmid isoform assessment (supercoiled form >80% DNA) and spectrophotometry (OD260/280nm 1.8–2.0, OD260/230nm 1.8–2.4).30 DNA concentration is verified by Qubit Fluorometric Quantitation.
Figure 1.
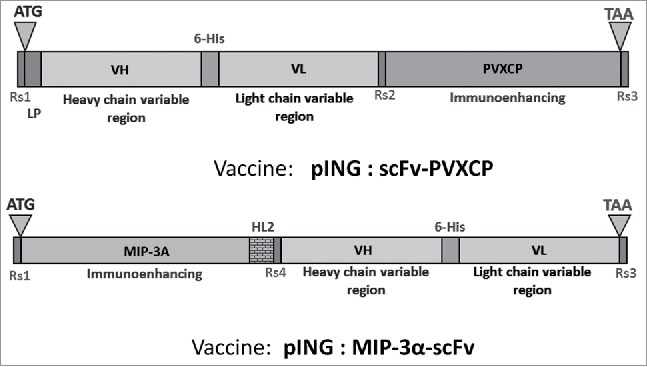
Constructions of DNA-vaccines. HL2 – helical linker 2: AEAAAKEAAAKEAAAK, LP – leader peptide, ATG и TAA – start- and stop-codons for protein translation, Restriction sites: Rs1 – BamHI or KpnI, Rs2 – SalI or HindIII, Rs3 – NotI, Rs4 – NdeI.
The study remains on-going until the completion of the study objectives. The study was approved by the ethics committee in the National Cancer Center of Belarus (protocol reg.# 20142755). After informed consent, patients undergo an excisional lymph node biopsy to confirm diagnosis and to provide the material for Id identification and cloning. Patients receive standard therapy following diagnosis and then remain untreated for a period of 2 to 6 months to allow for immune recovery (Fig. 2). Once a vaccine is prepared, the patient receives one or 2 courses, each consisting of 3 vaccinations administered monthly. One dose includes 500 μg of plasmid DNA in 1–2 ml of sterile DPBS buffer. Linear PEI (8 kDa) is used to prepare a complex with plasmid DNA in a ratio of 10:1 (PEI:DNA), as described previously.25 The required amount of a 10 μg/μl PEI stock solution is diluted with 5% glucose and added to an equal volume of DNA and rapidly mixed by pipetting. The mixture is kept for 10 minutes at room temperature to allow the formation of complexes before administration by intramuscular injection into the gluteal muscle. One of 2 vaccine constructs (scFv-PVXCP or MIP3A-scFv) is used per patient. One week after the last injection, peripheral blood is collected for evaluation of the immune response, if no specific anti-idiotypic response is detected, a second course of vaccination is administered.
Figure 2.
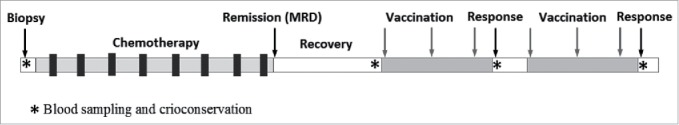
Overall treatment plan.
The study objectives are to examine (1) the safety and tolerability of DNA vaccination; (2) the immunological response to 2 separate DNA fusion vaccines; (3) the clinical response to DNA vaccination by monitoring of the minimal residual disease (MRD) by RQ-PCR and dissemination of tumor by MRT/PET.
The primary end point of this study is the assessment of the safety and tolerability of DNA vaccination and based on the published data, we assume that any adverse events will not exceed grade 1–2 according to Common Terminology Criteria for Adverse Events (CTCAE).
The secondary end point of this study is the evaluation of the immunologic response to vaccination (anti-Id cellular and humoral immune response). Peripheral blood mononuclear cells (PBMCs) and sera are collected from patients both before and after vaccination. Induction of cellular immunity will be assessed by cultured IFNγ ELISPOT. Pre- and post-vaccine PBMCs are cultured with antigenic proteins (PVXCP or Id-scFv) for 14 d before the cells are re-stimulated in an ELISPOT; a threefold increase in the number of IFNγ-producing T cells in the post-vaccine PBMC sample as compared with the pre-vaccine sample will be considered positive.
Proliferative responses are based on staining of PBMCs with a fluorescent dye PKH-26. Pre-stained PBMCs cultures are incubated with antigenic proteins (PVXCP or Id) for 6 d. T-cell proliferation is estimated by a decrease of PKH-26 fluorescence relative to the negative control. A threefold difference in number of proliferating cells in the post-vaccine PBMC samples as compared with the pre-vaccine samples is considered positive. Phytohaemagglutinin (PHA) is used as a positive control (0.2–0.5 μg/ml).
Humoral responses are analyzed by ELISA. Plates coated with antigenic proteins (PVXCP or Id-scFv) are incubated with serial dilutions of patient serum and after washing were stained with HRP-labeled anti-human immunoglobulin antibodies. A threefold increase of positive cells in post-immune serum compared with pre-immune serum is considered positive immune response.
MRD detection has been described previously.31 Clonal IgH and IgL gene rearrangements are identified during vaccine preparations. Allele-specific primers to CDR3 are used for the real-time quantitative polymerase chain reaction (RQ-PCR) measurement. DNA from primary removed lymph node is used for standard curve construction. DNA from follow up samples of peripheral blood and bone marrow (if available) are amplified in triplicate. For normalization, the same samples are amplified with primers to the albumin control gene. Interpretation of MRD analysis results is performed in accordance with the guidelines published by the European Study Group on MRD detection.32
Discussion
The immunogenicity and clinical efficacy of Id vaccination has been proven in many clinical trials. In the last decade, the study of DNA vaccination for cancer immunotherapy has intensified, as demonstrated by a rapidly increasing number of registered clinical trials. Plasmid DNA vaccines targeting cancer antigens have low immunogenicity and questionable efficacy when used in isolation.33 However, by tailoring the method of DNA delivery, the expression of antigen and its targeting into antigen-presenting cells may be enhanced. Electroporation is recognized as one powerful way in which this can be achieved in the clinic.34,35 However, electroporation requires the availability of specialized equipment. Combining plasmid DNA with a synthetic polymer to form a complex before administration to the patient can facilitate the entry of DNA into the cell, thus improving antigen expression, and represents an easily accessible and a convenient alternative.36-38 In this study, we plan to enhance transfection efficiency by complexing plasmid DNA with a cationic polymer – linear PEI with low molecular weight (8 kDa). To our knowledge, this is the first application of PEI as a carrier for DNA vaccines administered to human subjects. Many preclinical studies have shown a significant increase in immunogenicity of DNA-PEI conjugates, as well as a dramatic reduction in toxicity as the molecular weight of the polymer is reduced.22,23,25,26,38 The use of PEI in clinical vaccination has been approved by the regional regulatory authorities (State Committee on Science and Technology of the Republic of Belarus).
The model design of a DNA vaccine features a fusion of the target antigen to an immunostimulatory sequence. The majority of clinical trials to date have used fragment C (FrC) of tetanus toxin as the immunostimulatory sequence. However, more recent trials have reported the use of the human chemokine CCL20 (MIP3A) in which the resulting fusion protein is intended to provide enhanced recruitment and uptake by dendritic cells in vivo.39 However, it is unclear whether this design will be able to provide sufficient CD4+ T-cell help as is required for the induction of humoral and cytotoxic T cell responses.40 In this study, we are using PVXCP as the immune-enhancer and comparing its action to that of MIP3A. PVXCP is a potent inducer of Th1 help in preclinical studies,19,41 but has not yet been used clinically.
Immunity to DNA vaccination will be measured using methods that enable the quantitative evaluation of both humoral (ELISA) and T cellular (ELISPOT) responses. The Id vaccine encoding secretory protein is expected to induce a humoral immune response predominantly. However, evaluation of the cellular immune responses is necessary to compare the ability of the 2 different immunostimulatory sequences to induce the activation of T-helper cells, as measured by the production of IFN-γ and capture using ELISPOT assay.42
Conclusions
Previously published data on the preclinical and clinical use of DNA vaccination to target cancer indicates that they have real therapeutic efficacy. The clinical benefit of anti-cancer vaccination is limited by known and tested tumor antigens and routes of vaccine delivery. Idiotype is one of the most studied antigens for vaccination against lymphoma. Vaccination using protein is hampered by the need for the production of Id protein for each patient, a process which could be both long and complicated. Conversely, the production of DNA vaccine is relatively fast and inexpensive and therefore DNA vaccination provides and attractive alternative. The novelty of our study is twofold: first, we plan to enhance the immunogenicity of our DNA vaccine by using one of 2 immunostimulatory sequences fused to the target antigen; second, we will administer the DNA vaccine as a complex with the synthetic polymer linear PEI to enhance the transfection efficacy in vivo.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Dr. Katy McCann from Cancer Sciences, University of Southampton for a critical review of the manuscript.
Funding
The study was funded by the Belarus Ministry of Health (Grant #20142755).
References
- [1].Okeanov AE, Moiseyev PI, Levin LF, et al.. Статистика онкологических заболеваний в Республике Беларусь (2005–2014) [Statistics of oncological diseases in the Republic of Belarus (2005–2014)]. Minsk, Belarus: N.N. Alexandrov National Cancer Centre of Belarus; 2015. Russian. [Google Scholar]
- [2].Armitage JO. Non-Hodgkin lymphomas (2nd ed). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2010. [Google Scholar]
- [3].Kwak LW, Campbell MJ, Czerwinski DK, Hart S, Miller RA, Levy R. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327(17):1209-1215; PMID: 1406793; https://doi.org/ 10.1056/NEJM199210223271705 [DOI] [PubMed] [Google Scholar]
- [4].Bendandi M, Gocke CD, Kobrin CB, Benko FA, Sternas LA, Pennington R, Watson TM, Reynolds CW, Gause BL, Duffey PL, et al.. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5(10):1171-1177; PMID: 10502821; https://doi.org/ 10.1038/13928 [DOI] [PubMed] [Google Scholar]
- [5].Timmerman JM, Vose JM, Czerwinski DK, Weng WK, Ingolia D, Mayo M, Denney DW, Levy R. Tumor-specific recombinant idiotype immunisation after chemotherapy as initial treatment for follicular non-Hodgkin lymphoma. Leuk Lymphoma. 2009;50(1):37-46; PMID: 19125383; https://doi.org/ 10.1080/10428190802563355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Inogès S, Rodrìguez-Calvillo M, Zabalegui N, Lòpez-Dìaz de Cerio A, Villanueva H, Soria E, Suárez L, Rodríguez-Caballero A, Pastor F, García-Muñóz R et al.. Clinical benefit associated with idiotypic vaccination in patients with follicular lymphoma. J Natl Cancer Inst. 2006;98(18):1292-1301 [DOI] [PubMed] [Google Scholar]
- [7].Redfern CH, Guthrie TH, Bessudo A, Densmore JJ, Holman PR, Janakiraman N, Leonard JP, Levy RL, Just RG, Smith MR, et al.. Phase II trial of idiotype vaccination in previously treated patients with indolent non-Hodgkin's lymphoma resulting in durable clinical responses. J Clin Oncol. 2006;24(19):3107-3112 [DOI] [PubMed] [Google Scholar]
- [8].Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, Winter JN, Flowers CR, Nikcevich DA, Sotomayor EM, et al.. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29(20):2787-2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kretzschmar T, Aoustin L, Zingel O, Marangi M, Vonach B, Towbin H, Geiser M. High-level expression in insect cells and purification of secreted monomeric single-chain Fv antibodies. J Immunol Methods. 1996;195(1–2):93-101; PMID: 8814324; https://doi.org/ 10.1016/0022-1759(96)00093-2 [DOI] [PubMed] [Google Scholar]
- [10].McCormick AA, Reinl SJ, Cameron TI, Vojdani F, Fronefield M, Levy R, Tusé D. Individualized human scFv vaccines produced in plants: humoral anti-idiotype responses in vaccinated mice confirm relevance to the tumor Ig. J Immunol Methods. 2003; 278(1-2):95-104; PMID: 12957399; https://doi.org/ 10.1016/S0022-1759(03)00208-4 [DOI] [PubMed] [Google Scholar]
- [11].Tusé D, Ku N, Bendandi M, Becerra C, Collins R Jr, Langford N, Sancho SI, López-Díaz de Cerio A, Pastor F, Kandzia R et al.. Clinical safety and immunogenicity of tumor-targeted, plant-made Id-KLH conjugate vaccines for follicular lymphoma. Biomed Res Int. 2015; 2015:648143; PMID: 26425548; https://doi.org/ 10.1155/2015/648143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Coban C, Koyama S, Takeshita F, Akira S, Ishii KJ. Molecular and cellular mechanisms of DNA vaccines. Hum Vaccin. 2008; 4(6):453-457 [DOI] [PubMed] [Google Scholar]
- [13].Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8(2):108-120; PMID: 18219306; https://doi.org/ 10.1038/nrc2326 [DOI] [PubMed] [Google Scholar]
- [14].Stevenson FK, Rice J, Ottensmeier CH, Thirdborough SM, Zhu D. DNA fusion gene vaccines against cancer: from the laboratory to the clinic. Immunol Rev. 2004;199:156-180; PMID: 15233733; https://doi.org/ 10.1111/j.0105-2896.2004.00145.x [DOI] [PubMed] [Google Scholar]
- [15].Stevenson FK, Mander A, Chudley L, Ottensmeier CH. DNA fusion vaccines enter the clinic. Cancer Immunol Immunother. 2011;60(8):1147-1151; PMID: 21644035; https://doi.org/ 10.1007/s00262-011-1042-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Senovilla L, Vacchelli E, Garcia P, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: DNA vaccines for cancer therapy. Oncoimmunology. 2013;2(4):e23803; PMID: 23734328; https://doi.org/ 10.4161/onci.23803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Patel KG, Ng PP, Levy S, Levy R, Swartz JR. Escherichia coli-based production of a tumor idiotype antibody fragment-tetanus toxin fragment C fusion protein vaccine for B cell lymphoma. Protein Expr Purif. 2011; 75(1):15-20; https://doi.org/ 10.1016/j.pep.2010.09.005 [DOI] [PubMed] [Google Scholar]
- [18].Guo JH, Fan MW, Sun JH, Jia R. Fusion of antigen to chemokine CCL20 or CXCL13 strategy to enhance DNA vaccine potency. Int Immunopharmacol. 2009;9(7-8):925-930; https://doi.org/ 10.1016/j.intimp.2009.03.019 [DOI] [PubMed] [Google Scholar]
- [19].Savelyeva N, Munday R, Spellerberg MB, Lomonossoff GP, Stevenson FK. Plant viral genes in DNA idiotypic vaccines activate linked CD4+ T-cell mediated immunity against B-cell malignancies. Nat Biotechnol. 2001;19(8):760-764; PMID: 11479570; https://doi.org/ 10.1038/90816 [DOI] [PubMed] [Google Scholar]
- [20].Chen CG, Lu Y-T, Lin M, Savelyeva N, Stevenson FK, Zhu D. Amplification of immune responses against a DNA-delivered idiotypic lymphoma antigen by fusion to the B subunit of E. coli heat labile toxin. Vaccine. 2009; 27(32):4289-4296 [DOI] [PubMed] [Google Scholar]
- [21].Timmerman JM, Singh G, Hermanson G, Hobart P, Czerwinski DK, Taidi B, Rajapaksa R, Caspar CB, Van Beckhoven A, Levy R. Immunogenicity of a plasmid DNA vaccine encoding chimeric idiotype in patients with B-cell lymphoma. Cancer Res. 2002;62(20):5845-5852. PMID: 12384547 PMID: 12384547 PMID: 12384547 [PubMed] [Google Scholar]
- [22].Oh Y-K, Suh D, Kim JM, Choi HG, Shin K, Ko JJ. Polyethylenimine-mediated cellular uptake, nucleus trafficking and expression of cytokine plasmid DNA. Gene Ther. 2002;9(23):1627-1632; PMID: 12424615; https://doi.org/ 10.1038/sj.gt.3301735 [DOI] [PubMed] [Google Scholar]
- [23].Ma Y-F, Yang Y-W. Delivery of DNA-based cancer vaccine with polyethylenimine. Eur J Pharm Sci. 2010;40(2):75-83; https://doi.org/ 10.1016/j.ejps.2010.02.009 [DOI] [PubMed] [Google Scholar]
- [24].Garzon MR, Berraondo P, Crettaz J, Ochoa L, Vera M, Lasarte JJ, Vales A, Van Rooijen N, Ruiz J, Prieto J, et al.. Induction of gp120-specific protective immune responses by genetic vaccination with linear polyethylenimine-plasmid complex. Vaccine. 2005;23(11):1384-1392 [DOI] [PubMed] [Google Scholar]
- [25].Oh YK, Kim JP, Yoon H, Kim JM, Yang JS, Kim CK. Prolonged organ retention and safety of plasmid DNA administered in polyethylenimine complexes. Gene Ther. 2001;8:1587-1592; PMID: 11704820; https://doi.org/ 10.1038/sj.gt.3301516 [DOI] [PubMed] [Google Scholar]
- [26].Grant E V., Thomas M, Fortune J, Klibanov AM, Letvin NL. Enhancement of plasmid DNA immunogenicity with linear polyethylenimine. Eur J Immunol. 2012;42(11):2937-2948; PMID: 22886924; https://doi.org/ 10.1002/eji.201242410 [DOI] [PubMed] [Google Scholar]
- [27].Houot R, Levy R. Vaccines for lymphomas: idiotype vaccines and beyond. Blood Rev. 2009;23(3):137-142; https://doi.org/ 10.1016/j.blre.2008.09.001 [DOI] [PubMed] [Google Scholar]
- [28].Meleshko AN, Vashkevich KP, Fomina EG, Scheslenok EP, Schkolina TV, Sergeev GV. Cloning of variable fragments of tumor immunoglobulin, assembling and expressing of human SCFV protein in E. coli for anti-idiotype vaccination. Exp Oncol. 2013;35(1):8-14 [PubMed] [Google Scholar]
- [29].Jacobus AP, Gross J. Optimal cloning of PCR fragments by homologous recombination in Escherichia coli. PLoS One. 2015;10(3):1-17; https://doi.org/ 10.1371/journal.pone.0119221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Saltzman WM, Shen H, Brandsma JL. DNA Vaccines: Methods and Protocols SECOND EDI (Hackworth J, ed.). Totowa, New Jersey: Humana Press Inc; 2006 [Google Scholar]
- [31].Meleshko AN, Savva NN, Fedasenka UU, Romancova AS, Krasko OV, Eckert C, von Stackelberg A, Aleinikova OV. Prognostic value of MRD-dynamics in childhood acute lymphoblastic leukemia treated according to the MB-2002/2008 protocols. Leuk Res. 2011;35(10):1312-1320; https://doi.org/ 10.1016/j.leukres.2011.04.013 [DOI] [PubMed] [Google Scholar]
- [32].van der Velden VHJ, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER, Flohr T, Sutton R, Cave H, Madsen HO, et al.. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21(4):604-611; PMID: 17287850 [DOI] [PubMed] [Google Scholar]
- [33].Fioretti D, Iurescia S, Fazio VM, Rinaldi M. DNA vaccines: developing new strategies against cancer. J Biomed Biotechnol. 2010;2010:174378; PMID: 20368780; https://doi.org/ 10.1155/2010/174378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Best SR, Peng S, Juang CM, Hung CF, Hannaman D, Saunders JR, Wu TC, Pai SI. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27(40):5450-5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Low L, Mander A, McCann K, Dearnaley D, Tjelle T, Mathiesen I, Stevenson F, Ottensmeier CH. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum Gene Ther. 2009;20(11):1269-1278; PMID: 19619001; https://doi.org/ 10.1089/hum.2009.067 [DOI] [PubMed] [Google Scholar]
- [36].van den Berg JH, Nuijen B, Schumacher TN, Haanen JB, Storm G, Beijnen JH, Hennink WE.. Synthetic vehicles for DNA vaccination. J Drug Target. 2010;18(1):1-14; PMID: 19814658; https://doi.org/ 10.3109/10611860903278023 [DOI] [PubMed] [Google Scholar]
- [37].Bolhassani A, Safaiyan S, Rafati S. Improvement of different vaccine delivery systems for cancer therapy. Mol Cancer. 2011;10(1):3; PMID: 21211062; https://doi.org/ 10.1186/1476-4598-10-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Di Gioia S, Conese M. Polyethylenimine-mediated gene delivery to the lung and therapeutic applications. Drug Des Devel Ther. 2009;2(2):163-188; PMID: 19920904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Thomas SK, Kwak LW. Lymphoma vaccine therapy: next steps after a positive, controlled phase III clinical trial. Semin Oncol. 2012;39(3):253-262; https://doi.org/ 10.1053/j.seminoncol.2012.02.014 [DOI] [PubMed] [Google Scholar]
- [40].Savelyeva N, Allen A, Chotprakaikiat W, Harden E, Jobsri J, Godeseth R, Wang Y, Stevenson F, Ottensmeier C. Linked CD4 T Cell Help: Broadening Immune Attack Against Cancer by Vaccination. Curr Top Microbiol Immunol. 2016;351(January):139-157 [DOI] [PubMed] [Google Scholar]
- [41].Jobsri J, Allen A, Rajagopal D, Shipton M, Kanyuka K, Lomonossoff GP, Ottensmeier C, Diebold SS, Stevenson FK1, Savelyeva N1. Plant virus particles carrying tumour antigen activate TLR7 and Induce high levels of protective antibody. PLoS One. 2015;10(2):e0118096; PMID: 25692288; https://doi.org/ 10.1371/journal.pone.0118096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chudley L, McCann KJ, Coleman A, Cazaly AM, Bidmon N, Britten CM, van der Burg SH, Gouttefangeas C, Jandus C, Laske K, et al.. Harmonisation of short-term in vitro culture for the expansion of antigen-specific CD8+ T cells with detection by ELISPOT and HLA-multimer staining. Cancer Immunol Immunother. 2014;63(11):1199-1211; https://doi.org/ 10.1007/s00262-014-1593-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


