Abstract
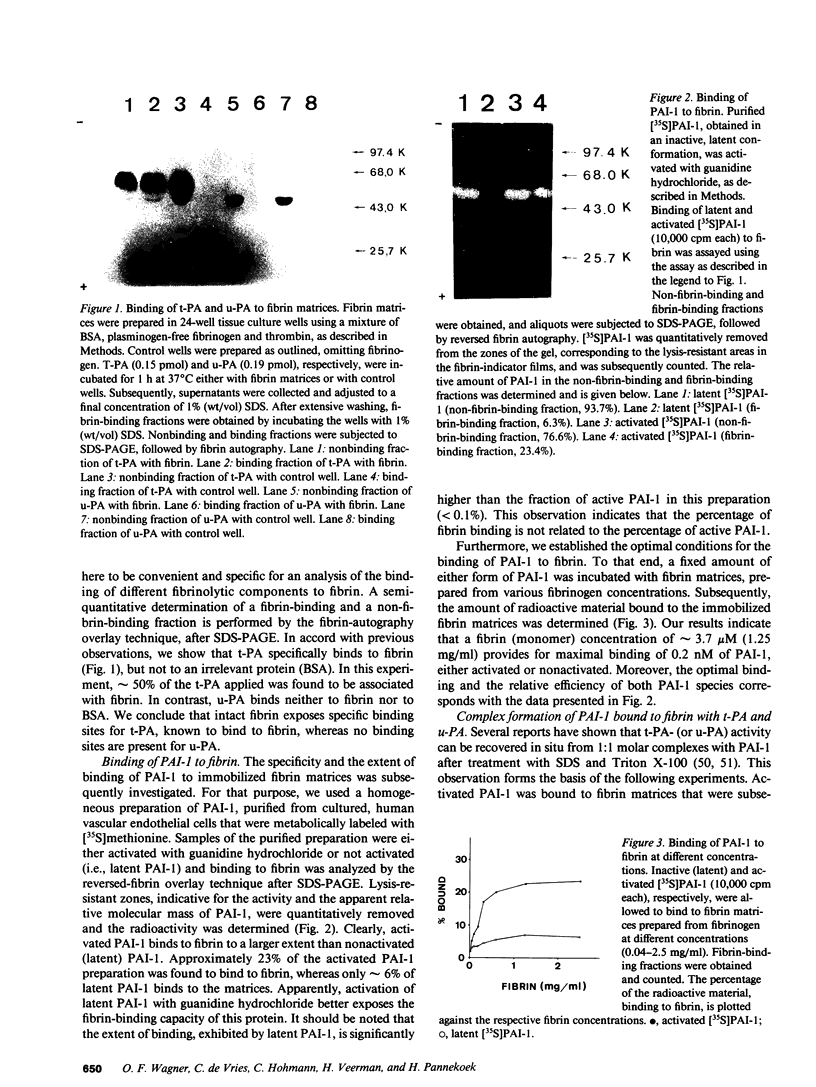
Plasminogen activation is catalyzed both by tissue-type-(t-PA) and by urokinase-type plasminogen activator (u-PA). This reaction is controlled by plasminogen activator inhibitor type 1 (PAI-1) that is either present in plasma or bound to fibrin, present in a thrombus. We studied the mechanism of in vitro inhibition of both t-PA and u-PA activity by PAI-1 bound to fibrin. It is shown that activation of latent PAI-1 unmasks a specific fibrin-binding site that is distinct from its reactive site. This reactive site of activated PAI-1 bound to fibrin is fully exposed to form complexes with t-PA and u-PA, that are unable to activate plasminogen. Upon complex formation with either one of the plasminogen activators, PAI-1 apparently undergoes a conformational change and loses its affinity for fibrin. Consequently, complexes of u-PA and PAI-1 dissociate from the fibrin matrix and are encountered in the fluid phase. In contrast, t-PA/PAI-1 complexes remain bound to fibrin. By employing recombinant t-PA deletion-mutant proteins, that precisely lack domains involved in fibrin binding, we demonstrate that binding of t-PA/PAI-1 complexes is mediated by both the "finger" (F) and the "kringle-2" (K2) domain of t-PA. A model is proposed that explains inhibition of the fibrinolytic process, at the level of plasminogen activation by t-PA, directed by PAI-1 bound to fibrin. An implication of the proposed model is that t-PA/PAI-1 complexes and free t-PA compete for the same binding sites on fibrin.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki N., Harpel P. C. Inhibitors of the fibrinolytic enzyme system. Semin Thromb Hemost. 1984 Jan;10(1):24–41. doi: 10.1055/s-2007-1004405. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Binder B. R., Spragg J., Austen K. F. Purification and characterization of human vascular plasminogen activator derived from blood vessel perfusates. J Biol Chem. 1979 Mar 25;254(6):1998–2003. [PubMed] [Google Scholar]
- Bányai L., Váradi A., Patthy L. Common evolutionary origin of the fibrin-binding structures of fibronectin and tissue-type plasminogen activator. FEBS Lett. 1983 Oct 31;163(1):37–41. doi: 10.1016/0014-5793(83)81157-0. [DOI] [PubMed] [Google Scholar]
- Colbere-Garapin F., Chousterman S., Horodniceanu F., Kourilsky P., Garapin A. C. Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3755–3759. doi: 10.1073/pnas.76.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980 Jun 18;43(2):77–89. [PubMed] [Google Scholar]
- Erickson L. A., Ginsberg M. H., Loskutoff D. J. Detection and partial characterization of an inhibitor of plasminogen activator in human platelets. J Clin Invest. 1984 Oct;74(4):1465–1472. doi: 10.1172/JCI111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson L. A., Lawrence D. A., Loskutoff D. J. Reverse fibrin autography: a method to detect and partially characterize protease inhibitors after sodium dodecyl sulfate--polyacrylamide gel electrophoresis. Anal Biochem. 1984 Mar;137(2):454–463. doi: 10.1016/0003-2697(84)90113-1. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Adler B., Boose J. A., Gerard R. D., Madison E. L., McGookey D., Meidell R. S., Roman L. M., Sambrook J. Variants of human tissue-type plasminogen activator that lack specific structural domains of the heavy chain. EMBO J. 1988 Sep;7(9):2731–2740. doi: 10.1002/j.1460-2075.1988.tb03127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg D., Zeheb R., Yang A. Y., Rafferty U. M., Andreasen P. A., Nielsen L., Dano K., Lebo R. V., Gelehrter T. D. cDNA cloning of human plasminogen activator-inhibitor from endothelial cells. J Clin Invest. 1986 Dec;78(6):1673–1680. doi: 10.1172/JCI112761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekman C. M., Loskutoff D. J. Endothelial cells produce a latent inhibitor of plasminogen activators that can be activated by denaturants. J Biol Chem. 1985 Sep 25;260(21):11581–11587. [PubMed] [Google Scholar]
- Hekman C. M., Loskutoff D. J. Kinetic analysis of the interactions between plasminogen activator inhibitor 1 and both urokinase and tissue plasminogen activator. Arch Biochem Biophys. 1988 Apr;262(1):199–210. doi: 10.1016/0003-9861(88)90182-8. [DOI] [PubMed] [Google Scholar]
- Higgins D. L., Vehar G. A. Interaction of one-chain and two-chain tissue plasminogen activator with intact and plasmin-degraded fibrin. Biochemistry. 1987 Dec 1;26(24):7786–7791. doi: 10.1021/bi00398a038. [DOI] [PubMed] [Google Scholar]
- Holmes W. E., Nelles L., Lijnen H. R., Collen D. Primary structure of human alpha 2-antiplasmin, a serine protease inhibitor (serpin). J Biol Chem. 1987 Feb 5;262(4):1659–1664. [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof E. K., Tran-Thang C., Bachmann F. Studies on the release of a plasminogen activator inhibitor by human platelets. Thromb Haemost. 1986 Apr 30;55(2):201–205. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambers J. W., Cammenga M., König B. W., Mertens K., Pannekoek H., van Mourik J. A. Activation of human endothelial cell-type plasminogen activator inhibitor (PAI-1) by negatively charged phospholipids. J Biol Chem. 1987 Dec 25;262(36):17492–17496. [PubMed] [Google Scholar]
- Loskutoff D. J., van Mourik J. A., Erickson L. A., Lawrence D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2956–2960. doi: 10.1073/pnas.80.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M. E., van Zonneveld A. J., Pannekoek H. Functional analysis of the human tissue-type plasminogen activator protein: the light chain. Gene. 1986;42(1):59–67. doi: 10.1016/0378-1119(86)90150-2. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Sawdey M., Lawrence D., Millan J. L., Loskutoff D. J. Cloning and sequence of a cDNA coding for the human beta-migrating endothelial-cell-type plasminogen activator inhibitor. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6776–6780. doi: 10.1073/pnas.83.18.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek H., Veerman H., Lambers H., Diergaarde P., Verweij C. L., van Zonneveld A. J., van Mourik J. A. Endothelial plasminogen activator inhibitor (PAI): a new member of the Serpin gene family. EMBO J. 1986 Oct;5(10):2539–2544. doi: 10.1002/j.1460-2075.1986.tb04532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Hoylaerts M., Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem. 1982 Mar 25;257(6):2920–2925. [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Welbergen J. Relationship between tissue plasminogen activator and the activators in blood and vascular wall. Thromb Res. 1980 Jun 15;18(6):815–830. doi: 10.1016/0049-3848(80)90204-2. [DOI] [PubMed] [Google Scholar]
- Rånby M. Studies on the kinetics of plasminogen activation by tissue plasminogen activator. Biochim Biophys Acta. 1982 Jun 24;704(3):461–469. doi: 10.1016/0167-4838(82)90068-1. [DOI] [PubMed] [Google Scholar]
- Sakata Y., Aoki N. Cross-linking of alpha 2-plasmin inhibitor to fibrin by fibrin-stabilizing factor. J Clin Invest. 1980 Feb;65(2):290–297. doi: 10.1172/JCI109671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengers E. D., Akkerman J. W., Jansen B. G. Blood platelet plasminogen activator inhibitor: two different pools of endothelial cell type plasminogen activator inhibitor in human blood. Thromb Haemost. 1986 Jun 30;55(3):325–329. [PubMed] [Google Scholar]
- Sprengers E. D., Princen H. M., Kooistra T., van Hinsbergh V. W. Inhibition of plasminogen activators by conditioned medium of human hepatocytes and hepatoma cell line Hep G2. J Lab Clin Med. 1985 Jun;105(6):751–758. [PubMed] [Google Scholar]
- Sumi Y., Nakamura Y., Aoki N., Sakai M., Muramatsu M. Structure of the carboxyl-terminal half of human alpha 2-plasmin inhibitor deduced from that of cDNA. J Biochem. 1986 Nov;100(5):1399–1402. doi: 10.1093/oxfordjournals.jbchem.a121846. [DOI] [PubMed] [Google Scholar]
- Thorsen S., Philips M. Isolation of tissue-type plasminogen activator-inhibitor complexes from human plasma. Evidence for a rapid plasminogen activator inhibitor. Biochim Biophys Acta. 1984 Nov 6;802(1):111–118. doi: 10.1016/0304-4165(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Tran-Thang C., Kruithof E. K., Atkinson J., Bachmann F. High-affinity binding sites for human Glu-plasminogen unveiled by limited plasmic degradation of human fibrin. Eur J Biochem. 1986 Nov 3;160(3):599–604. doi: 10.1111/j.1432-1033.1986.tb10080.x. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Wiman B., Mellbring G., Rånby M. Plasminogen activator release during venous stasis and exercise as determined by a new specific assay. Clin Chim Acta. 1983 Jan 24;127(2):279–288. doi: 10.1016/s0009-8981(83)80012-6. [DOI] [PubMed] [Google Scholar]
- Wun T. C., Capuano A. Initiation and regulation of fibrinolysis in human plasma at the plasminogen activator level. Blood. 1987 May;69(5):1354–1362. [PubMed] [Google Scholar]
- Wun T. C., Kretzmer K. K. cDNA cloning and expression in E. coli of a plasminogen activator inhibitor (PAI) related to a PAI produced by Hep G2 hepatoma cell. FEBS Lett. 1987 Jan 1;210(1):11–16. doi: 10.1016/0014-5793(87)81288-7. [DOI] [PubMed] [Google Scholar]
- van Mourik J. A., Leeksma O. C., Reinders J. H., de Groot P. G., Zandbergen-Spaargaren J. Vascular endothelial cells synthesize a plasma membrane protein indistinguishable from the platelet membrane glycoprotein IIa. J Biol Chem. 1985 Sep 15;260(20):11300–11306. [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., Brakenhoff J. P., Aarden L. A., Cajot J. F., Pannekoek H. Mapping of epitopes on human tissue-type plasminogen activator with recombinant deletion mutant proteins. Thromb Haemost. 1987 Feb 3;57(1):82–86. [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., MacDonald M. E., van Mourik J. A., Pannekoek H. Structure and function of human tissue-type plasminogen activator (t-PA). J Cell Biochem. 1986;32(3):169–178. doi: 10.1002/jcb.240320302. [DOI] [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., Pannekoek H. Autonomous functions of structural domains on human tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4670–4674. doi: 10.1073/pnas.83.13.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., Pannekoek H. On the interaction of the finger and the kringle-2 domain of tissue-type plasminogen activator with fibrin. Inhibition of kringle-2 binding to fibrin by epsilon-amino caproic acid. J Biol Chem. 1986 Oct 25;261(30):14214–14218. [PubMed] [Google Scholar]