ABSTRACT
Background: The type 2 component of the oral poliovirus vaccine is targeted for global withdrawal through a switch from the trivalent oral poliovirus vaccine (tOPV) to a bivalent oral poliovirus vaccine (bOPV). The switch is intended to prevent paralytic polio caused by circulating vaccine-derived poliovirus type 2. We aimed to assess the immunogenicity and safety profile of 6 vaccination schedules with different sequential doses of inactivated poliovirus vaccine (IPV), tOPV, or bOPV.
Methods: A randomized controlled trial was conducted in China in 2015. Healthy newborn babies randomly received one of the following 6 vaccination schedules: cIPV-bOPV-bOPV(I-B-B), cIPV-tOPV-tOPV(I-T-T), cIPV-cIPV-bOPV(I-I-B), cIPV-cIPV-tOPV(I-I-T), cIPV-cIPV-cIPV(I-I-I), or tOPV-tOPV-tOPV(T-T-T). Doses were administered sequentially at 4–6 week intervals after collecting baseline blood samples. Patients were proactively followed up for observation of adverse events after the first dose and 30 days after all doses. The primary study objective was to investigate the immunogenicity and safety profile of different vaccine schedules, evaluated by seroconversion, seroprotection and antibody titer against poliovirus types 1, 2, and 3 in the per-protocol population.
Results: Of 600 newborn babies enrolled, 504 (84.0%) were included in the per-protocol population. For type 1 poliovirus, the differences in the seroconversion were 1.17% (95% CI = −2.74%, 5.08%) between I-B-B and I-T-T and 0.00% (95% CI: −6.99%, 6.99%) between I-I-B and I-I-T; for type 3 poliovirus, differences in the seroconversion were 3.49% (95% CI: −1.50%, 8.48%) between I-B-B and I-T-T and −2.32% (95% CI: −5.51%, 0.86%) between I-I-B and I-I-T. The non-inferiority conclusion was achieved in both poliovirus type 1 and 3 with the margin of −10%. Of 24 serious adverse events reported, no one was vaccine-related.
Conclusions: The vaccination schedules with bOPV followed by one or 2 doses of IPV were recommended to substitute for vaccinations involving tOPV without compromising the immunogenicity and safety in the Chinese population. The findings will be essential for policy formulation by national and global authorities to facilitate polio elimination.
KEYWORDS: immunization, inactivated polio vaccine, infectious disease, oral polio vaccine, polio, poliomyelitis, poliovirus, vaccine, vaccine schedule
Introduction
Poliomyelitis, caused by a poliovirus infection, used to be one of most widespread childhood diseases worldwide. Poliomyelitis patients present the severe clinical symptoms, such as neuron damages, muscle weakness, paralysis, etc. Three serotypes of poliovirus, without cross-reactive of immunogenicity, have been identified in nature. Infection or vaccination with one poliovirus serotype does not confer immunity against the other serotypes.,1,2 Although no specific antiviral therapy is available to cure poliomyelitis, a trivalent oral poliovirus vaccine (tOPV) and a trivalent conventional inactivated poliovirus vaccine (cIPV) have been used effectively worldwide to combat poliovirus infections since the 1950s. Because of the good vaccine efficacy, a poliomyelitis eradication campaign was launched by the World Health Organization in 1988. Through intensive tOPV vaccination, the number of poliomyelitis-endemic countries has fallen from an estimated 125 in 1988 to 2 in 2015. The number of poliomyelitis cases fell from estimated 350,000 cases in 1988 to 39 in 2015.3,4 Currently, type 1 poliovirus (PV1) is the only serotype with a wild strain circulating in nature; and has caused 26 cases (9 in Afghanistan, 14 in Pakistan, and 3 in Nigeria) between January and September 2016.5 Outbreaks caused by wild strains of type 2 poliovirus (PV2) and type 3 poliovirus (PV3) has not been reported since 1999 and 2012, respectively.6
Poliovirus is transmitted by a fecal-oral cycle.4 Feeding the oral poliovirus vaccine (OPV) elicits strong intestinal and systemic immunity, which is effective at both preventing poliomyelitis and reducing viral fecal-oral transmission. In contrast, muscular injection of inactivated poliovirus vaccine (IPV) induces excellent systemic immunity However, IPV is incapable of establishing adequate mucosal immunity on gut epithelia against poliovirus fecal shedding. Nonetheless, in contrast to the inactivated viral particles in IPV, OPV consists of live attenuated polioviruses which might revert to virulent circulating vaccine-derived strains, thereby resulting in vaccine-derived paralytic poliomyelitis in vaccinated persons. As naturally occurring poliomyelitis cases have largely decreased, these OPV-related polioviruses have become potential causative agents in a re-established poliomyelitis endemic. For example, although PV2 is no longer a naturally occurring virus, hundreds of paralytic poliomyelitis cases have been caused by vaccine-derived PV2 since 2000.7,8 Natural circulation of vaccine-derived PV2 was still persistent in parts of northern Nigeria and Pakistan.9 Between August 2011 and February 2012, an outbreak caused by type 2 circulating vaccine-derived poliovirus (cVDPV) occurred in Sichuan Province, China.10 In Northeastern Nigeria, a cVDPV2 was identified in an environmental sample collected in March 2016, and the identification in August of polio cases caused by wild PV1 (WPV1) required further strengthening of surveillance and immunization. 11,12 As of 29 June 2016, the Global Polio Eradication Initiative (GPEI) had confirmed 12 cases of polio in Pakistan.13 Laos with VDPV transmission in 2015 has reported 3 additional cVDPV cases in 2016 to date.14 To avoid the threats by re-emergence of vaccine-derived poliomyelitis, a switch of prevention strategy from OPV to IPV has been implemented worldwide.2,15
To recognize the difficulty of synchronous switching to the exclusive global administering of IPV, the “Polio Eradication and Endgame Strategic Plan: 2013–2018,” was introduced by the Global Polio Eradication Initiative.2 By April of 2015, at least one dose of IPV must be introduced into routine immunization schedules. For removal of the potential threat from VDPV2 (naturally occurring PV2 has been eradicated since 1999), the trivalent OPV should be replaced by a bivalent OPV containing only serotypes 1 and 3 by April 2016.6 According to the September 2016 Morbidity and Mortality Weekly Report, all 155 countries and territories using OPV in their immunization programs in 2015 reported that they had completely ceased use of tOPV by mid-May 2016. As of August 31, 173 (89%) of 194 WHO (World Health Organization) countries had introduced IPV into their immunization programs and 29 countries are expected to deplete their supplies of IPV before being resupplied in 2017. 11
Given the blended dose of OPV and IPV is used in the transition period, the immunization schedules to maintain optimal safety and immunogenicity might have significant differences among regions and human populations worldwide. For Chinese infants, no studies on poliovirus vaccination schedule with bOPV have been reported for reference to date. Here, we aimed to evaluate the immunogenicity and safety profile of the bivalent types 1 and 3 oral poliovirus vaccine (bOPV). We then further compared the advantages and disadvantages of different childhood sequential vaccination schedules involving bOPV, trivalent types 1, 2, and 3 oral poliovirus vaccines (tOPV) and inactivated polio vaccine (IPV).
Results
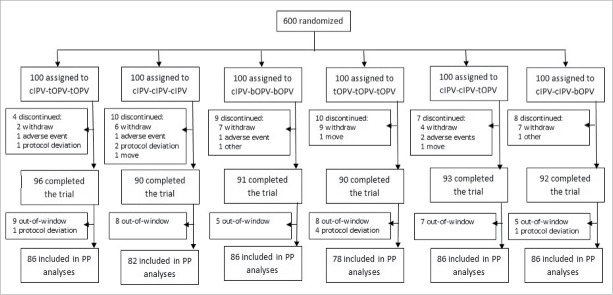
We enrolled 600 newborn babies randomized assigned into 6 sequential vaccination schedules as follows: cIPV-bOPV-bOPV (I-B-B), cIPV-tOPV-tOPV (I-T-T), cIPV-cIPV-bOPV (I-I-B), cIPV-cIPV-tOPV (I-I-T), cIPV-cIPV-cIPV (I-I-I), and tOPV-tOPV-tOPV (T-T-T). In total, 552 (92.00%) babies completed the trial (96 in I-T-T, 90 in I-I-I, 91 in I-B-B, 90 in T-T-T, 93 in I-I-T and 92 in I-I-B), while 48 (8.00%) discontinued (Fig. 1). In total, 504 (84.00%) of all randomized subjects were involved in the per-protocol (PP) population analyses (86 in I-T-T, 82 in I-I-I, 86 in I-B-B, 78 in T-T-T, 86 in I-I-T and 86 in I-I-B) according to the predefined requirements.
Figure 1.
Trial profile, intent-to-treat and per-protocol (PP) analyses, China, 2014 PP = per-protocol. cIPV = conventional inactivated poliovirus vaccine. bOPV = bivalent oral poliovirus vaccine. tOPV = trivalent oral poliovirus vaccine.
All 600 subjects who received at least one dose of study medication were included in the overall safety analyses and the safety analyses after the first dose of actual treatment as follows: 100 in I-T-T, 99 in I-I-I, 100 in I-B-B, 101 in T-T-T, 100 in I-I-T and 100 in I-I-B. There were 572 (95.33%) subjects in the safety analysis population for the second dose; and 565 (94.17%) subjects in the safety analysis population for the third dose.
Table 1 shows demographics, baseline characteristics, and seroprevalence in the various vaccine groups. There were no statistically significant differences among treatment groups.
Table 1.
Baseline characteristics and seroprevalence of per-protocol population by arms.
| cIPV-tOPV-tOPV (n = 86) | cIPV-cIPV-cIPV (n = 82) | cIPV-bOPV-bOPV (n = 86) | tOPV-tOPV-tOPV (n = 78) | cIPV-cIPV-tOPV (n = 86) | cIPV-cIPV-bOPV (n = 86) | |
|---|---|---|---|---|---|---|
| Age, mean ± SD (days) | 73.67 ± 9.24 | 72.32 ± 8.63 | 72.77 ± 8.21 | 73.60 ± 8.90 | 73.21 ± 9.37 | 74.79 ± 9.36 |
| Male sex, n (%) | 36(41.86) | 35(42.68) | 41(47.67) | 37(47.44) | 44(51.16) | 38(44.19) |
| Ethnicity, n (%) | ||||||
| Han | 68(79.07) | 73(89.02) | 70(81.40) | 58(74.36) | 74(86.05) | 73(84.88) |
| Miao | 5( 5.81) | 1( 1.22) | 3( 3.49) | 4( 5.13) | 0( 0.00) | 0( 0.00) |
| Zhuang | 5( 5.81) | 1( 1.22) | 5( 5.81) | 3( 3.85) | 2( 2.33) | 6( 6.98) |
| Yao | 8( 9.30) | 7( 8.54) | 6( 6.98) | 13(16.67) | 9(10.47) | 6( 6.98) |
| Others | 0( 0.00) | 0( 0.00) | 2( 2.33) | 0( 0.00) | 1( 1.16) | 1( 1.16) |
| Weight, mean ± SD (kg) | 5.33 ± 0.75 | 5.28 ± 0.66 | 5.26 ± 0.67 | 5.40 ± 0.64 | 5.23 ± 0.68 | 5.27 ± 0.69 |
| Length, mean ± SD (cm) | 58.97 ± 2.29 | 58.62 ± 2.44 | 58.63 ± 2.44 | 59.22 ± 2.32 | 58.72 ± 2.31 | 58.58 ± 2.26 |
| Type 1 Poliovirus | ||||||
| Seropositive, n(%) | 31( 36.05) | 43(52.44) | 46(53.49) | 41(52.56) | 45(52.33) | 45(52.33) |
| GMT(95%CI) | 8.27(6.39–10.71) | 10.43(8.06–13.51) | 11.27(8.86–14.35) | 9.48(7.36–12.21) | 9.99(7.85–12.71) | 10.87(8.36–14.13) |
| Type 2 Poliovirus | ||||||
| Seropositive, n(%) | 26(30.23) | 28(34.15) | 25(29.07) | 37(47.44) | 30(34.88) | 23(26.74) |
| GMT(95%CI) | 6.66(5.56–7.97) | 6.95(5.71–8.46) | 5.65(4.96–6.43) | 7.45(6.26–8.86) | 6.37(5.46–7.44) | 6.10(5.12–7.28) |
| Type 3 Poliovirus | ||||||
| Seropositive, n(%) | 14(16.28) | 14(17.07) | 8(9.30) | 11(14.10) | 10(11.63) | 18(20.93) |
| GMT(95%CI) | 4.87(4.37–5.44) | 5.12(4.47–5.85) | 4.56(4.11–5.07) | 5.04(4.36–5.82) | 4.85(4.21–5.58) | 5.79(4.85–6.92) |
cIPV = conventional inactivated poliovirus vaccine. bOPV = bivalent oral poliovirus vaccine. tOPV = trivalent oral poliovirus vaccine. GMT = geometric mean reciprocal titer.
Seroconversion, seroprotection, reciprocal titer and the times of reciprocal titer increase by vaccination groups based on PP population 30 days after the last vaccination are presented in Table 2. Differences in proportions of overall seroconversion to type 1 and 3 polioviruses were measured between I-T-T vs. I-B-B and I-I-T vs. I-I-B vaccination schedules, respectively, using 2-sided 95% confidence intervals (95% CIs) (Fig. 2).
Table 2.
Seroprotection, seroconversion and titer in per-protocol population 30 days after the last vaccination.
| cIPV-tOPV-tOPV (n = 86) | cIPV-cIPV-cIPV (n = 82) | cIPV-bOPV-bOPV (n = 86) | tOPV-tOPV-tOPV (n = 78) | cIPV-cIPV-tOPV (n = 86) | cIPV-cIPV-bOPV (n = 86) | |
|---|---|---|---|---|---|---|
| Type 1 Poliovirus | ||||||
| Seroconversion | 84(97.67, 91.85–99.72) | 75(91.46, 83.20–96.50) | 85(98.84, 93.69–99.97) | 75(96.15, 89.17–99.20) | 81(94.19, 86.95–98.09) | 81(94.19, 86.95–98.09) |
| Seroconversion in susceptible infants | 55(100.0, 93.51–100.0) | 39(100.0, 90.97–100.0) | 40(100.0, 91.19–100.0) | 37(100.0, 90.51–100.0) | 41(100.0, 91.40–100.0) | 41(100.0, 91.40–100.0) |
| Seroconversion in non-susceptible infants | 29(93.55, 78.58–99.21) | 36(83.72, 69.30–93.19) | 45(97.83, 88.47–99.94) | 38(92.68, 80.08–98.46) | 40(88.89, 75.95–96.29) | 40(88.89, 75.95–96.29) |
| Seroprotection | 86(100.0, 95.80–100.0) | 82(100.0, 95.50–100.0) | 86(100.0, 95.80–100.0) | 78(100.0, 95.38–100.0) | 86(100.0, 95.80–100.0) | 85(98.84, 93.69–99.97) |
| GMT | 1100.89(875.60–1384.2) | 301.42(246.33–368.85) | 1822.01(1444.3–2298.5) | 1459.89(1124.9–1894.6) | 864.44(630.70–1184.9) | 1167.98(849.70–1605.5) |
| GMI | 133.04(94.64–340.06) | 28.89(20.43–40.86) | 161.66(116.63–224.07) | 153.98(106.49–222.65) | 86.55(58.05–129.04) | 107.46(69.83–165.39) |
| Type 2 Poliovirus | ||||||
| Seroconversion | 82(95.35, 88.52–98.72) | 70(85.37, 75.83–92.20) | 48(55.81, 44.70–66.52) | 76(97.44, 91.04–99.69) | 81(94.19, 86.95–98.09) | 71(82.56, 72.87–89.90) |
| Seroconversion in susceptible infants | 60(100.0, 94.04–100.0) | 54(100.0, 93.40–100.0) | 47(77.05, 64.50–86.85) | 41(100.0, 91.40–100.0) | 56(100.0, 93.62–100.0) | 63(100.0, 94.31–100.0) |
| Seroconversion in non-susceptible infants | 22(84.62, 65.13–95.64) | 16(57.14, 37.18–75.54) | 1(4.00, 0.10–20.35) | 35(94.59, 81.81–99.34) | 25(83.33, 65.28–94.36) | 8(34.78, 16.38–57.27) |
| Seroprotection | 85(98.84, 93.69–99.97) | 82(100.0, 95.60–100.0) | 59(68.60, 57.70–78.19) | 78(100.0, 95.38–100.0) | 86(100.0, 95.80–100.0) | 85(98.84, 93.69–99.97) |
| GMT | 279.19 (218.50–356.74) | 136.94(106.68–175.77) | 11.01(8.97–13.51) | 271.39(216.87–339.62) | 662.66(480.02–914.79) | 53.49(43.82–65.29) |
| GMI | 41.94 (30.60–57.50) | 19.70 (3.40–28.96) | 1.95 (1.48–2.57) | 36.43 (27.56–48.16) | 103.99 (69.92–154.66) | 8.76 (6.42–11.97) |
| Type 3 Poliovirus | ||||||
| Seroconversion | 82(95.35, 88.52–98.72) | 80(97.56, 91.47–99.70) | 85(98.84, 93.69–99.97) | 78(100.0, 95.38–100.0) | 86(100.0, 95.80–100.0) | 84(97.67, 91.85–99.72) |
| Seroconversion in susceptible infants | 70(97.22, 90.32–99.66) | 68(100.0, 94.72–100.0) | 77(98.72, 93.06–99.97) | 67(100.0, 94.64–100.0) | 76(100.0, 95.26–100.0) | 68(100.0, 94.72–100.0) |
| Seroconversion in non-susceptible infants | 12(85.71, 57.19–98.22) | 12(85.71, 57.19–98.22) | 8(100.0, 63.06–100.0) | 11(100.0, 71.51–100.0) | 10(100.0, 69.15–100.0) | 16(88.89, 65.29–98.62) |
| Seroprotection | 84(97.67, 91.85–99.72) | 82(100.0, 95.60–100.0) | 85(98.84, 93.69–99.97) | 78(100.0, 95.38–100.0) | 86(100.0, 95.80–100.0) | 86(100.0, 95.80–100.0) |
| GMT | 480.91 (359.04–644.15) | 370.52(293.54–467.68) | 698.14(565.37–862.09) | 477.11(382.39–595.31) | 777.98(575.70–1051.4) | 1068.89(755.90–1511.4) |
| GMI | 98.68(72.31–134.66) | 72.43(55.02–95.36) | 153.00(121.20–193.14) | 94.73(74.40–120.63) | 160.38(116.90–220.03) | 184.55(123.75–275.22) |
Data for seroprotection and seroconversion are n(%, 95%CI); data for titer are GMT(95%CI). 95%CI was estimated by exact test. Seroprotection defined as neutralizing titer of at least 8. For susceptible infants who were seronegative (titer<8),seroconversion was defined as achieving an antibody titer of at least 8; for non-susceptible infants who were seropositive (titer> = 8),seroconversionwas defined as a titer four times higher than the expected fall in maternal antibody concentrations based on the pre-vaccination titer. cIPV = conventional inactivated poliovirus vaccine. bOPV = bivalent oral poliovirus vaccine. tOPV = trivalent oral poliovirus vaccine. GMT = geometric mean reciprocal titer. GMI = fold increase in GMT.
Figure 2.
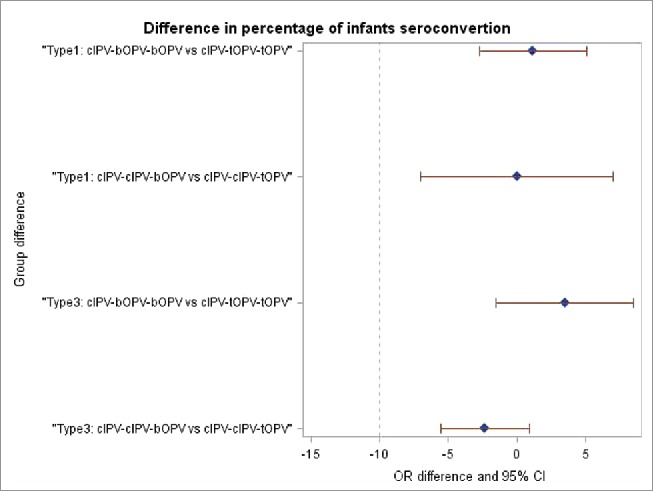
Differences in proportions of post-vaccination antibody seroconversion Differences in proportions of seroconversion to type 1 and 3 polioviruses were measured between cIPV-tOPV-tOPV vs. cIPV-tOPV-tOPV and cIPV-cIPV-tOPV vs. cIPV-cIPV-tOPV vaccination schedules using one-sided 95% CIs. cIPV = conventional inactivated poliovirus vaccine. bOPV = bivalent oral poliovirus vaccine. tOPV = trivalent oral poliovirus vaccine.
Type 1 poliovirus: Overall seroconversion rates to PV1 in the PP population at 30 d after the last vaccination were as follows: 97.67% for I-T-T, 91.46% for I-I-I, 98.84% for I-B-B, 96.25% for T-T-T, 94.29% for I-I-T, and 94.29% for I-I-B. No statistically significant differences among the 6 arms were detected using the Chi-Square Test (p = 0.2241). The difference between I-B-B and I-T-T seroconversion rates was 1.17%, with the lower 95%CI bound of −2.74%, and the difference between I-I-B and I-I-T in seroconversion rate was 0.00% with the lower bound of 95% CI −6.99%. Since the lower bounds of both 95% CIs were greater than the pre-specified margin −10%, non-inferiority was demonstrated.
The seroconversion rates of PV1 for susceptible infants after vaccination were 100.00% for all 6 arms. No statistically significant differences among the 6 arms were detected for the 4-fold increased rates among unsusceptible subjects (p = 0.2903).
Seroprotection rates for PV1 were 98.84% for I-I-B and 100.00% for the other 5 groups. No statistically significant differences among the 6 arms were detected using Chi-Square Test (p = 0.4319).
The overall geometric mean of the reciprocal antibody titer (GMT) of PV1 antibody was significantly higher in the I-B-B group than in the I-T-T (p = 0.0025), and numerically higher in I-I-B than in I-I-T. Increases of GMT were numerically greater after both schedules of bOPV than after tOPV.
Type 3 poliovirus: Seroconversion rates to PV3 in the PP population at 30 days after the last vaccination were as follows: 95.35% for I-T-T, 97.56% for I-I-I, 98.84% for I-B-B, 100.00% for T-T-T, 100.00% for I-I-T, and 97.67% for I-I-B. No statistically significant difference among the 6 arms were detected using the Chi-Square test (p = 0.1834). The difference between the I-B-B and I-T-T seroconversion rates was 3.49% with the 95%CI lower bound of −1.50%. The difference between the I-I-B and I-I-T seroconversion rates was −2.32% with a 95% CI lower bound of −5.51%. Since the lower bounds of both 95% CIs were greater than the pre-specified margin of −10%, non-inferiority was demonstrated.
The seroconversion rates of PV3 in susceptible infants after vaccination were 97.22% for I-T-T, 100.00% for I-I-I, 98.72% for I-B-B, 100.00% for T-T-T, I-I-T, and I-I-B arms. No statistically significant differences among the 6 arms were detected using Chi-Square test (p = 0.2339). No statistically significant differences among the 6 arms were detected for the 4-fold increase rates among unsusceptible subjects (p = 0.5124).
Seroprotection rates of PV3 were 97.67% for I-T-T, 98.84% for I-B-B, 100.00% for I-I-I, T-T-T, I-I-T, and I-I-B arms. No statistically significant differences among the 6 arms were detected using Chi-Square test (p = 0.2393).
The GMT of PV3 antibody was lower for the I-T-T group than for the I-B-B (p = 0.0413) and was numerically higher in the I-I-B group than in I-I-T (p = 0.1706). Group I-B-B exhibited a statistically higher increase of GMT (p = 0.0261).
Type 2 poliovirus: Seroconversion rates to PV2 in the PP population at 30 days after the last vaccination were as follows: 98.35% for I-T-T, 85.37% for I-I-I, 55.81% for I-B-B, 97.44% for T-T-T, 94.19% for I-I-T, and 82.56% for the I-I-B group. Seroconversion rates of PV2 in susceptible infants were 77.05%% for I-B-B, and 100.00% for the other 5 arms. Seroprotection rate of PV2 in PP population at 30 days after vaccination were 98.84% for I-T-T, 68.60% for I-B-B, 98.84% for I-I-B, 100.00% for I-I-I, T-T-T and I-I-T.
Differences among the 6 arms for above immunogenicity endpoints of PV2 were statistically significant (p<0.0001).
The increase of GMT against PV2 using the I-I-T vaccination schedule was numerically higher (103.99 folds, 95%CI = 69.92–154.66), followed by I-T-T, T-T-T, I-I-I, I-I-B, and I-B-B schedules.
The reverse cumulative distribution curves, which are a summary measure of antibody distribution, show similar curves for arms with similar type-specific seroconversion rates (Fig. 3).
Figure 3.
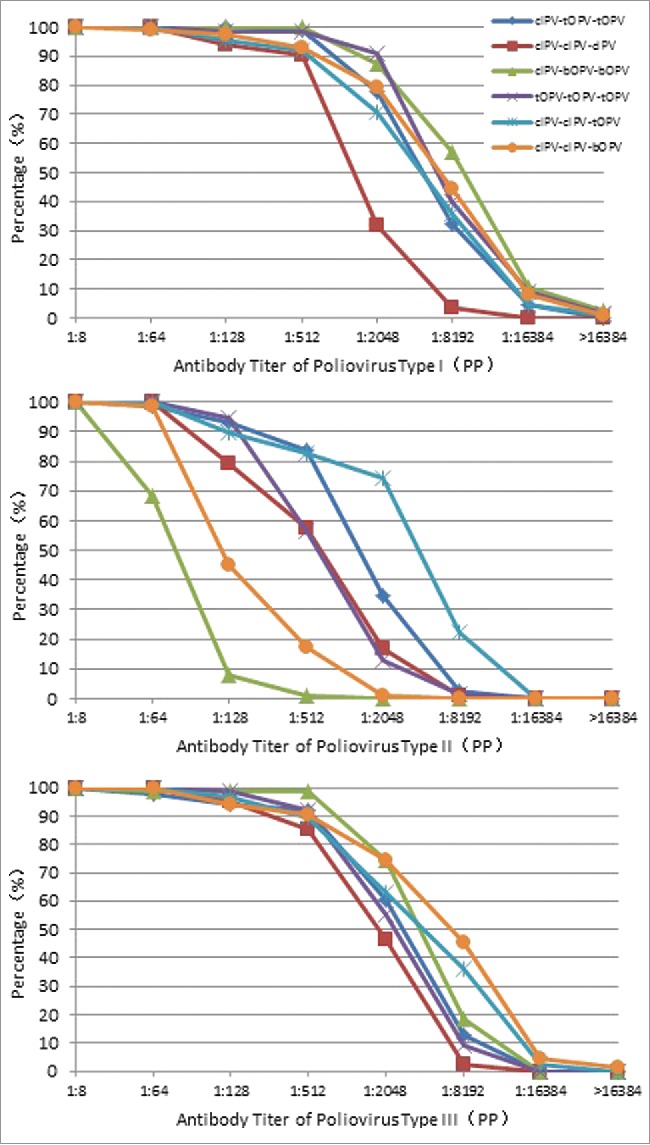
Reverse poliovirus antibody distribution curves PP = per-protocol. cIPV = conventional inactivated poliovirus vaccine. bOPV = bivalent oral poliovirus vaccine. tOPV = trivalent oral poliovirus vaccine.
Overall, all vaccines given during the study were well tolerated; and no vaccine-related serious adverse event (SAE) were reported (Table 3). Among 24 subjects with SAEs unrelated to the vaccine, 4 subjects (4.00%) had 4 SAEs were in the I-T-T group. Three subjects (3.03%) had 4 SAEs in I-I-I group. Five subjects (5.00%) had 7 SAEs in I-B-B group. One subject (0.99%) had one SAE in T-T-T group. Four subjects (4.00%) had 4 SAEs in I-I-T group. Seven subjects (7.00%) had 8 SAEs in I-I-T. There were no statistically significant differences in SAEs among the arms (p = 0.3628). Infectious pneumonia was the main SAE (10 subjects, 1.67%), followed by bronchitis (4 subjects, 0.67%) and hand-foot-and-mouth disease (4 subjects, 0.67%). The incidences of solicited and unsolicited AEs are presented in Table 3.
Table 3.
Summary of adverse events and subjects with adverse events.
| cIPV-tOPV-tOPV (n = 100) | cIPV-cIPV-cIPV (n = 99) | cIPV-bOPV-bOPV (n = 100) | tOPV-tOPV-tOPV (n = 101) | cIPV-cIPV-tOPV (n = 100) | cIPV-cIPV-bOPV (n = 100) | Total (n = 600) | |
|---|---|---|---|---|---|---|---|
| Serious adverse events | |||||||
| Number of AEs | 4 | 4 | 7 | 1 | 4 | 8 | 28 |
| Number of subjects with AEs (n/N%) | 4 (4.00%) | 3 (3.03%) | 5 (5.00%) | 1 (0.99%) | 4 (4.00%) | 7 (7.00%) | 24 (4.00%) |
| Systemic reactions* | |||||||
| Number of AEs | 248 | 256 | 242 | 239 | 289 | 257 | 1531 |
| Number of subjects with AEs (n/N%) | 83 (83.00%) | 76(76.77%) | 78 (78.00%) | 78 (77.23%) | 90 (90.00%) | 90 (90.00%) | 495 (82.50%) |
| Fever | |||||||
| Number of AEs | 186 | 188 | 173 | 170 | 202 | 180 | 1099 |
| Number of subjects with AEs (n/N%) | 80 (80.00%) | 70 (70.71%) | 71 (71.00%) | 75 (74.26%) | 86 (86.005) | 85 (85.00%) | 467 (77.83%) |
| Irritability/fussiness | |||||||
| Number of AEs | 10 | 16 | 13 | 15 | 20 | 20 | 94 |
| Number of subjects with AEs (n/N%) | 8 (8.005) | 10 (10.00%) | 9 (9.00%) | 11 (10.89%) | 15 (15.00%) | 13 (13.00%) | 66 (11.00%) |
| Somnolence | |||||||
| Number of AEs | 3 | 3 | 2 | 3 | 2 | 2 | 15 |
| Number of subjects with AEs (n/N%) | 3 (3.00%) | 3 (3.03%) | 2 (2.00%) | 2 (2.97%) | 2 (2.00%) | 2 (2.00%) | 15 (2.50%) |
| Vomit | |||||||
| Number of AEs | 7 | 7 | 8 | 3 | 14 | 3 | 42 |
| Number of subjects with AEs (n/N%) | 6 (6.00%) | 7 (7.07%) | 7 (7.00%) | 2 (2.97%) | 9 (9.00%) | 3 (3.00%) | 35 (5.83%) |
| Diarrhea | |||||||
| Number of AEs | 42 | 42 | 45 | 46 | 46 | 47 | 268 |
| Number of subjects with AEs (n/N%) | 34 (34.00%) | 28 (28.28%) | 31 (31.00%) | 29 (28.71%) | 29 (29.00%) | 29 (29.00%) | 180 (30.00%) |
| Allergic reaction | |||||||
| Number of AEs | 0 | 0 | 1 | 2 | 5 | 5 | 13 |
| Number of subjects with AEs (n/N%) | 0 | 0 | 1 (1.00%) | 1 (0.99%) | 5 (5.00%) | 5 (5.00%) | 12 (2.00%) |
| Local reaction: Redness on injection site | |||||||
| Number of AEs | 1 | 3 | 2 | 0 | 4 | 2 | 12 |
| Number of subjects with AEs (n/N%) | 1 (1.00%) | 3 (3.03%) | 2 (2.00%) | 0 | 3 (3.00%) | 2 (2.00%) | 11 (1.83%) |
Systemic reactions involve fever, easy irritation, somnolence, vomit, diarrhea and allergic reaction.
cIPV = conventional inactivated poliovirus vaccine. bOPV = bivalent oral poliovirus vaccine. tOPV = trivalent oral poliovirus vaccine.
Discussion
Eradication of wild type poliovirus has been achieved through intensive tOPV vaccination worldwide.16,17 Although the usage of tOPV effectively disrupts the transmission of the live virus, eradication of all circulating vaccine-derived polioviruses from the reverse mutated OPV Sabin strains requires the transfer of the vaccination schedule from OPV to IPV. Sequential removal of OPV-derived Sabin poliovirus strains from populations is a key objective to achieve the eradication goal. The first step toward this goal is the removal of vaccine-derived Sabin PV2, because naturally circulating wild PV2 was eradicated in 1999.6 Infection by vaccine-derived Sabin PV2 contributed to 26–31% of vaccine-associated paralytic poliomyelitis,7 which presented a significant public health burdens worldwide. Accordingly, the Global Polio Eradication Initiative raises a roadmap for new immunization schedule consisting of multiple doses of bOPV following at least one dose of IPV. Given the blended use of IPV and bOPV, the safety and immunogenicity of a new immunization schedule have urgently needed re-evaluation for different global regions and human populations. Previous studies have assessed the immunogenicity and safety of a blended IPV-OPV immunization schedule in Bangladesh18,19 Chile20 Panama,21 and India.22,23
In the current study, we have evaluated the immunogenicity and safety of bOPV by comparing different poliomyelitis vaccination schedules in China using a randomized controlled non-inferiority clinical trial. In general, bOPV was non-inferior to tOPV in terms of immunogenicity since the lower bounds of the 95% CIs for the differences between the treatment arms (I-B-B vs. I-T-T and I-I-B vs. I-I-T) were greater than pre-specified non-inferiority margin of −10%.
Reciprocal antibody titer against PV1 and PV3 were higher after both schedules with bOPV than after tOPV. The lowest reciprocal titer was identified in the I-I-I arm. A low GMT with PV1 was observed in the I-I-I arm. This finding was expected and was consistent with the results of previous studies using 3-dose cIPV manufactured by Sanofi Pasteur. In a comparison study among Chinese population, the GMT after vaccinated by cIPV was 386 (297–502), while the GMT after vaccine with tOPV was 3315 (2703–4064). 24
Compared with I-I-B, I-B-B possessed a significantly higher PV1 reciprocal titer, while the PV3 reciprocal antibody titer in I-I-B was higher than in the I-B-B group. In the context of the final stage of polio eradication, PV2 immunity is of concern as well. Both vaccination schedules with bOPV have shown certain efficacy of seroconversion and seropositivity against PV2 compared with other arms. The results favor I-I-B compared with the I-B-B vaccination schedule. Additionally, there was no significant difference in safety between the I-I-B and I-B-B vaccination schedules. Therefore, with at least one dose of IPV, adequate prevention of PV2 infection could be achieved, and the I-I-B vaccination schedule might be preferred choice if conditions allowed.
Our findings were consistent with the results of a study using different poliovirus vaccination schedules in Chile in which the seroconversion rates against PV1 (99% for I-B-B, 100% for I-I-B and 100% for I-I-I) and PV3 (98% for I-B-B, 100% for I-I-B and 99% for I-I-I) were non-inferior in vaccination schedules containing IPV and bOPV, compared with an all-IPV schedule, and proportions of infants with protective antibodies were high after all schedules.,21,15 Comparable results in an India Polio study showed that the seroconversion rates against PV1 were 99% for bOPV, bOPV-IPV and B-I-I arms; and the seroconversion rates against PV3 were 96%, 99% and 99% for bOPV, bOPV-IPV and B-I-I arms, respectively.22,23 The improved seroconversions to types 1 and 3 were also consistent with results from Bangladesh18,19 Panama21 and Pakistan.25
The safety results suggest that the immunization schedules with IPV and bOPV were well tolerated. No vaccine related SAEs were observed, and all local and systemic reactions were mild; which was consistent with previous studies globally. No SAEs were attributed to the vaccines in safety results in the studies in India, Bangladesh, Panama and Pakistan. Only one SAE was considered vaccine related (intestinal intussusception) in Chilean population.15
Current data indicated that the sequential schedules are suitable for China, as they achieved adequate immunogenicity, whereas the cIPV-only schedule showed lower GMTs; which is in line with expectations. These results were supported by the results from studies comparing immunogenicity and tolerability among different schedules with IPV and tOPV but not bOPV in China.26,27,28
Our study had 2 major limitations. First, vaccination arm assignment in the study could not be masked because of the different vaccination formulations used. This limitation may not impact on the immunogenicity results, but may have affected the reports of adverse events. Another limitation is the generalizability of our findings to all areas in China which covers 56 ethnic groups and broad areas with different socioeconomic status as well as other developing countries.
In developing countries, the acceptability of injection is limited compared with the oral route mainly due to poor clinical practices, higher cost, and occasional pain and bleeding, which may reduce the pace of introducing IPV globally and may ultimately impact the elimination of poliovirus.29,30 Increased availability and affordability of IPV in developing countries will be important prerequisites to ensure global withdrawal of the tOPV. The proportion of children using IPV is increasing each year in China, which is consistent with the introduction of IPV that is called for by the Polio Eradication Endgame Strategic Plan.31 GPEI report indicated that 7 countries which have not already received their first IPV shipment through UNICEF (United Nations Children's Fund) and were considered at low risk for polio outbreaks will be delayed to introduce IPV until the first quarter of 2017. 32
In conclusion, the current results support the application of the I-B-B and I-I-B vaccine schedules, substituting these vaccinations with pure tOPV or IPV and tOPV without compromising safety and immunogenicity. Our findings regarding bOPV and the different poliovirus vaccination schedules in the Chinese population would provide national and global policy makers with flexibility when choosing a vaccination schedule for eliminating vaccine-associated and vaccine-derived poliomyelitis.
Methods
Study design
This randomized controlled non-inferiority clinical trial was conducted between April 8 and August 23, 2015, at the Center of Diseases Control and Prevention (CDC) of Hezhou County and Zhongshan County in Guangxi Zhuang Autonomous Region of China. The study was approved by the ethics review committees of the CDC of Guangxi Zhuang Autonomous Region and authorized by the Center for Drug Evaluation of the China Food and Drug Administration for implementation (clinical trial authorization number: 2014L00500). The study was also registered in clinicaltrial.gov (NCT02785705).
Participants
Eligible participants were healthy full-term (37–42 weeks) infants aged 60–90 days who weighed more than 2.5 kg at birth with no obvious medical disorders, no polio vaccination, no immunoglobulin vaccination, with no other attenuated vaccine administered in the past 14 days and no other inactivated vaccine administered.
Participants were excluded if meet one or more of the following criteria: had or were at risk of immunodeficiency, severe allergic reaction, acute fever or infectious diseases, severe chronic diseases, family history of allergies, convulsions, seizures, encephalopathy or psychiatric diseases, oral steroids during at least 14 consecutive days of the preceding month, auxiliary temperature equal or greater than 38.0°C during the past 3 days, diarrhea (defection frequency equal or greater than 3 times per day) in the past 7 days, and participated in other drug clinical trials.
Participants could receive DTP vaccine during the study. However, they would need to have a 14-day interval from the polio vaccination.
Voluntary and informed consent for participation of newborn babies were obtained at enrollment from parents or guardians in accordance with ethical principles. Participants could withdraw from the study at any time point, on request by the mother or a legally acceptable representative. Reasons for withdrawal were collected where possible.
Randomization and masking
Serial numbers from 1–600 were equally randomized (1:1:1:1:1:1) into 6 sequential vaccination schedules as follows: cIPV-bOPV-bOPV(I-B-B), cIPV-tOPV-tOPV(I-T-T), cIPV-cIPV-bOPV(I-I-B), cIPV-cIPV-tOPV(I-I-T), cIPV-cIPV-cIPV(I-I-I), and tOPV-tOPV-tOPV(T-T-T). Sites were provided with sealed envelopes that contained the allocation assignments for emergency unblinding.
Considering that the formulations are different, the vaccines could not be completely masked (oral vs. injectable). cIPV, bOPV, and tOPV were coded as A, B, and C, respectively. However, the bOPV and tOPV vaccines could be masked; and laboratory investigators were blinded to group assignments. A statistician would analyze data unblinded with the allocation schedule after the database was locked.
Procedure
Healthy infants received following 3 doses sequentially at 4–6 weeks interval after collecting baseline blood sample as follows: I-B-B, I-T-T, I-I-B, I-I-T, I-I-I, and T-T-T. Subjects were proactively followed up for observation of adverse events from the first dose to 30 days after all doses. The blood samples were collected at pre-vaccination and 30 days after the third dose to test for antibodies to type 1, 2, and 3 polioviruses.
Serum samples were prepared within 24 hours after collection of blood (2.5mL). Half of the sample was used for antibodies testing, routine serum chemistry and hematological laboratory testing, and the other half was frozen and stored at −20°C using dry ice if needed. Immunogenicity was tested using the micro-neutralization assay, which has been described previously and applied, by National Institutes for Food and Drug Control. 18,33
At least 8 site visits were required as follows: 2 for each vaccination, one 30 days after the full immunization schedule, and another visit 6 months after all 3 vaccinations. Investigators followed up with a phone call or optional visits for any further information needed. Investigators recorded medical histories and provided training and a diary card for parents to record safety data and concomitant medications. Parents or guardians were asked at each site visits to provide information about any adverse event that occurred since the last visit, which were recorded on the diary card as well. The solicited adverse events were collected within 2 weeks and unsolicited adverse events were collected for 30 d after each vaccination. Serious adverse events were recorded for 6 months post all 3 vaccinations.
Tiantan Biological Product Co. (Beijing, China) manufactured the bOPV and tOPV vaccines used in this trial which is the pivotal study for bOPV registration in China. The cIPV was produced by Sanofi Pasteur (Lyon, France). Both tOPV and cIPV are licensed in China.
Outcomes
The primary immunogenicity outcome was the proportion of infants with seroconversion which was defined as antibody titers at 30 days after all 3 vaccinations equal or larger than 8 for susceptible infants or post-vaccination reciprocal titers 4 times higher than the pre-vaccination titers for unsusceptible infants. Here, susceptible infants are those whose pre-vaccination reciprocal titers were less than 8. Otherwise, the subjects were categorized as unsusceptible. The seroconversion rates of susceptible infants and 4-folder increased rate among unsusceptible infants were also calculated, respectively.
Other immunogenicity outcomes included the overall seroprotection rate, which was defined as the proportion of subjects with reciprocal titers of at least 8, the geometric mean of reciprocal antibody titer (GMT) and the fold increase in GMT (GMI).
The primary safety outcome was the proportion of infants with serious adverse events 6 months after the vaccination with the different vaccination schedules. Solicited adverse events, including both systemic reactions (including fever, irritability/fussiness, somnolence, vomiting, diarrhea, and allergic reactions) and local reactions (including tenderness, redness, swelling, and callous around the injection sites), within 30 days after the vaccination were presented and compared across all arms.
Statistical analyses
A sample size of at least 85 was needed for each arm based on the assumptions of 95% seroconversion rates for PV1 and PV3 and a 10% non-inferiority margin to achieve 85% power with one-sided α level of 0.025. The trial would be considered successful if both PV1 and PV3 achieved non-inferiority. It is planned that 100 subjects would be recruited for each arm in the case of any dropouts.
The PP population included all subjects who completed all 3 vaccinations, had complete pre- and post-vaccination immunogenicity data, met all inclusion criteria and no exclusion criteria, had good compliance, etc. The PP population was the primary population in the immunogenicity analyses presented in this article.
The non-inferiority analyses were conducted between the 2 arms with the same vaccination schedule. Non-inferiority was considered if all lower bounds of 95% CIs of the differences between the I-B-B arm and I-T-T arm, I-I-B arm and I-I-T arm for both PV1 and PV2 are greater than −10%. No α adjustments were needed for these multiple comparisons.
The Clopper-Pearson method 34 was used to compute the 95% confidence intervals (CIs) for the rates. Their differences among the groups were compared using Fisher's exact test or the Chi-Square test. GMT and GMI were compared using an analysis of variance (ANOVA) after a log-transformation. Two-sided p-values and 95% CIs for immunogenicity assessments were provided.
For safety assessment, serious adverse events (SAEs) within 6 months and adverse events (AEs) within 30 days after the vaccination were assessed and compared among the 6 arms using Fisher's exact test.
We conducted the statistical analyses using SAS software (version 9.2).
Clinical trials registration
Abbreviations
- AEs
Adverse Events
- bOPV
bivalent Oral Poliovirus Vaccine
- cIPV
trivalent conventional Inactivated Poliovirus Vaccine
- CDC
Center of Diseases Control and Prevention
- cVDPV
circulating Vaccine-Derived Poliovirus
- DTP
Diphtheria-Tetanus-Pertussis vaccine
- GPEI
Global Polio Eradication Initiative
- GMT
Geometric Mean of Reciprocal Antibody Titer
- GMI
Fold Increase in GMT
- IPV
Inactivate Poliovirus Vaccine
- I-B-B
cIPV-bOPV-bOPV
- I-T-T
cIPV-tOPV-tOPV
- I-I-B
cIPV-cIPV-bOPV
- I-I-T
cIPV-cIPV-tOPV
- I-I-I
cIPV-cIPV-cIPV
- OPV
Oral Poliovirus Vaccine
- PP
Per-Protocol
- PV1
Type 1 Poliovirus
- PV2
Type 2 Poliovirus
- PV3
Type 3 Poliovirus
- SAEs
Serious Adverse Events
- T-T-T
tOPV-tOPV-tOPV
- tOPV
trivalent Oral Poliovirus Vaccine
- UNICEF
United Nations Children's Fund
- WPV1
Wild Type 1 Poliovirus
- WHO
World Health Organization
- 95% CIs
95% Confidence Intervals
Disclosure of potential conflicts of interest
The authors declare no conflicts of interests.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of Center of Disease Control and other contributing agencies. We sincerely thank all investigators and the families who participated in the study.
Funding
This work was supported by National Natural Science Foundation of China (Grant No. 81273176; 81473069), National Major Scientific and Technological Special Project for Significant New Drugs Development of China (Grant No. 2015ZX09501008–004), and Technological Special Project for Significant New Drugs Development of China (Grant No.2013ZX09402302–003).
Author contributions
Jingjun Qiu: drafted the manuscript and provided statistical analysis.
Yunkai Yang: trial design and coordinate trial conduction
Ling Wang: trial design and statistical analysis
Zhiwei Jiang: provided the main part of statistical analysis.
Wei Wang: trial conduction
Hongyan Wang: process optimization
Shaohong Guo: trial conduction
Chanjuan Li: trial design and statistical analysis
Prof. Jielai Xia: contributed to the study concept and design.
Dr. Zhaojun Mo: trial design, conduct and evaluation
Prof. Shuyuan Wei: contributed to the study concept and design
All authors contributed to the interpretation of data, final review and approval of the manuscript.
References
- [1].Kew OM, Sutter RW, De Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Ann Rev Microbiol. 2005; 59:587-635; https://doi.org/ 10.1146/annurev.micro.58.030603.123625 [DOI] [PubMed] [Google Scholar]
- [2].Global Polio Eradication Initiative (GPEI) Polio Eradication and Endgame Strategic Plan: 2013–2018. 2013. Available at: http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf [Google Scholar]
- [3].Global Polio Eradication Initiative (GPEI) Wild poliovirus list. n.d.. Available at: http://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/
- [4].Garon JR and Orenstein WA. A worldwide shift in polio vaccines for routine immunization. Lancet 2015; 386(10011):2375-77; PMID:26388533; https://doi.org/ 10.1016/S0140-6736(15)00243-3 [DOI] [PubMed] [Google Scholar]
- [5].Lopalco PL. Wild and vaccine-derived poliovirus circulation, and implications for polio eradication. Epidemiol Infect 2016; 21:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].WHO Polio vaccines: WHO position paper. Wkly Epidemiol Rec 2014; 89:73-92; PMID:24707513 [PubMed] [Google Scholar]
- [7].Hogle J. Poliovirus cell entry: common structural themes in viral cell entry pathways. Annu Rev Microbiol 2002; 56:677-702; PMID:12142481; https://doi.org/ 10.1146/annurev.micro.56.012302.160757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Platt LR, Estívariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis 2014; 210 (suppl 1):S380-89; PMID:25316859; https://doi.org/ 10.1093/infdis/jiu184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Patel M, Zipursky S, Orenstein W, Garon J, Zaffran M. Polio endgame: the global introduction of inactivated polio vaccine. Expert Rev Vaccines 2015; 14:749-62; PMID:25597843; https://doi.org/ 10.1586/14760584.2015.1001750 [DOI] [PubMed] [Google Scholar]
- [10].Yan D, Zhang Y, Zhu S, Chen N, Li X, Wang D, Ma X, Zhu H, Tong W, Xu W. Limited and localized outbreak of newly emergent type 2 vaccine-derived poliovirus in Sichuan, China. Clin Vaccine Immunol 2014; 21(7):1012-8; PMID:24850620; https://doi.org/ 10.1128/CVI.00196-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, Rubin J, Garon J, Harris J, Hyde T, et al.. Cessation of Trivalent Oral Poliovirus Vaccine and Introduction of Inactivated Poliovirus Vaccine - Worldwide 2016. MMWR Morb Mortal Wkly Rep 2016; Sep 9;65(35):934-8; https://doi.org/ 10.15585/mmwr.mm6535a3 [DOI] [PubMed] [Google Scholar]
- [12].Nasir UN, Bandyopadhyay AS, Montagnani F, Akite JE, Mungu EB, Uche IV, Ismaila AM. Polio elimination in Nigeria: A review. Hum Vaccin Immunother. 2016; 12(3):658-63; PMID:26383769; https://doi.org/ 10.1080/21645515.2015.1088617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].United States Aid for International Development (USAID) Pakistan - Complex Emergency. Fact Sheet #3, Fiscal Year 2016. 2017. Available at: http://www.usaid.gov/what-we-do/working-crises-and-conflict/responding-times-crisis/where-we-work [Google Scholar]
- [14].Morales M, Tangermann RH, Wassilak SG. Progress Toward Polio Eradication — Worldwide, 2015–2016. MMWR Morb Mortal Wkly Rep 2016; 13;65(18):470-3; https://doi.org/ 10.15585/mmwr.mm6518a4 [DOI] [PubMed] [Google Scholar]
- [15].Garon J, Seib K, Orenstein WA, Ramirez Gonzalez A, Chang Blanc D, Zaffran M, Patel M. Polio endgame: the global switch from tOPV to bOPV. Expert Rev Vaccines 2016 Jun; 15(6):693-708; https://doi.org/ 10.1586/14760584.2016.1140041 [DOI] [PubMed] [Google Scholar]
- [16].Hagan JE, Wassilak SG, Craig AS, Tangermann RH, Diop OM, Burns CC. Quddus A; Centers for Disease Control and Prevention (CDC). Progress toward polio eradication-worldwide, 2014–2015. MMWR-Morbidity and mortality weekly report 2015; 64(19):527-31; PMID:25996095 [PMC free article] [PubMed] [Google Scholar]
- [17].Hampton LM. Introduction of Inactivated Poliovirus Vaccine and Switch from Trivalent to Bivalent Oral Poliovirus Vaccine-Worldwide, 2013–2016. MMWR- Morbidity and mortality weekly report 2015; 64(25):699-702; PMID:26135591 [PMC free article] [PubMed] [Google Scholar]
- [18].Estívariz CF, Anand A, Gary HE, Rahman M, Islam J, Bari TI, Wassilak SG, Chu SY, Weldon WC, Pallansch MA, et al.. Immunogenicity of three doses of bivalent, trivalent, or type 1 monovalent oral poliovirus vaccines with a 2 week interval between doses in Bangladesh: an open-label, non-inferiority, randomised, controlled trial. Lancet Infect Dis 2015; 15(8):898-904; PMID:26093980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mychaleckyj JC, Haque R, Carmolli M, Zhang D, Colgate ER, Nayak U, Taniuchi M, Dickson D, Weldon WC, Oberste MS, et al.. Effect of substituting IPV for tOPV on immunity to poliovirus in Bangladeshi infants: An open-label randomized controlled trial. Vaccine 2016; 34(3):358-66; PMID:26643930 [DOI] [PubMed] [Google Scholar]
- [20].O'Ryan M, Bandyopadhyay AS, Villena R, Espinoza M, Novoa J, Weldon WC, Oberste MS, Self S, Borate BR, Asturias EJ, et al.. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study. Lancet Infect Dis 2015; 15(11):1273-82; PMID:26318714 [DOI] [PubMed] [Google Scholar]
- [21].Sáez-Llorens X, Clemens R, Leroux-Roels G, Jimeno J, Clemens SA, Weldon WC, Oberste MS, Molina N, Bandyopadhyay AS. Immunogenicity and safety of a novel monovalent high-dose inactivated poliovirus type 2 vaccine in infants: a comparative, observer-blind, randomised, controlled trial. Lancet Infect Dis 2015; 16(3):321-30; PMID:26719058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sutter RW, Bahl S, Deshpande JM, et al.. Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label, randomised controlled trial. Lancet 2015; 386(10011):2413-21; PMID:26388534 [DOI] [PubMed] [Google Scholar]
- [23].Sutter RW, John TJ, Jain H, Agarkhedkar S, Ramanan PV, Verma H, Deshpande J, Singh AP, Sreevatsava M, Malankar P, et al.. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet 2010; 376(9753):1682-88; PMID:20980048 [DOI] [PubMed] [Google Scholar]
- [24].Liao G, Li R, Li C, Sun M, Li Y, Chu J, Jiang S, Li Q. Safety and immunogenicity of inactivated poliovirus vaccine made from Sabin strains: a phase II, randomized, positive-controlled trial. J Infect Dis 2012; 15;205(2):237-43. [DOI] [PubMed] [Google Scholar]
- [25].Mir F, Quadri F, Mach O, Ahmed I, Bhatti Z, Khan A, Rehman NU, Durry E, Salama M, Oberste SM, Weldon WC, Sutter RW, Zaidi AK. Monovalent type-1 oral poliovirus vaccine given at short intervals in Pakistan: a randomised controlled, four-arm, open-label, non-inferiority trial. Lancet Infect Dis 2015; 15(8):889-97; PMID:26093979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lu L, Li X, Zhang H, Liu D, Zhang Z, Wang H, Liu F, Ning Z, Li J, Pang X. Immunogenicity and persistence from different 3-dose schedules of live and inactivated polio vaccines in Chinese infants. Vaccine 2015; 33(36):4653-8; PMID:25681659 [DOI] [PubMed] [Google Scholar]
- [27].Li R, Li CG, Li Y, Liu Y, Zhao H, Chen X, Kuriyakose S, Van Der Meeren O, Hardt K, Hezareh M, et al.. Primary and booster vaccination with an inactivated poliovirus vaccine (IPV) is immunogenic and well-tolerated in infants and toddlers in China. Vaccine 2016; 34(12):1436-43; PMID:26873055 [DOI] [PubMed] [Google Scholar]
- [28].Li RC, Li CG, Wang HB, Luo HM, Li YP, Wang JF, Ying ZF, Yu WZ, Shu JD, Wen N, et al.. Immunogenicity of Two Different Sequential Schedules of Inactivated Polio Vaccine Followed by Oral Polio Vaccine Versus Oral Polio Vaccine Alone in Healthy Infants in China. J Pediatric Infect Dis Soc 2015; 5(3):287-96 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [29].Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nature Medicine. 2003; 9, 99-103; PMID:12514720 [DOI] [PubMed] [Google Scholar]
- [30].Mitragotri S. Immunization without needles. Nature Reviews Immunology. 2005; 5, 905-916 [DOI] [PubMed] [Google Scholar]
- [31].Chang C, Zhang J, Zhou J, Cao R, Song K, Liu C, Zhang X, Geng X, Liu X, Li C. Coverage estimates and patterns of inactivated poliovirus vaccine (IPV) use prior to and during the polioeradication endgame, Jinan City, China, 2010–2015. Hum Vaccin Innumother. 2016; 12(11):2749-52; PMID:16239901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Global Polio Eradication Initiative (GPEI) Update on the OPV switch and the supply constraints for IPV. 2016. Available at: http://www.who.int/immunization/diseases/poliomyelitis/endgame_objective2/inactivated_polio_vaccine/Information_Note_on_short_term_supply_constraints_for_IPV-Nov_2015.pdf
- [33].Albrecht P, van Steenis G, van Wezel AL, Salk J. Standardization of poliovirus neutralizing antibody tests. Rev Infect Dis 1984; 6(Supplement_2): S540-S544. [DOI] [PubMed] [Google Scholar]
- [34].Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of binomial. Biometrika 1934; 26:404-13; https://doi.org/ 10.1093/biomet/26.4.404 [DOI] [Google Scholar]