ABSTRACT
A universal vaccine that provides long-lasting protection from both epidemic and pandemic influenza viruses remains the “holy grail” of influenza vaccine research. Though virus neutralization assays are the current benchmark of measuring vaccine effectiveness, it is clear that Fc-receptor functions can drastically improve the effectiveness of antibodies and vaccines in vivo. Antibodies that kill virus-infected cells and/or elicit an antiviral environment, termed antibody-dependent cellular cytotoxicity (ADCC)-mediating antibodies, provide a link between the innate and adaptive immune response. New technologies allowing the rapid isolation and characterization of monoclonal antibodies (mAb) have yielded a plethora of mAbs which target conserved regions of influenza virus, such as the hemagglutinin (HA) stem region. Many such mAbs have been used to gain a better understanding of Fc-receptor functions in vivo. In parallel, several studies have characterized the induction of polyclonal ADCC following influenza vaccination and infection in humans. Taken together, these studies suggest that ADCC-mediating antibodies (ADCC-Abs) significantly contribute to host immunity against influenza virus and may be a mechanism to exploit for rational vaccine and therapeutic design. We discuss recent research on influenza-specific ADCC and potential future avenues to extend our understanding.
KEYWORDS: antibody-dependent cellular cytotoxicity, influenza, monoclonal antibodies, universal vaccine
Introduction
Seasonal influenza epidemics and pandemics result in extensive global morbidity and mortality in humans. Seasonal influenza viruses infect approximately 10–20% of world's population and result in 250–500,000 deaths per year, with deaths occurring disproportionately in lower income countries.1 Influenza also imposes an immense economic and public health burden, with total spending estimated to be in excess of $US 26.8–87.1 billion per year in the US alone.2 Yearly influenza vaccination strategies act to reduce the severity influenza epidemics on the human population. Unfortunately, the effectiveness of seasonal influenza vaccines is significantly reduced by the continual accumulation of amino acid substitutions and differential glycosylation patterns at regions targeted by the vaccine-mediated antibody response, combined with waning serological concentrations of protective antibodies in each individual over time.3,4 This very effective immune evasion strategy by the virus necessitates globally coordinated strategies to actively survey for “drifted” virus strains and yearly efforts to reformulate the vaccine with antigenically relevant strains. Since seasonal influenza vaccines provide limited protection against novel influenza subtypes, substantial resources and effort have been expended to develop broadly protective vaccines and therapeutics effective against multiple subtypes of influenza viruses. Such strategies include, but are not limited to, eliciting antibodies targeting conserved regions of influenza surface proteins HA-stem,5-8 M2 protein,9-14 NA protein.15,16 as well as T cell-based vaccine approaches17 A more detailed understanding of the immunological mechanisms of vaccine-mediated protection will be beneficial for rational immunogen and vaccine design.
Influenza vaccine effectiveness studies have generally focused on the induction of antibodies that mediate neutralization, a proven correlate of protection. However antibodies that target the HA-head region, while potently neutralizing, generally only recognize related viruses within a narrow range of antigenic diversity, whereas, antibodies that bind to the conserved stem region have been found to provide broadly cross-reactive immunity, but are less potent at neutralizing virus in vitro.18,19 In addition to neutralization, influenza-specific antibodies may mediate a number of Fc-receptor dependent functions, including complement-mediated lysis,20-24 phagocytosis,25,26 and ADCC.27,28 Indeed, the Fc-receptor binding activity of antibodies has been shown to increase the protective efficacy of broadly neutralizing antibodies and has been associated with protection against experimental influenza challenge for a number of candidate universal vaccines.5,6,27,29,30 In this review, we highlight recent key studies on the characterization of targets of influenza-specific ADCC and discuss potential future avenues of investigation.
The mechanisms of ADCC function
ADCC relies upon cross-talk and synergism between the innate and adaptive immune response. Innate immune cells such as NK cells, macrophages and neutrophils possess Fc-receptors (FcRs) that can engage the Fc-region of particular antibody isotypes. Bound antibodies provide the specificity lacking from innate immune cells, allowing them to recognize pathogens specifically through the engagement of their Fc receptors. Antibody Fc regions can engage a combination of either activating FcRs (murine; FcγRI, FcγRIII, FcγRIV and human; FcγRI and FcγRIIIa) or inhibitory FcRs (murine; FcγRIIb and human; FcγRIIa/b/c) which are expressed on the surface of immune cells.31 The ligation of a combination of these activating or inhibitory FcRs determines the overall cellular effector function. In particular, FcyRIIIa (CD16) receptor (the ortholog of the mouse FcγRIV receptor which has the highest affinity for IgG2a) found on NK cells can bind to the Fc-region of a surface-bound Ab (IgG1 and IgG3). Crosslinking of the CD16 receptor leads to phosphorlyation of the C-terminal ITAM to activate the downstream calcium-dependent signaling pathway.32 This results in the release of pre-formed granzyme B and perforin from endosomes, which together facilitate DNA fragmentation and apoptosis of the target cell. Activation of innate immune cells such as NK can have a number of other indirect consequences including the secretion of antiviral cytokines and chemokines, including IFN-γ and TNF, which have important antiviral and immunopathological properties.
Influenza ADCC following vaccination and infection
Antibodies that mediate cytotoxicity have been studied in children and adults for many years. Early studies by Greenberg et al. found that lymphocytes from subjects vaccinated with inactivated influenza vaccine or experimentally inoculated with live influenza viruses developed cytotoxicity toward virus infected target cells via an antibody-dependent mechanism.33,34 Subsequent studies by Hashimoto et al. found that NK cells (HNK-1+ cells) present in PBMCs mediated cytotoxicity to virus-infected target cells in the presence of sera from children after vaccination (either inactivated or live-attenuated influenza vaccine (LAIV)) or following natural influenza infection.35 Additionally, Hashimoto and colleagues showed that immunisation with a LAIV was more durable in inducing ADCC-mediating antibodies (ADCC-Abs) whereas the inactivated influenza vaccine induced ADCC-Ab only in some of the children.35 In contrast, more recent studies have found that unlike TIV (trivalent influenza vaccine), which generate a modest rise in ADCC-Ab titers in children, LAIV failed to generate significant changes in ADCC-Ab titers.36,37 It is likely that ADCC-Abs are present early during life, with cord blood plasma from newborns shown to have detectable ADCC-Ab titers.38 Broadly cross-reactive ADCC-Abs have been detected against antigenically novel strains such as H5N1, H7N9 and H1N1pdm09 (before the 2009) early during infancy, even in the absence of any detectable neutralizing antibodies to these viruses.22,39-42 These cross-reactive ADCC-Ab titers increase with age, with higher levels found in older adults than infants.22,40,41,43 The generation of broadly cross-reactive ADCC-Abs is likely a result of repeated influenza infections and vaccinations throughout life. Cross-reactive ADCC-Ab in the absence of any detectable neutralizing antibodies, suggests that ADCC-Ab may target regions conserved and not classically neutralizing. This should be of great interest to understand potential strategies for universal immunogen design.
The generation and protective potential of cross-reactive ADCC-Abs has been studied in both humans and non-human primates. Macaques administered either seasonal H1N1, H1N1pmd09 or H3N2 generate robust serological titers of ADCC-Abs following infection.44-46 However, detecting ADCC-Abs following confirmed influenza infection in humans is confounded by the rapid boost in ADCC-Abs before patients presenting to the clinic with symptoms. To this end, we observed a modest increase in homologous ADCC-Ab titers when adult subjects were experimentally infected with influenza virus,36,47 with increases in ADCC-Ab titers associated with higher virus replication and symptom score.36 There was no correlation between pre-existing homologous ADCC-Ab titers and subsequent viral load or clinical symptoms following challenge.36 However, when subjects were stratified based upon if they had a “high” or “low” baseline ADCC titers, subjects with high ADCC titers before influenza challenge had lower viral loads and significantly lower total symptom scores. It should be noted that this study used a limited cohort size, with only 3 individuals with ADCC-Ab titers ≥ 320. Despite this, these findings provide a good rationale to initiate larger cohort based studies to clarify if pre-existing ADCC-titers contribute to protection. Studies such as these will provide useful data to inform therapeutic and vaccine design, as well as informing licensing criteria.
The ability of influenza vaccines to induce robust ADCC-Abs has been investigated in some detail. Vaccination of non-human primates with 2 doses of TIV failed to induce detectable ADCC-Ab, whereas influenza infection with either H1N1 or H3N2 was capable of inducing robust ADCC-Abs. In contrast, studies in human adults have shown that pre-existing cross-reactive HA-specific ADCC-Abs can be boosted following seasonal inactivated influenza vaccination,36,43,48-50 in most cases independent of increases in neutralizing antibodies. Priming of the ADCC-Ab response, before inactivated vaccine administration, seems to be important for the generation of robust ADCC-Abs; with the generation of robust H7-specific ADCC-Ab responses boosted by prior H7N9 pLAIV vaccination.51 Vaccination of adults with seasonal TIV boosts responses to a number of antigenically distinct influenza subtypes.43,48 including drifted strains49 In contrast, vaccination of adults with LAIV alone fails to induce increases in ADCC-Ab titers.36,51 ADCC-Ab have been also shown to be generated following vaccination with a number of novel vaccine constructs including MVA52 and stable trimeric stem constructs.5 An interesting study performed by Goodier et al. suggests that the CD16 receptor on NK cells is significantly downregulated following TIV partially via ADAM17 matrix metalloprotease mediated cleavage.53 This data suggests that vaccination may increase the level of ADCC-Abs available, however, circulating NK cells (or other CD16 expressing cells) may have a reduced ability to mediate ADCC upon influenza infection. That ADCC-Ab titers can be elicited somewhat independently of neutralizing antibody titers suggests that standard neutralization assays used for measuring vaccine immunogenicity and effectiveness provide an accurate measurement of only a narrow subset of potentially protective antibodies induced by vaccination.
A role for ADCC in protection by HA-specific antibodies
The advent of high-throughput molecular biological techniques to clone and express human immunoglobulins has facilitated the isolation of numerous monoclonal antibodies (mAbs) that target a highly conserved region of the influenza HA stem. Studies have shown that the Fc-receptor function of antibodies is important for the potency and protective capacity of broadly binding HA-specific antibodies in vivo.27,29 An initial study by Corti et al. showed that mice administered the 3 mg/kg of a FcR-binding deficient mutant of the neutralising FI6 mAb (FI6-LALA) were 60% less likely to survive a lethal dose of PR8 virus compared with unmutated FI6 mAb.29 This observation was subsequently confirmed by DiLillo et al. where mice survived lethal challenge with PR8 when administered 4 mg/kg of FI6 in the context of a murine IgG2a Fc (an Fc shown previously to be associated with ADCC function in mice31) but succumbed to infection when mice were administered the an IgG1 variant of FI6 (not ADCC capable).27 DiLillo and colleagues went on to confirm this result was FcγR-dependent, by showing the protection afforded by FI6- IgG2a was abolished when administered to FcerIγ−/− deficient mice.27 Using the several alternative broadly neutralizing stem-antibodies, they showed that FcγR binding activity was critical for the potent protection provided by broadly neutralizing stem-mAbs in vivo. In particular, a 6F12 DA265 mutant mAb (which lacked Fc-receptor binding activity), had to be administered at concentrations >16 mg/kg for protection of mice from lethal challenge whereas the unmodified IgG2a form provided protection at only 4 mg/kg. These experiments highlight Fc receptor function underpins the protective potency of stem-specific antibodies.
A surprising finding from experiments performed by DiLillo and colleagues was that stem-specific mAbs could engage FcRs and mediate ADCC activity whereas head-specific antibodies (binding canonical sites surrounding the receptor binding site) were limited in their ADCC capacity.27,54 A number of recent studies confirmed this result using in vitro ADCC assays whereby numerous stem-specific mAb can induce robust ADCC30 whereas, head-specific, HAI+ antibodies are not capable of inducing ADCC-activity.55-57 In particular, Leon et al. elegantly demonstrated, using FLAG-epitopes inserted into either the stem or head region of the influenza HA, that anti-FLAG antibodies binding within the stem-region induce 2–4-fold higher ADCC than the same antibodies binding within the HA head region (adjacent to the sialic acid binding site).55 Furthermore, antibodies that mediate HAI+ activity are capable of antagonizing the ability of stem-specific antibodies to mediate ADCC. He et al. showed using NK cell lines and primary human NK cells that ADCC-induced by stem-specific antibody (CR8020) is reduced in the presence of an HAI+ HA-specific antibody (C05). Several groups have performed competitive titrations with stem-specific and head-specific mAbs, showing that HAI+ head-specific antibodies inhibit in vitro ADCC induction.55-57 These studies also highlight that ADCC induction by a polyclonal IgG can be inhibited by the addition of a HAI+ HA-specific antibody. In particular, experiments by Cox et al. have shown that the presence of high titers of HAI antibodies or the addition of mAbs with HAI activity to sera from vaccinees can dramatically inhibit ADCC induction. The binding of head-specific antibodies does not directly prevent the binding of stem-specific antibodies to HA56 however, mutation of HA residues critical to sialic acid binding (Y108F HA or K195F HA,) has been shown to lead to a marked reduction in ADCC activity. This suggests that the sialic acid binding activity of HA is imperative for stem-specific ADCC activity. In addition, recent data from Mullarkey et al. have extended this observation to show that stem-specific mAbs can induce Fc-dependent ROS release and phagocytosis by neutrophils, whereas head-specific mAbs could not.25 It is not clear at present whether sialic acid binding is stabilizing the HA protein conformation or whether alternative co-receptor interactions between HA and effector cells are augmenting ADCC activity. These results have wide implications for vaccination strategies that aim to generate stem-specific antibodies (a summary of this is shown in Fig. 1A).
Figure 1.
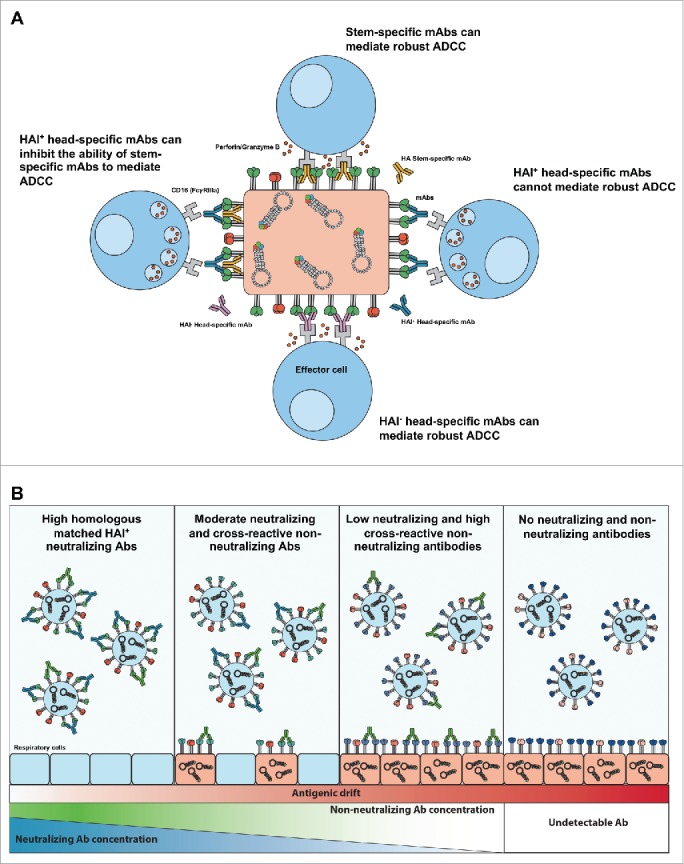
Possible mechanisms of influenza-specific ADCC. (A) Differential ability of HA-specific mAbs to mediate ADCC. Mabs targeting regions of influenza virus HA (stem or head region) have the ability to mediate ADCC (HAI− head-specific mAbs or stem-specific mAbs), cannot mediate ADCC (HAI+ head-specific mAbs) or inhibit ADCC (HAI + head-specific with stem-specific mAbs). (B) Potential role of neutralizing and non-neutralizing (including ADCC-Abs) antibodies against seasonal influenza viruses that antigenically drift through influenza seasons. High concentractions of neutralizing antibodies against seasonal influenza virus entry before infection is established, such as following "matched" seasonal influenza vaccination or homologous influenza control of virus infection. Moderate neutralization and cross-reactive non-nuetralizing antibodies may lead to some infection but provide rapid control of virus infection and clearance, as maybe the case following vaccine miss-match or heterologous influenza infection. Low neutralizing and high cross-reactive non-neutralizing antibodies may not prevent influenza virus infection but reduce the severity of influenza infection.
The inability of head-specific mAbs to mediate effective ADCC is still contentious. It is clear that strain-specific antibodies with HAI activity induce lower ADCC activity and can even antagonize stem-specific ADCC activity.55-57 However there exists some broadly conserved epitopes within the HA-head region that are non-neutralizing but facilitate potent ADCC activity. The study by DiLillo shows that 2 broadly neutralizing anti-head mAbs (4G05 1 mg/kg and 1F05 4 mg/kg) provided protection from lethal Neth/09 challenge.54 Additionally, they also show that 3 non-neutralizing, head-specific mAbs (1A01, 1A05 and 4G01) can similarly protect from Neth/09 lethal challenge in an FcR mediated manner. This is somewhat contrary to in vitro data by He et al. where 2 HA head-binding antibodies (FEE8 and 5E02) had diminished ADCC activity using a FcγRIV reporter cell assay. It is clear that in addition to the preliminary studies to date, a comprehensive approach combining detailed antigenic characterization of mAbs with in vivo passive transfer studies is necessary to conclusively define the capacity for particular epitopes in the HA globular head to induce ADCC activity and provide protection. Such an antigenic map of ADCC antigenicity would be invalable for vaccine design to target broadly conserved non-neutralizing regions of the influenza head region.
ADCC to other viral proteins
While HA epitopes have been extensively studied, other influenza proteins can also be targeted by ADCC-Abs. Hashimoto et al. first described the surface glycoprotein NA as a target of ADCC over 30 y ago.35 A recent study by DiLillo and colleagues confirmed that broadly neutralizing NA-specific mAbs, like broadly neutralizing HA-specific antibodies, also require Fc-receptor interactions to mediate protection in vivo.54 Concurrently, He et al. used an in vitro ADCC reporter assay to show that NA-specific antibodies can only induce modest ADCC, but could cooperatively enhance ADCC activity when combined with HA stalk antibodies.55 Further, there evidence that both influenza infection and vaccination with TIV can induce low levels of NA-specific ADCC-Abs.36,58 Indeed, anti-NA antibodies may be an important component of the polyclonal ADCC response to influenza, which in combination with HA-specific antibodies, exert pressure on influenza virus to undergo significant antigenic drift. Combinatoral strategies simultaneously targeting conserved non-neutralizing HA-specific and NA-specific epitopes may elicit synergistic humoral immunity to maximise vaccine-elicited protection, or in the case of mAbs, novel therapeutics for influenza treatment.
There has been a renewed interest in ADCC-Abs targeting highly conserved influenza antigens like nucleoprotein (NP) and matrix proteins. NP is detectable on the surface of influenza-infected cells in vitro,59,60 and may provide a conserved target for ADCC-Abs. NP-specific antibodies have been shown to provide robust Fc-mediated protection in mice passively transferred antibodies before lethal heterosubtypic influenza challenge.61,62 Supporting the idea that NP-specific antibodies can mediate ADCC, recent work by our group has shown that NP-specific antibodies from influenza immunized or infected humans can crosslink FcγRIIIa and activate primary NK cells.45,47 Terajima et al. found high titers of ADCC-Abs in children and adults, but not infants, that could kill A549 target cells infected with avian influenza viruses of the H7N9 and H5N1 subtypes.22 Further analysis showed that subjects had low to undetectable ADCC-Ab titers against H7N9 HA or NA, but high ADCC-Ab titers against H7N9 NP.40 A strong correlation was observed between ADCC-Ab titers against H7N9 NP and H7N9 virus-infected cells.40 Collectively, these findings suggest that influenza infection and immunization induces cross-reactive NP antibodies with the ability to mediate ADCC against a wide array of influenza viruses.
Influenza matrix protein 2 (M2) also localizes to the membrane of influenza-infected cells63 and the extracellular region (M2e) is accessible to ADCC-Abs. There is mounting evidence that M2e-specific antibodies require Fc-mediated effector functions for protection. Infusion of M2e-specific immune serum can protect wild type mice from lethal PR8 infection, while FcγR knockout mice were not protected.64 Prophylactic and therapeutic administration of a human M2e-specific mAb (Z3G1) protected mice from lethal influenza challenge and decreased viral load in the lungs through FcγR- and complement-dependent mechanisms.65 Simhadri et al. showed that another human M2e-specific mAb (Ab1–10) can activate NK cells and mediate antibody-dependent killing of M2 expressing or influenza-infected cells.66 Furthermore, vaccination studies in mice have demonstrated that M2e-based vaccines are capable of generating improved FcγR-mediated cross-protection compared with commercially available split virion vaccines.67-71 Broadly reactive ADCC-Abs targeting NP, M2e or other conserved influenza proteins could therefore be important targets in the development of a universally protective influenza vaccines.
Effector cells mediating ADCC in vivo
Human NK cells, monocytes, macrophages and neutrophils all express FcyRIII on the cell surface,72,73 and have the potential to mediate ADCC in vivo. An early study by Hashimoto et al. showed that human NK cells, monocytes and neutrophils can mediate ADCC of influenza-infected cells ex vivo.35 NK- and monocyte-mediated ADCC was rapidly detected within 2–6 hours, whereas neutrophil-mediated ADCC was detected between 6–10 hours.35 NK cells induced ADCC of influenza-infected target cells at lower antibody concentrations (detectable at a 1:45,000 serum dilution) than monocytes and neutrophils.35 Based on these findings, NK cells are seen as the primary effector cells in vivo and the focus of most influenza ADCC research to date. Degranulation of primary human and non-human primate NK cells has been studied in vitro using a variety of surrogate influenza ADCC assays.27,36,44,45,54,74,75 A single FcγR (FcγRIIIa) is expressed on the surface of NK cells,72,73 and the underlying mechanism of NK cell-mediated ADCC through perforin/granzyme and Fas/FasL pathways is well characterized.76,77 However, emerging evidence suggests that other innate effector cells, with a broader range Fc functions, also contribute to influenza-specific ADCC in vivo.
Neutrophils are the most abundant subset of blood leukocytes in humans78 and they express a myriad of both activating and inhibitory FcγRs (mice constitutively express mFcγRIIb, mFcγRIII and mFcγRIV; humans constitutively express hFcγRIIa, hFcγRIIb and hFcγRIIIb).72,73 A recent study has shown that ROS production and ADP against influenza virus-infected cells requires FcγR engagement.25 Though, there has been limited studies on neutrophils mediating ADP and ADCC, in the HIV, neutrophils have been shown to capable of killing HIV-infected CD4 T-cells in an antibody-dependent manner.79 Interestingly ROS production by neutrophils and monocytes is not requirement for ADCC of HIV-infected cells.80-82 Since, neutrophils lack granzymes and perforin83 other methods have been proposed for neutrophil-mediated killing including neutrophil extracellular traps82 and secretion of CD63+ azurphilic granules.84 The ability of neutrophils to mediate ADCC and their relative importance in controlling influenza virus replication remains undefined and warrants further investigation.
Monocytes and macrophages, like neutrophils, express a variety of FcγRs on their cell surface (mice constitutively express mFcγRI, mFcγRIIb, mFcγRIII and mFcγRIV; humans constitutively express hFcγRI, hFcγRIIa, hFcγRIIb and hFcγRIIIa).72,73 A human monocytic cell line, THP-1, has been shown to mediate ADP of influenza virions and monocytes may act to limit the spread of influenza infection in vivo.26 In fact, ADP was recently proposed as the primary mechanism of Fc-mediated protection by non-neutralizing HA-specific mAbs in a mouse model of H7N9 infection.85 Monocyte-mediated ADCC has also been described against both influenza- and HIV-infected cells,35,82 but the precise mechanism remains elusive. FcγRIIIa expression is essential for ADCC by human monocytes86 and enhanced granzyme B expression leads to enhanced monocyte-mediated ADCC,87 suggesting some mechanistic conservation between monocyte and NK cell ADCC pathways.
Studying the Fc-mediated effector functions of monocytes, macrophages and neutrophils presents a unique challenge because these cell types are capable of mediating both ADP and ADCC. As such, experimental models of influenza infection using LALA mutant mAbs, pan-FcγR blocking or knockout animals and cell type depletion do not allow for the assessment of ADCC independently from ADP. Future experiments should make use of knockout (FcγRIV−/− mice) or knockdown (RNAi or FcγRIII blocking mAb) methods targeting specific FcγRs to assess whether neutrophils, monocytes and macrophages participate in the ADCC response to influenza infection.
Conclusions/future directions
The HAI assay has been a mainstay of research into humoral immunity to influenza and remains an important correlate for protection elicited by conventional influenza vaccines. However an overreliance upon HAI titers has led to an underappreciation of antibodies that bind outside the canonical HAI epitopes and the roles that they may play in limiting influenza aquisition and disease severity. By binding to conserved epitopes outside the canonical neutralizing epitopes of HA, or to viral antigens with high conservation such as NA and NP, non-neutralizing antibodies display much greater breadth of influenza recognition than classical neutralizing antibodies. As such, polyclonal ADCC-Abs may act synergistically to kill influenza virus-infected cells and provide broad protection from disease caused by epidemic and pandemic influenza strains. A role for ADCC as a correlate of protection from severe influenza infection is supported by pre-clincal testing of current universal influenza vaccine candidates, which protect by a FcR dependent mechanism.5,6 Moreover, this is not limited to influenza, with studies following the HIV RV144 trial showing an association of ADCC-Abs with protection from HIV acquisition.88 Indeed, if potent strain specific protection is all that required, then conventional seasonal vaccines are appropriate and can provide robust HAI antibodies that are protective against the circulating “matched” seasonal influenza strains (Fig. 1B). But to provide robust protection against drift variants and novel emergent influenza subtypes, novel vaccines that rely upon non-neutralizing effector functions may be appropriate mechanism to reduce the severity of illness in the human population (Fig. 1B).
Despite the increasing interest in the field, there is still much to learn about influenza-specific ADCC. Noteable gaps in the knowledge include; (1) Which epitopes within the HA head region and NA are protective and capable of mediating ADCC? This information would provide important insights for vaccines and therapeutics to potentially target broadly cross-reactive regions. (2) Why are stem-specific mAbs inhibited by HAI+ HA-specific mAbs? This could be because of interactions between the HA sialic acid binding site and a co-receptor on effector cells or another unknown mechanism. (3) What are the effector cells that mediate ADCC in vivo in humans? Although, mouse models have been very informative, differential Fc-receptor expression between humans and mice may profoundly influence which cells are the major contributors to ADCC. (4) Does the presence of ADCC-Ab in humans confer any protection against influenza infection? If so, what is the degree of protection provided by ADCC-Ab and how is this influenced by the functionality of the effector cells within each individual? The answer to this question will require additional human clinical trials or larger human influenza challenge studies. Upcoming pre-clinical studies of next-generation HA stem and other vaccines will clarify the limitations and hurdles surrounding Fc-receptor mediated protection. However, the tremendous potential that ADCC-Abs have for expanding the breadth of antibody-based protection against influenza is heartening for the development of a truly universal influenza vaccine.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
We would like to acknowledge that we are supported by Australian NHMRC award (1052979) and Australian Early Career Fellowship (1072127).
References
- [1].WHO Influenza (Seasonal), Fact sheet. http://www.who.int/mediacentre/factsheets/fs211/en/, 2016 [Google Scholar]
- [2].Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086-96; PMID:17544181; https://doi.org/ 10.1016/j.vaccine.2007.03.046 [DOI] [PubMed] [Google Scholar]
- [3].Radin JM, Hawksworth AW, Myers CA, Ricketts MN, Hansen EA, Brice GT. Influenza vaccine effectiveness: Maintained protection throughout the duration of influenza seasons 2010-2011 through 2013-2014. Vaccine 2016; 34:3907-12; PMID:27265447; https://doi.org/ 10.1016/j.vaccine.2016.05.034 [DOI] [PubMed] [Google Scholar]
- [4].Bedford T, Suchard MA, Lemey P, Dudas G, Gregory V, Hay AJ, McCauley JW, Russell CA, Smith DJ, Rambaut A. Integrating influenza antigenic dynamics with molecular evolution. Elife 2014; 3:e01914; https://doi.org/ 10.7554/eLife.01914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, et al.. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015; 349:1301-6; PMID:26303961; https://doi.org/ 10.1126/science.aac7263 [DOI] [PubMed] [Google Scholar]
- [6].Yassine HM, Boyington JC, McTamney PM, Wei CJ, Kanekiyo M, Kong WP, Gallagher JR, Wang L, Zhang Y, Joyce MG, et al.. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med 2015; 21:1065-70; PMID:26301691; https://doi.org/ 10.1038/nm.3927 [DOI] [PubMed] [Google Scholar]
- [7].Mallajosyula VV, Citron M, Ferrara F, Lu X, Callahan C, Heidecker GJ, Sarma SP, Flynn JA, Temperton NJ, Liang X, et al.. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc Natl Acad Sci U S A 2014; 111:E2514-23; PMID:24927560; https://doi.org/ 10.1073/pnas.1402766111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Valkenburg SA, Mallajosyula VV, Li OT, Chin AW, Carnell G, Temperton N, Varadarajan R, Poon LL. Stalking influenza by vaccination with pre-fusion headless HA mini-stem. Sci Rep 2016; 6:22666; PMID:26947245; https://doi.org/ 10.1038/srep22666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A 2011; 108:757-61; PMID:21187388; https://doi.org/ 10.1073/pnas.1012199108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med 1999; 5:1157-63; PMID:10502819; https://doi.org/ 10.1038/13484 [DOI] [PubMed] [Google Scholar]
- [11].De Filette M, Martens W, Smet A, Schotsaert M, Birkett A, Londono-Arcila P, Fiers W, Saelens X. Universal influenza A M2e-HBc vaccine protects against disease even in the presence of pre-existing anti-HBc antibodies. Vaccine 2008; 26:6503-7; PMID:18835315; https://doi.org/ 10.1016/j.vaccine.2008.09.038 [DOI] [PubMed] [Google Scholar]
- [12].De Filette M, Min Jou W, Birkett A, Lyons K, Schultz B, Tonkyro A, Resch S, Fiers W. Universal influenza A vaccine: optimization of M2-based constructs. Virology 2005; 337:149-61; PMID:15914228; https://doi.org/ 10.1016/j.virol.2005.04.004 [DOI] [PubMed] [Google Scholar]
- [13].Huleatt JW, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, Tang J, McDonald W, Song L, Evans RK, et al.. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine 2008; 26:201-14; PMID:18063235; https://doi.org/ 10.1016/j.vaccine.2007.10.062 [DOI] [PubMed] [Google Scholar]
- [14].Kolpe A, Schepens B, Fiers W, Saelens X. M2-based influenza vaccines: recent advances and clinical potential. Expert Rev Vaccines 2016:16(2):123-136 [DOI] [PubMed] [Google Scholar]
- [15].Liu WC, Lin CY, Tsou YT, Jan JT, Wu SC. Cross-reactive neuraminidase-inhibiting antibodies elicited by immunization with recombinant neuraminidase proteins of H5N1 and pandemic H1N1 influenza a viruses. J Virol 2015; 89:7224-34; PMID:25948745; https://doi.org/ 10.1128/JVI.00585-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eichelberger MC, Wan H. Influenza neuraminidase as a vaccine antigen. Curr Top Microbiol Immunol 2015; 386:275-99; PMID:25033754 [DOI] [PubMed] [Google Scholar]
- [17].Sridhar S. Heterosubtypic T-Cell Immunity to Influenza in Humans: Challenges for Universal T-Cell Influenza Vaccines. Front Immunol 2016; 7:195; PMID:27242800; https://doi.org/ 10.3389/fimmu.2016.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].He W, Mullarkey CE, Duty JA, Moran TM, Palese P, Miller MS. Broadly neutralizing anti-influenza virus antibodies: enhancement of neutralizing potency in polyclonal mixtures and IgA backbones. J Virol 2015; 89:3610-8; PMID:25589655; https://doi.org/ 10.1128/JVI.03099-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].He W, Mullarkey CE, Miller MS. Measuring the neutralization potency of influenza A virus hemagglutinin stalk/stem-binding antibodies in polyclonal preparations by microneutralization assay. Methods 2015; 90:95-100; PMID:25957674; https://doi.org/ 10.1016/j.ymeth.2015.04.037 [DOI] [PubMed] [Google Scholar]
- [20].O'Brien KB, Morrison TE, Dundore DY, Heise MT, Schultz-Cherry S. A protective role for complement C3 protein during pandemic 2009 H1N1 and H5N1 influenza A virus infection. PLoS One 2011; 6:e17377; https://doi.org/ 10.1371/journal.pone.0017377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ohta R, Torii Y, Imai M, Kimura H, Okada N, Ito Y. Serum concentrations of complement anaphylatoxins and proinflammatory mediators in patients with 2009 H1N1 influenza. Microbiol Immunol 2011; 55:191-8; https://doi.org/ 10.1111/j.1348-0421.2011.00309.x [DOI] [PubMed] [Google Scholar]
- [22].Terajima M, Co MD, Cruz J, Ennis FA. High Antibody-Dependent Cellular Cytotoxicity Antibody Titers to H5N1 and H7N9 Avian Influenza A Viruses in Healthy US Adults and Older Children. J Infect Dis 2015; 212:1052-60; PMID:25795791; https://doi.org/ 10.1093/infdis/jiv181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Quinnan GV, Ennis FA, Tuazon CU, Wells MA, Butchko GM, Armstrong R, McLaren C, Manischewitz JF, Kiley S. Cytotoxic lymphocytes and antibody-dependent complement-mediated cytotoxicity induced by administration of influenza vaccine. Infect Immun 1980; 30:362-9; PMID:7439982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Verbonitz MW, Ennis FA, Hicks JT, Albrecht P. Hemagglutinin-specific complement-dependent cytolytic antibody response to influenza infection. J Exp Med 1978; 147:265-70; PMID:627837; https://doi.org/ 10.1084/jem.147.1.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mullarkey CE, Bailey MJ, Golubeva DA, Tan GS, Nachbagauer R, He W, Novakowski KE, Bowdish DM, Miller MS, Palese P. Broadly Neutralizing Hemagglutinin Stalk-Specific Antibodies Induce Potent Phagocytosis of Immune Complexes by Neutrophils in an Fc-Dependent Manner. mBio 2016; 7:e01624-16; PMID:27703076; https://doi.org/ 10.1128/mBio.01624-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ana-Sosa-Batiz F, Vanderven H, Jegaskanda S, Johnston A, Rockman S, Laurie K, Barr I, Reading P, Lichtfuss M, Kent SJ. Influenza-Specific Antibody-Dependent Phagocytosis. PLoS One 2016; 11:e0154461; PMID:27124730; https://doi.org/ 10.1371/journal.pone.0154461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 2014; 20:143-51; PMID:24412922; https://doi.org/ 10.1038/nm.3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jegaskanda S, Reading PC, Kent SJ. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J Immunol 2014; 193:469-75; PMID:24994909; https://doi.org/ 10.4049/jimmunol.1400432 [DOI] [PubMed] [Google Scholar]
- [29].Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, et al.. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 2011; 333:850-6; PMID:21798894; https://doi.org/ 10.1126/science.1205669 [DOI] [PubMed] [Google Scholar]
- [30].Fu Y, Zhang Z, Sheehan J, Avnir Y, Ridenour C, Sachnik T, Sun J, Hossain MJ, Chen LM, Zhu Q, et al.. A broadly neutralizing anti-influenza antibody reveals ongoing capacity of haemagglutinin-specific memory B cells to evolve. Nat Commun 2016; 7:12780; PMID:27619409; https://doi.org/ 10.1038/ncomms12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bournazos S, DiLillo DJ, Ravetch JV. The role of Fc-FcgammaR interactions in IgG-mediated microbial neutralization. J Exp Med 2015; 212:1361-9; PMID:26282878; https://doi.org/ 10.1084/jem.20151267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol 2001; 19:275-90; PMID:11244038; https://doi.org/ 10.1146/annurev.immunol.19.1.275 [DOI] [PubMed] [Google Scholar]
- [33].Greenberg SB, Criswell BS, Six HR, Couch RB. Lymphocyte cytotoxicity to influenza virus-infected cells: response to vaccination and virus infection. Infect Immun 1978; 20:640-5; PMID:669816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Greenberg SB, Criswell BS, Six HR, Couch RB. Lymphocyte cytotoxicity to influenza virus-infected cells. II. Requirement for antibody and non-T lymphocytes. J Immunol 1977; 119:2100-6 [PubMed] [Google Scholar]
- [35].Hashimoto G, Wright PF, Karzon DT. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J Infect Dis 1983; 148:785-94; PMID:6605395; https://doi.org/ 10.1093/infdis/148.5.785 [DOI] [PubMed] [Google Scholar]
- [36].Jegaskanda S, Luke C, Hickman HD, Sangster MY, Wieland-Alter WF, McBride JM, Yewdell JW, Wright PF, Treanor J, Rosenberger CM, et al.. Generation and protective ability of influenza virus-specific antibody-dependent cellular cytotoxicity in humans elicited by vaccination, natural infection, and experimental challenge. J Infect Dis 2016; 214:945-52; PMID:27354365; https://doi.org/ 10.1093/infdis/jiw262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wright PF, Hoen AG, Ilyushina NA, Brown EP, Ackerman ME, Wieland-Alter W, Connor RI, Jegaskanda S, Rosenberg-Hasson Y, Haynes BC, et al.. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016; 3:ofw108; PMID:27419180; https://doi.org/ 10.1093/ofid/ofw108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hashimoto G, Wright PF, Karzon DT. Ability of human cord blood lymphocytes to mediate antibody-dependent cellular cytotoxicity against influenza virus-infected cells. Infect Immun 1983; 42:214-8; PMID:6604697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Co MD, Terajima M, Thomas SJ, Jarman RG, Rungrojcharoenkit K, Fernandez S, Yoon IK, Buddhari D, Cruz J, Ennis FA. Relationship of preexisting influenza hemagglutination inhibition, complement-dependent lytic, and antibody-dependent cellular cytotoxicity antibodies to the development of clinical illness in a prospective study of A(H1N1)pdm09 Influenza in children. Viral Immunol 2014; 27:375-82; PMID:25141276; https://doi.org/ 10.1089/vim.2014.0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jegaskanda S, Co MD, Cruz J, Subbarao K, Ennis FA, Terajima M. Human seasonal influenza A viruses induce H7N9-cross-reactive antibody-dependent cellular cytotoxicity (ADCC) antibodies that are directed towards the nucleoprotein. J Infect Dis 2016; https://doi.org/10.1093/infdis/jiw629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jegaskanda S, Laurie KL, Amarasena TH, Winnall WR, Kramski M, De Rose R, Barr IG, Brooks AG, Reading PC, Kent SJ. Age-associated cross-reactive antibody-dependent cellular cytotoxicity toward 2009 pandemic influenza A virus subtype H1N1. J Infect Dis 2013; 208:1051-61; PMID:23812238; https://doi.org/ 10.1093/infdis/jit294 [DOI] [PubMed] [Google Scholar]
- [42].Mesman AW, Westerhuis BM, Ten Hulscher HI, Jacobi RH, de Bruin E, van Beek J, Buisman AM, Koopmans MP, van Binnendijk RS. Influenza virus A(H1N1)2009 antibody-dependent cellular cytotoxicity in young children prior to the H1N1 pandemic. J Gen Virol 2016; 97:2157-65; PMID:27412007; https://doi.org/ 10.1099/jgv.0.000552 [DOI] [PubMed] [Google Scholar]
- [43].de Vries RD, Nieuwkoop NJ, Pronk M, de Bruin E, Leroux-Roels G, Huijskens EG, van Binnendijk RS, Krammer F, Koopmans MP, Rimmelzwaan GF. Influenza virus-specific antibody dependent cellular cytoxicity induced by vaccination or natural infection. Vaccine 2017; 35:238-47; PMID:27914742; https://doi.org/ 10.1016/j.vaccine.2016.11.082 [DOI] [PubMed] [Google Scholar]
- [44].Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol 2013; 87:5512-22; PMID:23468501; https://doi.org/ 10.1128/JVI.03030-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jegaskanda S, Amarasena TH, Laurie KL, Tan HX, Butler J, Parsons MS, Alcantara S, Petravic J, Davenport MP, Hurt AC, et al.. Standard trivalent influenza virus protein vaccination does not prime antibody-dependent cellular cytotoxicity in macaques. J Virol 2013; 87:13706-18; PMID:24109221; https://doi.org/ 10.1128/JVI.01666-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Koopman G, Mooij P, Dekking L, Mortier D, Nieuwenhuis IG, van Heteren M, Kuipers H, Remarque EJ, Radošević K, Bogers WM. Correlation between Virus Replication and Antibody Responses in Macaques following Infection with Pandemic Influenza A Virus. J Virol 2015; 90:1023-33; PMID:26537681; https://doi.org/ 10.1128/JVI.02757-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vanderven HA, Ana-Sosa-Batiz F, Jegaskanda S, Rockman S, Laurie K, Barr I, Chen W, Wines B, Hogarth PM, Lambe T, et al.. What Lies Beneath: Antibody Dependent Natural Killer Cell Activation by Antibodies to Internal Influenza Virus Proteins. EBioMedicine 2016; 8:277-90; PMID:27428437; https://doi.org/ 10.1016/j.ebiom.2016.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kristensen AB, Lay WN, Ana-Sosa-Batiz F, Vanderven HA, Madhavi V, Laurie KL, Carolan L, Wines BD, Hogarth M, Wheatley AK, et al.. Antibody Responses with Fc-Mediated Functions after Vaccination of HIV-Infected Subjects with Trivalent Influenza Vaccine. J Virol 2016; 90:5724-34; PMID:27053553; https://doi.org/ 10.1128/JVI.00285-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhong W, Gross FL, Holiday C, Jefferson SN, Bai Y, Liu F, Katz JM, Levine MZ. Vaccination with 2014-15 Seasonal Inactivated Influenza Vaccine Elicits Cross-Reactive Anti-HA Antibodies with Strong ADCC Against Antigenically Drifted Circulating H3N2 Virus in Humans. Viral Immunol 2016; 29:259-62; PMID:26950058; https://doi.org/ 10.1089/vim.2016.0003 [DOI] [PubMed] [Google Scholar]
- [50].Zhong W, Liu F, Wilson JR, Holiday C, Li ZN, Bai Y, Tzeng WP, Stevens J, York IA, Levine MZ. Antibody-Dependent Cell-Mediated Cytotoxicity to Hemagglutinin of Influenza A Viruses After Influenza Vaccination in Humans. Open Forum Infect Dis 2016; 3:ofw102; PMID:27419174; https://doi.org/ 10.1093/ofid/ofw102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sobhanie M, Matsuoka Y, Jegaskanda S, Fitzgerald T, Mallory R, Chen Z, Luke C, Treanor J, Subbarao K. Evaluation of the Safety and Immunogenicity of a Candidate Pandemic Live Attenuated Influenza Vaccine (pLAIV) Against Influenza A(H7N9). J Infect Dis 2016; 213:922-9; PMID:26655841; https://doi.org/ 10.1093/infdis/jiv526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Florek NW, Weinfurter JT, Jegaskanda S, Brewoo JN, Powell TD, Young GR, Das SC, Hatta M, Broman KW, Hungnes O, et al.. Modified vaccinia virus Ankara encoding influenza virus hemagglutinin induces heterosubtypic immunity in macaques. J Virol 2014; 88:13418-28; PMID:25210172; https://doi.org/ 10.1128/JVI.01219-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Goodier MR, Lusa C, Sherratt S, Rodriguez-Galan A, Behrens R, Riley EM. Sustained Immune Complex-Mediated Reduction in CD16 Expression after Vaccination Regulates NK Cell Function. Front Immunol 2016; 7:384; PMID:27725819; https://doi.org/ 10.3389/fimmu.2016.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].DiLillo DJ, Palese P, Wilson PC, Ravetch JV. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest 2016; 126:605-10; PMID:26731473; https://doi.org/ 10.1172/JCI84428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].He W, Tan GS, Mullarkey CE, Lee AJ, Lam MM, Krammer F, Henry C, Wilson PC, Ashkar AA, Palese P, et al.. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc Natl Acad Sci U S A 2016; 113:11931-6; PMID:27698132; https://doi.org/ 10.1073/pnas.1609316113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Leon PE, He W, Mullarkey CE, Bailey MJ, Miller MS, Krammer F, Palese P, Tan GS. Optimal activation of Fc-mediated effector functions by influenza virus hemagglutinin antibodies requires two points of contact. Proc Natl Acad Sci U S A 2016; 113:E5944-E51; PMID:27647907; https://doi.org/ 10.1073/pnas.1613225113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cox F, Kwaks T, Brandenburg B, Koldijk MH, Klaren V, Smal B, Korse HJ, Geelen E, Tettero L, Zuijdgeest D, et al.. HA Antibody-Mediated FcgammaRIIIa Activity Is Both Dependent on FcR Engagement and Interactions between HA and Sialic Acids. Front Immunol 2016; 7:399; PMID:27746785; https://doi.org/ 10.3389/fimmu.2016.00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jegaskanda S, Vandenberg K, Laurie KL, Loh L, Kramski M, Winnall WR, Kedzierska K, Rockman S, Kent SJ. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity in intravenous immunoglobulin as a potential therapeutic against emerging influenza viruses. J Infect Dis 2014; 210:1811-22; PMID:24916185; https://doi.org/ 10.1093/infdis/jiu334 [DOI] [PubMed] [Google Scholar]
- [59].Bodewes R, Geelhoed-Mieras MM, Wrammert J, Ahmed R, Wilson PC, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. In vitro assessment of the immunological significance of a human monoclonal antibody directed to the influenza a virus nucleoprotein. Clin Vaccine Immunol 2013; 20:1333-7; PMID:23761662; https://doi.org/ 10.1128/CVI.00339-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yewdell JW, Frank E, Gerhard W. Expression of influenza A virus internal antigens on the surface of infected P815 cells. J Immunol 1981; 126:1814-9 [PubMed] [Google Scholar]
- [61].Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol 2008; 181:4168-76; https://doi.org/ 10.4049/jimmunol.181.6.4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].LaMere MW, Lam HT, Moquin A, Haynes L, Lund FE, Randall TD, Kaminski DA. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol 2011; 186:4331-9; https://doi.org/ 10.4049/jimmunol.1003057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell 1985; 40:627-33; PMID:3882238; https://doi.org/ 10.1016/0092-8674(85)90211-9 [DOI] [PubMed] [Google Scholar]
- [64].El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, Van Rooijen N, Verbeek S, Fiers W, Saelens X. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol 2011; 186:1022-31; https://doi.org/ 10.4049/jimmunol.0902147 [DOI] [PubMed] [Google Scholar]
- [65].Wang R, Song A, Levin J, Dennis D, Zhang NJ, Yoshida H, Koriazova L, Madura L, Shapiro L, Matsumoto A, et al.. Therapeutic potential of a fully human monoclonal antibody against influenza A virus M2 protein. Antiviral Res 2008; 80:168-77; PMID:18598723; https://doi.org/ 10.1016/j.antiviral.2008.06.002 [DOI] [PubMed] [Google Scholar]
- [66].Simhadri VR, Dimitrova M, Mariano JL, Zenarruzabeitia O, Zhong W, Ozawa T, Muraguchi A, Kishi H, Eichelberger MC, Borrego F. A Human Anti-M2 Antibody Mediates Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) and Cytokine Secretion by Resting and Cytokine-Preactivated Natural Killer (NK) Cells. PLoS One 2015; 10:e0124677; PMID:25915748; https://doi.org/ 10.1371/journal.pone.0124677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol 2004; 172:5598-605; https://doi.org/ 10.4049/jimmunol.172.9.5598 [DOI] [PubMed] [Google Scholar]
- [68].Kim MC, Lee YN, Hwang HS, Lee YT, Ko EJ, Jung YJ, Cho MK, Kim YJ, Lee JS, Ha SH, et al.. Influenza M2 virus-like particles confer a broader range of cross protection to the strain-specific pre-existing immunity. Vaccine 2014; 32:5824-31; PMID:25171841; https://doi.org/ 10.1016/j.vaccine.2014.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lee YN, Kim MC, Lee YT, Hwang HS, Lee J, Kim C, Kang SM. Cross Protection against Influenza A Virus by Yeast-Expressed Heterologous Tandem Repeat M2 Extracellular Proteins. PLoS One 2015; 10:e0137822; PMID:26366729; https://doi.org/ 10.1371/journal.pone.0137822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lee YN, Lee YT, Kim MC, Hwang HS, Lee JS, Kim KH, Kang SM. Fc receptor is not required for inducing antibodies but plays a critical role in conferring protection after influenza M2 vaccination. Immunology 2014; 143:300-9; PMID:24773389; https://doi.org/ 10.1111/imm.12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, Jin HT, Pekosz A, Compans RW, Kang SM.. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One 2011; 6:e14538; PMID:21267073; https://doi.org/ 10.1371/journal.pone.0014538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012; 119:5640-9; PMID:22535666; https://doi.org/ 10.1182/blood-2012-01-380121 [DOI] [PubMed] [Google Scholar]
- [73].Bruhns P, Jonsson F. Mouse and human FcR effector functions. Immunol Rev 2015; 268:25-51; PMID:26497511; https://doi.org/ 10.1111/imr.12350 [DOI] [PubMed] [Google Scholar]
- [74].Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, Winnall WR, Stratov I, Brooks AG, Reading PC, et al.. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 2013; 190:1837-48; https://doi.org/ 10.4049/jimmunol.1201574 [DOI] [PubMed] [Google Scholar]
- [75].Wines BD, Vanderven HA, Esparon SE, Kristensen AB, Kent SJ, Hogarth PM. Dimeric FcgammaR Ectodomains as Probes of the Fc Receptor Function of Anti-Influenza Virus IgG. J Immunol 2016; 197:1507-16; https://doi.org/ 10.4049/jimmunol.1502551 [DOI] [PubMed] [Google Scholar]
- [76].Smalls-Mantey A, Doria-Rose N, Klein R, Patamawenu A, Migueles SA, Ko SY, Hallahan CW, Wong H, Liu B, You L, et al.. Antibody-dependent cellular cytotoxicity against primary HIV-infected CD4+ T cells is directly associated with the magnitude of surface IgG binding. J Virol 2012; 86:8672-80; PMID:22674985; https://doi.org/ 10.1128/JVI.00287-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti MA, Hayakawa Y. Activation of NK cell cytotoxicity. Mol Immunol 2005; 42:501-10; PMID:15607806; https://doi.org/ 10.1016/j.molimm.2004.07.034 [DOI] [PubMed] [Google Scholar]
- [78].Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 2013; 210:1283-99; PMID:23825232; https://doi.org/ 10.1084/jem.20122220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Smalls-Mantey A, Connors M, Sattentau QJ. Comparative efficiency of HIV-1-infected T cell killing by NK cells, monocytes and neutrophils. PLoS One 2013; 8:e74858; PMID:24040353; https://doi.org/ 10.1371/journal.pone.0074858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Horner H, Frank C, Dechant C, Repp R, Glennie M, Herrmann M, Stockmeyer B. Intimate cell conjugate formation and exchange of membrane lipids precede apoptosis induction in target cells during antibody-dependent, granulocyte-mediated cytotoxicity. J Immunol 2007; 179:337-45; https://doi.org/ 10.4049/jimmunol.179.1.337 [DOI] [PubMed] [Google Scholar]
- [81].Koller CA, LoBuglio AF. Monocyte-mediated antibody-dependent cell-mediated cytotoxicity: the role of the metabolic burst. Blood 1981; 58:293-9; PMID:6264996 [PubMed] [Google Scholar]
- [82].Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, et al.. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 2012; 12:109-16; https://doi.org/ 10.1016/j.chom.2012.05.015 [DOI] [PubMed] [Google Scholar]
- [83].Metkar SS, Froelich CJ. Human neutrophils lack granzyme A, granzyme B, and perforin. Blood 2004; 104:905-6; author reply 7-8; PMID:15265799; https://doi.org/ 10.1182/blood-2004-03-0888 [DOI] [PubMed] [Google Scholar]
- [84].Metelitsa LS, Gillies SD, Super M, Shimada H, Reynolds CP, Seeger RC. Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcgammaRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood 2002; 99:4166-73; PMID:12010822; https://doi.org/ 10.1182/blood.V99.11.4166 [DOI] [PubMed] [Google Scholar]
- [85].Henry Dunand CJ, Leon PE, Huang M, Choi A, Chromikova V, Ho IY, Tan GS, Cruz J, Hirsh A, Zheng NY, et al.. Both Neutralizing and Non-Neutralizing Human H7N9 Influenza Vaccine-Induced Monoclonal Antibodies Confer Protection. Cell Host Microbe 2016; 19:800-13; https://doi.org/ 10.1016/j.chom.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yeap WH, Wong KL, Shimasaki N, Teo EC, Quek JK, Yong HX, Diong CP, Bertoletti A, Linn YC, Wong SC. CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes. Sci Rep 2016; 6:34310; PMID:27670158; https://doi.org/ 10.1038/srep34310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].lavazhagan S, Fatehchand K, Santhanam V, Fang H, Ren L, Gautam S, Reader B, Mo X, Cheney C, Briercheck E, et al.. Granzyme B expression is enhanced in human monocytes by TLR8 agonists and contributes to antibody-dependent cellular cytotoxicity. J Immunol 2015; 194:2786-95; https://doi.org/ 10.4049/jimmunol.1402316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, et al.. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol 2011; 85:7029-36; PMID:21543485; https://doi.org/ 10.1128/JVI.00171-11 [DOI] [PMC free article] [PubMed] [Google Scholar]


