ABSTRACT
Objective: This study aimed to evaluate the immunogenicity and protective efficacy of recombinant bacille calmette-guerin (rBCG) strains expressing Ag85A (A), CFP10 (C), ESAT6 (E), IL-12p70 (I), and fusion protein GM-CSF (G).
Method: rBCGs were established by integrating of A, C, E, I, G, AE, CE, IE, GC, GE and GCE into Mycobacterium bovis BCG-1173 and BCG-SH. The macro-effects of rBCGs on mice were evaluated by phenotype and weight. The immunogenicity of rBCGs was analyzed by lgG, lgG1 and lgG2a antibody titers, and IFN-γ and IL-4 contents through Enzyme-linked immunosorbent assay (ELISA). Meanwhile, the proportions of CD4+ and CD8+ T splenic lymphocytes were determined using flow cytometry. The protective efficacy of rBCGs was evaluated by bacterial load in spleen and lung tissues from immunized mice.
Results: rBCGs exhibited no obvious side effects on mice. The antibody titers of lgG, lgG1 and lgG2a, proportion of CD4+ and CD8+ T cells, and concentrations of IFN-γ were found to be significantly higher in multiple-gene rBCGs than that in single-gene rBCGs (P < 0.05). Bacterial load in both spleen and lung tissues from mice infected with M. tuberculosis H37Rv were significantly reduced by rBCGs. A significantly lower bacterial load was revealed in rBCG-1173:A compared with multiple-gene rBCGs (P < 0.05).
Conclusion: Immunogenicity was better on multiple-gene rBCGs than on single-gene rBCGs, while excellent protective efficacy was exhibited on rBCG-1173:A and BCG-1173.
KEYWORDS: Immunogenicity, Protective efficacy, Recombinant bacille calmette-guerin, Tuberculosis
Introduction
Tuberculosis (TB) is a serious infectious disease caused by bacteria Mycobacterium tuberculosis (M. tuberculosis).1 It is estimated that one-third of the world's population have been infected by M. tuberculosis, and 5–10% infectious accompanied with TB.2 Recently, TB has become the second-most common cause of death in patients with infectious diseases, resulting in more than 2 million deaths annually worldwide.3 Meanwhile, the morbidity of TB has increased because of the emergence of multi- and extensively drug-resistant causative bacilli and co-infection with M. tuberculosis and pandemic human immunodeficiency virus (HIV).4,5 Therefore, TB should be considered as a global emergency, which urgently needs to be solved.
Attenuated strain of Mycobacterium bovis (M. bovis) bacille calmette-guerin (BCG) used to be considered as the only available and effective vaccine in prevention of TB in clinical. However, the protective efficacy of BCG on TB varies in different populations, ranging from 0% to 80%.6,7 Thus, improved TB vaccines are urgently needed as an alternative to BCG. By insertion of various immunostimulatory cytokines, recombinant BCG (rBCG) can be used as an effective modified vaccine to activate immune response and protect against severe forms of TB.8,9 It has been reported, rBCG expressing PPE protein Rv3425 could induce Th1 immune response and provide long-term protection against TB by expanding lymphocytes, increasing IL-2 production and reducing IL-6 production10; By overexpressing Ag85A, rBCG:Ag85A could induce stronger antigen-specific IFN-γ response and higher antibody titer against H37Rv infection than BCG11; rBCG strain expressing pro-apoptotic BAX was able to induce macrophages apoptosis, increase Ag85B-specific IgG2b/IgG1 ratio, promote IFN-γ and IL-2 secretion, and reduce Ag85B-specific IL-4 content.12 In addition, more and more studies began to put emphasis on combination use of immunostimulatory factors in establishment of rBCGs. For example, compared with BCG, rBCG:XB (Ag85B and HspX) was reported to be able to provide stronger and longer-lasting protection effects against M. tuberculosis H37Rv by eliciting more durable multistage antigen-specific CD4(+) Th1-biased immune response13; rBCG expressing Ag85B-ESAT6-Rv3620c could significantly induce strong Th1 immune response and antigen-specific humoral response characterized by increased ratio of antigen-specific IgG2b/IgG1, high expression of Th1 cytokines (IFN-γ, TNF-α and IL-2) and decreased secretion of Th2 cytokine IL-1014; rBCG co-expressing Ag85B, CFP10 and IL-12 could not only elicit greater IFN-γ and TNF-α production than parental BCG, but also limit M. tuberculosis H37Rv multiplication in macrophages.15 All these rBCGs were considered as preferential antigenic targets for vaccine development against TB, while their application in clinical was still limited.16
In this study, a specific antigen of M. tuberculosis Ag85A, BCG defective antigen CFP10 and ESAT-6, as well as immune regulation cytokines GM-CSF and IL-12p70 were integrated into BCG, respectively. The immunogenicity and protective efficacy of these rBCGs were evaluated. Our findings may not only reveal the differences between multiple gene-rBCGs and single-gene rBCGs, but also provide a foundation for the clinical application of rBCGs against TB.
Results
Macro-effects of rBCGs on mice
As the safety of rBCG has always been the basis of its application, the macro-effects of rBCGs established in this study was first evaluated. As a result, no obvious vaccine-related serious adverse events (such as skin infection or skin ulcers) and death could be observed in mice immunized with all kinds of rBCGs for 12 weeks. In addition, no significant change was found on the phenotype of mice immunized with single-gene rBCGs (rBCG-1173:A, rBCG-1173:E, rBCG-1173:I, rBCG-1173:C and rBCG-1173:G) or control BCGs (PBST, BCG-1173 and BCG-1173:361). Although the hair gloss and mental status of mice immunized with multiple-gene rBCGs (rBCG-1173:AE, rBCG-1173:IE, rBCG-1173:GC, rBCG-1173:GE and rBCG-1173:GCE) were slightly worse than that in the control group, the activity and stimuli-response of these mice were considered to be normal. Besides, the weight of mice in each groups was significantly increased in a time-dependent manner (P < 0.05), and no significant differences were revealed among single-gene rBCGs, multiple-gene rBCGs and the control group (Fig. 1). These phenomena indicated that these rBCGs exhibited no obvious side effects on the phenotype and growth state of mice.
Figure 1.
The weight of mice immunized with recombinant bacille calmette-guerins (rBCGs), including rBCG-1173:A, rBCG-1173:E, rBCG-1173:I, rBCG-1173:C, rBCG-1173:G, rBCG-1173:AE, rBCG-1173:IE, rBCG-1173:GC, rBCG-1173:GE and rBCG-1173:GCE. Mice immunized with PBST and BCG-1173 were considered to be the control groups. These mice were weighted every 2 weeks after immunization.
Effects of rBCGs on lgG titers
To evaluate the immunogenicity of rBCGs in mice, the antibody titers of lgG, lgG1 and lgG2a were detected. As shown in Fig. 2, the titers of lgG, lgG1 and lgG2 in mice were significantly increased with the intervention times of rBCGs form 6th to 10th week and then reduced in 12th week (a peak at 10th week) (P < 0.05). This phenomenon indicated that rBCGs could induce strong humoral immune response in mice, particularly in the peak intervention time of 10 weeks.
Figure 2.
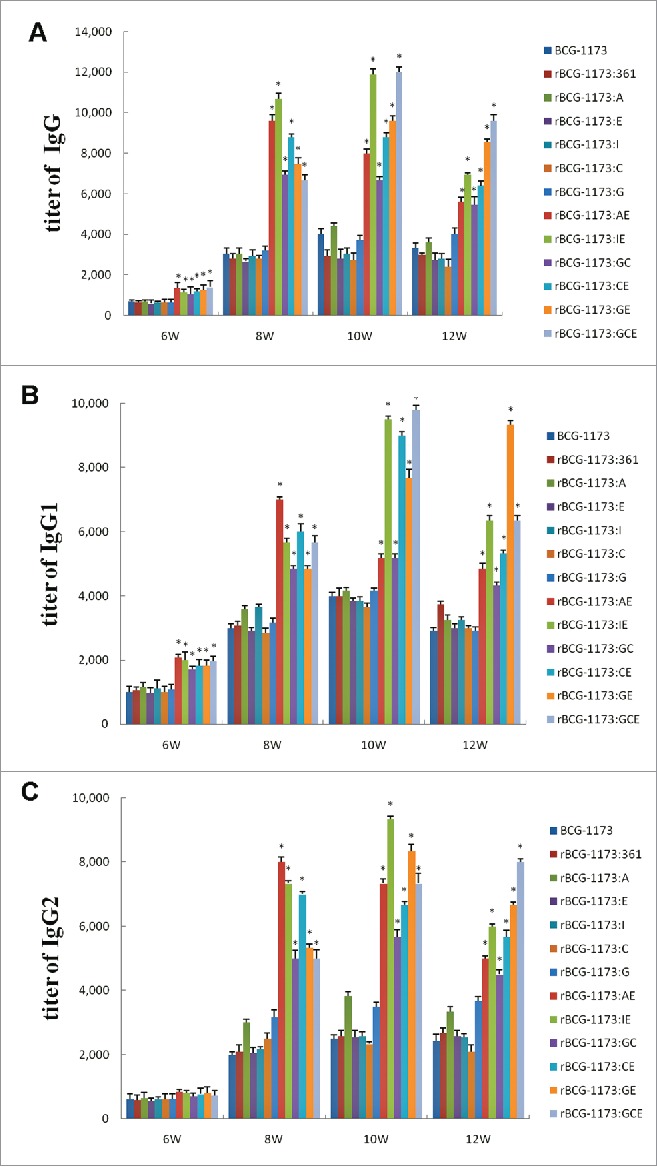
Serum titers of lgG (A), lgG1 (B) and lgG2 (C) in mice immunized with recombinant bacille calmette-guerins (rBCGs), including rBCG-1173:A, rBCG-1173:E, rBCG-1173:I, rBCG-1173:C, rBCG-1173:G, rBCG-1173:AE, rBCG-1173:IE, rBCG-1173:GC, rBCG-1173:GE and rBCG-1173:GCE. Mice immunized with BCG-1173 and BCG-1173:361 were considered to be the control groups. *P < 0.05 vs. BCG-1173, rBCG-1173:361, rBCG-1173:A, rBCG-1173:C, rBCG-1173:E, rBCG-1173:I and rBCG-1173:G group.
For lgG, lgG1 and lgG2 in single-gene rBCGs, most of them were not significantly different from the control groups (BCG-1173 and BCG-1173:361). Although lgG, lgG1 and lgG2 diverse in different multiple-gene rBCGs, they were all found to be significantly higher than single-gene rBCGs (P < 0.05). These results indicated that humoral immune response could be obviously enhanced by multiple-gene rBCGs, while a similar immune response with BCG-1173 was exhibited on single-gene rBCGs.
Effects of rBCGs on splenic lymphocyte
To further reveal the immunogenicity of rBCGs in vivo, the number of CD4+ and CD8+ T splenic lymphocytes in mice was detected by fluorescence intensity. As shown in Tables 1 and 2, the proportion of CD4+ and CD8+ T cells significantly increased in mice with the intervention times of rBCGs (a peak at 8th, 10th or 12th week) (P < 0.05). This result was similar to the trend of lgG titers and further illustrated that rBCGs could induce strong immune response in mice.
Table 1.
The percentages of CD4+ T splenic lymphocytes in mice immunized with recombinant bacille calmette-guerins (rBCGs).
| Groups | 6w | 8w | 10w | 12w |
|---|---|---|---|---|
| PBST | 23.40 ± 0.80 | 22.50 ± 0.71 | 23.20 ± 1.02 | 23.5 ± 0.63 |
| BCG-1173 | 30.51 ± 1.72 | 33.43 ± 0.94a | 35.74 ± 1.81b | 35.14 ± 2.32 |
| rBCG-1173:361 | 29.85 ± 2.50 | 33.65 ± 2.59a | 35.80 ± 1.19b | 34.98 ± 1.68 |
| rBCG-1173:A | 32.34 ± 3.32# | 35.77 ± 2.20a# | 36.95 ± 1.45b# | 36.55 ± 3.07# |
| rBCG-1173:E | 31.40 ± 0.23# | 34.80 ± 1.49a# | 36.90 ± 1.22b# | 36.35 ± 3.49# |
| rBCG-1173:I | 31.58 ± 1.92# | 34.45 ± 2.77a# | 36.50 ± 0.96b# | 35.87 ± 1.07# |
| rBCG-1173:C | 31.80 ± 1.93# | 34.58 ± 1.02a# | 36.18 ± 1.12b# | 35.65 ± 0.93# |
| rBCG-1173:G | 31.05 ± 2.77# | 34.61 ± 0.79a# | 36.43 ± 0.92b# | 36.63 ± 2.25# |
| rBCG-1173:AE | 35.76 ± 0.68* | 39.18 ± 1.24a* | 42.33 ± 2.34b* | 40.45 ± 1.97c* |
| rBCG-1173:IE | 35.10 ± 1.41* | 38.48 ± 1.17a* | 42.68 ± 2.50b* | 41.50 ± 1.07* |
| rBCG-1173:GC | 35.15 ± 1.10* | 37.88 ± 0.47a* | 39.15 ± 1.10b* | 38.78 ± 0.65* |
| rBCG-1173:CE | 34.83 ± 0.47* | 37.15 ± 0.45a* | 40.43 ± 1.33b* | 38.60 ± 0.98c* |
| rBCG-1173:GE | 35.36 ± 0.63* | 37.85 ± 2.25a* | 40.25 ± 1.48b* | 41.45 ± 1.48c* |
| rBCG-1173:GCE | 34.23 ± 2.50* | 38.93 ± 3.18a* | 40.58 ± 0.87b* | 42.63 ± 6.76c* |
a, b and c represent significantly differences at P < 0.05 when compared with the intervention time of 6 w, 8 w and 10 w.
P < 0.05 vs. BCG-1173, rBCG-1173:361, rBCG-1173:A, rBCG-1173:E, rBCG-1173:I, rBCG-1173:C and rBCG-1173:G groups.
P < 0.05 vs. BCG-1173 and rBCG-1173:361 groups.
Table 2.
The percentages of CD8+ T splenic lymphocytes in mice immunized with recombinant bacille calmette-guerins (rBCGs).
| Groups | 6 w | 8 w | 10 w | 12 w |
|---|---|---|---|---|
| PBST | 18.61 ± 0.92 | 17.40 ± 0.63 | 18.32 ± 0.64 | 17.22 ± 0.80 |
| BCG-1173 | 24.71 ± 1.40 | 28.52 ± 1.53a | 30.42 ± 0.94b | 29.51 ± 2.23 |
| rBCG-1173:361 | 24.78 ± 0.39 | 28.13 ± 0.67a | 30.10 ± 1.47b | 29.58 ± 0.79 |
| rBCG-1173:A | 25.83 ± 0.63# | 31.90 ± 0.79a# | 31.02 ± 0.83# | 30.65 ± 0.64c# |
| rBCG-1173:E | 25.23 ± 0.90# | 29.33 ± 2.77a# | 30.98 ± 2.41b | 30.20 ± 0.91# |
| rBCG-1173:I | 25.85 ± 0.82# | 29.75 ± 1.61a# | 31.30 ± 1.24b# | 30.33 ± 1.30c# |
| rBCG-1173:C | 24.93 ± 0.79 | 31.43 ± 1.43a# | 30.52 ± 1.14 | 30.03 ± 0.75# |
| rBCG-1173:G | 25.70 ± 1.27# | 29.88 ± 0.79a# | 31.25 ± 0.61b# | 31.15 ± 0.60# |
| rBCG-1173:AE | 28.85 ± 1.25*,# | 35.66 ± 0.58a* | 35.18 ± 0.33* | 34.63 ± 0.53* |
| rBCG-1173:IE | 28.35 ± 1.62*,# | 34.35 ± 1.34a*,# | 36.90 ± 0.65b* | 34.58 ± 0.64c* |
| rBCG-1173:GC | 27.86 ± 0.63*,# | 33.33 ± 0.86a*,# | 34.92 ± 0.84b* | 33.78 ± 2.55c*,# |
| rBCG-1173:CE | 28.03 ± 1.65*,# | 33.55 ± 0.61a*,# | 34.66 ± 0.55b* | 33.23 ± 1.36c*,# |
| rBCG-1173:GE | 27.88 ± 1.33*,# | 34.21 ± 1.23a*,# | 35.87 ± 1.26b* | 34.65 ± 0.57c* |
| rBCG-1173:GCE | 27.72 ± 0.79*,# | 33.57 ± 1.14a*,# | 35.33 ± 1.22b* | 36.83 ± 1.28c* |
a, b and c represent significantly differences at P < 0.05 when compared with the intervention time of 6 w, 8 w and 10 w.
P < 0.05 vs. BCG-1173, rBCG-1173:361, rBCG-1173:A, rBCG-1173:E, rBCG-1173:I, rBCG-1173:C and rBCG-1173:G groups.
P < 0.05 vs. BCG-1173 and rBCG-1173:361 groups.
Compared with the control group (BCG-1173 and rBCG-1173:361), more CD4+ and CD8+ T cells were found in single-gene rBCGs (P < 0.05). Meanwhile, multiple-gene rBCGs exhibited higher levels of CD4+ and CD8+ T cells than that of single-gene rBCGs (P < 0.05) (Tables 1 and 2). These phenomena indicated that both single-gene rBCGs and multiple-gene rBCGs could enhance the immune response in spleen of mice, and the immune response was stronger in multiple-gene rBCGs than single-gene rBCGs.
On the other hand, a stimulation index (SI) was further used to evaluate the effects of rBCGs on the proliferation of splenic lymphocytes. As a result, SI values were all significantly increased with the intervention of rBCGs (a peak at 8th or 10th weeks) (P < 0.05). Meanwhile, a similar SI value with BCG-1173 was exhibited on single-gene rBCGs, which indicated that single-gene rBCGs could not influence the proliferation of splenic lymphocytes. However, the promotional effects of multiple-gene rBCGs on splenic lymphocytes were obvious, which exhibited significantly higher SI values than in single-gene rBCGs (P < 0.05) (Fig. 3A).
Figure 3.
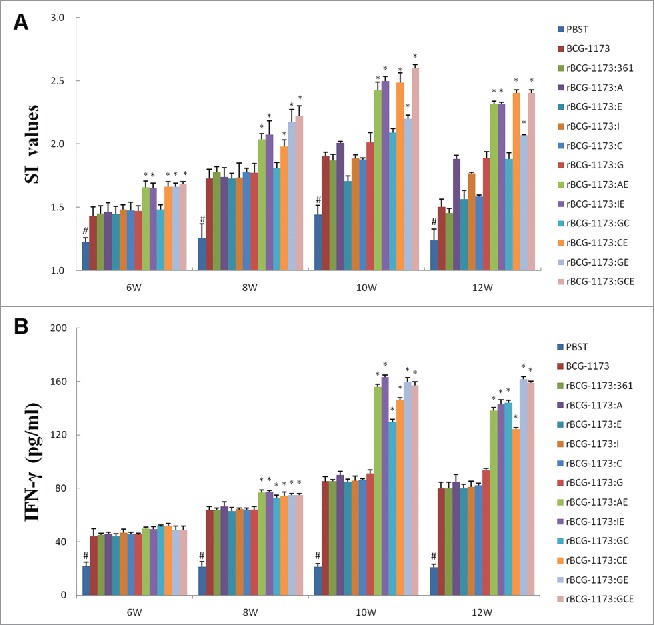
Stimulation index (SI) (A) and IFN-γ content (B) in mice immunized with recombinant bacille calmette-guerins (rBCGs), including rBCG-1173:A, rBCG-1173:E, rBCG-1173:I, rBCG-1173:C, rBCG-1173:G, rBCG-1173:AE, rBCG-1173:IE, rBCG-1173:GC, rBCG-1173:GE and rBCG-1173:GCE. Mice immunized with PBST, BCG-1173 and BCG-1173:361 were considered to be the control groups. * P < 0.05 vs. BCG-1173, rBCG-1173:361, rBCG-1173:A, rBCG-1173:C, rBCG-1173:E, rBCG-1173:I and rBCG-1173:G group; # P < 0.05 vs. mice immunized with rBCGs.
Effects of rBCG on IFN-γ and IL-4
To understand the immune mechanisms within the action of rBCGs, the concentrations of IFN-γ and IL-4 in splenocytes were also analyzed. In consistent with the changed trend of lgG titers and CD4+ and CD8+ T cell numbers, the content of IFN-γ was significantly increased in mice immunized with rBCGs from 6th to 10th week (P < 0.05). In addition, no significant differences on IFN-γ concentrations were revealed among different single-gene rBCGs, while IFN-γ diverse in different multiple-gene rBCGs. What's more, significantly higher IFN-γ was revealed in multiple-gene rBCGs than in single-gene rBCGs (P < 0.05). However, no significant difference was found in the content of IL-4 between rBCGs groups and control groups (data not shown). As IFN-γ was an indicator of Th1-biased immune response, our results just illustrated that Th1 cytokines IFN-γ was essential for protective immunity against M. tuberculosis infection, while Th2 cytokines IL-4 was not.
Protective efficacy of rBCGs
As improved immunogenicity of rBCGs has been identified by the above results, the protective efficacy of rBCGs was further evaluated by bacterial load in spleen and lung of mice infected with M. tuberculosis H37Rv. As shown in Fig. 4, the bacterial load was significantly reduced in spleen and lung from mice immunized with rBCGs when compared with the control group (PBST) (P < 0.05). This result illustrated that rBCGs were conducive to protect against TB, while the protective degree diverse in different types of rBCGs.
Figure 4.
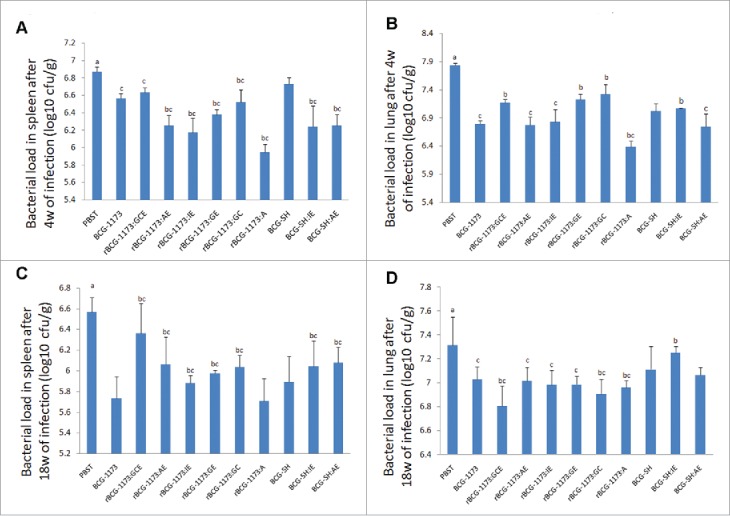
The bacterial load in spleen and lung of mice immunized with recombinant bacille calmette-guerins (rBCGs), including rBCG-1173:A, rBCG-1173:AE, rBCG-1173:IE, rBCG-1173:GC, rBCG-1173:GE, rBCG-1173:GCE, BCG-SH:IE and BCG-SH:AE (log10 CFU/g). Mice immunized with PBST, BCG-1173 and BCG-SH were considered to be the control groups. a P < 0.05 vs. all BCGs; b P < 0.05 vs. BCG-1173; c P < 0.05 vs. BCG-SH.
After 4 weeks of infection, the bacterial load in spleen was significantly lower in various rBCG-1173s than in BCG-1173, except rBCG-1173:GCE (P < 0.05). However, the bacterial load in lung was higher in rBCG-1173:GC, rBCG-1173:GE and rBCG-1173:GCE, and only rBCG-1173:A exhibited a lower bacterial load in lung than in control group (BCG-1173) (P < 0.05). For rBCG-SHs (rBCG-SH:IE and rBCG-SH:AE), the bacterial load in spleen was significantly lower compared with BCG-SH, whereas significantly lower bacterial load in lung was only found in rBCG-SH:AE (P < 0.05). Meanwhile, the bacterial load in spleen was significantly lower in BCG-1173 and rBCG-1173s compared with BCG-SH (P < 0.05). However, the bacterial load in lung was only found to be lower in BCG-1173, rBCG-1173:AE and rBCG-1173:IE than in BCG-SH (P < 0.05). Besides, the bacterial load in spleen and lung did not change significantly between rBCG-SH:IE/AE and rBCG-1173:IE/AE (Fig. 4A,B).
After 18 weeks of infection, the bacterial load in spleen and lung was significantly reduced compared with 4th weeks (P < 0.05), which indicated the infection of M. tuberculosis relieved with prolonged intervention of rBCGs. In spleen, the bacterial load in most multiple-gene rBCG-1173s were significantly higher than that of BCG-1173 (P < 0.05), and only rBCG-1173:A exhibited a similar bacterial load with BCG-1173. On the contrary, the bacterial load in lung was significantly lower in rBCG-1173:GC, rBCG-1173:GCE and rBCG-1173:A than in BCG-1173 (P < 0.05), and no significant differences were revealed on rBCG-1173:AE, rBCG-1173:IE and rBCG-1173:GE. When compared with BCG-SH, significantly higher bacterial load in spleen was found in rBCG-SHs (P < 0.05), while the bacterial load in lung did not change significantly. Meanwhile, the bacterial load in spleen and lung was significantly higher in rBCG-1173s than in BCG-SH (P < 0.05). However, the bacterial load in lung was significantly lower in BCG-1173/rBCG-1173:IE than in BCG-SH/rBCG-SH:IE (P < 0.05). No significantly changes were found in bacterial load of spleen between rBCG-SH/rBCG-SH:IE/rBCG-SH:AE and rBCG-1173/rBCG-1173:IE/ rBCG-1173:AE (Fig. 4C,D).
The pathology of lung, spleen and liver tissues from infected mice were also observed. As a result, the pathologic changes were obviously alleviated in these tissues by various rBCGs. However, there were no significant histopathological differences observed among these rBCGs (data not shown).
Discussion
Vaccination is known as an effective method in prevention of infectious diseases.17 Although BCG vaccine has been widely used against TB in clinical, its application dose not significantly contribute to reduce the incidence of TB.18 Meanwhile, the effects of BCG vaccine always decrease with time since vaccination.19 Based on traditional BCG, rBCG is developed and revealed to be a complete antigen with enhanced immune protection effects.20 In this study, single-gene rBCGs and multiple-gene rBCGs were successfully established by integration of Ag85A, CFP10, ESAT-6, GM-CSF and IL-12p70. After 12 weeks of immunization, no obvious vaccine-related adverse events, including abnormal activity, reduced weight and death were revealed on mice immunized with rBCGs. This result illustrated that rBCGs constructed in our study were safe to mice, which laid a foundation for their clinical application.
The immune responses of rBCGs always depend on the exogenous gene imported.21 For example, Ag85A is known as a specific antigen of M. tuberculosis, which can induce strong T-cell proliferation and antigen-specific IFN-γ response.11 M. tuberculosis secreted protein CFP10 and ESAT-6 also confer strong cell-mediated immunity through T-cell proliferation and IFN-γ production, which has been considered to be effective mycobacterial antigen diagnostic reagents.22 Meanwhile, CFP10 and ESAT-6 are defective in BCG, and the virulence and immunogenicity of BCG could be enhanced by incorporation of RD1 region to restore the expression of CFP10 and ESAT6.23 Besides, many immune regulatory cytokines also has been used in establishment of novel rBCGs due to their ability on improvement of T cell immunity.24 To consistent with previous studies, the integration of Ag85A, CFP10, ESAT-6, GM-CSF and IL-12p70 in single-gene rBCGs significantly promoted the generation of CD4+ and CD8+ T cells in this study, which further illustrated that these integrated factors were beneficial for the improvement of BCG induced immune response.
Although single-gene rBCGs were found to be able to increase the level of CD4+ and CD8+ T cells, the contents of lgG, lgG1, lgG2 did not significantly change in this study. This phenomenon indicated that single-gene rBCGs could enhance the immune response in vivo by various immune protective antigens or immune regulation cytokines in some degree, whereas the generation of antibody against M. tuberculosis were still unsatisfactory and limited. To address this issue, combined application of immune protective antigens and immune regulation cytokines was developed to generate more effective immune response against M. tuberculosis infection than single factors. It has been reported, rBCG-AE (Ag85B and ESAT-6) and rBCG:GE (GM-CSF and ESAT6) were able to induce higher titer of antibody and elicit longer-lasting and stronger Th1 type cellular immune responses than either in rBCG-A or rBCG-E alone25,26; rBCG expressing Ag85C, MPT51 and HspX could induce high amounts of Th1, Th17 and polyfunctional specific T cells, and reduce the extension of lung lesions caused by M. tuberculosis challenge as well as bacterial load in lung27; rBCG co-expressing CFP10, ESAT6, Ag85A and Ag85B could induce a potent antigen-specific immune response characterized by enhanced T cell response and increased production of cytokines28 Similar to the above studies, significantly stronger immune response and antibody generation on multiple-gene rBCGs were revealed than in single-gene rBCGs, including higher levels of IgG2/IgG1, CD4+ and CD8+ T cells and SI values. Our results further illustrated the combined integration of immune protective antigens and immune regulation cytokines in rBCGs was an effective method to improve the immune response against TB.
Although multiple-gene rBCGs were revealed to be able to induce strong immune response against M. tuberculosis, the mechanisms underlying the action of rBCGs were still not fully understood. In this study, IFN-γ was significantly increased in multiple-gene rBCGs than in single-gene rBCGs, while no changes were found on IL-4. As cytokines were always important in controlling M. tuberculosis infection, Th1 cytokines, such as IFN-γ, TNF-a and IL-12 were considered to be essential in the regulation of TB immunity, which have been used as indicators of vaccine efficacy.29 Therefore, the increased IFN-γ in multiple-gene rBCGs may explain the phenomenon of enhanced Th1 immune response, and the efficacy of multiple-gene rBCGs vaccination may be partially reflected by the level of IFN-γ. However, Th2 cytokine IL-4 associated with chronic and progressive phase of tuberculosis may not directly contribute to the immune response induced by multiple-gene rBCGs.
The immune protective effects of rBCGs on M. tuberculosis are not always consistent with their immune response. It has been reported, vaccination with rBCG over-expressing ESAT-6 could induce stronger IFN-γ response than BCG, but exhibited no significant improvement in protective efficacy against M. tuberculosis infection over BCG vaccine30; rBCG-IA was able to elicit stronger humoral and cellular immune responses, while similar protective efficacy with parental BCG was revealed.31 In this study, the bacterial load in spleen and lung was significantly reduced by immunization of various rBCGs, but the protective degree of diverse rBCGs varied from their immune response. In the short term, significantly lower bacterial load in spleen than parental BCG was exhibited on rBCG-1173s, which indicated that these rBCGs could obviously decrease the infection of M. tuberculosis. However, low level of bacterial load in lung was only found in rBCG-1173:A, and other multiple-gene rBCG-1173s all showed significantly higher bacterial load than in parental BCG. This phenomenon may be explained by that Ag85A was an endogenous gene in M. tuberculosis, thus could not induce any side effects.32 By contrast, the toxicity of various exogenous genes (CFP10, ESAT-6, GM-CSF, IL-12p70) may directly contribute to limited immune protective effects of multiple-gene rBCGs. After long-term infection, the bacterial load in spleen was still higher in rBCG-1173s than parental BCG except rBCG-1173:A, while the bacterial load in lung were significantly reduced to a similar level as parental BCG. These results illustrated a better protective immunity of rBCG-1173s in the tissue of lung compare with that in spleen. The lung tissue of immunized mice may adapt to these exogenous genes by long time infection induced immune response, thereby produce better protective immunity against M. tuberculosis. However, the protective efficiency of these rBCG-1173s were not considered to any better than parental BCG. On the other hand, a significantly lower bacterial load in spleen and lung was also revealed in BCG-1173 than BCG-SH, which has been proved by previous studies.33 To pay attention to, similar level of bacterial load between rBCG-SH:IE/AE and rBCG-1173:IE/AE indicated that IE and AE could promote the efficiency of protective immunity obviously regardless of parental differences.
In conclusion, all fusion protein of Ag85A, CFP10, ESAT6, IL-12p70 and GM-CSF could enhance immunogenicity of rBCGs. Compared to single-gene rBCGs, stronger immune response against M. tuberculosis infection was exhibited on multiple-gene rBCGs, while the protective immunity was more effective in rBCG-1173:A and BCG-1173. As protective immunity was still limited in multiple-gene rBCGs, further researches on the development and application of novel rBCGs were still needed.
Materials and methods
Establishment of rBCGs
As to express Ag85A (A), CFP10 (C), ESAT6 (E), 12p70 (I), GM-CSF (G) in BCG, the encoding sequence of these genes were first amplified by PCR and cloned into pMV361 plasmid vector (preserved in our laboratory). After direct sequencing identification, these recombinant plasmids were transformed into M. bovis BCG Pasteur Strain(1173P) and BCG Shanghai Strain (SH) through electroporation as described previously.34 These established rBCGs were cultured in Sauton medium (1.1 g sodium citrate, 4.0 g L-sodium glutamate, 0.33 g K2HPO4, 0.12 g anhydrous magnesium sulfate, 250µl 1% ammonium ferric citrate and 30 ml glycerol; adjust the volume to 500 ml with ddH2O and the PH to 7.2–7.4 with ammonia) at 37°C. After 3–4 weeks of growth, individual colonies were isolated and cultured in Sauton medium containing 50 μg/ml kanamycin. These rBCGs were finally identified by DNA sequencing and Western-blotting.
Immunization of rBCGs on mice
Pathogen-free BALB/c female mice at 6 to 8 weeks old (obtained from Sichuan Antibiotics Institute) were immunized subcutaneously with 100µl 1 × 106 CFU single-gene rBCGs (rBCG-1173:A, rBCG-1173:E, rBCG-1173:I, rBCG-1173:C and rBCG-1173:G) rBCGs and multiple-gene rBCGs (rBCG-1173:AE, rBCG-1173:IE, rBCG-1173:GC, rBCG-1173:GE and rBCG-1173:GCE) on the back. PBST, BCG and BCG:361 in a same volume were used as control groups (PBST, BCG-1173 and BCG-1173). After 6, 8, 10 and 12 weeks of immunization, the phenotype of mice was observed and the weight was measured, respectively. This study was approved by the local Animal Research Committee.
Detection of antigen-specific antibody levels by ELISA
The antigen-specific antibody levels of lgG, lgG1 and lgG2a in mice immunized with single-gene rBCGs and multiple-gene rBCGs were detected by Enzyme-linked immunosorbent assay (ELISA). Simply, plates were first coated with 0.1 ml culture filtrate proteins of M. tuberculosis H37Rv (5 µg/ml) overnight at 4°C. Then 1% BSA-PBS was added to block nonspecific binding sites. The serum samples from mice immunized with rBCGs were analyzed in twofold dilutions using horseradish peroxidase-conjugated goat anti-mouse lgG, lgG1 and lgG2a at 1:1000 (Zhongshan Biotech, Ltd., Beijing, China), and the antibody titers were expressed as reciprocal end point titers.
Detection of splenocytes subsets by flow cytometry
Lymphocytes from spleen of mice immunized with single-gene rBCGs and multiple-gene rBCGs were obtained as described previously,35 and resuspended in complete RPMI 1640 medium with 10% fetal calf serum, 1% L-glutamine, 50 µM 2-mercaptoethanol and 1% penicillin-streptomycin. These lymphocytes at a density of 7 × 105 cells were added into 96-well plates and cultured with 25 µg/ml culture filtrate proteins of M. tuberculosis H37Rv. After 72h of culturing, a part of these cells were pooled by centrifugation and incubated with rabbit anti-mouse CD4 and CD8 MAbs. These cells were washed with PBS, and continuously incubated with goat anti-rabbit IgG-fluorescein isothiocyanate. The proportions of CD4+ and CD8+ T splenic lymphocytes were determined by flow cytometry. On the other hand, the supernatants of the remaining part of lymphocyte cultures were collected, and the contents of IFN-γ and IL-4 were measured by ELISA using paired MAbs (Jingmei Biotech, Ltd., Shenzhen, China) according to the manufacturer's instructions.
Challenge infection
After 8 weeks of immunization with various rBCGs, 10 mice were challenged withby 1 × 105 CFU M. tuberculosis H37Rv through vena caudalis injection. These mice were killed st 8 and 18 weeks postchallenge. The spleen and lung tissues were isolated, then homogenated with physiologic saline and cultured in 7H11 agar medium at 37°C with 5% CO2 for 4 weeks. The bacterial load was finally calculated by log10 CFU of M. tuberculosis (CFU/g). Besides, histopathological examination was performed on the lung, spleen and liver tissues by sequentially fixed in 15% phosphate-buffered formalin, embedded in paraffin, sectioned, stained with hematoxylin and eosin (HE) and Ziehl-Neelsen, and observed under microscope.
Statistical analysis
Statistical analysis was performed by SPSS version 18.0 (SPSS Inc., Chicago, IL). All data were expressed as mean ± standard deviation (SD). Comparison between different groups was determined by one-way ANOVA. A p-value less than 0.05 was considered to be significantly different.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Grants from the Chinese National Key Project of Infectious Disease (2012ZX10003008004) and the Fund of Doctoral Scientific Research of MOE (20110181110046).
References
- [1].Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis 2004; 84:29-44; PMID: 14670344; https://doi.org/ 10.1016/j.tube.2003.08.003 [DOI] [PubMed] [Google Scholar]
- [2].Sinha M, Jain N, Sudevan K. In silico diagnosis of tuberculosis in multiple species using single nucleotide polymorphism as biological marker. Int J Adv Tech Eng Sci 2014; 2:2348-7550. [Google Scholar]
- [3].Masters BR. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, Eighth Edition (2015). John E. Bennett, Raphael Dolin, Martin J. Blaser (eds.). ISBN: 13-978-1-4557-4801-3, Elsevier Saunders. [Google Scholar]
- [4].Migliori GB, Centis R, Lange C, Richardson MDA, Sotgiu G. Emerging epidemic of drug-resistant tuberculosis in Europe, Russia, China, South America and Asia: current status and global perspectives. Curr Opin Pulm Med 2010; 16:171-9; PMID: 20134324 [DOI] [PubMed] [Google Scholar]
- [5].Lazarus J, Olsen M, Ditiu L, Matic S. Tuberculosis–HIV co‐infection: policy and epidemiology in 25 countries in the WHO European region. HIV Med 2008; 9:406-14; PMID: 18410353; https://doi.org/ 10.1111/j.1468-1293.2008.00567.x [DOI] [PubMed] [Google Scholar]
- [6].Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 1995; 346:1339-45; PMID: 7475776; https://doi.org/ 10.1016/S0140-6736(95)92348-9 [DOI] [PubMed] [Google Scholar]
- [7].Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994; 271:698-702; PMID: 8309034; https://doi.org/ 10.1001/jama.1994.03510330076038 [DOI] [PubMed] [Google Scholar]
- [8].Zheng YQ, Naguib YW, Dong Y, Shi YC, Bou S, Cui Z. Applications of bacillus Calmette-Guerin and recombinant bacillus Calmette-Guerin in vaccine development and tumor immunotherapy. Expert Rev Vaccines 2015; 14:1255-75; PMID:26268434 [PMC free article] [PubMed] [Google Scholar]
- [9].Ahsan MJ. Recent advances in the development of vaccines for tuberculosis. Ther Adv Vaccines 2015; 3:66-75; PMID: 26288734; https://doi.org/ 10.1177/2051013615593891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang E, Gu J, Wang F, Wang H, Shen H, Chen ZW. Recombinant BCG prime and PPE protein boost provides potent protection against acute Mycobacterium tuberculosis infection in mice. Microb Pathog 2016; 93:1-7; PMID: 26792673; https://doi.org/ 10.1016/j.micpath.2016.01.006 [DOI] [PubMed] [Google Scholar]
- [11].Xu ZZ, Chen X, Hu T, Meng C, Wang XB, Rao Y, Zhang XM, Yin YL, Pan ZM, Jiao XA. Evaluation of immunogenicity and protective efficacy elicited by mycobacterium bovis BCG Overexpressing Ag85A protein against mycobacterium tuberculosis aerosol infection. Front Cell Infect Microbiol 2016; 6:6; PMID: 26913242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li G, Liu G, Song N, Kong C, Huang Q, Su H, Bi A, Luo L, Zhu L, Xu Y, et al.. A novel recombinant BCG-expressing pro-apoptotic protein BAX enhances Th1 protective immune responses in mice. Mol Immunol 2015; 66:346-56; PMID: 25942359; https://doi.org/ 10.1016/j.molimm.2015.04.003 [DOI] [PubMed] [Google Scholar]
- [13].Yuan X, Teng X, Jing Y, Ma J, Tian M, Yu Q, Zhou L, Wang R, Wang W, Li L, et al.. A live attenuated BCG vaccine overexpressing multistage antigens Ag85B and HspX provides superior protection against Mycobacterium tuberculosis infection. Appl Microbiol Biotechnol 2015; 99:10587-95; PMID: 26363555; https://doi.org/ 10.1007/s00253-015-6962-x [DOI] [PubMed] [Google Scholar]
- [14].Yang E, Lu Y, Xu Y, Liang Q, Wang C, Wang H, Shen H. Recombinant BCG coexpressing Ag85B, ESAT-6 and Rv3620c elicits specific Th1 immune responses in C57BL/6 mice. Microb Pathog 2014; 69:53-9; PMID: 24726737; https://doi.org/ 10.1016/j.micpath.2014.03.011 [DOI] [PubMed] [Google Scholar]
- [15].Chen Y-Y, Lin C-W, Huang W-F, Chang J-R, Su I-J, Hsu C-H, et al.. Recombinant bacille Calmette–Guerin coexpressing Ag85b, CFP10, and interleukin-12 elicits effective protection against Mycobacterium tuberculosis. J Microbiol Immunol Infect 2014; S1684-1182(14)00250-3; PMID: 25732698; 23257069https://doi.org/10.1016/j.jmii.2014.11.019 [DOI] [PubMed] [Google Scholar]
- [16].Pitt JM, Blankley S, McShane H, O'Garra A. Vaccination against tuberculosis: how can we better BCG? Microbial pathogenesis 2013; 58:2-16; PMID: 23257069; https://doi.org/ 10.1016/j.micpath.2012.12.002 [DOI] [PubMed] [Google Scholar]
- [17].Arrazola MP, Serrano A, Lopez-Velez R Vaccination for international travelers. Enferm Infecc Microbiol Clin 2016; 34(5):315-23; PMID: 26920587; https://doi.org/10.1016/j.eimc.20 [DOI] [PubMed] [Google Scholar]
- [18].Marais BJ, Gie RP, Schaaf HS, Beyers N, Donald PR, Starke JR. Childhood pulmonary tuberculosis: old wisdom and new challenges. Am J Respir Crit Care Med 2006; 173:1078-90; PMID: 16484674; https://doi.org/ 10.1164/rccm.200511-1809SO [DOI] [PubMed] [Google Scholar]
- [19].Subramani R, Datta M, Swaminathan S. Does effect of BCG vaccine decrease with time since vaccination and increase tuberculin skin test reaction? Indian J Tuberc 2016; 62(4):226-9; PMID: 26970464; 17584076https://doi.org/10.1016/j.ijtb.2015.08.002 [DOI] [PubMed] [Google Scholar]
- [20].Hernandez-Pando R, Castanon M, Espitia C, Lopez-Vidal Y. Recombinant BCG vaccine candidates. Curr Mol Med 2007; 7:365-72; PMID: 17584076; https://doi.org/ 10.2174/156652407780831610 [DOI] [PubMed] [Google Scholar]
- [21].Singh V, Srivastava R, Srivastava B. Manipulation of BCG vaccine: a double-edged sword. Eur J Clin Microbiol Infect Dis 2016; 35:535-43; ; https://doi.org/ 10.1007/s10096-016-2579-y [DOI] [PubMed] [Google Scholar]
- [22].Tang X-L, Zhou Y-X, Wu S-M, Pan Q, Xia B, Zhang X-L. CFP10 and ESAT6 aptamers as effective Mycobacterial antigen diagnostic reagents. J Infect 2014; 69:569-80; PMID: 24968239; https://doi.org/ 10.1016/j.jinf.2014.05.015 [DOI] [PubMed] [Google Scholar]
- [23].Guo S, Xue R, Li Y, Wang SM, Ren L, Xu JJ. The CFP10/ESAT6 complex of Mycobacterium tuberculosis may function as a regulator of macrophage cell death at different stages of tuberculosis infection. Med Hypotheses 2012; 78:389-92; PMID: 22192908; https://doi.org/ 10.1016/j.mehy.2011.11.022 [DOI] [PubMed] [Google Scholar]
- [24].Millán O, Brunet M. Cytokine-based immune monitoring. Clin Biochem 2016; 49(4-5):338; PMID: 26800778; 20883318https://doi.org/10.1016/j.clinbiochem.2016.01.004 [DOI] [PubMed] [Google Scholar]
- [25].Deng Y, Sun Z, Yang X, Bao L. Improved Immunogenicity of recombinant mycobacterium bovis bacillus calmette‐guérin strains expressing fusion protein Ag85A-ESAT-6 of mycobacterium tuberculosis. Scand J Immunol 2010; 72:332-8; PMID: 20883318; https://doi.org/ 10.1111/j.1365-3083.2010.02444.x [DOI] [PubMed] [Google Scholar]
- [26].Yang X, Bao L, Deng Y. A novel recombinant Mycobacterium bovis bacillus Calmette-Guerin strain expressing human granulocyte macrophage colony-stimulating factor and Mycobacterium tuberculosis early secretory antigenic target 6 complex augments Th1 immunity. Acta Biochim Biophys Sin (Shanghai) 2011; 43:511-8; PMID: 21676888; https://doi.org/ 10.1093/abbs/gmr045 [DOI] [PubMed] [Google Scholar]
- [27].da Costa AC, de Oliveira Costa-Junior A, de Oliveira FM, Nogueira SV, Rosa JD, Resende DP, Kipnis A, Junqueira-Kipnis AP. A new recombinant BCG vaccine induces specific Th17 and Th1 effector cells with higher protective efficacy against tuberculosis. PLoS One 2014; 9:e112848; PMID: 25398087; https://doi.org/ 10.1371/journal.pone.0112848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li W, Li M, Deng G, Zhao L, Liu X, Wang Y. Prime-boost vaccination with Bacillus Calmette Guerin and a recombinant adenovirus co-expressing CFP10, ESAT6, Ag85A and Ag85B of Mycobacterium tuberculosis induces robust antigen-specific immune responses in mice. Mol Med Rep 2015; 12:3073-80; PMID:25962477 [DOI] [PubMed] [Google Scholar]
- [29].Li W, Huang H, Hua W, Ben S, Liu H, Xu B, Xian Q, Tang Z, Shen H. Neonatal revaccination with Bacillus Calmette–Guérin elicits improved, early protection against Mycobacterium tuberculosis in mice. Vaccine 2012; 30:3223-30; PMID: 22342709; https://doi.org/ 10.1016/j.vaccine.2012.02.014 [DOI] [PubMed] [Google Scholar]
- [30].Dey B, Jain R, Khera A, Rao V, Dhar N, Gupta UD, Katoch VM, Ramanathan VD, Tyagi AK. Boosting with a DNA vaccine expressing ESAT-6 (DNAE6) obliterates the protection imparted by recombinant BCG (rBCGE6) against aerosol Mycobacterium tuberculosis infection in guinea pigs. Vaccine 2009; 28:63-70; PMID: 19835824; https://doi.org/ 10.1016/j.vaccine.2009.09.121 [DOI] [PubMed] [Google Scholar]
- [31].Deng YH, He HY, Zhang FJ. Immunogenicity and protective efficacy conferred by a novel recombinant Mycobacterium bovis bacillus Calmette-Guerin strain expressing interleukin-12p70 of human cytokine and Ag85A of Mycobacterium tuberculosis fusion protein. Scand J Immunol 2013; 78:497-506; PMID: 24283772; https://doi.org/ 10.1111/sji.12116 [DOI] [PubMed] [Google Scholar]
- [32].Tang X, Deng W, Xie J. Novel insights into Mycobacterium antigen Ag85 biology and implications in countermeasures for M. tuberculosis. Crit Rev Eukaryot Gene Expr 2012; 22:179-87 [DOI] [PubMed] [Google Scholar]
- [33].Wang J-F, Dai F-Y, Gong X-L, Bao L. Commonly administered bacille Calmette-Guerin strains induce comparable immune response. Int J Clin Exp Med 2015; 8:15834; PMID:26629084 [PMC free article] [PubMed] [Google Scholar]
- [34].Stover CK, Bansal GP, Hanson MS, Burlein J, Palaszynski S, Young J, Koenig S, Young DB, Sadziene A, Barbour AG.. Protective immunity elicited by recombinant bacille Calmette-Guerin (BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J Exp Med 1993; 178:197-209; PMID: 8315378; https://doi.org/ 10.1084/jem.178.1.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Andersen P, Askgaard D, Ljungqvist L, Bentzon MW, Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun 1991; 59:1558-63; PMID:1900811 [DOI] [PMC free article] [PubMed] [Google Scholar]


