ABSTRACT
Hepatitis E virus still poses a great threat to public health worldwide. To date, Hecolin® is the only licensed HEV vaccine in China. Total anti-HEV antibody has been used to reflect vaccine induced immune response in clinical trials for the lack of robust HEV neutralizing antibody detection methods. In this study, we applied a broad neutralizing mouse monoclonal antibody 8G12 to develop a competitive ELSIA assay and quantified 8G12 competitive antibody (8G12-like antibody) in serum samples. The presence of 8G12-like antibody was detected both from participants from HEV vaccine clinical trial and mice immunized with HEV vaccine. Furthermore, 8G12-like antibody was found to have a similar dynamic pattern as anti-HEV antibody during “prime-boost” vaccination, and the proportion of 8G12-like antibody in anti-HEV antibody increased along boost vaccination. Together with previously reported finding that 8G12 could block the most binding of HEV vaccine induced serum antibody to vaccine antigen, we proposed that 8G12-like antibody might be a promising surrogate for vaccine induced HEV neutralizing antibody and had potential to be used as a convenient indicator for HEV vaccine potency evaluation.
KEYWORDS: 8G12, Hecolin®, Hepatitis E virus, neutralizing antibody, vaccine
Introduction
Hepatitis E virus is a nonenveloped virus with a positive-sense, single-stranded RNA genome and belongs to genus Hepevirus of the Hepeviridae family.1 An estimated 35 million HEV infections occur annually worldwide, resulting in more than 70,000 deaths.2 Average mortality rate is between 0.2–4%, while it can reach to 10–25% in pregnant women who are at higher risk for HEV infection.3-5 Although replication of HEV in nonhepatic, hepatic cell lines and even stem cell derived cells has been reported,6-9 robust in vitro culture system is still unavailable. Fortunately, recombinant antigens derived from nucleocapsid protein ORF2 are antigenic and can protect macaque and human from HEV infection.10-15
Early truncation analysis of ORF2 protein (660 aa in length) has found that N-terminal truncated ORF2 can form virus-like particles (VLPs) (112–608aa), while N-terminal 112 residues are responsible for viral RNA genome package.16,17 Extensive structural analysis has identified 3 distinct domains: the S domain (129–319 aa) which forms viral shell; the M domain (320–455 aa) which interacts with S domain and forms the 2-fold, 3-fold and 5-fold icosahedral symmetries of HEV capsid; and the P domain (456–606 aa). P domain which is also known as E2s can form tight homodimers protruding outward from the shell and is essential for viral-host interaction.18 Immune-dominant epitopes have been founded in E2s and it has been identified as the minimum segment with the capability to induce HEV neutralizing antibodies.19-21 Therefore most current HEV vaccine development projects have been focused on various E2s domain containing truncated ORF2 recombinant proteins and different expression systems. Of 3 HEV candidates that have been evaluated in clinical trials (ClinicalTrials.gov Identifier: NCT00287469; NCT01014845; NCT02603055), one HEV vaccine developed by GlaxoSmithKline was ceased after Phase II clinical trial study despite of good safety and efficacy;14 another HEV vaccine, developed by Xiamen Innovax Biotech Co.,Ltd (China) with a trade name as Hecolin®, also showed good safety and efficacy in clinical trials and got successfully licensed in China in 2012;15 while the other HEV vaccine developed by Changchun Institute of Biological Products Co. Ltd., China National Biotech Corporation (CCIBP) has just completed Phase I clinical trial.
Monoclonal antibodies (mAbs) against ORF2 have been raised as probes to study the antigenic sites on E2s and understand host humoral response to HEV ORF2 based vaccines.20,22-25 Comprehensive epitope mapping and clustering have founded a tool box with representative mAbs recognizing conformational and linear epitopes.22,26 Among these mAbs developed by Zhang J and Gu Y. et.al, broad neutralizing mAbs 8H3, 8C11, 8G12 recognize the conserved residues in the E2s dimerization region on ORF2 and can protect macaque rhesus from HEV challenge.23,27 Previous studies showed that 8G12 can significantly block the binding of HEV convalescent sera as well as Hecolin® vaccinated sera from human and macaques rhesus to HEV ORF2 protein.27 The rare predominant existence of 8G12 competitive antibodies (8G12-like antibodies) in serum indicated that most neutralizing antibodies elicited by HEV natural infection or Hecolin® vaccination might recognize similar epitope as 8G12 or epitopes in the vicinity. These findings led to a very intriguing question that whether 8G12-like antibody played a prominent protective role in people vaccinated with Hecolin®. The answer to that question would greatly increase our understanding of the protection mechanism of HEV vaccines.
In this study, we developed an 8G12 competitive ELISA assay to quantify 8G12-like antibody, and analyzed the dynamics of 8G12-like antibody in HEV vaccine induced immune response. Besides, we explored the theoretical feasibility to use 8G12-like antibody as a surrogate for HEV neutralizing antibody, and the possible application of 8G12-like antibody in HEV vaccine development and quality control.
Results
Development of quantitative method for 8G12-like antibody detection
With 3 reported neutralizing mAb 8H3, 8C11, 8G12 kindly provided by Dr.Ningshao Xia, we first analyzed their binding affinity with 3 HEV vaccine candidates in development along with Hecolin® and found that only 8G12 could bind to all known HEV vaccines with high affinity (Table 1). Given the good reactivity to aforementioned HEV vaccines, 8G12 was chosen for the following competitive ELISA assay optimization. Competitive binding of ORF2 truncation protein (394–606aa, genotype I) with horseradish peroxidase labeled 8G12 monoclonal antibody (HRP-8G12) was performed in 96-well MaxiSorp microtiter plates. We first got the response curve of HRP-8G12 binding to immobilized antigen and determined the optimal input of HRP-8G12 (Fig. 1A), and then optimized the procedures of this assay using unlabeled 8G12 diluted in fetal bovine serum as reference (Fig. 1B).
Table 1.
Reactivity of mAbs with different HEV vaccines.
| Binding affinity |
|||
|---|---|---|---|
| HEV vaccine manufacturer | 8H3 | 8C11 | 8G12 |
| National Vaccine & Serum Institute (NVSI) | - | +++ | +++ |
| Changchun Institute of Biological Products (CCIBP) | - | + | +++ |
| Xiamen Innovax Biotech Co., Ltd | + | +++ | +++ |
| Lanzhou Institute of Biological Products (LIBP) | - | +++ | +++ |
"-": No binding; "+": Weak binding; "+++": Strong binding.
Figure 1.
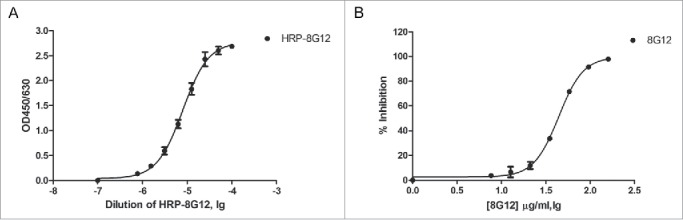
Establishment of a competitive ELISA assay for 8G12-like antibody detection. A) Dose dependent binding of horseradish peroxidase labeled 8G12 (HRP-8G12) to immobilized ORF2 truncation protein (394–606aa, genotype I). The optimal concentration of HRP-8G12 used in competitive ELISA assay was empirically determined. B) Dose dependent competition of unlabeled 8G12 with the binding of HRP-8G12 to immobilized ORF2 truncation protein (394–606aa, genotype I). Data was analyzed with Graphpad Prism. All samples were at least measured for 3 times and representative data from one experiment was shown.
Dynamics of HEV vaccination induced 8G12-like antibody in healthy individuals
Prime-boost vaccination schedule has been often applied in recombinant vaccines to achieve adequate humoral immune response for defenses against infection. General antibody response during “prime-boost vaccination” has been well established. 8G12-like antibody is a subclass of antibodies recognizing dominant epitope on HEV ORF2, we wondered if 8G12-like antibody also adopted similar pattern during HEV vaccination. Both anti-HEV IgG antibody titers and 8G12-like antibody titers from serum samples collected at different time points during HEV vaccination were quantified (Fig. 2), respectively. Though with various titers, 8G12-like antibody could be detected from subjects vaccinated with either Hecolin® or HEV vaccine (Changchun), showing that 8G12-like antibody could be also induced by genotype IV vaccine, and also indicating that the epitope recognized by 8G12 was a dominant epitope recognized by human adaptive immune system. Although a limited amount of human serum samples were analyzed in this study, a positive correlation between titers of 8G12-like antibody and titers of anti-HEV IgG was revealed with linear regression analysis(Hecolin®: r2 = 0.67; Changchun: r2 = 0.80) and Pearson correlation analysis (P<0.001) (Figure S1).The dynamic changes of 8G12-like antibody (Fig. 2, B&D) was similar to anti-HEV IgG (Fig. 2, A&C), and their patterns were consistent with our current knowledge on vaccine immunology.
Figure 2.
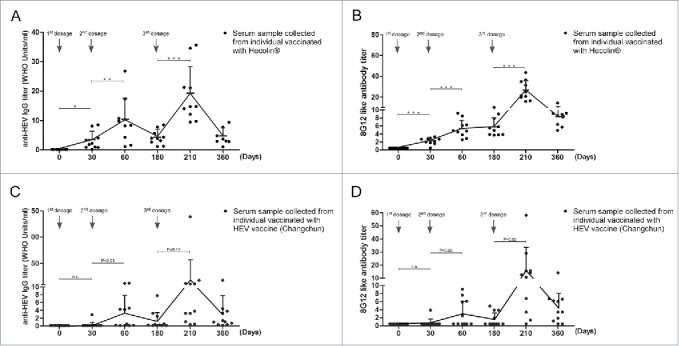
Dynamics of anti-HEV IgG antibodies and 8G12-like antibodies during HEV vaccine vaccination. A) anti-HEV IgG antibodies induced by Hecolin®. B) 8G12-like antibody titers induced by Hecolin®. C) anti-HEV IgG antibodies induced by HEV vaccine (Changchun). D) 8G12-like antibody titers induced by HEV vaccine (Changchun). Antibody titers of each individual were shown in black dots; the overall dynamic pattern of antibody titers (antibody mean titers) along with vaccination time were shown with connecting lines, and antibody mean titers were indicated by connecting points. SD was indicated by error bar. Three vaccine administration time points were indicated with arrow Heads. P value from Student's test was shown in number or asterisk (*** P<0.001; ** P<0.01; * P<0.05). All samples were at least measured for 3 times and representative data from one experiment was shown.
Secondary antibody response resulted from booster antigen exposure achieves a markedly higher level, and produces higher affinity antibodies, also known as affinity maturation. Although only a rather small group of subjects were analyzed in this study, the affinity maturation of 8G12-like antibody along with Hecolin® boost vaccination might be revealed by the increasing ratio of 8G12-like antibody in anti-HEV IgG antibodies in serum samples (Fig. 3).
Figure 3.
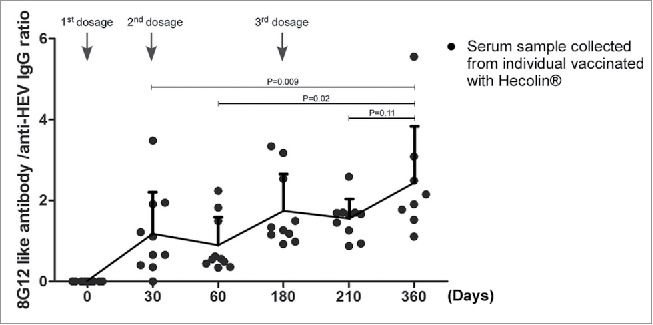
Affinity maturation of 8G12-like antibody during Hecolin® vaccination. The ratio of 8G12-like antibody titer and anti-HEV IgG titer collected from subjects vaccinated with Hecolin® (shown in Fig. 2 A and B) were plotted against vaccination time. Titer ratio of each individual were shown in black dots; the overall dynamic pattern of antibody titers ratio (mean ratio) along with vaccination time were shown with connecting lines, and mean ratio was indicated by connecting point. SD was indicated by error bar. Three vaccine administration time points were indicated with arrow Heads. P value from Student's test was shown.
Dynamics of 8G12-like antibody in mice vaccinated with HEV vaccine
Although mouse model is widely used in vaccine potency evaluation, existing discrepancies in immune response between mouse and human could not be overlooked. So we next wanted to find out if 8G12-like antibody also had the similar pattern as anti-HEV IgG antibody in mice. Both 8G12-like antibody and anti-HEV IgG antibody from BALB/c mice vaccinated with Hecolin® were quantified (Fig. 4). The overall 8G12-like antibody increased along with vaccination course as anti-HEV IgG antibody. To our surprise, both 8G12-like antibody titers and anti-HEV IgG antibody titers varied greatly among vaccinated mice. Although BALB/c mouse is an inbred strain, their immune response to HEV vaccine in terms of vaccine induced antibody titer seemed to be disparate and this may be attributed to the unique characteristics of HEV vaccine per se which need more researches in the future.
Figure 4.
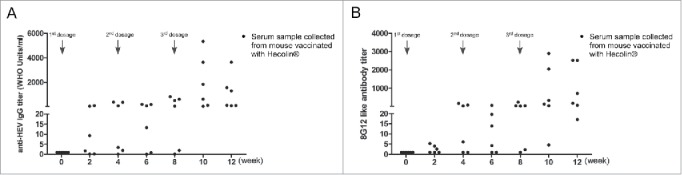
Dynamics of anti-HEV IgG antibodies and 8G12-like antibodies in mice during Hecolin® vaccination. A) anti-HEV IgG antibodies induced by Hecolin®. B) 8G12-like IgG antibody induced by Hecolin®. Antibody titers of each mouse were shown in black dots; 3 vaccine administration time points were indicated with arrow Heads. All samples were at least measured for 3 times and representative data from one experiment was shown.
Discussion
In this work, a well characterized mouse neutralizing monoclonal antibody 8G12, which was shown to efficiently block the binding of naturally acquired anti-HEV antibodies to Hecolin® antigen p239,27 was applied to develop a competitive ELISA assay to detect 8G12-like antibodies in serum samples. With serial serum samples collected from participants enrolled in a HEV vaccine clinical trial, we could detect 8G12-like antibody in all participants vaccinated from Hecolin® and HEV vaccine (Changchun), through the titers at prime vaccination varied in each participant. Dynamic pattern of 8G12-like antibody was similar to that of anti-HEV IgG antibody and relative titer increased after each boost vaccination. Similar correlation of anti-HEV IgG antibody and 8G12-like antibody were also found in Hecolin® immunized mice. These findings indicated that 8G12-like antibody was predominant in vaccine induced anti-HEV antibody both in human and mouse. It was extremely difficult to provide direct evidence to prove that 8G12-like antibody was correlated with in vivo efficacy, so far our findings and good efficacy of Hecolin® together all favored the idea that 8G12-like antibody might be important to in vivo protection of Hecolin®.
Given the difficulties in establishment of robust in vitro HEV infection, there is no convenient way to directly measure HEV neutralizing antibody. Methods detecting neutralizing antibody titers which are relevant to in vivo protection efficacy can provide predictive insights to preclinical and clinical studies, and also are pivotal to vaccine quality control. Real-time PCR and immunofluorescence foci assay (IFA) based methods have been reported to quantify HEV neutralizing antibody titers.29,30 However, these methods are not only laborious, but also restricted by the limited HEV infection efficiency in vitro. Our findings leaded us to bring up the hypothesis that 8G12-like antibody might as well be used as a more relevant surrogate for HEV neutralizing antibody than anti-HEV antibody. There was a similar scenario in respiratory syncytial virus: 4 major neutralizing antigenic sites (I, II,IV, and Ø) have been identified in the fusion protein (F protein); a humanized monoclonal antibody palivizumab which has been approved for prophalytic use in high-risk infants can neutralize RSV infection by binding to the dominant neutralizing site II;31 palivizumab competitive antibody has been proposed as a potential serologic indicator for effective immune responses in RSV vaccine clinical trials.32-34 Though it is unorthodox to use a subclass of antibodies to represent the whole antibody repertoire, the predominant existence of 8G12-like antibody in HEV vaccine induced antibodies could make it a feasible indicator for the neutralizing activity of HEV specific antibodies. Ideally, if more broad neutralizing mAbs which recognize distinct epitopes from 8G12 could be identified and included, together they would be more representative for the whole repertoire. Besides that, similar assays could be developed to stratify the diverse antibody repertoire induced by vaccination in individuals.
Though serum samples from HEV infected patients were unavailable in this work, it would be interesting to investigate the dynamic changes of 8G12-like antibody along with disease progression and resolution. Our study indicated that 8G12-like antibody could be a more relevant surrogate of HEV neutralizing antibody; however, further studies are needed to provide evidences showing the correlation between 8G12-like antibody titer and HEV neutralizing antibody titer. Also, it would be informative to follow the duration of 8G12-like antibody in vaccinees several years after vaccination and find if there is any correlation with HEV infection incidence rate in vaccines group.
Materials and methods
HEV vaccines and vaccine antigens
Hecolin® was derived from ORF2 (368–606aa, genotype I) and provided by Xiamen Innovax Biotech Co.,Ltd (China). The vaccine contains 30μg of the purified protein absorbed to 0.8mg of aluminum hydroxide suspended in 0.5ml of buffered saline. The HEV vaccine candidate used in this study was provided by CCIBP and based on HEVp179 purified from E.coli expression system (ORF2 439–617aa, genotype IV, GenBank accession number AY789228). The vaccine contains 30μg or 40μg of the purified protein HEVp179 absorbed to 0.5mg of aluminum hydroxide suspended in 0.5ml of buffered saline and was designated as HEV vaccine (Changchun).
HEV vaccine antigens were provided by vaccine manufactures, which are CCIBP, National Vaccine & Serum Institute (NVSI), China National Biotechnology Group (Beijing) and Lanzhou Institute of Biological Products, China National Biotech Corporation (LIBP) (Lanzhou).
Monoclonal antibodies
Mouse monoclonal antibody 8G12, 8H3, 8C11 were kindly provided by Dr. Ningshao Xia
Clinical serum samples
Serum samples were collected from healthy participants who were enrolled in phase Ia clinical trial on HEV vaccine developed by CCIBP. Three doses of HEV vaccine (Changchun), Hecolin® reference or placebo were given intramuscularly at 0, 1, and 6 months. Serum samples were taken before the first vaccine dose (Day 0), before the second dose (Day 30), one month after the second dose (Day 60), before the third dose (Day 180), one month (Day 210) and 6 months (Day 360) after the third dose. Serum samples from 10 individuals received Hecolin® as well as 11 individuals vaccinated with 30μg HEV vaccine (Changchun) were analyzed in this work. Written informed consent was obtained from each subject. Independent Ethics Committee approvals were obtained from the Ethics Committee of the Jiangsu Provincial Center for Disease Prevention and Control.
Immunization
Specific-pathogen-free (SPF) female BALB/c mice, weighing 18–22 g and aged 6–7 weeks, were provided by NIFDC. Prior to immunization, all mice were tested for anti-HEV IgG antibodies with ELISA and confirmed to be seronegative for HEV infection. Mice were immunized intraperitoneally (i.p.) with a dose of 1μg Hecolin® at 4-week interval for 3 times. Blood samples were collected on each immunization day and 14 d after each immunization. All the mice were housed and handled strictly in accordance with the institutional (National Institute for Food and Drug Control) guidelines for animal care and use.
Serum references
A WHO reference serum (NIBSC code: 95/584) obtained from the National Institute for Biologic Standards and Control, UK was used as human reference serum for human anti-HEV IgG quantification. It was assigned with an anti-HEV antibody level of 100 WHO units/ml. An in-house mouse reference serum, which was used for mouse anti-HEV IgG quantification in this study, was prepared from sera of Hecolin® vaccinated mice and with an anti-HEV antibody level of 30.9 WHO Units/ml calibrated against the WHO reference serum using a commercial Sandwich Elisa kit purchased from Wantai Co.,Ltd (China).28
Binding affinity comparison of HEV vaccine antigens with mAbs
HEV vaccine antigens were immobilized on 96 well maxisorp microtiter plate at the concentration of 0.5μg/ml or 1μg/ml and incubated with 5μg/ml mAbs (8H3, 8C11, 8G12) in duplicates. Then the bound mAbs were detected with Horseradish peroxidase labeled goat anti-mouse IgG secondary antibody. Binding affinity of mAbs to different HEV vaccine antigens were was arbitrarily defined by OD450/630: strong binding was defined by OD450/630≥ 2.0 and represented with “+++,” medium binding affinity was 1.0≤OD450/630<2.0 with “++,” weak binding affinity was 0.1≤OD450/630<0.5 with “+,” and no binding affinity was OD450/630<0.1 with “−.“
Quantification of anti-ORF2 IgG titers with sandwich ELISA
Anti-ORF2 antibody titer was conventionally used as a surrogate for anti-HEV antibody titer. In this work, anti-ORF2 IgG titer was referred as anti-HEV IgG titer unless stated otherwise. Anti-HEV IgG titers in human serum samples were quantified with commercial HEV IgG detection kit produced by Wantai Co.,Ltd (China). Briefly, serum samples as well as WHO HEV IgG reference were serially diluted with Fetal Bovine serum (Gibco) and incubated with truncated ORF2 (394–606aa, genotype I) which was immobilized on 96 well maxisorp microtiter plate. Captured IgGs were further detected with Horseradish peroxidase labeled mouse anti-human IgG secondary antibody. Standard curve was generated by plotting OD450/630 against the reference concentration (WHO units/ml). Anti-HEV IgG concentration in serum samples was calculated by multiplying the dilution factor with concentration obtained from the standard curve.
Anti-HEV IgG titers in mouse serum samples were quantified with an in-house ELISA assay modified from aforementioned commercial HEV IgG detection kit. Horseradish peroxidase labeled goat anti-mouse IgG secondary antibody was used for bound mouse HEV IgG detection. Meanwhile, the reference was replaced with the in-house calibrated mouse HEV IgG reference.
Quantification of 8G12-like antibody titers with 8G12 competitive ELISA
Serum samples were serially diluted with fetal bovine serum and incubated with horseradish peroxidase labeled 8G12, then incubated with immobilized ORF2 truncated protein (394–606aa, genotype I). Percent inhibition titers were calculated for each serum dilution using the following formula: (OD450/630 8G12 - OD450/630 sample/ OD450/630 8G12) × 100%. Using a Four Parameter Logistic (4PL) curve fit, the titers were determined as the reciprocal value of the serum dilution that resulted in 50% inhibition of 8G12 binding.
Data analysis
Standard curves of ELISA were generated via a 4PL curve fit and concentrations of samples were also extrapolated from standard curves with this software. All statistical analysis in this study was performed with Graphpad Prism software package.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National High Technology Research and Development Program<“863” program, No.2012AA02A402>from the Ministry of Science and Technology of the People's Republic of China.
References
- [1].Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, Reyes GR. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology 1991; 185:120-31; PMID:1926770; https://doi.org/ 10.1016/0042-6822(91)90760-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aggarwal R. The global prevalence of hepatitis E virus infection and susceptibility: a systematic review. Geneva, Switzerland, World Health Organization; 2010 [Google Scholar]
- [3].Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med 1981; 70:252-5; PMID:6781338; https://doi.org/ 10.1016/0002-9343(81)90758-0 [DOI] [PubMed] [Google Scholar]
- [4].Kumar A, Beniwal M, Kar P, Sharma JB, Murthy NS. Hepatitis E in pregnancy. Int J Gynaecol Obstet 2004; 85:240-4; PMID:15145258; https://doi.org/ 10.1016/j.ijgo.2003.11.018 [DOI] [PubMed] [Google Scholar]
- [5].Khaskheli MN, Baloch S, Sheeba A. Acute Hepatitis E Viral infection in pregnancy and maternal morbidity. J Coll Physicians Surg Pak 2015; 25:734-7; PMID:26454389 [DOI] [PubMed] [Google Scholar]
- [6].Emerson SU, Nguyen H, Graff J, Stephany DA, Brockington A, Purcell RH. In vitro replication of hepatitis E virus (HEV) genomes and of an HEV replicon expressing green fluorescent protein. J Virol 2004; 78:4838-46; PMID:15078965; https://doi.org/ 10.1128/JVI.78.9.4838-4846.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Emerson SU, Nguyen H, Torian U, Purcell RH. ORF3 protein of hepatitis E virus is not required for replication, virion assembly, or infection of hepatoma cells in vitro. J Virol 2006; 80:10457-64; PMID:16928762; https://doi.org/ 10.1128/JVI.00892-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Helsen N, Debing Y, Paeshuyse J, Dallmeier K, Boon R, Coll M, Sancho-Bru P, Claes C, Neyts J, Verfaillie CM. Stem cell-derived hepatocytes: A novel model for hepatitis E virus replication. J Hepatol 2016; 64:565-73; PMID:26626494; https://doi.org/ 10.1016/j.jhep.2015.11.013 [DOI] [PubMed] [Google Scholar]
- [9].Okamoto H. Efficient cell culture systems for hepatitis E virus strains in feces and circulating blood. Rev Med Virol 2011; 21:18-31; PMID:21294213; https://doi.org/ 10.1002/rmv.678 [DOI] [PubMed] [Google Scholar]
- [10].Purdy MA, McCaustland KA, Krawczynski K, Spelbring J, Reyes GR, Bradley DW. Preliminary evidence that a trpE-HEV fusion protein protects cynomolgus macaques against challenge with wild-type hepatitis E virus (HEV). J Med Virol 1993; 41:90-4; PMID:8228944; https://doi.org/ 10.1002/jmv.1890410118 [DOI] [PubMed] [Google Scholar]
- [11].Tsarev SA, Tsareva TS, Emerson SU, Govindarajan S, Shapiro M, Gerin JL, Purcell RH. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Natl Acad Sci U S A 1994; 91:10198-202; PMID:7937861; https://doi.org/ 10.1073/pnas.91.21.10198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tsarev SA, Tsareva TS, Emerson SU, Govindarajan S, Shapiro M, Gerin JL, Purcell RH. Recombinant vaccine against hepatitis E: dose response and protection against heterologous challenge. Vaccine 1997; 15:1834-8; PMID:9413090; https://doi.org/ 10.1016/S0264-410X(97)00145-X [DOI] [PubMed] [Google Scholar]
- [13].Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, Ge SX, Xian YL, Pang SQ, Ng MH, et al.. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine 2005; 23:2893-901; PMID:15780738; https://doi.org/ 10.1016/j.vaccine.2004.11.064 [DOI] [PubMed] [Google Scholar]
- [14].Shrestha MP, Scott RM, Joshi DM, Mammen MP Jr., Thapa GB, Thapa N, Myint KS, Fourneau M, Kuschner RA, Shrestha SK, et al.. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med 2007; 356:895-903; PMID:17329696; https://doi.org/ 10.1056/NEJMoa061847 [DOI] [PubMed] [Google Scholar]
- [15].Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, et al.. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010; 376:895-902; PMID:20728932; https://doi.org/ 10.1016/S0140-6736(10)61030-6 [DOI] [PubMed] [Google Scholar]
- [16].Li TC, Yamakawa Y, Suzuki K, Tatsumi M, Razak MA, Uchida T, Takeda N, Miyamura T. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol 1997; 71:7207-13; PMID:9311793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xing L, Kato K, Li T, Takeda N, Miyamura T, Hammar L, Cheng RH. Recombinant hepatitis E capsid protein self-assembles into a dual-domain T = 1 particle presenting native virus epitopes. Virology 1999; 265:35-45; PMID:10603315; https://doi.org/ 10.1006/viro.1999.0005 [DOI] [PubMed] [Google Scholar]
- [18].Li S, Tang X, Seetharaman J, Yang C, Gu Y, Zhang J, Du H, Shih JW, Hew CL, Sivaraman J, et al.. Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus-host interaction. PLoS Pathogens 2009; 5:e1000537; PMID:19662165; https://doi.org/ 10.1371/journal.ppat.1000537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Khudyakov Yu E, Favorov MO, Jue DL, Hine TK, Fields HA. Immunodominant antigenic regions in a structural protein of the hepatitis E virus. Virology 1994; 198:390-3; PMID:8259678; https://doi.org/ 10.1006/viro.1994.1048 [DOI] [PubMed] [Google Scholar]
- [20].Schofield DJ, Glamann J, Emerson SU, Purcell RH. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J Virol 2000; 74:5548-55; PMID:10823861; https://doi.org/ 10.1128/JVI.74.12.5548-5555.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Meng J, Dai X, Chang JC, Lopareva E, Pillot J, Fields HA, Khudyakov YE. Identification and characterization of the neutralization epitope(s) of the hepatitis E virus. Virology 2001; 288:203-11; PMID:11601892; https://doi.org/ 10.1006/viro.2001.1093 [DOI] [PubMed] [Google Scholar]
- [22].Zhao M, Li XJ, Tang ZM, Yang F, Wang SL, Cai W, Zhang K, Xia NS, Zheng ZZ. A comprehensive study of neutralizing antigenic sites on the hepatitis E virus (HEV) capsid by constructing, clustering, and characterizing a tool box. J Biol Chem 2015; 290:19910-22; PMID:26085097; https://doi.org/ 10.1074/jbc.M115.649764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang J, Gu Y, Ge SX, Li SW, He ZQ, Huang GY, Zhuang H, Ng MH, Xia NS. Analysis of hepatitis E virus neutralization sites using monoclonal antibodies directed against a virus capsid protein. Vaccine 2005; 23:2881-92; PMID:15780737; https://doi.org/ 10.1016/j.vaccine.2004.11.065 [DOI] [PubMed] [Google Scholar]
- [24].He J, Kuschner RA, Dewar V, Voet P, Asher LV, Vaughn DW. Characterization of monoclonal antibodies to hepatitis E virus (HEV) capsid protein and identification of binding activity. J Biomed Sci 2007; 14:555-63; PMID:17487571; https://doi.org/ 10.1007/s11373-007-9172-4 [DOI] [PubMed] [Google Scholar]
- [25].Schofield DJ, Purcell RH, Nguyen HT, Emerson SU. Monoclonal antibodies that neutralize HEV recognize an antigenic site at the carboxyterminus of an ORF2 protein vaccine. Vaccine 2003; 22:257-67; PMID:14615154; https://doi.org/ 10.1016/j.vaccine.2003.07.008 [DOI] [PubMed] [Google Scholar]
- [26].Tang X, Yang C, Gu Y, Song C, Zhang X, Wang Y, Zhang J, Hew CL, Li S, Xia N, et al.. Structural basis for the neutralization and genotype specificity of hepatitis E virus. Proc Natl Acad Sci U S A 2011; 108:10266-71; PMID:21642534; https://doi.org/ 10.1073/pnas.1101309108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gu Y, Tang X, Zhang X, Song C, Zheng M, Wang K, Zhang J, Ng MH, Hew CL, Li S, et al.. Structural basis for the neutralization of hepatitis E virus by a cross-genotype antibody. Cell Res 2015; 25:604-20; PMID:25793314; https://doi.org/ 10.1038/cr.2015.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lin HJ WX, Gao F, Chen P, Hao XT, Zhou X, Liang ZL. Development and application of a quantitative method in detection of mouse anti-HEV IgG. Prog Microbiol Immunol 2016; 44:17-20 [Google Scholar]
- [29].Xing L, Li TC, Mayazaki N, Simon MN, Wall JS, Moore M, Wang CY, Takeda N, Wakita T, Miyamura T, et al.. Structure of hepatitis E virion-sized particle reveals an RNA-dependent viral assembly pathway. J Biol Chem 2010; 285:33175-83; PMID:20720013; https://doi.org/ 10.1074/jbc.M110.106336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Meng J, Dubreuil P, Pillot J. A new PCR-based seroneutralization assay in cell culture for diagnosis of hepatitis E. J Clin Microbiol 1997; 35:1373-7. PMID:9163446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].IMpact-RSV Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics 1998; 102:531-7 [PubMed] [Google Scholar]
- [32].Glenn GM, Fries LF, Thomas DN, Smith G, Kpamegan E, Lu H, Flyer D, Jani D, Hickman SP, Piedra PA. A Randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis 2016; 213:411-22; PMID:26259809; https://doi.org/ 10.1093/infdis/jiv406 [DOI] [PubMed] [Google Scholar]
- [33].Smith G, Raghunandan R, Wu Y, Liu Y, Massare M, Nathan M, Zhou B, Lu H, Boddapati S, Li J, et al.. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PloS One 2012; 7:e50852; PMID:23226404; https://doi.org/ 10.1371/journal.pone.0050852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Raghunandan R, Lu H, Zhou B, Xabier MG, Massare MJ, Flyer DC, Fries LF, Smith GE, Glenn GM. An insect cell derived respiratory syncytial virus (RSV) F nanoparticle vaccine induces antigenic site II antibodies and protects against RSV challenge in cotton rats by active and passive immunization. Vaccine 2014; 32:6485-92; PMID:25269094; https://doi.org/ 10.1016/j.vaccine.2014.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


