ABSTRACT
Aim: To compare the safety, immunogenicity and long-term effect of a purified vero cell cultured rabies vaccine in post-exposure subjects following 2 intramuscular regimens, Zagreb or Essen regimen. Methods: Serum samples were collected before vaccination and on days 7, 14, 42, 180 and 365 post vaccination. Solicited adverse events were recorded for 7 d following each vaccine dose, and unsolicited adverse events throughout the entire study period. This study was registered with ClinicalTrials.gov (NCT01821911 and NCT01827917). Results: No serious adverse events were reported. Although Zagreb regimen had a higher incidence of adverse reactions than Essen regimen at the first and second injection, the incidence was similar at the third and fourth injection between these 2 groups as well. At day 42, 100% subjects developed adequate rabies virus neutralizing antibody concentrations (≥ 0.5IU/ml) for both regimens. At days 180 and 365, the antibody level decreased dramatically, however, the percentage of subjects with adequate antibody concentrations still remained high (above 75% and 50% respectively). None of confirmed rabies virus exposured subjects had rabies one year later, and percentage of subjects with adequate antibody concentrations reached 100% at days 14 and 42. Conclusions: Rabies post-exposure prophylaxis vaccination with PVRV following a Zagreb regimen had a similar safety, immunogenicity and long-term effect to the Essen regimen in China.
KEYWORDS: Post-exposure, Protective effect, Rabies vaccine, Safety, Immunogenicity
Introduction
Rabies is a rabid infectious zoonosis disease caused by rabies virus with almost 100% fatality rate.1 According to the World Health Organization (WHO), rabies poses a potential threat to 3.3 billion people worldwide and causes 55,000 deaths each year, most of whom occurred in developing countries.2 China is one of countries threated severely by rabies, where more than 40 million people were bitten by animals every year. More human deaths due to rabies have been reported annually (about 2,000) during the last 5 years, which was the third leading cause of death due to infectious diseases.3-5
Contrary to many other human infectious diseases, timely rabies vaccination can prevent the development of rabies even after exposure to the virus. Therefore, post-exposure vaccination is recognized as an effective method to prevent rabies. Now the intramuscular post exposure prophylaxis (PEP) including 2 immunization schedules, namely Essen regimen (1–1–1–1–1) and Zagreb regimen (2–1–1), was recommended by WHO.6 For Essen regimen, the 5 doses are administered at days 0, 3, 7, 14, 28. As for Zagreb regimen, the first 2 doses are injected at day 0, followed by additional 2 doses given at days 7 and 21. Both regimens performed well in safety and immunogenicity according to previous research.7-9 Since Zagreb regimen entails fewer injections, earlier protective titers, cost-effective option and convenient operation, it was actively promoted in clinical application.10-11
The purified vero cell cultured rabies vaccine (PVRV) manufactured by Chengda Biotechnology has been most widely used in China. And a total of 25 million doses vaccines have been used until now. But this vaccine was not permitted to be used under the Zagreb regimen, until 2010.12 Although the Zagreb regimen has been approved by SFDA later, there were still lacking of direct evidence to confirm the safety, immunogenicity and antibody persistence of this regimen. In this study, we aimed to reevaluate the safety, immunogenicity and antibody persistence of this PVRV administered by the Zagreb regimen by comparing with Essen regimen for post-exposure prophylaxis in 10560 subjects. 37 patients exposed to laboratory-proven rabies-infected dogs were selected to evaluate the indicative of a protective immune response of this vaccine after vaccinated by the Zagreb regimen.
Results
Study population
The trial profile was shown in Fig. 1. 10,560 participants were enrolled, of whom 10,559 received at least one dose of the rabies vaccine and were included in safety analysis. 10386 of these subjects completed the study. Serum samples were collected from 558, 537, 527, 522, 516 and 335 participants at days 0, 7, 14, 42, 180 and 365, respectively. 552 participants were included in the according-to-protocol immunogenic analysis. Reasons why some participants were not included in according-to-protocol immunogenic analysis are: antibody level higher in pre-vaccination than post-vaccination; positive antibody level before vaccination; receipt of anti-rabies passive immunization preparations; and treatment with harmonic drugs.
Figure 1.
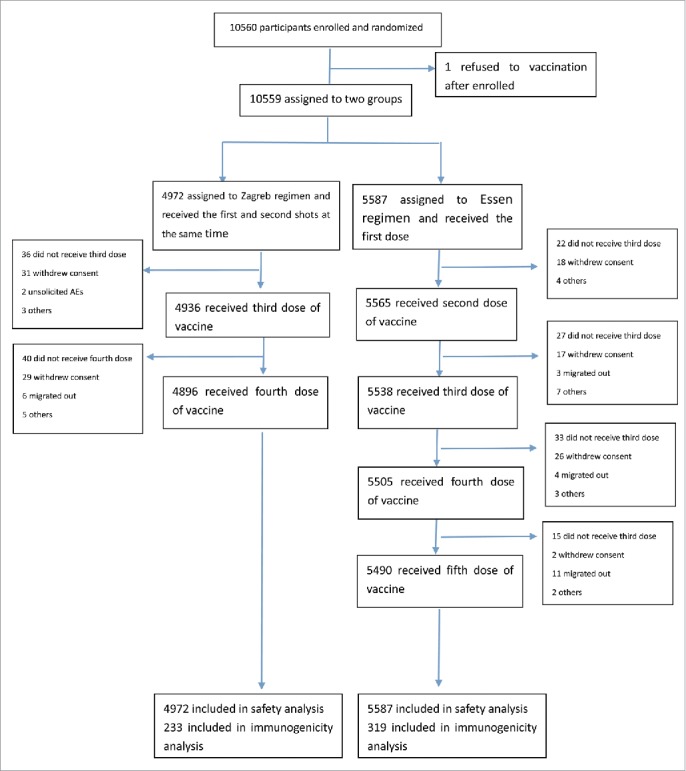
Flow chart of immunogenicity and safety of the vaccine in participants throughout the trial.
The baseline demographic characteristics are presented in Table 1. In the Zagreb group, the average age is 33.7 ± 19.8 years, the male to female sex ratio is 1.10; as for Essen group, the average age is 33.5 ± 20.4 years, sex ratio is 1.06. There is no statistical difference for age and gender distribution in both immune procedures, and both groups are comparable. When comparing different age subgroups, average age in Zagreb group was older than that in Essen group (χ2 = 33.453, p< = 0.0001) in the subgroup of ≤ 20 y. Meanwhile, the inter-group differences of age and gender distribution are insignificant, which is showing better comparability.
Table 1.
Baseline demographic characteristics of participants.
| Zagreb group |
Essen group |
||||||
|---|---|---|---|---|---|---|---|
| ≤ 20 | 21–50 | ≥ 51 | ≤ 20 | 21–50 | ≥ 51 | Total | |
| N | 1426 | 2474 | 1072 | 1600 | 2740 | 1247 | 10559 |
| Age (years) | |||||||
| Mean ± SD | 9.8 ± 5.7 | 35.7 ± 9.3 | 61.0 ± 8.1 | 8.6 ± 5.8 | 35.6 ± 9.4 | 60.9 ± 8.1 | 33.6 ± 20.1 |
| Min;max | 0∼20 | 21∼50 | 51∼90 | 0∼20 | 21∼50 | 51∼89 | 0∼90 |
| Median | 9.0 | 36.0 | 59.0 | 7.0 | 36.0 | 59.0 | 33.0 |
| F | 33.453 | 0.137 | 0.094 | 0.294 | |||
| P value | 0.000 | 0.711 | 0.759 | 0.587 | |||
| Sex | |||||||
| Male n (%) | 859 (60.2) | 1236 (50.0) | 513 (47.9) | 958 (59.9) | 1319 (48.1) | 601 (48.2) | 5486 (52.0) |
| Female n (%) | 567 (39.8) | 1238 (50.0) | 559 (52.1) | 642 (40.1) | 1421 (51.9) | 646 (51.8) | 5073 (48.0) |
| Male/Female | 1.51 | 1.00 | 0.92 | 1.49 | 0.93 | 0.93 | 1.08 |
| F | 0.042 | 1.725 | 0.027 | 0.934 | |||
| P value | 0.839 | 0.189 | 0.870 | 0.334 | |||
Safety
In the study, 2820 out of 10559 (26.7%) participants reported injection-site or systemic adverse reactions considered to be related to vaccination, with 1469 (29.5%) in Zagreb group and 1351 (24.2%) in Essen group (Table 2). Overall, the incidence of adverse reactions was slightly higher in Zagreb group than in Essen group (χ2 = 38.675, P = 0.000). 61 (1.2%) subjects in Zagreb group and 21 (0.4%) subjects in Essen group had adverse events of grade 3. No serious adverse events (SAE) were reported.
Table 2.
Incidence of adverse reactions within 28 d after the last vaccination.
| Zagreb group |
Essen group |
|||||
|---|---|---|---|---|---|---|
| N | % | N | % | χ2 | P | |
| N | 4972 | 100.0 | 5587 | 100.0 | ||
| Any | 1469 | 29.5(28.3∼30.8) | 1351 | 24.2(23.1∼25.3) | 38.675 | <0.0001 |
| Grade 1 | 1217 | 24.5(23.3∼25.7) | 1237 | 22.1(21.1∼23.2) | ||
| Grade 2 | 191 | 3.8(3.3∼4.4) | 93 | 1.7(1.4∼2.0) | ||
| Grade 3 | 61 | 1.2(0.9∼1.6) | 21 | 0.4(0.2∼0.6) | ||
Incidence of adverse reactions for each injection differed between the 2 groups. 1262 of 4972 (12.7%) participants in Zagreb group who received 2 doses at the same time had adverse reactions comparing with 994 of 11152 (8.9%) in Essen group who received the first and second injections successively (χ2 = 78.558, P<0.0001). The incidence of adverse reactions did not differ much between the 2 groups after the third or fourth injections (Table 3).
Table 3.
Incidence of adverse reactions of each vaccination in 2 regimen groups.
| Vaccination injection | Zagreb group(%) | Essen group(%) | χ2 | P |
|---|---|---|---|---|
| The first/second injection | 12.7 (1262/9944) | 8.9(994/11152) | 78.558 | 0.000 |
| The third injection | 9.2(455/4936) | 8.5(469/5538) | 1.821 | 0.177 |
| The fourth injection | 5.5(271/4896) | 5.9(325/5505) | 0.652 | 0.419 |
| The fifth injection | 2.3(124/5490) |
Overall, 1988 and 1214 participants reported injection-site and systemic reactions, respectively, most of which were reported at day 0∼3 after vaccination. The most common systemic adverse reactions were fever and fatigue (Table 4). The incidence of local reactions was 20.6% in the Zagreb regimen and 17.3% in the Essen regimen. The most common injection-site adverse reactions were pain, swelling and itching. Significantly more participants in the Zagreb regimen had injection-site pain than did those in the Essen regimen. However, incidence of local reactions, such as swelling and itching did not differ much between the 2 groups.
Table 4.
Adverse reactions list within 28 d after the last vaccination.
| Zagreb group |
Essen group |
|||||
|---|---|---|---|---|---|---|
| Age(years) | ≤ 20 N = 1426 | 21–50 N = 2474 | ≥ 51 N = 1072 | ≤ 20 N = 1600 | 21–50 N = 2740 | ≥ 51 N = 1247 |
| Total incidence of adverse reactions (%) | 463(32.4) | 736(29.7) | 271(25.2) | 276(17.3) | 803(29.3) | 272(21.8) |
| Systemic adverse reactions | ||||||
| Incidence (%) | 278(19.5) | 243(9.8) | 110(10.3) | 189(11.8) | 280(10.2) | 114(9.1) |
| Fever | 240(16.8) | 59(2.4) | 17(1.6) | 128(8.0) | 42(1.5) | 13(1.0) |
| fatigue | 33(2.3) | 132(5.3) | 64(6.0) | 25(1.6) | 147(5.4) | 71(5.7) |
| Allergy | 8(0.6) | 6(0.2) | 5(0.5) | 11(0.7) | 21(0.8) | 8(0.6) |
| Dysphoria | 1(0.1) | 6(0.2) | 4(0.4) | 2(0.1) | 4(0.1) | 1(0.1) |
| Loss of appetite | 1(0.1) | 2(0.1) | 4(0.4) | 12(0.8) | 3(0.1) | 6(0.5) |
| Nausea | 13(0.9) | 12(0.5) | 5(0.5) | 6(0.4) | 24(0.9) | 6(0.5) |
| Diarrhea | 2(0.1) | 4(0.2) | 1(0.2) | 6(0.4) | 5(0.2) | 6(0.5) |
| Dizziness | 13(0.9) | 35(1.4) | 16(1.5) | 4(0.2) | 44(1.6) | 12(1.0) |
| Headache | 6(0.5) | 16(0.6) | 6(0.6) | 8(0.5) | 22(0.8) | 10(0.8) |
| Myalgia | 0(0.0) | 12(0.5) | 5(0.5) | 2(0.1) | 11(0.4) | 5(0.4) |
| Cough | 3(0.2) | 1(0.0) | 0 | 4(0.3) | 1(0.0) | 0(0.0) |
| Others | 9(0.6) | 14(0.6) | 12(1.1) | 6(0.4) | 31(1.1) | 11(0.9) |
| Local reactions | ||||||
| Incidence (%) | 234(16.4) | 587(23.7) | 201(18.8) | 125(7.8) | 648(23.6) | 193(15.5) |
| Pain | 190(13.3) | 497(20.1) | 170(15.9) | 107(6.7) | 558(20.4) | 168(13.5) |
| Swelling | 25(1.8) | 77(3.1) | 23(2.1) | 15(0.9) | 104(3.8) | 37(3.0) |
| Itching | 13(0.9) | 39(1.6) | 26(2.4) | 6(0.4) | 72(2.6) | 23(1.8) |
| Induration | 9(0.6) | 28(1.1) | 9(0.8) | 5(0.3) | 45(1.6) | 18(1.4) |
| Erythema | 14(1.0) | 20(0.8) | 8(0.7) | 15(0.9) | 58(2.1) | 13(1.0) |
| Others | 6(0.4) | 16(0.6) | 4(0.4) | 1(0.1) | 15(0.5) | 3(0.2) |
Immunogenicity
The baseline antibody level is shown in Table 5. Overall, there were no significant differences in GMC between the 2 regimens (F = 0.997, P = 0.408). The antibody concentration in the age subgroup were similar between the 2 regimens (P > 0.05).
Table 5.
Baseline antibody level of participants.
| Zagreb group |
Essen group |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age(years) | ≤ 10 | 11–20 | 21–50 | 51–60 | >61 | Total | ≤ 10 | 11–20 | 21–50 | 51–60 | >61 | Total |
| N | 34 | 36 | 92 | 35 | 36 | 233 | 56 | 31 | 129 | 67 | 36 | 319 |
| GMC(IU/ml) | ||||||||||||
| Mean ± SD | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.03 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.02 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.04 | 0.04 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.03 |
| Min-max | 0.04–0.04 | 0.04–0.04 | 0.04–0.30 | 0.04–0.04 | 0.04–0.04 | 0.04 – 0.30 | 0.04–0.04 | 0.04–0.04 | 0.04–0.41 | 0.04–0.10 | 0.04–0.04 | 0.04 – 0.41 |
| 95%CI | 0.04–0.04 | 0.04–0.04 | 0.04–0.05 | 0.04–0.04 | 0.04–0.04 | 0.04 – 0.04 | 0.04–0.04 | 0.04–0.04 | 0.04–0.05 | 0.04–0.04 | 0.04–0.04 | 0.04 – 0.05 |
| F | 0.000 | 0.000 | 0.186 | 0.520 | 0.000 | 0.997 | ||||||
| P | 1.000 | 1.000 | 0.667 | 0.473 | 1.000 | 0.408 | ||||||
A significant neutralizing antibody response was elicited after vaccination under both regimens. The percentage of subjects with adequate antibody concentrations (≥ 0.5IU/ml) in Zagreb group was 24.3% higher than that in Essen group at day 7 (Fig. 2). 100% of participants developed adequate rabies virus neutralizing antibody (RVNA) concentrations at day 14 with a GMC of 6.15 IU/ml (95%CI, 5.00 to 7.30 IU/ml) for Zagreb group, while 99% of participants developed adequate RVNA concentrations at day 14 with a GMC of 7.63 IU/ml (95%CI, 6.48 to 8.79 IU/ml) for Essen group (Figs. 2 and 3). At day 42, both regimens elicited peak immune response with mean GMC of 9.59 ± 8.66IU/ml (95% CI, 8.45 to 10.73IU/ml) and 12.29 ± 9.75IU/ml (95% CI, 11.16 to 13.41IU/ml) for Zagreb and Essen groups, respectively (Figs. 2 and 3). Antibody level in Essen group was significantly higher than that in Zagreb group (χ2 = −3.321, P = 0.001). However, at day 42, 100% subjects developed adequate RVNA concentrations for both regimens (Fig. 2). Vaccination with PVRV following a Zagreb regimen induced immune responses at days 7, 14 and 42 non-inferior to those of Essen regimen (P>0.05).
Figure 2.
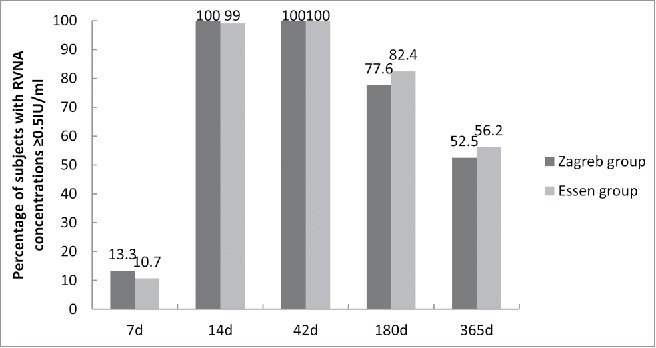
Percentage of subjects with RVNA concentrations ≥ 0.5IU/ml of the vaccine on days 7, 14, 42, 180, 365. Percentage of rate difference was 24.3%, 95%CI: 26.5 to 112.5 on day 7.
Figure 3.
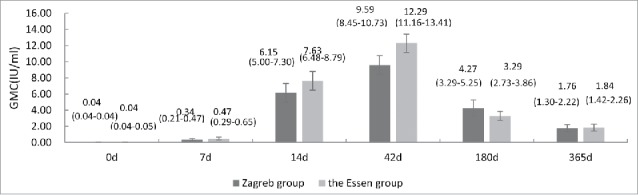
Rabies virus neutralizing antibody concentrations (GMC) in the Zagreb and Essen regimens on days 7, 14, 42, 180, 365. Error bars and values in parenthesis represent 95% CI.
To assess antibody persistence, we tested antibody concentration at months 6 and 12. The results showed that the GMCs decreased dramatically in both groups, 95%CI 1.30 to 2.22 IU/ml in Zagreb group and 95%CI 1.42 to 2.26 IU/ml in Essen group at month 12, respectively. However, the percentage of subjects with adequate antibody concentrations remained high in both groups, 77.6% in Zagreb group and 82.4% in Essen group at month 6. One year after vaccination, more than 50% of participants remained showed adequate RVNA concentrations (Figs. 2 and 3).
Protective effect in patients exposed to laboratory-proven rabies infected dogs by the Zagreb regimen
PVRV had a good protective effect by the Essen regimen, however, little is known about the protective effect by the Zagreb regimen. In this study, these 37 subjects exposed to laboratory-proven rabies infected dogs were followed up for more than 12 months to monitor actual protective effect of this vaccine. And consecutive serum samples from these participants on days 0, 7 14 and 42 were tested for systemic evaluation antibody level.
Up to 12 months, none of 37 subjects had rabies. Compared with concentrations measured on day 0, we noted a significant increase in anti-rabies virus antibody concentration on days 14 (22.75 IU/ml, 95%CI 5.01 to 40.49 IU/ml, p = 0.016) and 42(16.44 IU/ml, 95%CI 11.39 to 21.48 IU/ml, p = 0.000) (Fig. 4). The percentage of subjects with adequate antibody concentrations was statistically differences at days 7(21.7%, 95%CI 8.4 to 41.0%), 14(100%) and 42(100%) (Fig. 5).
Figure 4.
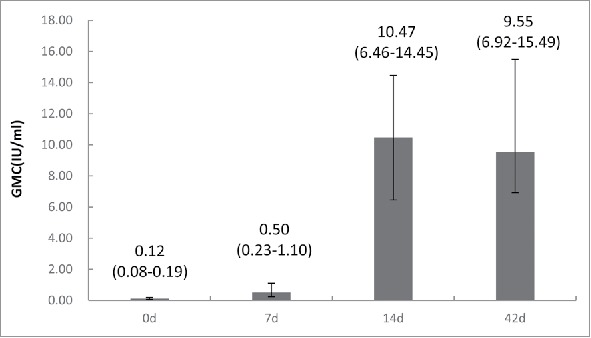
Rabies virus neutralizing antibody concentrations (GMC) of patients exposed to laboratory-proven rabies infected dogs in the Zagreb regimen on days 7,14 and 42. Error bars and values in parenthesis represent 95% CI. Y-axis is logarithmic scale of GMC.
Figure 5.
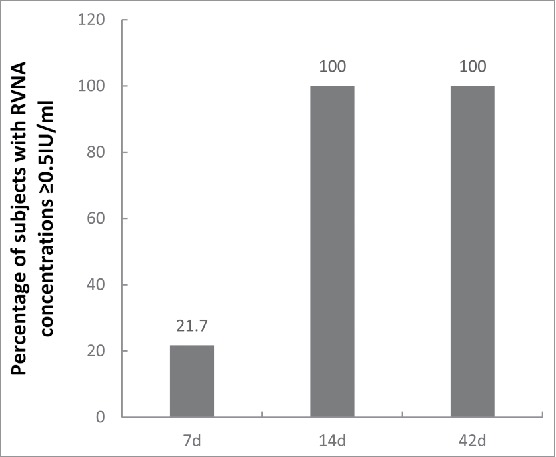
Percentage of subjects exposed to laboratory-proven rabies infected dogs with RVNA concentrations ≥ 0.5IU/ml in the Zagreb regimen on days 7, 14 and 42. 95% CI on day 7 is 8.4 to 41.0, while on days 14 and 42 is 100 to 100.
Discussion
Rabies is a fatal disease with almost 100% of fatality rate.1 PEP is the most important way to prevent and control rabies. 5-dose Essen regimen has been recommended by WHO for PEP vaccination for several decades,6 which was ever the only schedule approved in China. However, exposed individuals often withdrew vaccination midway, on the other hand, full vaccination compliance rate of category II, III exposure was only 77.1% and 78%,10 remaining great risk in those peoples. The effectiveness of the 4-dose Zagreb regimen was first investigated by Vodopija et al in 1986.13 They compared the immunogenicity of 4 different cell culture vaccines administered post-exposure according to the Zagreb regimen. In their study, each PEP vaccine resulted in 100% subjects attaining high RVNA levels (≥ 0.5 IU/ml) by day 14. WHO have recommended Zagreb vaccination regimen since 1992 due to it reduce cost and increase full vaccination compliance rate markedly.6,10,14 Numerous studies over the years have served to verify that cell culture vaccines administered under the Zagreb regimen are immunogenic with an acceptable safety profile.12-13,15-18 However, Zagreb regimen was not approved for purified chick embryo cell rabies vaccine (PCECV) and PVRV until 2010 in China.
The PVRV for intramuscular pre- and post-exposure rabies prophylaxis manufactured by Chengda Biotechnology has been proven to be well tolerated and immunogenic with an acceptable safety profile in China.19-23 It has been approved initially by SFDA to use under the Essen regimen in 2005, and permitted to use Zagreb regimen later. Until now, the vaccine has been used 3 million under Zagreb regimen, however, it remained far below than Essen regimen.
In this study, we conducted a post-marketing evaluation for the safety, immunogenicity, and antibody persistence of PVRV given by the Zagreb regimen by comparing with Essen regimen. The most frequent injection-site AEs were pain, swelling and itching, which was the same as the results reported by Hu11 and Liu.12 And most frequent systemic AEs were fever and fatigue, consisted with the finding reported by Hu,11 but different from that of Liu.12 The reason may be we performed the research in people of all ages while Liu performed the research in healthy adults. Zagreb regimen caused slightly more AEs than Essen regimen did, which may be associated with the different doses of vaccine at first time immunization. However, most of them were mild or moderate and SAE were uncommon. Similar results were also observed in the research of Yuan24 and Hu.11 To the immunogenicity, both 2 regimens induced significantly antibody response at days 14 and 42 with peak antibody concentration of more than 9 IU/mL at day 42, and the percentage of subjects with adequate antibody concentrations of both groups were 100% at day 42, consistent with the previous research results of this product.1,12 Though antibody concentration in Essen group was significantly higher than that in Zagreb group on day 42, no significant difference was observed between the 2 groups in terms of adequate antibody concentrations rates. And the percentage of subjects with adequate antibody concentrations in Zagreb group was 24.3% higher than that in Essen group at day 7, which indicating Zagreb regimen being non-inferior to Essen regimen. These results were in agreement with previous results obtained with healthy adult subjects vaccinated by the Zagreb regimen with PCECV or other licensed vaccines.7,11-12 Our results confirmed again that a 4-dose regimen was sufficient for RVNA titers to reach effective levels of protection antibody concentrations significantly declined at month 6 and 12, but the percentage of subjects with adequate antibody concentrations in these 2 groups were very close. Essen regimen has been demonstrated the persistence of immunogenicity for up to 5 years,25 so we speculated Zagreb regimen had good long-term effect. In general, the Zagreb regimen showed a good safety, immunogenicity profile and long-term effects as well as Essen regimen.
Studies showed rabies virus neutralizing antibody has protection when its concentration higher than 0.5 IU/ml tested by rapid fluorescent focus inhibition test (RFFIT),26 However, little is known about the actual protective effect after vaccination with this vaccine especially under the Zagreb regimen. In this study, we identified cohort exposed to laboratory-proven rabies infected dogs and evaluated the protective effect after vaccination under Zagreb regimen. Our results showed 37 severely bitten patients were all survived one year later, after injection with immune globulin and PVRV by the Zagreb regimen. The percentage of subjects with adequate antibody concentrations on days 14 and 42 was 100%, and the antibody concentration was 22.75IU/ml and 16.44 IU/ml. This results suggested Zagreb regimen was likely to be “protective.”
In addition to intramuscular(IM) regimens, intradermal (ID) regimens, like TRC ID regimen (2.2.2.0.2) and some of the promising new ID regimens (e.g., one week 4.4.4), were also recommended by WHO.27-28 Several of these were regarded as scientifically rational, highly immunogenic, safe and economical vaccination for PEP. However, China did not approved any ID regimens used in rabies vaccine vaccination. We believe it might be interesting to explore Speeda administered according to the ID regimen in future.
The present study may have several limitations. First, the 37 patients bitten by proven rabid animals is not big enough, so to confirm the protective effect of Speeda used in the Zagreb regimen need to expand the sample size for further study. Second, the potency (7.5IU/dose) was higher than the required 4.5 IU/dose. This maybe have an impact on the longevity of RVNA concentrations. The issue of long-term immunogenicity of these 2 regimens is important to perform in the future.
In conclusion, this study showed that rabies PEP vaccination with PVRV following a Zagreb regimen had a similar safety, immunogenicity and antibody persistence to Essen regimen in China. PVRV administered by the Zagreb regimen also showed good protective effect. Based on calculations of the direct and indirect costs of rabies immunization in China, the Essen regimen costs about $104 (694RMB) per patient, whereas the Zagreb regimen cost $72 (482.2RMB) per patient, respectively. About an average of 14 million patients receive rabies immunizations each year in China. Adopting the Zagreb regimen could save approximately $442(2963RMB) million plus the cost of clinic visits.10 So comparing with Essen regimen, Zagreb regimen was a more convenient, cost-effective and compliant vaccination schedule.
Methods
Study design and participants
This trial was conducted in 5 centers in China (Chaoyang district in Beijing, Wuhan city in Hubei, Guangdong, Guizhou and Hunan province). We defined participants who were bitten or scratched by animals suspected or confirmed of rabies virus as post-exposure subjects. There were no age limit for subjects. The main exclusion criteria included: history of rabies vaccination; receipt of anti-rabies immunoglobulin; allergy to any vaccine component; treatment with immunosuppressive or immunoenhancer drugs; fever higher than 37°C (axillary); acute or chronic infectious diseases; receipt of any vaccines or investigational medicines in the previous week; history of eclampsia, epilepsy, encephalopathy and spiritual disease; immunodeficiency; congenital defects; other conditions being unable to comply with the study schedule.
The study was approved by the institutional review board of Chaoyang District Center of Disease Control and Prevention and conducted in accordance with Good Clinical Practice. All subjects or the subjects' parents/legal guardian (minor subjects <18 y of age) provided written informed consent before enrollment.
Procedures
The rabies vaccines (vero cell, Lot no.201108227) used in this study were manufactured in Liaoning Chengda Biotechnology (Shenyang, China). Potency of the vaccine was 7.5IU/ml as provided by the manufacturer using the NIH test. 0.5 ml of vaccine was administered intramuscularly as each dose. The vaccine used in this clinical trial originated from normal commercial circulation and were produced in the same lot. The subjects paid for their own vaccine.
Subjects were enrolled in conventional rabies outpatient clinic. Rabies-clinics were randomly allocated into 2 groups in a 1:1 ratio to inject rabies vaccine for subjects by the Zagreb regimen or Essen regimen. Randomization list was generated with SAS 9.1 statistical software by means of randomized blocking. The size of the block was set as variable with the length of the smallest block being 4. 10560 participants were enrolled. The vaccine was injected intramuscularly into the anterolateral side of thigh in infants of 0–11 months and the deltoid region in other subjects. The occurrence of immediate adverse events was monitored for 30 minutes after each injection. All solicited adverse events were recorded for up to 7 d following each vaccine dose, and unsolicited AEs were recorded throughout the entire study period up to day 28. Adverse events were graded according to the scale issued by the China Food and Drug Administration.29
Blood samples for immunogenicity testing were collected from a subset of 558 participants before vaccination (day 0) and days 7, 14, 42, 180 and 365 post vaccination. In the second phase of the study, 37 subjects exposed to laboratory-proven rabies infected dogs were enrolled, cleaned up the wound, injected with the human rabies immune globulin (HRIG, Wuhan Biotechnology, Lot no.20120303, China), and vaccinated with the rabies vaccine by the Zagreb regimen. HRIG was infiltrated into the wound by the body weight, 20IU/kg. Serum samples were taken from these participants at days 0, 7, 14, 42. Determination of RVNA using the RFFIT with CVS-11 as the challenge virus for the assay was performed at the National Institute for Food and Drug Control (Beijing, China). Base on WHO criteria, an rabies virus neutralizing antibody concentration ≥ 0.5IU/mL was considered as adequate,28 and 0.5IU/ml also was taken as the cut-off value of indicative of a protective immune responses.26
Dogs' specimens collection and detection
In this study, dogs suspected to carry rabies virus and that infected more than 3 people were captured and then humanely executed by Guizhou Provincial Center of Disease Control and Prevention. About 1–3 g specimens were taken from dogs' brain homogenates and frozen at −20°C, then sent to Chinese Center for Disease Control and Prevention to tested for presence of the rabies virus using a direct immunofluorescence assay (DFA).30 Green dot fluorescence was judged as positive and then carry out the follow-up test.
The DFA positive specimens would be tested by reverse transcription PCR (RT-PCR) to confirm the rabies virus infection according to Lang's method.31 A total of 3 rabies virus carrying dogs were identified, and 44 persons were bitten by these dogs. 37 out of 44 subjects were informed consent and participated in this study.
Statistical analysis
The primary end point for immunogenicity was the percentage of subjects with adequate antibody concentrations at day 42. Secondary end point for immunogenicity was rabies virus neutralizing antibody GMC at day 42.
The safety analysis was expressed as the incidence and severity of injection-site and systemic adverse events, and the incidence of SAE. The incidence and grade of an adverse event were calculated based on the highest intensity if same symptom with 2 or more intensity was reported. The safety analysis was performed on the total vaccinated cohort.
To compare safety and immunogenicity profile between these 2 groups, Student's t test for log-transformed GMC and χ2 for categorical data were used. The criterion for non-inferiority was defined as the upper bound to the 2-sided 95% CI of difference on the percentage of subjects with adequate antibody concentration slower than 5%. The statistical analysis was conducted by an independent statistician. Hypothesis testing was 2-sided with α value of 0.05.
Abbreviations
- WHO
World Health Organization
- PEP
post exposure prophylaxis
- PVRV
purified vero cell cultured rabies vaccine
- RFFIT
rapid fluorescent focus inhibition test
- DFA
direct immunofluorescence assay
- GMC
geometric mean concentration
- SAE
serious adverse events
- RVNA
Rabies virus neutralizing antibody
- AE
adverse event
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This Work was supported by the National 12th Five Major Special Projects Funding Program (No. 2011ZX09304) from the Ministry of Science and Technology of the People's Republic of China. And Liaoning Chengda Biotechnology provided financial support.
References
- [1].Wang LY, Sun MP, Zhang XC, Suo LD, Xu RH, Zou YJ, Zuo LB, Qi H. Safety and immunogenicity of two freeze-dried vero cell rabies vaccines for human use in post-exposure prophylaxis. Vaccine 2011; 29:2679-81; PMID:21296694; https://doi.org/ 10.1016/j.vaccine.2011.01.053 [DOI] [PubMed] [Google Scholar]
- [2].WHO Expert consultation on rabies: first report WHO technical report series, 931. Geneva Switzerland: World Health Organization; 2005. [PubMed] [Google Scholar]
- [3].National Health and Family Planning Commission of the People's Republic of China Available at: http://www.nhfpc.gov.cn/.
- [4].Yu J, Li H, Tang Q, Rayner S, Han N, Guo Z, Liu H, Adams J, Fang W, Tao X, et al.. The spatial and temporal dynamics of rabies in China. PLos Negl Trop Dis 2012; 6:e1640; PMID:22563518; https://doi.org/ 10.1371/journal.pntd.0001640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Si H, Guo ZM, Hao YT, Liu YG, Zhang DM, Rao SQ, Lu JH. Rabies trend in China (1990-2007) and post-exposure prophylaxis in the Guangdong province. BMC Infect Dis 2008; 8:113; PMID:18717989; https://doi.org/ 10.1186/1471-2334-8-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].WHO Expert Committee on Rabies Guide for post-exposure treatment. Eighth report. WHO technical report 824. Geneva Switzerland: World Health Organization; 1992. [Google Scholar]
- [7].Ma J, Wang H, Li J, Chang L, Xie Y, Liu Z, Zhao Y, Malerczyk C. A randomized open-labeled study to demonstrate the non-inferiority of purified chick-embryo cell rabies vaccine administered in the zagreb regimen (2-1-1) compared with the essen regimen in chinese adults. Hum Vaccin Immunother 2014; 10:2805-12; PMID:25483635; https://doi.org/ 10.4161/21645515.2014.972773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mahendra BJ, Narayana DA, Agarkhedkar S, Ravish HS, Harish BR, Agarkhedkar S, Madhusudana SN, Belludi A, Ahmed K, Jonnalagedda R, et al.. Comparative study on the immunogenicity and safety of a purified chick embryo cell rabies vaccine(PCECV) administered according to two different simulated post exposure intramuscular regimens (Zagreb versus Essen). Hum Vaccin Immunother 2015;11:428-34; PMID:25692792; https://doi.org/ 10.4161/21645515.2014.995059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li R, Li Y, Wen S, Wen H, Nong Y, Mo Z, Xie F, Pellegrini M. Immunogenicity and safety of purified chick-embryo cell rabies vaccine under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese children 6 to 17 years old and adults over 50 years:a randomized open-label study. Hum Vaccin Immunother 2015; 11:435-42; PMID:25692350; https://doi.org/ 10.4161/21645515.2014.994460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang CL, Zhang XW, Yu YX. Study on the Compliance and economic cost of rabies vaccination. Zhong guo Yi Miao He Mian Yi 2010; 16:254-7; PMID:20726270 [PubMed] [Google Scholar]
- [11].Hu Q, Liu MQ, Zhu ZG, Zhu ZR, Lu S. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum Vaccin Immunother 2014;10:1645-9; PMID:24632727; https://doi.org/ 10.4161/hv.28420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu H, Huang G, Tang Q, Li J, Cao S, Fu C, Cao Q, Liu B, Pan H, Wang M. The immunogenicity and safety of vaccination with purified vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vaccin 2011; 7:220-4; PMID:21311216; https://doi.org/ 10.4161/hv.7.2.14003 [DOI] [PubMed] [Google Scholar]
- [13].Vodopija I, Sureau P, Lafon M, Baklaic Z, Ljubicić M, Svjetlicić M, Smerdel S. An evaluation of second generation tissue culture rabies vaccines for use in man: a four-vaccine comparative immunogenicity study using a pre-exposure vaccination schedule and an abbreviated 2-1-1 postexposure schedule. Vaccine 1986; 4:245-8; PMID:3541428; https://doi.org/ 10.1016/0264-410X(86)90138-6 [DOI] [PubMed] [Google Scholar]
- [14].Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett SM, Levis R, Meltzer MI, Schaffner W, et al.. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep 2010; 59:1-9; PMID:20300058 [PubMed] [Google Scholar]
- [15].Chutivongse S, Wilde H, Fishbein DB, Baer GM, Hemachudha T. One-year study of the 2-1-1 intramuscular post exposure rabies vaccine regimen in 100 severely exposed Thai patients using rabies immune globulin and Vero cell rabies vaccine. Vaccine 1991; 9:573-6; PMID:1771970; https://doi.org/ 10.1016/0264-410X(91)90244-Z [DOI] [PubMed] [Google Scholar]
- [16].Colnot F, Sureau P, Alexandre JL, Arnaudo JP, Hesse JY, Jeanmaire H. Post-exposure antirabies vaccination. Early serological response to vaccine cultivated on VERO cells using a reduced 2-1-1 schedule. Presse Med 1994; 23:1609-12; PMID:7831241 [PubMed] [Google Scholar]
- [17].Vodopija I, Baklaic Z, Vodopija R. Rabipur: a reliable vaccine for rabies protection. Vaccine 1999; 17:1739-41; PMID:10194832; https://doi.org/ 10.1016/S0264-410X(98)00427-7 [DOI] [PubMed] [Google Scholar]
- [18].Vodopija I, Sureau P, Smerdel S, Lafon M, Baklaić Z, Ljubicić M, Svjetlicić M. Interaction of rabies vaccine with human rabies immunoglobulin and reliability of a 2-1-1 schedule application for postexposure treatment. Vaccine 1988; 6:283-6; PMID:3420976; https://doi.org/ 10.1016/0264-410X(88)90225-3 [DOI] [PubMed] [Google Scholar]
- [19].Wang C, Zhang X, Song Q, Tang K. Promising rabies vaccine for post exposure prophylaxis in developing countries, a purified Vero cell vaccine produced in China. Clin Vaccine Immunol 2010; 17:688-90; PMID:20147495; https://doi.org/ 10.1128/CVI.00433-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang X, Zhu Z, Wang C. Persistence of rabies antibody 5 years after post exposure prophylaxis with Vero Cell anti-rabies vaccine and antibody response to a single booster dose. Clin Vaccine Immunol 2011; 18:1477-9; PMID:21752947; https://doi.org/ 10.1128/CVI.05090-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yu P, Huang Y, Zhang Y, Tang Q, Liang G. Production and evaluation of a chromatographically purified Vero cell rabies vaccine (PVRV) in China using microcarrier technology. Hum Vaccin Immunother 2012; 8:1230-5; PMID:22894963; https://doi.org/ 10.4161/hv.20985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huang G, Liu H, Cao Q, Liu B, Pan H, Fu C. Safety of post-exposure rabies prophylaxis during pregnancy: A follow-up study from Guangzhou, China. Hum Vaccin Immunother 2013; 9:177-83; PMID:23442589; https://doi.org/ 10.4161/hv.22377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang G, Liu H, Tang Q, Yu P, Shen X, Zhang Y, Liu X, Cao Q, Fu C, Liu B, et al.. Making rabies prophylaxis more economical:immunogenicity and safety results from a preliminary study using a 2-1 intramuscular regimen in healthy volunteers. Hum Vaccin Immunother 2014; 10:114-9; PMID:24008819; https://doi.org/ 10.4161/hv.26264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fang Y, Chen L, Liu MQ, Zhu ZG, Zhu ZR, Hu Q. Comparison of safety and immunogenicity of PVRV and PCECV immunized in patients with WHO category II animal exposure: a study based on different age groups. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang X, Zhu Z, Wang C. Persistence of Rabies Antibody 5 Years after Postexposure Prophylaxis with Vero Cell Antirabies Vaccine and Antibody Response to a Single Booster Dose. Clin Vaccine Immunol 2011; 18:1477-9; PMID:21752947; https://doi.org/ 10.1128/CVI.05090-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zalan E, Wilson C, Pukitis D. A microtest for the quantitation of rabies virus neutralizing antibodies. J Biol Stand 1979; 7:213-20; PMID:387798; https://doi.org/ 10.1016/S0092-1157(79)80024-4 [DOI] [PubMed] [Google Scholar]
- [27].World Health Organization Rabies vaccine: WHO position paper. Weekly Epidemiol Rec 2010;85:309-20. [Google Scholar]
- [28].World Health Organization WHO Expert Consultation on Rabies. Second report. World Health Organ Tech Rep Ser 2013:1-139, back cover. [PubMed] [Google Scholar]
- [29].China Food and Drug Administration The standard guidelines for adverse reactions grading of vaccine clinical trials, Oct 14, 2005. Available at: http://www.sda.gov.cn/WS01/CL0844/9350_5.html.
- [30].Tao XY, Tang Q, Li H, Mo ZJ, Zhang H, Wang DM, Zhang Q, Song M, Velasco-Villa A, Wu X, et al.. Molecular epidemiology of rabies in Southern People's Republic of China. Emerg Infect Dis 2009; 15:1192-8; PMID:19751579; https://doi.org/ 10.3201/eid1508.081551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lang SL, Tao XY, Guo ZY, Tang Q, Li H, Yin CP, Li Y, Liang GD. Molecular characterization of viral G gene in emerging and re-emerging areas of rabies in China, 2007 to 2011. Virol Sin 2012; 27:194-203; PMID:22684474; https://doi.org/ 10.1007/s12250-012-3248-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


