Abstract
Purpose
Mismatch repair deficient (dMMR) colorectal cancer (CRC) is caused by Lynch syndrome (LS) in 3% and sporadic inactivation of MLH1 by hypermethylation (MLH1-hm) in 12% of CRC cases. It is not clear whether outcomes between LS-associated and MLH1-hm CRC differ. The objective of this study was to explore differences in clinical factors and outcomes in these two groups.
Methods
Patients with dMMR CRC by immunohistochemistry staining treated at a single institution from 1998 to 2012 were included. MLH1-hm was established with BRAF mutational analysis or hypermethylation testing. Patients’ charts were accessed for information on pathology, germline MMR mutation testing and clinical course.
Results
A total of 143 patients had CRC associated with LS (37 pts, 26%) or MLH1-hm (106 pts, 74%). Patients with LS were younger, more often male, presented more often with stage III disease and had more metachronous disease than patients with MLH1-hm tumors. There was no difference in cancer-specific survival (CSS) between the groups while overall survival (OS) was longer in patients with LS but this difference was minimal after adjusting for age and stage at diagnosis.
Conclusion
CSS did not differ in LS-associated CRC compared to MLH1-hm CRC suggesting that they carry a similar prognosis.
Keywords: Deficient mismatch repair system, survival, Lynch syndrome, hypermethylation, colorectal cancer
INTRODUCTION
Deficient mismatch repair activity (dMMR) is found in 15% of colorectal cancers (CRC). MMR genes remove errors in the form of deletions or insertions of DNA nucleotides that occur during the mitotic process. In the presence of defective MMR activity, mismatched nucleotides are incorporated into cells thus predisposing them to malignant transformation with a hypermutated phenotype.1 Microsatellite instability (MSI) is the hallmark of mismatch repair deficiency. In 3% of CRC cases dMMR is caused by Lynch syndrome (LS)2 through germline mutations in the MLH1, MSH2, MSH6, PMS2 or EPCAM3 genes and in 12% of cases it is caused by sporadic inactivation of MLH1 (hypermethylation of the MLH1 gene promoter, MLH1-hm). In rare cases, it is caused by bi-allelic germline MMR mutations (Constitutional dMMR Deficiency syndrome)4 and more recently, the bi-allelic occurrence of two somatic MMR mutations were shown to explain some dMMR cases.5,6
Tumors with dMMR have been associated with specific characteristics such as right-sided location, poor differentiation, lymphocytic infiltration, and mucinous features.7 They are also less likely to metastasize and tend to have a better overall survival in the early stages.8–10 Differences in clinical features and outcomes between LS-associated and MLH1-hm tumors have not been well explored, and many studies categorize the two subtypes without regard to their divergent origins as MSI-high tumors. Poynter et al. examined the incidence of MLH1-hm in MSI-high CRC in a population-based cohort and found MSI-high hypermethylated tumors to be significantly associated with older age, female gender, and a right-sided location when compared to MSI-high non-methylated tumors.11 Tumor genomic studies suggest that there is a molecular difference between tumors that develop in the setting of an inherited MMR mutation when compared with sporadic MLH1-hm tumors, most notably with regard to the association of a BRAF mutation. BRAF mutations are observed in up to 60–70% of MLH1-hm tumors but very rarely occur in LS-associated tumors.12,13
Universal immunohistochemistry (IHC) screening for MMR proteins in all CRC tumors was recommended by the Evaluation of Genomic Applications in Practice and Prevention workgroup of the CDC in 200912, by the National Comprehensive Cancer Network in March 201414 and by the US Multi-Society Task Force in August 2014.15 Data for MMR IHC in CRC patients is available at The Ohio State University since 1998 and has been performed routinely on all CRC tumors since 2006. The objective of this study was to retrospectively explore differences in clinical presentation and outcomes in patients with dMMR CRC related to LS vs. sporadic MLH1-hm.
MATERIALS AND METHODS
Patients
Consecutive patients with CRC who had dMMR on IHC performed from May 1998 to May 2012 were included in the study. This consisted of patients enrolled in the Columbus LS study2, and also included all CRC patients diagnosed after 2006. Patients with MLH1-hm tumors were identified by either absent MLH1 protein on IHC and BRAF mutation or hypermethylation of the MLH1 promoter region. Patients classified as having LS had confirmed germline MMR mutations. Patients with dMMR tumors who did not have conclusive testing (i.e. not diagnosed as either LS or MLH1-hm tumor) were excluded from further analysis.
Recurrent disease was defined as a recurrent tumor at the anastomotic site or distant metastasis that developed within 5 years of a primary diagnosis at stage I–III. Patients with less than 2 years of follow-up were excluded from this analysis. Synchronous tumors were defined as two colorectal tumors that were discovered simultaneously or within 6 months of each other. Metachronous colorectal tumors were discovered more than 6 months from each other.16
Baseline information on demographics, tumor characteristics, treatment and survival were obtained from medical charts. All tumors were pathologically reported according to the American Joint Committee on Cancer, 7th edition for CRC.17 For patients with synchronous tumors, the tumor with higher stage was documented as the primary. The institutional review board at the Ohio State University approved this study.
MSI, Immunohistochemistry and MLH1 hypermethylation testing
For patients on the Columbus LS study, DNA was extracted from paraffin-embedded tumor, normal adjacent tissue and blood. IHC for the four MMR proteins, MSI testing, MLH1-hm testing, and germline genetic testing (sequencing and multiplex ligation probe assay) for the four MMR genes was performed as previously described.2,18–20 Tumor tissue was stained for MLH1 (Novacastra, Buffalo Grove, IL; NCL-L-MLH-1), MSH2 (Calbiochem, [Merck Biosciences AG], Basel-Land, Switzerland; NA27), MSH6 (Epitomics Inc, Burlingame, CA; AC-0047) and PMS2 (BD Pharmingen, San Jose, CA; 556415) proteins with IHC. For patients enrolled onto the Columbus LS study, the promoter region of MLH1 was assessed for methylation with methylation-specific polymerase chain reaction.21 In clinical cases since 2005, DNA was modified with sodium bisulfite and the bisulfite treated DNA was sequenced by PyroMark MD (Quiagen) for MLH1 methylation analysis. Sequencing of exon 15 of the BRAF gene was performed in some clinical cases to identify any activating mutations. Approximately 25 to 50 ng of tumor DNA was amplified in a 15-μL polymerase chain reaction (PCR) using Promega’s GoTaq master mix (Promega, Madison, WI). PCR products were analyzed using an ABI3700 sequencer (Life Technologies, Grand Island, NY) following suitable amplification.
Survival analysis
Age at diagnosis was defined as the age when a CRC diagnosis was confirmed. Overall survival (OS) was defined as time from diagnosis to death from any cause. Patients who developed a metachronous primary CRC were censored at the time of diagnosis of the second tumor. Patients who were alive were censored at their last follow-up appointment date. Cancer-specific survival (CSS) was defined as time from diagnosis to a CRC related death and we chose to censor patients who developed a second cancer (except for non-melanoma skin cancer), died from other causes or were lost to follow-up.
Statistical Analysis
Descriptive statistics (median with quartiles for age, follow-up time and CEA and mean with standard deviation for other continuous variables and frequency for categorical variables) were provided to summarize the patient population. Student’s t-test and Chi-square were used to compare the difference of continuous and categorical parameters in LS vs. MLH1-hm groups respectively. Kaplan Meier estimation and log-rank test were used to compare the difference of OS and CSS between the two groups. Stage of cancer and age at diagnosis were considered as a covariate in the Cox proportional hazards model in the survival analysis. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Patient characteristics, stage at diagnosis and location of tumor
A total of 189 patients with CRC and documented MMR deficiency by IHC were identified for the study period. Full testing (IHC, MLH1-hm or BRAF mutation testing and germline sequencing) with conclusive results was obtained in 143 patients. MLH1-hm tumors were found in 106 patients (74.1% of the cohort) and a germline MMR mutation was found in 37 patients (25.9% of the cohort). In the other 46 patients with dMMR tumors, further testing was either not feasible or was negative; these patients were excluded from further analysis. Patient characteristics are described in Table 1. Forty-eight patients were enrolled on the Columbus LS study (33.6%) and 95 patients (66.4%) were found on routine clinical screening, and there were no statistically significant differences in the patient characteristics in the two patient populations. Of the 106 patients with MLH1-hm tumors, 25 patients had confirmed BRAF mutation analysis and the remainder had MLH1 hypermethylation testing. Patients with LS-associated tumors were significantly younger (median 47 vs. 70.5 years at diagnosis, p<0.0001) and predominantly male (59.5% vs. 42.5% p=0.077) compared to MLH1-hm patients. Most patients (72.9% and 71.7% in the LS and MLH1-hm groups) were diagnosed at stage II or III, with only 8.2% and 13.2% diagnosed at stage IV in the two groups, respectively. Patients with LS were significantly more often diagnosed with stage III disease compared to patients with MLH1-hm tumors (46.0% vs. 26.4%, p=0.022) with a higher likelihood of recurrence (24.3% vs. 11.3%, p=0.040). The division by stage and germline mutation was as follows: For MLH1 mutations stages I-IV at diagnoses were 0%, 27.3%, 54.5% and 9.1%; for MSH2 mutations 5.9%, 17.6%, 52.9% and 11.8%; for MSH6 mutations 40.0%, 60.0%, 0% and 0%; and for PMS2 mutations 0%, 33.3%, 66.7% and 0%. Table 1 shows the number of patients with LS and MLH1-hm tumors receiving 5-FU based adjuvant chemotherapy in stage II and III disease, respectively. Median duration of follow-up was longer for patients with LS (27.5 months vs. 25.0 months, p=0.032).
Table 1.
Patient characteristics, stage and tumor location
| Lynch syndrome (n=37) | MLH1-hm (n=106) | p-value | |
|---|---|---|---|
| Age (median; Q1,Q3) | 47 (35, 58) | 70.5 (63,80) | p< 0.0001 |
| Sex | |||
| Male | 22 (59.5%) | 45 (42.5%) | p=0.074 |
| Female | 15 (40.5%) | 61 (57.5%) | |
| Race | |||
| Caucasian | 33 (89.2%) | 88 (89.8%) | p=0.775 |
| AA | 3 (8.1%) | 9 (9.2%) | |
| Hispanic | 1 (2.7%) | 1 (1.0%) | |
| Stage | |||
| I | 4 (10.8%) | 15 (14.2%) | p=0.022 |
| II | 10 (27.0%) | 48 (45.3%) | |
| III | 17 (46.0%) | 28 (26.4%) | |
| IV | 3 (8.1%) | 14 (13.2%) | |
| Stage I–III (unknown) | 3 (8.1%) | 1 (0.9%) | |
| Location | |||
| Right | 27 (73.0%) | 87 (82.9%) | p=0.457 |
| Left | 7 (18.9%) | 11 (10.5%) | |
| Rectum | 3 (8.1%) | 7 (6.7%) | |
| Synchronous | 4 (10.8%) | 6 (5.7%) | p=0.459 |
| Metachronous | 7 (18.9%) | 4 (3.8%) | p=0.009 |
| Received adjuvant chemotherapy | |||
| Stage II | 3 (30%) | 4 (8.3%) | p=0.058 |
| Stage III | 10 (59%) | 11 (39%) | P=0.091 |
| Recurrence (%) | 9 (24.3%) | 12 (11.3%) | p=0.040 |
| Other cancer diagnosis (%) | 13 (34.2%) | 27 (25.5%) | p=0.301 |
| Follow-up time (months) | |||
| (median; Q1,Q3) | 30.0 (16, 117) | 25 (5, 54) | p=0.02 |
| CEA (n, median; Q1, Q3) | 1.2 (0.7, 2.6) | 1.9 (1.0, 5.6) | P=0.070 |
AA = African-American; Q = quartiles; Statistical significance was evaluated by Chi-square for categorical variables and t-test for continuous variables. Significance was set at p<0.05.
Tumor pathology and type of surgery
Table 2 describes tumor pathology and type of surgery performed in the two groups. There were no differences in any of the pathology factors between the two groups. Patients with LS had total colectomies performed significantly more often than patients with MLH1-hm CRC (27.8% vs. 5.1%, p=0.0047). There were no statistically significant differences in the locations of the tumors with the majority found in the right colon (73.0% and 82.1% in the two groups, p=0.51).
Table 2.
Tumor characteristics and type of surgery
| Lynch syndrome (n=37) | MLH1-hm (n=106) | p-value | |
|---|---|---|---|
| Tumor type | |||
| Adenocarcinoma | 27 (77.1%) | 78 (74.3%) | |
| Mucinous (>50%) | 5 (14.3%) | 21 (20.0%) | p=0.473 |
| Signet ring | 3 (8.6%) | 3 (2.9%) | |
| Medullary | 0 | 2 (1.9%) | |
| Undifferentiated | 0 | 1 (1.0%) | |
| Grade | |||
| Well | 2 (5.9%) | 4 (3.9%) | p=0.641 |
| Moderately | 21 (61.8%) | 53 (51.5%) | |
| Poorly | 11 (32.3%) | 45 (43.7%) | |
| Undifferentiated | 0 | 1 (1.0%) | |
| Border | |||
| Infiltrative | 22 (91.7%) | 66 (89.2%) | |
| Pushing | 0 | 5 (6.7%) | p=0.32 |
| Focally both | 2 (8.3%) | 3 (4.1%) | |
| Lymph nodes examined | |||
| Mean (SD) | 24.8 (16.0) | 22.3 (17.5) | p=0.436 |
| Lymph nodes positive | |||
| Mean (SD) | 2.1 (3.7) | 1.8 (3.9) | p=0.619 |
| Size (largest tumor) | |||
| Mean (SD) | 5.8 (3.2) | 5.9 (2.6) | p=0.922 |
| Surgery | |||
| R hemicolectomy | 16 (44.4%) | 71 (72.5%) | |
| L hemicolectomy | 6 (16.7%) | 11 (11.2%) | p=0.0047 |
| Subtotal colectomy | 2 (5.6%) | 8 (8.2%) | |
| Total colectomy | 10 (27.8%) | 5 (5.1%) | |
| LAR | 2 (5.5%) | 3 (3.0%) | |
R = right; L = left; LAR = low anterior resection; SD = standard deviation; Statistical significance was evaluated by Chi-square for categorical variables and t-test for continuous variables. Significance was set at p<0.05.
Survival
OS and CSS in the two groups are presented in Figures 1 and 2 and Table 3. Supplementary Figures S3–S5 show CSS in the two groups broken down by stage at diagnosis. CSS events were not observed in patients with stage I disease and are not depicted in a figure. Table 3 presents median survival and hazard ratios for OS and CSS by stage and overall while adjusting for age and stage at diagnosis. The difference in CSS between the two groups was not statistically significant (HR=1.33, p=0.60) after adjusting for the effect of age and stage. Most CSS events occurred in the first 24 months after CRC diagnosis and there was no significant difference between the groups when broken down by stage. OS was significantly longer for patients with LS and remained borderline significant after adjusting for the effect of age and stage (HR=1.96, p=0.095). This was mostly driven by survival difference seen in stage II when broken down by stage (HR=3.97, p=0.079). No difference was seen in OS and CSS when the LS-group was limited to comparing MLH1 germline mutated patients (n=11) to patients with MLH1-hm tumors (n=106) (calculations not shown). Patients with stage II or III cancer who received adjuvant chemotherapy had improved CSS, although not statistically significant (HR=0.48, p=0.25), as compared to those without chemotherapy when including stage and age as covariates. There were better but not significant differences in CSS when comparing the LS-group that received adjuvant chemotherapy to the MLH1-hm group receiving adjuvant chemotherapy after adjusting for age and stage (HR:2.72, 95%CI: 0.32, 23.2, p=0.36). There was no significant difference in CSS when comparing patients diagnosed before or after year 2007 (HR: 0.90, p=0.81), and the results of patients with LS vs MLH1-hm tumors were similar (HR=1.38, p=0.57 for CSS) after adjusting for time of diagnosis. Supplementary Table S1 describes the germline mutations in the LS-group.
Figure 1.
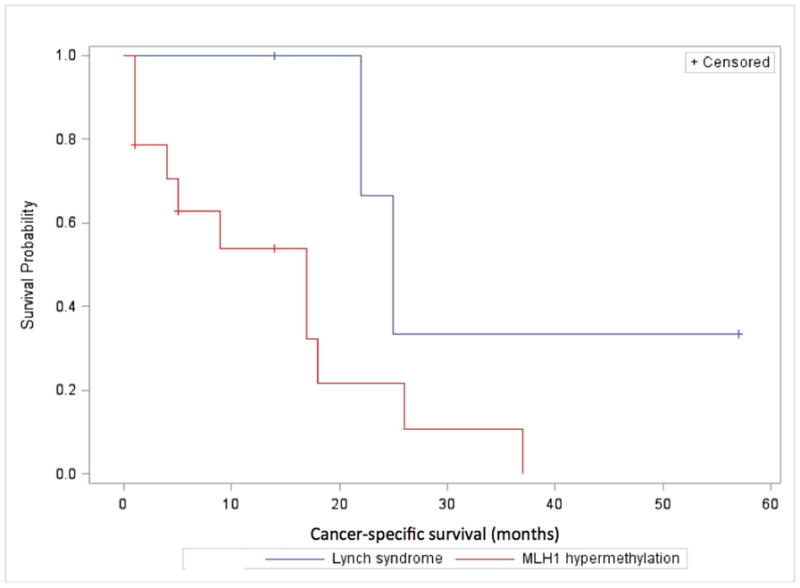
Overall survival in all stages in patients with LS-associated CRC and MLH1-hm CRC in months.
Figure 2.
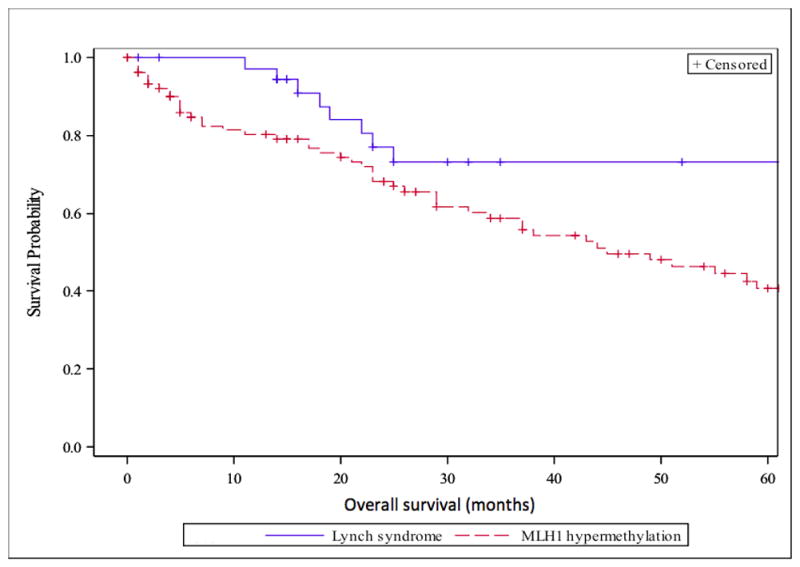
Cancer-specific survival in all stages in patients with LS-associated CRC and MLH1-hm CRC in months.
Table 3.
Median overall survival, cancer-specific survival and hazard ratios corrected for age and stage at diagnosis
| Overall survival (mo) | HR (95%CI) | p-value | Cancer-specific survival (mo) | HR (95%CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| LS | MLH1-hm | LS | MLH1-hm | |||||
| Stage I | NR | 51 | * | * | NR | NR | * | * |
| Stage II | NR | 59 | 3.97 (0.85–18.5) | 0.079 | NR | NR | 1.07 (0.08–13.6) | 0.96 |
| Stage III | 71 | 32 | 1.08 (0.28–4.18) | 0.91 | 71 | NR | 1.11 (0.22–5.64) | 0.90 |
| Stage IV | 23.5 | 17 | 2.07 (0.32–13.5) | 0.45 | 23.5 | 17 | 2.07 (0.32–13.5) | 0.45 |
| Overall | NR | 45 | 1.96 (0.89–4.92) | 0.095 | NR | NR | 1.33 (0.46–3.83) | 0.60 |
HR>1 signifies that LS has better survival than MLH1-hm patients.
Not enough events to calculate.
CI=confidence interval; HR=hazard ratio; Mo=months; NR=not reached.
DISCUSSION
In this study, CSS is similar in patients with LS-associated CRC and sporadic MLH1-hm CRC. The lack of difference in CSS suggests that the prognosis for CRC is similar in patients with dMMR tumors, whether it is related to germline MMR mutations or sporadic MLH1 inactivation. OS was longer in patients with LS as shown in Figure 1 but after correcting for age and stage at diagnosis the difference was of borderline significance and seems to be driven by patients with stage II disease. Both LS and MLH1-hm patients had exceptionally good survival for stage I (5-yr CSS 100%) and stage II (5-yr CSS >90%) disease; this is better than expected for CRC with proficient mismatch repair activity as has been previously reported.9 Five-year CSS in stage III was 60% in both groups and similar to prognosis in mismatch repair proficient tumors. Benatti et al. looked at the prognostic impact of MSI-high CRC and the efficacy of chemotherapy in a cohort of 1263 CRC patients. In a sub-analysis, patients with germline MMR mutations had better CSS as compared to sporadic MSI-high patients but this impact disappeared in a multivariate analysis where only age and stage at diagnosis predicted CSS.22 Sinicrope et al. looked at germline MMR mutations vs. MLH1-hm tumors in a study looking at the benefit of adjuvant 5-fluorouracil (5-FU) chemotherapy. Patients with dMMR tumors suspected to have germline mutations (based on clinical and IHC criteria) had improved survival compared with sporadic dMMR tumors but this association was lost after adjusting for age.23 Although more patients with LS received adjuvant therapy in our study, CSS was not significantly different between the 2 groups according to whether or not they received adjuvant chemotherapy. The interpretation of this analysis is limited due to a small sample size. Not surprisingly, patients with MLH1-hm tumors tend to be older at diagnosis and more often female. Methylation and inactivation of genes is believed to be part of normal aging but it is unclear why these tumors occur more frequently in females.
LS-associated tumors were more frequently diagnosed at stage III compared to MLH1-hm tumors. This could explain the higher recurrence rates in the LS-associated CRC group. It is possible that higher stage at presentation in younger patients with CRC is related to lack of awareness of this disease in the younger population and lower rates of routine screening colonoscopies. It is possible that some patients with LS were diagnosed before entering this study but many were diagnosed upon their CRC diagnosis and had no knowledge of the germline mutation.
There was no observed difference in pathologic features between the two groups in our study. A study by Hartman et al. analyzed grade, histology and tumor location in sporadic MSI-high tumors vs. LS/probable LS-associated tumors (based on BRAFV600E mutation, MLH1-hm, cancer history and germline MMR mutations). They found left-sided MSI-high tumors to be more frequently associated with LS. Sporadic MSI-high tumors demonstrated tumor-infiltrating lymphocytes more often (81% vs. 61%) as compared to LS-associated tumors with other pathologic factors being similar.24
In concordance with other studies, very few patients presented with stage IV disease in the two groups. The median OS in stage IV was similar to what would be expected for proficient MMR tumors (23.5 months) in the LS-group but worse in the MLH1-hm group (17 months). After adjusting for age there was no significant difference between the two groups (HR 1.96, 95%CI 0.89–4.29). The interpretation of this is limited due to low patient numbers. Venderbosch et al. published survival data on dMMR CRC combining the CAIRO, CAIRO2, COIN and FOCUS clinical trial datasets in patients with stage IV disease. They found that patients with dMMR tumors had worse OS than patients with pMMR tumors (13.6 vs. 16.8 months, HR 1.35 (95% CI 1.13–1.61). The main cause of dMMR in the studies was MLH1-hm (30 out of 45 patients) and 73% of them had BRAF mutations.15
Our study has some limitations including its retrospective nature and the relatively short median length of follow-up (2 years). Also, the relatively low number of patients gives us limited power to detect small differences in CSS between the two groups, particularly when measuring the effect of adjuvant chemotherapy. However, one of the strengths of our study is the fact that it was limited to patients with either LS or MLH1-hm tumors only by definitive molecular diagnosis.
In conclusion, we have shown that there is no statistically significant difference in CSS between LS-associated and MLH1-hm CRC in this cohort of patients. The observed difference in median OS became non-significant after correcting for age and stage at diagnosis and is therefore likely confounded by the differences in median age and disease stages between the two groups.
Supplementary Material
Supplemental Figure 1: Cancer-specific survival in stage II in patients with LS-associated CRC and MLH1-hm CRC in months.
Supplemental Figure 2: Cancer-specific survival in stage III in patients with LS-associated CRC and MLH1-hm CRC in months.
Supplemental Figure 3: Cancer-specific survival in stage IV in patients with LS-associated CRC and MLH1-hm CRC in months.
Acknowledgments
This study was supported by grants from the Ohio State University Comprehensive Cancer Center Core Grant (CA16058) and the National Cancer Institute (CA67941).
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
Supplementary information is available at the Genetics in Medicine website.
References
- 1.Parsons R, Li G-M, Longley MJ, et al. Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell. 1993;75(6):1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- 2.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) New England Journal of Medicine. 2005;352(18):1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 3.Ligtenberg MJL, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3[prime] exons of TACSTD1. Nat Genet. 2009;41(1):112–117. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 4.Bandipalliam P. Syndrome of Early Onset Colon Cancers, Hematologic Malignancies & Features of Neurofibromatosis in HNPCC Families with Homozygous Mismatch Repair Gene Mutations. Familial Cancer. 2005;4(4):323–333. doi: 10.1007/s10689-005-8351-6. [DOI] [PubMed] [Google Scholar]
- 5.Mensenkamp AR, Vogelaar IP, van Zelst–Stams WAG, et al. Somatic Mutations in MLH1 and MSH2 Are a Frequent Cause of Mismatch-Repair Deficiency in Lynch Syndrome-Like Tumors. Gastroenterology. 2014;146(3):643–646. e648. doi: 10.1053/j.gastro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Haraldsdottir S, Hampel H, Tomsic J, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014;147(6):1308–1316. e1301. doi: 10.1053/j.gastro.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994;145(1):148–156. [PMC free article] [PubMed] [Google Scholar]
- 8.Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10:917–923. [PubMed] [Google Scholar]
- 9.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 10.Gryfe R, Gallinger S. Microsatellite instability, mismatch repair deficiency, and colorectal cancer. Surgery. 2001;130(1):17–20. doi: 10.1067/msy.2001.112738. [DOI] [PubMed] [Google Scholar]
- 11.Poynter JN, Siegmund KD, Weisenberger DJ, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17:3208–3215. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009;11(1):42–65. doi: 10.1097/GIM.0b013e31818fa2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons MT, Buchanan DD, Thompson B, Young JP, Spurdle AB. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification. Journal of Medical Genetics. 2012;49(3):151–157. doi: 10.1136/jmedgenet-2011-100714. [DOI] [PubMed] [Google Scholar]
- 14.Network NCC. NCCN Genetic/Familial High-Risk Assessment: Colorectal. Version 1.2014. 2014. [Accessed 04/30/2014]. [Google Scholar]
- 15.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on Genetic Evaluation and Management of Lynch Syndrome: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2014;147(2):502–526. doi: 10.1053/j.gastro.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Moertel C. Multiple primary malignant neoplasms. Historical perspectives. Cancer. 1977;40(S4):1786–1792. doi: 10.1002/1097-0142(197710)40:4+<1786::aid-cncr2820400803>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Edge S, Byrd D, Compton CC, Fritz A, Greene F, Trotti A. Colon and rectum. 7. New York: Springer-Verlag; 2010. [Google Scholar]
- 18.Hampel H, Frankel W, Panescu J, et al. Screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Research. 2006;66(15):7810–7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 19.Hampel H, Panescu J, Lockman J, et al. Comment on: Screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Research. 2007;67(19):9603. doi: 10.1158/0008-5472.CAN-07-2308. [DOI] [PubMed] [Google Scholar]
- 20.Hampel H, Frankel WL, Martin E, et al. Feasibility of Screening for Lynch Syndrome Among Patients With Colorectal Cancer. Journal of Clinical Oncology. 2008;26(35):5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proceedings of the National Academy of Sciences. 1998;95(12):6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benatti P, Gafa R, Barana D, et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res. 2005;11:8332–8340. doi: 10.1158/1078-0432.CCR-05-1030. [DOI] [PubMed] [Google Scholar]
- 23.Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA Mismatch Repair Status and Colon Cancer Recurrence and Survival in Clinical Trials of 5-Fluorouracil-Based Adjuvant Therapy. Journal of the National Cancer Institute. 2011;103(11):863–875. doi: 10.1093/jnci/djr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartman DJ, Brand RE, Hu H, et al. Lynch syndrome–associated colorectal carcinoma: frequent involvement of the left colon and rectum and late-onset presentation supports a universal screening approach. Human Pathology. 2013;44(11):2518–2528. doi: 10.1016/j.humpath.2013.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Cancer-specific survival in stage II in patients with LS-associated CRC and MLH1-hm CRC in months.
Supplemental Figure 2: Cancer-specific survival in stage III in patients with LS-associated CRC and MLH1-hm CRC in months.
Supplemental Figure 3: Cancer-specific survival in stage IV in patients with LS-associated CRC and MLH1-hm CRC in months.


