Abstract
The Drosophila and mammalian digestive systems bear striking similarities in genetic control and cellular composition, and the Drosophila midgut has emerged as an amenable model for dissecting the mechanisms of tissue homeostasis. The Drosophila midgut is maintained by multipotent intestinal stem cells (ISCs) that give rise to all cell types in the intestinal epithelium and are required for long-term tissue homeostasis. ISC proliferation rate increases in response to a myriad of chemical and bacterial insults through the release of JAK-STAT and EGFR ligands from dying enterocytes that activate the JAK-STAT and EGFR pathways in ISCs. The Hippo and JNK pathways converge upon JAK-STAT and EGFR signaling, presumably in response to specific stresses, and JNK and insulin signaling have been shown to be critical in response to age-related stresses. This review details these emerging mechanisms of tissue homeostasis and the proliferative response of ISCs to epithelial damage, environmental stresses, and aging.
INTRODUCTION
Longevity is both driven and limited by stem cell proliferation—regenerative potential is necessary to maintain tissue homeostasis, but uncontrolled regenerative potential can lead to hyperproliferative diseases such as cancer. Maintenance of tissue homeostasis is particularly critical in high turnover tissues such as the intestinal epithelium, which faces constant cellular damage due to both its digestive function as well as its function as a barrier to chemical and bacterial insults. Extensive similarities in the genetic control and cellular composition of the mammalian and Drosophila digestive systems and the availability of cell specific markers and ease of clonal analysis have made Drosophila a particularly amenable model organism to uncover mechanistic insight and new paradigms in how intestinal homeostasis is maintained. In addition, exploitation of damaging agents and induction of oral infection have proved invaluable in uncovering pathways involved in the activation of intestinal stem cell (ISC) proliferation following epithelial injury. In this review, we highlight the emerging mechanisms of tissue homeostasis and ISC response to epithelial damage, environmental challenges, and aging.
MULTIPOTENT ISCs GIVE RISE TO ALL CELL TYPES IN THE INTESTINAL EPITHELIUM AND ARE REQUIRED FOR TISSUE HOMEOSTASIS
The Drosophila midgut corresponds to the mammalian small intestine. Like the vertebrate gut, the Drosophila midgut is largely comprised of absorptive enterocytes (EC) with interspersed hormone-producing enteroendocrine (ee) cells.
The presence of ISCs and the fate of ISCs and their progeny were determined by clonal analysis.1,2 ISCs are multipotent, giving rise to all cell types in the intestinal epithelium1,2 (Figure 1(a)). ISCs divide to give rise to an enteroblast (EB) daughter cell and self-renew1,2 (Figure 1(a)). ISCs contain cytoplasmic vesicular Delta (Dl) and activate the Notch (N) signaling pathway in EB daughters. Transcriptional repression of N target genes is necessary for ISC maintenance,3 and both Dl4 and N1,2 signaling are necessary to regulate ISC daughter differentiation, as loss of Dl in the ISC or N in the EB leads to aberrant daughter differentiation.1,2 While an ISC division gives rise to an asymmetric outcome, it is currently unknown how asymmetry is generated. It has recently been proposed that ISCs also undergo symmetric divisions within the first 4 days after eclosion, as an increase in ISC number was observed in newly eclosed animals to give rise to the final number of ISCs that is maintained throughout most of the lifetime of the animal.5 However, it is unknown what drives symmetric versus asymmetric divisions.
FIGURE 1.
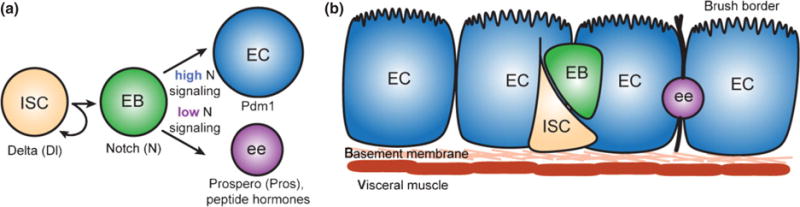
Intestinal stem cells (ISCs) are multipotent and give rise to all cell types in the intestinal epithelium. (a) ISCs divide asymmetrically to self-renew and give rise to an enteroblast (EB) daughter cell. The EB goes on to differentiate into either an enterocyte (EC) or enteroendocrince (ee) cell, depending on the level of N signaling in the EB. (b) ISCs are located along the basement membrane and the EB daughter moves away from the basement membrane upon division. The underlying visceral muscle contacts the entire epithelium.
EBs differentiate into one of two cell types based on levels of N signaling.4 Commitment of an ISC daughter to differentiate into an EB requires N signaling1,4 presumably mediated by GDP-mannose 4,6-dehydratase, while downstream fate decisions requires lower N signaling.6 ISCs with high levels of vesicular Dl send a strong N signal to their daughters, which then differentiate into polyploid ECs, a cell type identified by nuclear size and the POU domain protein Pdm1. In contrast, EB daughters of ISCs with low levels of vesicular Dl receive a weak N signal and differentiate into diploid ees, which can be identified by the transcription factor Prospero as well as peptide hormones such as Allatostatin and Tachykinin. In addition to N signaling, Janus Kinas-Signal Transducer and Activator of Transcription (JAK-STAT) signaling is necessary to make the EB competent for multilineage differentiation.7–9 Approximately 90% of ISC progeny are ECs and 10% of ISC progeny are ees. Like the vertebrate intestine, ECs and ees turnover weekly.1
During larval development, a transient niche called the peripheral cell was identified that maintains adult midgut precursors in an undifferentiated state.10 However, in contrast to the Drosophila larval gut, germline stem cells and the mammalian gut, ISCs in the adult Drosophila midgut adjoin the basement membrane and are not associated with stromal cells at specific anatomical sites serving as a niche (Figure 1(b)), making identification of the niche elusive. As Wnt/β-catenin signaling is required for proliferative maintenance in the mammalian gut, expression of the Wnt ligand Wingless (Wg) was determined in the Drosophila midgut to be expressed in visceral muscle by in situ hybridization and antibody staining.11,12 Given that mutant clones of Frizzled (Fz), Disheveled (Dsh), and Armadillo, all acting downstream of Wg, show clone loss over time, it was concluded that Wg is an integral part of the ISC niche.11,12 While manipulation of the Wnt signaling pathway alludes to a necessity for Wnt signaling from the visceral muscle in maintaining ISC number, two unresolved problems arise from Wnt signaling results. First, as the entire epithelium is in contact with the underlying visceral muscle, it is unclear how specific anatomical niche sites for ISC maintenance are achieved. Second, if Wg is a necessary niche signal, one would anticipate a complete loss of ISCs in Wg mutants, which was not observed.13 Furthermore, mutant clones of Apc, also downstream of Wg, show a proliferation defect, not an ISC maintenance defect.13 Why differences were observed in Apc mutants versus Fz and Dsh mutants remains unanswered and may be due to divergence in the Wnt signaling pathway. In addition, how ISC spacing, position, and number are maintained remains unresolved, as the muscle layer contacts the entire epithelium, not just the anatomical position of the ISC (Figure 1(b)).
ISCs and EBs, collectively referred to as progenitor cells are required for long-term midgut maintenance. Enhancers from the transcription factor escargot (esg), belonging to the Snail/Slug family, drive expression of Gal4 in both the ISC and its EB daughter.2 Upon ISC and EB ablation using esgGal4 driving the expression of p53 or ricin A, significant loss of EC and ee progeny is found in 30-day old animals suggesting that tissue homeostasis is dependent on the presence of functional ISCs.7
ISC PROLIFERATION INCREASES IN RESPONSE TO APOPTOSIS OF ECs, CHEMICAL DAMAGING AGENTS, AND BACTERIAL INFECTION
The intestinal epithelium of both vertebrate and invertebrate organisms is constantly exposed to both chemical and bacterial aggressions and cellular stress. Use of damaging agents and induction of bacterial infection or apoptosis of ECs demonstrated that ISC proliferation increases in response to tissue damage.
Dextran sodium sulfate (DSS) provided through drinking water causes epithelial damage in the mammalian gut and excessive inflammation that resembles ulcerative colitis.14 Similarly in Drosophila, DSS results in mortality in a dose-dependent manner and disrupts the organizational structure of the basement membrane. DSS treatment induced a 20-fold increase in the number ISCs positive for phosphorylated histone-H3 (PH3), indicative of mitosis, and fourfold accumulation of EBs.15 Interestingly, EC turnover did not increase suggesting that the factors in the basement membrane act to limit ISC proliferation. In accordance, mosaic analysis with a repressible cell marker (MARCM) clones generated in animals fed DSS contained one ISC but a greater total number of cells per clone, largely EBs, than control animals.15
Bleomycin, an anti-cancer drug and DNA damaging agent, causes mortality in a dose-dependent manner in Drosophila and extensive DNA damage in ECs as detected by the increased expression of phospho-histone 2A variant D.15 Bleomycin induced a striking dose-dependent increase in ISC proliferation, detected by an increase in PH3-positive cells and clonal analysis. Like with DSS treatment, MARCM clones generated in animals fed bleomycin contained one ISC but a greater total number of cells per clone. However, unlike DSS damage, bleomycin does not result in the accumulation of EB daughters, but rather EBs go on to differentiate into mature ECs.15 In addition, EC specific expression of apoptotic inducers Hid and Reaper mimicked the phenotype seen with bleomycin feeding, suggesting that damaged and dying ECs might be the source of signals that feedback to ISCs to initiate their proliferation.15
Independent studies using guts infected by Pseudomonas entomophila,16 Erwinia carotovora 15,17 and Serratia marcescens18 demonstrated an ISC response to bacterial infection. Following infection, a marked increase in ISC proliferation was observed by PH3 detection and was corroborated by clonal analysis.16,17 Clones from infected guts contained more cells relative to controls, but only one Dl positive ISC per clone, indicating an increase in proliferation rate but no increase in stem cell number. EB number increased by two to fourfold,16 indicating that the increase in ISC proliferation leads to an accumulation of EB daughters. Increase in ISC proliferation rates were determined to be independent from the Imd pathway mediated immune response,17 which was previously shown to be activated in response to detection of invading bacteria by Gram-negative binding protein.19 Rather, quantification of transcriptome variations,17 a genome-wide RNAi screen,18 and subsequent genetic experiments identified that the increase in ISC proliferation is due to activated JAK-STAT signaling in response to dying EC.17,18 In addition, production of reactive oxygen species (ROS) of by the NADPH oxidase enzyme Duox activates the Jun N-terminal kinase (JNK) pathway,20 which in turn activates JAK-STAT signaling in ISCs and results in increased proliferation.
TISSUE HOMEOSTASIS IS MAINTAINED BY RELEASE OF JAK-STAT AND EGFR LIGANDS FROM DYING ECs THAT PROMOTE ISC PROLIFERATION
EC damage-induced upregulation of ISC proliferation in conjunction with the identification of key pathways involved in response to damage fueled the detailing of the intricacies of enterocyte feedback on ISC proliferation. Epidermal Growth Factor Receptor/Mitogen-Activated Protein Kinase (EGFR/MAPK) and JAK-STAT signaling have been identified as the central signaling pathways in EC damage-induced ISC proliferation (Figure 2). Positive feedback from dying EC on ISCs confirms that the intestinal epithelium in not a static tissue in which ISCs proliferate in a conveyor belt manner. Rather, ISC proliferation is a dynamically regulated process that can directly respond to positive feedback from lost differentiated cells when rapid regeneration due to tissue injury is needed.
FIGURE 2.

Enterocyte (EC) damage elicits a proliferative response in intestinal stem cells (ISCs). Upon EC damage, Unpaired (Upd) and Epidermal Growth Factor Receptor (EGFR) ligands are released and initiate the Janus Kinas-Signal Transducer and Activator of Transcription (JAK-STAT) and EGFR pathways, respectively, in the ISC which induced ISC proliferation. Both the Jun N-terminal kinase (JNK) and Hippo (Hpo) pathways feed into JAK-STAT and EGFR signaling though the activation or inhibition of Yorkie (Yki), respectively.
EGFR signaling is involved in human colorectal cancer,21,22 and is critical in the development of colonic crypts in vertebrates23 and during development of the Drosophila intestine.24 Drosophila contains the EGFR ligands Vein (Vn), Keren (Krn), and Spitz (Spi) and a single EGF receptor. EGFR signaling is transduced by the MAPK signaling pathway containing the small GTPase RAS and the MAP kinase ERK. The EGFR ligands are detected in various cell types—Krn is detected in ECs, Vn in ECs and visceral muscle, and Spi in progenitor cells.25 Upon EC damage, Krn and Vn are secreted from the dying EC and elicit EGFR signaling in the ISC to promote proliferation.25
In addition to the role in feedback from dying EC, EGFR signaling is necessary for ISC proliferation, as ISCs in EGFR null ISC clones do not divide.26 Vn from the visceral muscle was identified as the permissive signal for ISC proliferation.26 However, EGFR signaling has been shown to have no role in differentiation of the EB daughter.26
JAK-STAT is an evolutionary conserved pathway in vertebrates and invertebrates that transduces cytokine signals. Drosophila contains the interleukin-6 family Unpaired (Upd) ligands Upd, Upd2, and Upd3, the transmembrane receptor Domeless (Dome), the receptor-associated kinase Hopscotch (Hop), and the transcription factor Stat92E. Binding of Upd ligands to Dome leads to the activation of Hop. Stat92E binds the phosphorylated Dome/Hop complex, resulting in the phosphorylation of Stat92E and translocation of phosphorylated Stat92E into the nucleus. Stat92E is expressed in both ISCs and EBs,7,8,27 and is located within the nucleus of a subset of ISCs and EBs, indicating active Stat92E in these cells.8 Expression of Upd ligands in either the EC or the ISC and EB of uninjured guts induces a rapid increase in ISC mitoses and significant hyperplasia. Hyperplasia is reversible, with normal morphology returning 2 weeks after the silencing of Upd overexpression. In conjunction with the identification of JAK-STAT pathway activation in response to injury,17,18 this result suggested that ECs may release Upd ligands that activate JAK-STAT in the ISC. Indeed, dying ECs presumably produce Upd, Upd2, and Upd3 cytokines, which are subsequently released and positively feedback to promote ISC proliferation,7 although it has not been determined if Upd ligands come from the dying ECs themselves or neighboring healthy ECs that sense damage. In addition to release of Upd cytokines from ECs, JAK-STAT signaling also promotes ISC proliferation through induction of Vn in the visceral muscle28 (Figure 2).
JAK-STAT signaling is also required for differentiation of EB daughters under homeostatic conditions. Stat92E8,27 and Hop8 mutant clones rarely contain Pdm1-positive polyploid EC or ee cells, demonstrating the necessity for JAK-STAT signaling for the differentiation of EB daughters. This additional level of regulation may serve to coordinate ISC proliferation and EB differentiation in regenerative responses.
THE HIPPO AND JNK PATHWAYS CONVERGE UPON JAK-STAT AND EGFR SIGNALING, PRESUMABLY IN RESPONSE TO VARIOUS STRESSES
The Hippo pathway regulates growth and proliferation29 and its misregulation has been implicated in a variety of cancer types.30,31 In Drosophila, Salvador (Sav) and Mob as tumor suppressor (Mats) form a complex with Hippo (Hpo) and Warts (Wts). Upon complex formation, the kinase activities of Hpo and Wts are activated, and the membrane-associated proteins Merlin (Mer) and Expanded (Ex) activate the phosphorylation of Wts by Hpo. Phosphorylated Wts inactivates Yorkie (Yki), which regulates the transcription of genes involved in cell proliferation and apoptosis. In the Drosophila midgut, loss of Hippo signaling stimulates ISC proliferation through the production of Upd cytokines and EGFR ligands in the EC that activates the JAK-STAT and EGFR pathways in the ISC.32,33 Hippo downregulation results in increased levels of Yki, which in turn stimulates the production and release of JAK-STAT and EGFR ligands. Activation of Yki in ECs results in a 25-fold increase in upd3 expression and a fourfold increase in upd and upd2 expression.33
In addition to a non-cell autonomous role for Hippo signaling in the EC, it is disputed that there may be an addition cell-autonomous role in the ISC itself. Staley et al. observed a negligible increase in ISC proliferation upon Yki activation in the ISC and EB, concluding there is no significant cell-autonomous role.33 In contrast, Karpowicz et al. and Ren et al. observed a cell-autonomous role for Hippo signaling in the ISC, as loss of Hippo or overexpression of Yki specifically in the ISC and EB led to increased ISC proliferation.32,34 Furthermore, Yki is necessary for the increased ISC proliferation observed upon DSS damage which leads to disruption of the basement membrane but no observable EC death.32
Janus Kinase (JNK) is a stress-activated protein kinase induced by a range of damaging agents including UV irradiation, ROS, DNA damage, bacterial infection, and inflammatory cytokines. Drosophila contains a single JNK, Basket (Bsk), and two JNK Kinases, Hemipterous (Hep) and dMKK4, which acts in parallel with Hep to induce immune responses and apoptosis.35–37 A target of JNK signaling, puckered (puc), restricts JNK activity in a negative feedback loop38,39 and is often used as a marker of stress response. JNK signaling is required in ECs of the intestinal epithelium to prevent sensitivity to oxidative stress,40 and promotes ISC proliferation non-autonomously by the induction and release of Upd1, Upd2, and Upd3 from stressed ECs,7 which in turn activate the JAK-STAT pathway in ISCs. JNK signaling may also have an autonomous role in the ISC, as removal of the dJun transcription factor in progenitor cells results in progenitor cell loss.17
AGING, OXIDATIVE STRESS, AND INSULIN SIGNALING AFFECT ISC PROLIFERATION
In addition to a general role in inducing ISC proliferation in response to oxidative stress and EC damage throughout the life of the animal, the cytoprotective role of JNK is critical in aging animals. Aging is associated with a progressive decline in tissue function and, in rapidly cycling tissues, a decline in regenerative potential. Environmental stresses and oxidative damage contribute to this age-related functional decline and cancer development in tissues.41,42
In aged animals, dyplasia is observed and is accompanied by an increase in the number of cells expressing green fluorescent protein (GFP) driven by esgGal4 (esg>GFP) and a general disruption of tissue morphology.40,43,44 esg>GFP positive cells are often found in clusters, and several positively marked cells are polyploid,40,43 suggesting that esgGal4 in aged animals is no longer ISC and EB specific. Dl expression is also widespread within these clusters and increases up to threefold in 60-day old animals,44 although typically only one cell per cluster solely expresses Dl and not Su(H)GBE-lacZ, a N response reporter. The esg>GFP positive polyploid cells also do not express Fer1HCH, a marker of EC differentiation, and are therefore thought to be misdifferentiated cells.40 Oxidative damage induced by paraquat treatment in young animals mimics the dysplasia and general phenotype seen in old animals,40,43,44 suggesting that oxidative damage accumulated with age contributes to this phenotype. In addition, an increase in ISC proliferation is observed both upon paraquat treatment and in aged animals.40,43,44
JNK promotes tolerance to oxidative damage and longevity in both Drosophila and Caenorhabditis elegans,45,46 and is a key regulator of the age-related decline in ISC function and dysplasia in the Drosophila midgut.40,43 In aged animals or in animals exposed to oxidative stress, puc-lacZ levels dramatically increase in ECs, ees, and in polyploid esg>GFP positive cells with abnormal morphology.40 Moderate activation of JNK signaling results in a decrease in age-induced dysplasia and esg>GFP positive clusters.43 In addition to the role of JNK in the EC, JNK signaling also appears to act cell autonomously in aged animals, as expressing a dominant-negative Bsk in the ISC and EB also inhibits aberrant esg>GFP expression and dysplasia.20,40 N signaling in the ISC and EB is required to restrict JNK induced proliferation under stress conditions.40 The excessive increase in ISC proliferation mediated by JNK signaling in aging flies is hypothesized to be the result of age-related changes in the intestinal microflora that induce chronic inflammation.20 This response may be linked to p38, which is also required for ISC proliferation and mediates EC response to bacterial antigens.47
Insulin signaling and nutrient availability have also been intimately linked to growth and aging, and a requirement for insulin signaling in progenitor cells has recently been shown.15,40,48 Increased insulin signaling in the ISC and EB decreases lifespan similarly to activation of JNK signaling in these cells.43 In contrast, reduced insulin signaling in the ISC and EB shortens lifespan, as ISC proliferation and regenerative potential is dramatically decreased.43 Yet, dampening insulin signaling delays dysplasia,43 showing an intricate balance between maintaining regenerative potential while delaying age-related morphological abnormalities.
As in old animals, reduced insulin signaling suppresses ISC proliferation in young animals and dampens the increase in ISC proliferation observed upon DSS or bleomycin treatment.15 Reduced insulin signaling in the EB alone results in prolonged contact between the EB daughter cell and ISC and significantly dampens ISC proliferation, demonstrating a non-autonomous role for insulin signaling in the EB.49 Disruption of cell contact between the ISC and EB by knockdown of DE-Cadherin (DE-Cad) rescues this proliferation defect, suggesting that ISCs directly sense changes in nutritional status through contact with their recent daughter cell.49 In contrast, use of esgGal4 to drive an active form of the insulin receptor (dInR) in the ISC and the EB in young animals leads to an increase in ISC proliferation and build-up of small diploid cells.15 However, esgGal4 is no longer ISC and EB specific under these conditions, and a sixfold increase in apoptotic ECs suggest that the increase in ISC proliferation could be a result of injury to the midgut.49
Similar phenotypes are observed with changes in nutrient availability. Protein deprivation leads to a decrease in progenitor cells in 16–17-day old animals, and the number of progenitor cells returns to that of age-matched controls upon re-feeding of protein.48 While protein deprivation affects the rate of ISC proliferation, it does not affect ISC number in animals up to 14 days old,49 and the decrease in progenitor cells observed in 16–17-day old animals is presumably due to a loss of EBs.
In addition to JNK and insulin signaling, age-related changes in the midgut have been linked to PDGF- and VEGF-related factor 2 (Pvf2), the Drosophila homolog of the human platelet-derived growth factor/vascular endothelial growth factor (PDGF/VEGF)-like growth factor. VEGF responds to oxidative stress and displays increased expression in response to increased ROS present in human cancer cells. In the Drosophila midgut, Pvf2-lacZ expression colocalizes with esg>GFP expression in the midgut, and Pvf2 mRNA levels increase upon aging or oxidative stress induced by paraquat treatment.44 Overexpression of Pvf2 or its receptor Pvr in the ISC and EB leads to an increase in proliferation, as seen with aging and oxidative stress.44 How Pvf2/Pvr signaling interplays with other pathways regulating stem cell proliferation in young animals and during aging remains to be determined.
CONCLUSION
As with the vertebrate intestine, the Drosophila midgut epithelium is a highly dynamic tissue in which ISCs respond to a wide variety of damage and adjust their proliferation rate accordingly. How ISCs respond to their environment and the way cell fate choices are made are not only conserved at the cellular level between Drosophila and vertebrates but the molecular pathways utilized are remarkably similar. Given these similarities the Drosophila midgut is likely to continue to be an invaluable model system for the elucidation of regenerative mechanisms in both insects and vertebrates alike.
References
- 1.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 2.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 3.Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147:603–614. doi: 10.1016/j.cell.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perdigoto CN, Schweisguth F, Bardin AJ. Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development. 2011;138:4585–4595. doi: 10.1242/dev.065292. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beebe K, Lee W-C, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Developmental Biology. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of Drosophila intestinal stem cells. Journal of Molecular Cell Biology. 2010;2:37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- 10.Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin G, Xi R. Intestinal stem cell, muscular niche and wingless signaling. Fly. 2008;2:310–312. doi: 10.4161/fly.7428. [DOI] [PubMed] [Google Scholar]
- 12.Lin G, Xu N, Xi R. Paracrine wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:U1119–U1111. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- 13.Lee W-C, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- 14.Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World Journal of Gastroenterology. 2007;13:5581–5593. doi: 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee M, Ip YT. Pathogenic stimulation of intestinal stem cell response in Drosophila. Journal of Cellular Physiology. 2009;220:664–671. doi: 10.1002/jcp.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host & Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Cronin SJF, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, de Matos Simoes R, Gruber S, Puc U, et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nehme NT, Liegeois S, Kele B, Giammarinaro P, Pradel E, Hoffmann JA, Ewbank JJ, Ferrandon D. A model of bacterial intestinal infections in Drosophila melanogaster. Plos Pathogens. 2007;3:1694–1709. doi: 10.1371/journal.ppat.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes & Development. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilger A, Sullivan R, Prunuske AJ, Clipson L, Drinkwater NR, Dove WF. Widespread hyperplasia induced by transgenic TGF alpha in Apc(Min) mice is associated with only regional effects on tumorigenesis. Carcinogenesis. 2008;29:1825–1830. doi: 10.1093/carcin/bgn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A, Sebolt-Leopold J, Shannon KM, et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nature Genetics. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, et al. Targeted disruption of mouse egf receptor - effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang H, Grenley MO, Bravo M-J, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138:1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, Singh SR, Hou SX. JAK-STAT is restrained by notch to control cell proliferation of the Drosophila intestinal stem cells. Journal of Cellular Biochemistry. 2010;109:992–999. doi: 10.1002/jcb.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. Bmc Biology. 2010;8:152–171. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao B, Tumaneng K, Guan K-L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nature Cell Biology. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badouel C, Garg A, McNeill H. Herding Hippos: regulating growth in flies and man. Current Opinion in Cell Biology. 2009;21:837–843. doi: 10.1016/j.ceb.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nature Reviews Molecular Cell Biology. 2007;8:613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 32.Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Current Biology. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boutros M, Agaisse H, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Developmental Cell. 2002;3:711–722. doi: 10.1016/s1534-5807(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, White MA, Cobb MH. Stimulus-specific requirements for MAP3 kinases in activating the JNK pathway. Journal of Biological Chemistry. 2002;277:49105–49110. doi: 10.1074/jbc.M204934200. [DOI] [PubMed] [Google Scholar]
- 37.Geuking P, Narasimamurthy R, Lemaitre B, Basler K, Leulier F. A non-redundant role for Drosophila Mkk4 and Hemipterous/Mkk7 in TAK1-mediated activation of JNK. Plos One. 2009;4:e7709. doi: 10.1371/journal.pone.0007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEwen DG, Peifer M. Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development. 2005;132:3935–3946. doi: 10.1242/dev.01949. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes & Development. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila GUT. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beckman KB, Ames BN. The free radical theory of aging matures. Physiological Reviews. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 42.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 43.Biteau B, Karpac J, Supoyo S, DeGennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. Plos Genetics. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi N-H, Kim J-G, Yang D-J, Kim Y-S, Yoo M-A. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Developmental Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- 47.Park J-S, Kim Y-S, Yoo M-A. The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging-Us. 2009;1:637–651. doi: 10.18632/aging.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Current Biology. 2010;20:2100–2105. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi NH, Lucchetta E, Ohlstein B. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18702–18707. doi: 10.1073/pnas.1109348108. [DOI] [PMC free article] [PubMed] [Google Scholar]


