Summary
The innate immune system is essential for the initial detection of invading viruses and subsequent activation of adaptive immunity. Three classes of receptors, designated retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), Toll-like receptors (TLRs) and NOD-like receptors (NLRs), sense viral components, such as double-stranded (ds) RNA, single-stranded RNA and DNA. RLRs and TLRs play essential roles in the production of type I interferons (IFNs) and proinflammatory cytokines in cell type-specific manners. While the RLRs play essential roles in the recognition of RNA viruses in various cells, plasmacytoid dendritic cells utilize TLRs for detecting virus invasion. On the other hand, NLRs play a role in the production of mature interleukin-1β to dsRNA stimulation. Activation of innate immune cells is critical for mounting adaptive immune responses. In this review, we will discuss recent advances in our understanding of the mechanisms of viral RNA recognition by these different types of receptors, and its relation to acquired immune responses.
Keywords: Type I Interferon, Toll-like receptor, RIG-I-like receptor, signaling
Introduction
Host cells recognize the invasion of viruses and mount strong antiviral responses. Viruses initially activate the innate immune system, which recognizes viral components through pattern-recognition receptors (PRRs) (1-3). On the other hand, acquired immunity plays a major role in the responses to re-infection with viruses. Host PRRs detect viral components, such as genomic DNA, single-stranded (ss) RNA, double-stranded (ds) RNA, RNA with 5′-triphosphate ends and viral proteins. Currently, three classes of PRRs have been shown to be involved in the recognition of virus-specific components in innate immune cells, namely Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) and NOD-like receptors (NLRs). Among these receptor types, TLRs and RLRs are important for the production of type I interferons (IFNs) and various cytokines, whereas NLRs are known to regulate interleukin-1β (IL-1β) maturation through activation of caspase-1 (4, 5).
Detection of viral components by RLRs and TLRs in immune cells activates intracellular signaling cascades, leading to the secretion of type I IFNs, proinflammatory cytokines and chemokines, and increased expression of co-stimulatory molecules such as CD40, CD80 and CD86. Type I IFNs activate intracellular signaling pathways via a type I IFN receptor, and regulate the expression of a set of genes. The IFN-inducible genes, such as protein kinase R and 2′5′-oligoadenylate synthase, are involved in eliminating viral components from infected cells, inducing apoptosis of infected cells and conferring resistance to viral infection on uninfected cells. Type I IFNs are produced not only by professional innate immune cells, including dendritic cells (DCs) and macrophages, but also by non-professional cells, such as fibroblasts. Proinflammatory cytokines and chemokines are also critical for eliminating virus infection by provoking inflammation and recruiting innate and acquired immune cells. Co-stimulatory molecules are essential for the activation of T cells, leading to acquired immune reactions.
In this review, we focus on the roles of these PRRs in the recognition of viruses and initiation of antiviral immune responses, as well as their mechanisms for recognizing viral components.
The RLR family
RLRs comprise a family of cytoplasmic proteins consisting of three members, RIG-I (also known as DDX58), melanoma differentiation-associated gene 5 (MDA5; also known as helicard or IFIH1) and LGP2 (6-8) (9, 10). RIG-I and MDA5 consist of two N-terminal caspase-recruitment domains (CARDs), a DExD/H box RNA helicase domain and a C-terminal repressor domain (RD), whereas LGP2 lacks a CARD. The helicase domains of the RLR family members are highly similar to that of mammalian Dicer, a dsRNA-specific nuclease required for micro RNA (miRNA) and small interfering RNA (siRNA) biogenesis. Although a report showed that presence of the helicase domain suppressed Dicer RNase activity, the role of the Dicer helicase domain in its function in miRNA maturation is not well understood (11).
The RLRs recognize viral RNAs in the cytoplasm. RNA virus infection leads to the generation of dsRNA and RNAs with 5′-triphosphate ends in infected cells. Long dsRNA is not normally present in cells, and the 5′ ends of host RNAs are typically capped. The helicase domain and RD are important for the recognition of these RNAs, while the CARDs are essential for triggering intracellular signaling cascades (6, 12). LGP2 lacks a CARD, and is suggested to function as a negative regulator of RIG-I/MDA5 signaling. Overexpression of LGP2 inhibits Sendai virus (SeV) and Newcastle disease virus signaling. Lgp2-/- mice show highly elevated induction of type I IFNs in response to polyinosinic polycytidylic acid (poly I:C) stimulation, and modestly increased IFN production in response to vesicular stomatitis virus (VSV) infection (9, 10). On the other hand, Lgp2-/- mice show partially impaired type I IFN production in response to EMCV infection (13). In this study, it was shown that LGP2 was a negative regulator of RIG-I, but not of MDA5. However, given that both poly I:C and EMCV are recognized by MDA5, the difference cannot be fully explained by differential usage of LGP2 for RIG-I and MDA5 signaling.
Recognition of RNA viruses by RLRs
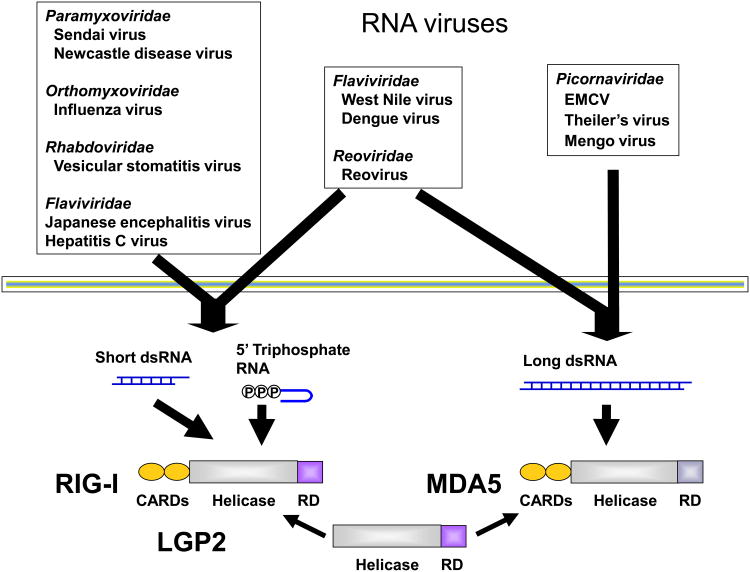
Studies using RLR-deficient mice have revealed that these proteins are essential for the production of type I IFNs and proinflammatory cytokines in response to RNA virus infection. Cell lacking RIG-I do not produce type I IFNs in response to various RNA viruses, including paramyxoviruses, VSV and influenza virus (Figure 1) (14-16). In contrast, MDA5-deficient mice do not respond to infection with picornaviruses, such as encephalomyocarditis virus (EMCV) and Theiler's virus (15, 17). Consistent with the defect in type I IFN production, RIG-I-/- and MDA5-/- mice are highly susceptible to inoculation with VSV and EMCV, respectively. Japanese encephalitis virus and hepatitis C virus (HCV), which belong to the Flaviviridae family, are both recognized by RIG-I (15, 18). Huh7 cells harboring a RIG-I mutant were found to be permissive to HCV infection (18). GB virus, a mouse model of human HCV, is also recognized by RIG-I (19). However, Dengue virus and West Nile virus, which also belong to the Flaviviridae family, were shown to induce type I IFN production, even in the absence of RIG-I or MDA5 (20, 21). siRNA experiments suggested that Dengue virus is recognized by the combination of RIG-I and MDA5. Vaccine strains of measles virus were found to activate cells in a RIG-I/MDA5-dependent manner, whereas wild-type measles virus failed to induce type I IFN production (22). However, poliovirus is expected to be recognized by MDA5 because it is a member of the Picornaviridae family. Interestingly, MDA5 was reported to be cleaved by a poliovirus protease in infected cells. Thus, poliovirus may subvert the MDA5-mediated recognition system to establish its infection (23). Infection with dsRNA virus, Reovirus, induced IFN-β production mainly through MDA5, however, absence of both RIG-I and MDA5 completely abrogated IFN production, suggesting that both RIG-I and MDA5 are involved in the recognition of Reovirus (24). It was reported that a DNA virus, EBV, produced small RNAs which induced RIG-I-mediated IFN-β production. However, since the study utilized artificial RNA synthesized by T7 polymerase which can activate RIG-I irrespective of the sequence, it is not clear if small RNA encoded by EBV truly activate RIG-I in the cells (25, 26).
Figure 1.
Differential roles of RIG-I and MDA5 in RNA virus recognition.
RIG-I recognizes 5′-triphosphate RNA and short dsRNA, whereas MDA5 discriminates long dsRNA generated during the course of virus infection. RNA viruses are differentially recognized by RIG-I and MDA5. RIG-I is responsible for detecting Paramyxoviridae, Orthomyxoviridae, Rhabdoviridae and some Flaviviridae family members. On the other hand, MDA5 recognizes Picornaviridae family members. Some viruses such as West Nile virus and Reovirus are detected by both RIG-I and MDA5. LGP2 is reported to be a genitive regulator for the RIG-I and MDA5 signaling.
In summary, RNA viruses are recognized by either RIG-I, MDA5 or combination of RIG-I and MDA5 for inducing type I IFNs in various cells. Given that type I IFN production to various RNA viruses was totally abrogated in cells lacking RIG-I and MDA5, it is assumed that there is no other receptor recognizing RNA viruses to induce type I IFNs present.
Recognition of RNAs by RIG-I and MDA5
dsRNA is present in cells infected with dsRNA viruses as well as being generated during the course of ssRNA virus replication. Since host cells do not produce dsRNA, the innate immune system discriminates between host and viral RNAs by the presence of dsRNA. Initially, both RIG-I and MDA5 were implicated in the recognition of poly I:C, a synthetic analogue of viral dsRNA (6, 9, 10). However, analyses of RIG-I-/- and MDA5-/- mice revealed that MDA5, but not RIG-I, is responsible for the IFN response to poly I:C stimulation (15). Reciprocally, RIG-I, but not MDA5, is essential for IFN production in response to ssRNA with 5′-triphosphate ends (27, 28). RNAs from some viruses are known to be 5′-triphosphorylated and uncapped, whereas the 5′ ends of host mRNAs are capped. A previous report showed that 5′ triphosphate ssRNAs of >19 nt in length efficiently induced IFN-α production in a RIG-I-dependent fashion, and that the RNA sequence did not affect the ability of RNAs to induce IFNs (27). However, a recent report showed that a poly U-containing RNA sequence corresponding to the 3′ non-translated region of Hepatitis C virus (HCV) genomic RNA preferentially activated RIG-I compared to other sequences of HCV (29). Given the RNAs in the study was synthesized by the T7 polymerase, it is not clear if the phenomenon recapitulates HCV infection in vivo. Further studies will clarify the roles of RNA sequences in RIG-I-mediated responses to 5′ triphosphate RNAs.
The next issues are whether RIG-I recognizes dsRNAs without 5′-triphosphate ends and the identification of the molecular structures of MDA5 ligands. Poly I:C is an artificial dsRNA generated by annealing between poly I and poly C. Poly I and poly C are synthesized by polynucleotide phosphorylase, which catalyzes the polymerization of nucleotide diphosphate (30). Thus, poly I:C does not harbor a 5′ triphosphate end. By electrophoresis, we found that untreated poly I:C migrated as a smeared band corresponding to the mobilities of 4-8-kbp dsDNA fragments (24). Partial digestion of poly I:C with a dsRNA-specific endonuclease, RNase III, led to the generation of short trimmed poly I:C of about 300 bp. By treating RIG-I-/- and MDA5-/- mouse embryonic fibroblasts with untreated and shortened poly I:C, we found that poly I:C was converted from a MDA5 ligand into a RIG-I ligand in a dsRNA length-dependent manner. In addition, chemically synthesized monophosphate end dsRNA of 70 bp induced production of type I IFNs in a RIG-I-dependent manner. Notably, complete digestion of poly I:C produced 10-20-bp dsRNAs that failed to stimulate even wild-type cells, consistent with a previous observation. In addition, long and short poly I:Cs preferentially bind to MDA5 and RIG-I proteins, respectively, leading to the activation of ATPase activity. It seems that dsRNAs of up to 1 kb are completely recognized by RIG-I, but not by MDA5. On the other hand, dsRNAs of >2 kb can be recognized by MDA5. These observations indicate that RIG-I and MDA5 proteins directly discriminate the lengths of dsRNA.
Not only synthesized dsRNAs, but also viral dsRNAs differentially activate RIG-I and MDA5 depending on their length (24). The genome of Reovirus, a dsRNA virus, consists of 10 segments in three distinct classes called L, M and S corresponding to their sizes of 3.9, 2.2-2.3 and 1.2-1.4 kbp, respectively. While the introduction of S segments into cells induced IFN-β in a RIG-I-dependent manner, both RIG-I and MDA5 contributed to the recognition of L segments.
EMCV produces high levels of dsRNA in infected cells, whereas dsRNA was barely detected in influenza virus-infected cells (28, 31). Genomic RNA from influenza virus harbors a 5′ triphosphate end, whereas the 5′ end of EMCV genomic RNA is covalently linked to a small protein, VPg (32). Thus, it was thought that the presence of dsRNA or 5′ triphosphate RNA was responsible for the recognition of viruses by MDA5 or RIG-I. However, VSV, a ssRNA virus recognized by RIG-I, was found to produce dsRNA in infected cells (24). Disruption of dsRNA among RNAs from VSV-infected cells reduced IFN-β-inducing activity, suggesting that the presence of dsRNA in VSV-infected cells is important for recognition by RIG-I. Interestingly, the dsRNA fragments produced by VSV infection were about 2.0-2.5 kbp, and much shorter than those produced by EMCV infection. Given that the length of the VSV genomic RNA is 11 kb, the dsRNA fragments were not replication intermediates of VSV. It has been reported that defective interfering (DI) particles are generated in VSV-infected cells, and that the sizes of DI particle snap-back dsRNAs are about 2.2 kb (33). Thus, dsRNA generated during the course of VSV replication may be derived from DI particles, although further studies are needed to clarify the source of the dsRNA. Since DI particles are known to strongly induce type I IFNs, RIG-I may have a role in detecting the presence of dsRNA in DI particles. Collectively, these findings demonstrate that RIG-I recognizes 5′ triphosphate RNA and dsRNA during the course of RNA virus infection, and that the length of the dsRNA generated during the course of the infection is important for differential recognition by RIG-I and MDA5.
Both the helicase domain and RD of RLRs potentially associate with viral RNA. Structural analyses of the RIG-I RD by X-ray crystallography and NMR revealed that 5′ triphosphate ssRNA and dsRNA directly bind to the basic surface of the RIG-I RD (34, 35). Interestingly, the RIG-I RD resembles a zinc-binding domain that is structurally related to the GDP/GTP exchange factors of Rab-like GTPases. Although the RIG-I helicase domain has an activity to unwind dsRNA with 3′ overhands, in vitro studies suggest that the helicase activity of RIG-I is not correlated with its function to induce type I IFNs (35). On the other hand, a point mutation in Walker's ATP-binding motif in the RIG-I helicase domain abolished the IFN-β-inducing ability. Therefore, it is assumed that the RIG-I helicase domain is required not for actually unwinding dsRNAs, but for changing the conformation of RIG-I to facilitate signaling through the CARDs.
Modulation of RIG-I-mediated recognition
RIG-I-mediated signaling is positively and negatively controlled by ubiquitination of RIG-I. First, the CARDs of RIG-I undergo Lys 63-linked ubiquitination by tripartite motif (TRIM) 25, a ubiquitin E3 ligase composed of a RING finger domain, B box/coiled-coil domain and SPRY domain (36). This ubiquitination is necessary for efficient activation of the RIG-I signaling pathway, and TRIM25-/- cells display impaired production of type I IFNs in response to viral infection. RIG-I also undergoes ubiquitination by the ubiquitin ligase RNF125, which leads to its proteasomal degradation (37). Thus, RIG-1 ubiquitination by RNF125 is considered to inhibit aberrant activation of RIG-I signaling.
RNase L, an endonuclease originally thought to cleave viral ssRNA, was reported to be involved in the production of IFN-β in response to RNA virus infection or dsRNA stimulation (38). Furthermore, 2′,5′-linked oligoadenylate generated by virus infection was found to activate RNase L for cleavage of self-RNA, resulting in the generation of small RNA products that are responsible for RIG-I/MDA5-mediated recognition and subsequent production of type I IFNs. However, the precise structures of these small RNAs generated by RNase L require further investigation.
The RLR signaling pathway
In response to detection of viral RNAs, RIG-I and MDA5 associate with an adaptor protein, designated IFN-β promoter stimulator-1 (IPS-1; also known as MAVS, VISA or CARDIF; Figure 2) (39-42). IPS-1 contains a CARD in its N-terminus, and crystal structurure analyses revealed that this CARD adopts the classic CARD fold with an asymmetric surface charge distribution, and shares homology with the first CARDs of RIG-I and MDA5 for homotypic CARD-CARD interaction (43). IPS-1-/- MEFs and cDCS are defective in producing type I IFNs and proinflammatory cytokines in response to all RNA viruses recognized by RIG-I or MDA5, and IPS-1-/- mice were susceptible to infection with various RNA viruses (44, 45). These findings indicate that IPS-1 plays essential roles in RIG-I/MDA5 signaling. This protein is present in the outer mitochondrial membrane, suggesting that mitochondria may be important for IFN responses, in addition to their roles in metabolism and cell death. IPS-1 is known to be cleaved by the HCV protease NS3/4A in HCV-infected cells (41, 46). NLRX1 (also known as NOD9) was reported to associate with IPS-1 (47). NLRX1 is comprised of a nucleotide-binding domain and leucine-rich repeats (LRRs), and is localized on the mitochondrial outer membrane. Overexpression of NLRX1 inhibits virus-induced IFN-β promoter activation by disrupting RIG-I/MDA5-IPS-1 interactions. Reciprocally, knockdown of NLRX1 leads to augmentation of virus-induced type I IFN production. Thus, NLRX1 is suggested to function as a modifier of IPS-1.
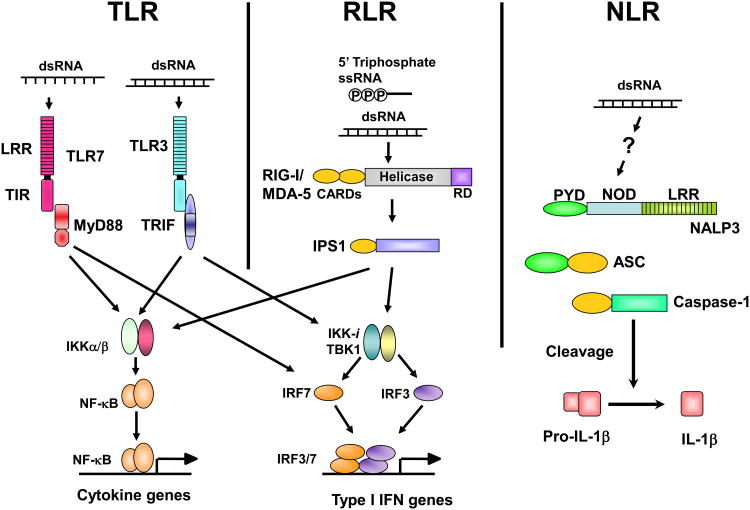
Figure 2.
Three classes of PRRs for RNA virus recognition.
ssRNA from viruses is recognized by TLR7 in pDCs, whereas dsRNA is detected by TLR3 in cDCs. TLR7 and TLR3 trigger signaling cascades via the adaptors MyD88 and TRIF, respectively. RIG-I and MDA5 recruit another adaptor protein, IPS-1. TRIF and IPS-1 share signaling molecules for phosphorylation of IRF-3 and IRF-7 by TBK1/IKK/i. MyD88-dependent signaling directly activates IRF-7 in pDCs. Phosphorylated IRF-3 and IRF-7 activate the expression of type I IFN genes. Simultaneously, TLRs and RLRs induce the translocation of NF-κB to induce the expression of cytokine genes via distinct adaptor proteins. On the other hand, one of the NLR proteins, NALP3, detects the presence of dsRNA and induces the catalytic activity of caspase-1 via an adaptor, ASC. Caspase-1 is essential for cleavage of pro-IL-1β to the mature form.
The RLR and tumor necrosis factor receptor (TNFRI) signaling pathways share molecules for activating transduction. TNFR-associated death domain (TRADD) protein, an essential adaptor for TNFR signaling, is recruited to IPS-1 upon stimulation, and is also important for RLR signaling (48). TRADD forms a complex with FAS-associated death domain-containing protein (FADD) and a death domain kinase, receptor-interacting protein 1 (RIP1), in addition to TNF-receptor associated factor (TRAF) 3, an E3 ubiquitin ligase that assembles a lysine 63-linked polyubiquitin chain. TRAF3 is essential for RLR-mediated type I IFN responses, and its function is regulated by the deubiquitinase DUBA (49-51).
Downstream of TRAF3, two IKK-related kinases, TANK-binding kinase 1 (TBK1) and inducible IκB kinase (IKKi; also known as IKKε), which phosphorylate IFN-regulatory factor (IRF)-3 and IRF-7, are activated (52-54). Phosphorylation of IRF-3/-7 by these kinases induces the formation of homodimers and/or heterodimers. Next, the IRF-3/-7 homodimers and/or heterodimers translocate into the nucleus and bind to ISREs, resulting in the expression of type I IFNs and a set of IFN-inducible genes (55, 56). Cells lacking both IRF-3 and IRF-7 did not produce type I IFNs in response to viral infection.
TBK1 and IKK-i interact with TRAF family member-associated NF-κB activator (TANK), NAK-associated protein 1 (NAP1) and similar to NAP1 TBK1 adaptor (SINTBAD) (57-59). These molecules contain a TBK1-binding motif, and show similarities among their coiled-coil domains. Although knockdown of either TANK, NAP1 or SINTBAD impairs RLH signaling, the relationship between these molecules in RLH signaling is not yet fully understood.
The RLR signaling pathway activates another transcription factor, NF-κB, for the expression of proinflammatory genes. IPS-1, TRADD and FADD are important for activating both IRFs and NF-κB (60). FADD then interacts with caspase-8/-10 and the catalytic activities of these caspases are critical for the subsequent nuclear translocation of NF-κB.
A recent study identified a novel protein named stimulator of IFN genes (STING) as an important molecule for RIG-I/MDA5 signaling (61). Overexpression of STING activated NF-κB and ISRE via TBK1/IKKi. STING-deficient mice showed impaired production of IFN-β to VSV infection. Interestingly, STING is a protein with 5 transmembrane regions localizing endoplasmic reticulum (ER) membrane, and interacts with SSR2/TRAPβ, a member of translocon-associated protein (TRAP) complex. This protein complex is required for protein translocation across the ER membrane. Given IPS-1 localizes on mitochondrial membrane, how the RIG-I signaling transduces through different organelles is interesting topics for future studies.
Recognition of viral components by the TLR system
In addition to the RLRs, TLRs are important for recognizing virus infection. TLRs are comprised of LRRs, a transmembrane domain and a cytoplasmic domain designated the Toll/IL-1 receptor (IL-1R) homology (TIR) domain (1). TLRs are transmembrane proteins suitable for detecting viral components outside of cells as well as in cytoplasmic vacuoles after phagocytosis or endocytosis. Among the >10 TLRs present in mammals, TLR2, TLR3, TLR4, TLR7 and TLR9 are involved in the recognition of viral components. TLR2 and TLR4, present on plasma membrane, are involved in the recognition of viral envelope proteins on the cell surface, while TLR2 and TLR4 are critical for the recognition of bacterial components, lipoproteins and lipopolysaccharide, respectively. In contrast, TLR3, TLR7 and TLR9 are localized on cytoplasmic vesicles, such as endosomes and the endoplasmic reticulum (ER), and recognize microbial nucleotides. TLR3 recognizes dsRNA, while TLR7 and TLR9 recognize ssRNA and DNA with CpG motifs, respectively. While TLR3 recognizes dsRNA in conventional DCs and possibly epithelial cells, TLR7 and TLR9 are highly expressed in plasmacytoid DCs (pDCs), a cell type known to produce extremely high levels of type I IFNs in response to virus infection. Crystal structure analyses of TLR3 clarified that the ectodomain of TLR3 containing the LRRs is dimerized in the presence of 40-50-bp dsRNA (62-64). The ectodomains of TLRs exhibit a horseshoe shape, and dsRNAs bind to the N- and C-terminal portions of the TLR3 ectodomain. Ligand association with the TLR ectodomains stabilizes dimer formation, thereby leading to dimerization of the TIR domains and the initiation of signal transduction.
TLR signaling
All TLRs except TLR3 activate a common signaling pathway leading to the production of proinflammatory cytokines via MyD88, a protein comprised of a N-terminal death domain (DD) and a C-terminal TIR domain. Upon ligand stimulation, MyD88 interacts with IL-1R-associated kinase (IRAK)-4. Human or mouse has 4 IRAK family members, called IRAK-1, IRAK-2, IRAK-M and IRAK-4. The IRAKs are characterized by an N-terminal DD and a C-terminal serine/threonine kinase domain. Recent studies revealed that IRAK-4 is an upstream kinase that phosphorylates IRAK-1 and IRAK-2 (65-67). IRAK-1 rapidly interacts with IRAK-4 and is phosphorylated after TLR activation, and then IRAK-1 undergoes degradation by the ubiquitin-proteasome pathway. In contrast, IRAK-2 interacts with IRAK-4 later than IRAK-1, and stayed phosphorylated for a long time. IRAK-2-/- macrophages failed to sustain cytokine gene expression in response to TLR stimulation, and cells lacking both IRAK-1 and IRAK-2 show abrogated TLR-mediated cytokine production as well as severe impairment in NF-κB activation(67). These results indicate that IRAK-1 and IRAK-2 are sequentially activated by IRAK-4, and are essential for the TLR signaling. On the other hand, IRAK-M is reported to be a negative regulator of the TLR signaling (68).
Downstream of IRAKs, TRAF6 is activated and catalyzes the formation of a K63-linked polyubiquitin chain on TRAF6 and on IKK-γ/NF-κB essential modulator (NEMO), together with an ubiquitination E2 enzyme complex consisting of UBC13 and UEV1A (69). TRAF6 also activates TGF-β-activated kinase 1 (TAK1), which phosphorylates IKK-β and MAP kinase kinase 6, which modulates the activation of NF-κB and MAP kinases that results in induction of genes involved in inflammatory responses. Deletion of TAK1 and UBC13 in mice revealed that these molecules play a critical role in TLR-mediated cytokine production, in addition to their role in embryonic development (70, 71). TAK1 is essential for both NF-κB and MAP kinases, whereas UBC13 was dispensable for NF-κB activation.
In response to stimulation with dsRNA, TLR3 recruits another adaptor protein, TIR domain-containing adaptor inducing IFN-β (TRIF; also known as TICAM-1; Figure 2) (72-74). TRIF associates with TRAF3 and TRAF6 through TRAF-binding motifs present in its N-terminal portion, and TRIF contains a C-terminal receptor-interacting protein (RIP) homotypic interaction motif (RHIM), and interacts with RIP1 and RIP3 via the RHIM (75, 76). Recent studies showed that TRADD is also involved in the TRIF-dependent signaling pathway (77, 78). The downstream signaling molecules for the expression of IFN-inducible genes are shared between the TLR3 and RLR signaling pathways. Simultaneously, TRAF6 and RIP1 are responsible for activating NF-κB through IκB kinase-α and -β (IKKα/β), leading to the expression of proinflammatory cytokines.
TLR7 and TLR9 activate distinct signaling pathways in response to viral RNA or DNA in pDCs. TLR7 and TLR9 recruit MyD88, which forms a complex with IRAK-1, IRAK-4 and IRF-7 in this cell type(79, 80). IRAK-1 and IKKα have been identified as potential IRF-7 kinases (81, 82). Phosphorylated IRF-7 translocates into the nucleus to activate the promoters of type I IFN and IFN-inducible genes. The MyD88-dependent pathway is also critical for NF-κB leading to the production of cytokines including IL-12 and IL-6.
The localizations of TLR proteins are critical for the recognition of their ligands. An ER membrane protein, UNC93B, was identified as an essential molecule for the translocation of TLR7 and TLR9 from the ER to endosomes (83, 84). In cells from 3d mice harboring a missense point mutation in UNC93B, signaling by TLR3, TLR7 and TLR9 was abrogated. In addition, an autosomal recessive mutation in UNC93B in humans results in impaired immune responses against herpes simplex virus (HSV)-1 encephalitis (85). It will be interesting to further clarify the mechanisms underlying the UNC93B-mediated regulation of TLR trafficking. Another ER protein, protein associated with TLR4 (PRAT4A), also controls TLR9 trafficking from the ER to endosomes/lysosomes (86, 87). In lysosomes, a cysteine protease, cathepsin K and cathepsin B/L, was found to be important for TLR9 signaling, although the mechanism is not yet fully understood (88, 89).
A previous report showed that autophagosome formation is required for TLR7-mediated VSV recognition in pDCs (90). It was hypothesized that viral RNAs were taken up into autophagosomes, which then fuse with lysosomes where TLR7 is localized. pDCs from mice with defective autophagosome formation show impaired type I IFN production in response to VSV infection.
Collectively, recognition of viral nucleotides by TLRs in endosomes/lysosomes is controlled by the localizations of TLRs as well as their ligands. This elaborate mechanism may be essential for preventing autoimmune diseases caused by aberrant initiation of TLR signaling. In this regard, understanding the entire mechanism of TLR trafficking will lead to the development of ways to manipulate the immune system.
Production of IL-1β in response to RNA virus infection
Among the proinflammatory cytokines, IL-1β production is regulated not only by its mRNA synthesis but also by cleavage of pro-IL-1β via caspase-1(4, 5). Recent studies have revealed that the processing of pro-IL-1β is mediated by a large protein complex containing caspase-1, known as the inflammasome. The activation of caspase-1 is triggered by cytoplasmic NOD-LRR receptors, NALP3 (also known as cryopyrin or CIAS1) and ICE-protease-activating factor (IPAF). These receptors together with an adaptor, ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), are components of the inflammasome (Figure 2). NALP3 is responsible for sensing presence of ATP as well as various crystals, such as monosodium urate, silica, asbestos and aluminum salts, by phagocytosis(91-94). Although mechanisms of crystal recognition by NALP3 is not fully understood, recent studies suggest that NALP3 activation is triggered by reaction oxygen species produced by a NADPH oxidase, or by lysosomal destabilization which may release protease Cathepsin B to the cytosol(93, 95). IPAF is known to be activated by infections with bacteria, such as Salmonella, Pseudomonas and Legionella, possibly by recognizing flagellin in the infected cells(96-98). dsRNA and poly I:C were reported to activate the inflammasome via a NALP3 -dependent pathway, but it remains unclear whether NALP3 directly recognizes dsRNA in the cytoplasm(99). NALP3 is also critical for IL-1β processing in response to adenovirus infection. Although adenoviruses are DNA viruses, introduction of dsDNA into cells was found to activate IL-1β in a NALP3-independent manner, suggesting that adenovirus-induced IL-1β production is not induced by recognition of genomic DNA (100). However, ASC and caspase-1 are essential for dsDNA-induced IL-1β production, suggesting that one of the unknown NALP family members functions as a cytoplasmic DNA sensor.
Type I IFN-producing cells in response to viral infection
pDCs are known to produce vast amounts of type I IFNs to virus infection, and the importance of pDCs as the IFN-inducer has been emphasized. Nevertheless, cells other than pDCs are potent to produce type I IFNs as described above. Although RLRs play essential roles in the production of type I IFNs and cytokines in various cell types, such as fibroblasts and cDCs, pDCs produce these cytokines in the absence of RLH signaling(14). To identify IFN-producing cells in vivo, a reporter mouse strain expressing green fluorescent protein (GFP) under the control of the IFN-α6 gene (Ifna6GFP/+) has been generated(101). In response to systemic NDV infection, pDCs were highly potent in expressing GFP, although cDCs and macrophages also produced IFN-α6. However, lung local infection of Ifna6GFP/+ mice with NDV resulted in increased numbers of GFP+ alveolar macrophages and cDCs, but not pDCs. pDCs started to produce IFN-α when alveolar macrophages were depleted or IPS-1-deficient mice were infected. NDV is non-pathogenic to wild-type mice, and NDV is almost cleared by 96 h post infection. On the other hand, alveolar macrophage-depleted mice and IPS-1-/- mice showed increased viral burden, suggesting that failure of the first line of defense led to production of type I IFNs from pDCs. In addition, lung infection with pathogenic Sendai virus also induced production of type I IFNs from pDCs. Thus, RLR-mediated IFN responses function as the first line of defense against respiratory infection, and pDCs started to be activated when the defense is broken.
Roles of RLRs and TLRs in the activation of adaptive immune responses to viruses
Innate immediate immune responses are important for mounting acquired immune responses to viral infections. However, it is not clear how the innate PRRs are involved in the activation of acquired immunity. Recently, two different virus infection models have been analyzed to examine the roles of RLRs and TLRs in the activation of acquired immune responses. The first model virus is lymphocytoid choriomeningitis virus (LCMV), an ambisense ssRNA virus belonging to the Arenaviridae family, which is known to induce a cytotoxic T lymphocyte (CTL) response in a type I IFN-dependent manner(102). Analyses of MyD88-/- and IPS-1-/- mice revealed that the serum levels of type I IFNs and pro-inflammatory cytokines are mainly dependent on the presence of MyD88, but not IPS-1. Furthermore, the generation of virus-specific CTLs is critically dependent on MyD88, but not IPS-1. Analysis of Ifna6+/GFP reporter mice revealed that pDCs are the major source of IFN-α in LCMV infection. These results suggest that recognition of LCMV by pDCs via TLRs is responsible for the production of type I IFNs in vivo. Furthermore, TLRs, but not RLRs, appear to be important for mounting CTL responses to LCMV infection.
Influenza virus has also been used to study the activation of adaptive immune responses. Induction of type I IFNs in response to intranasal influenza A virus infection was found to be abrogated in the absence of both MyD88 and IPS-1, although mice lacking either of these molecules were capable of producing IFNs(103). Induction of B cells or CD4 T cells specific to viral proteins was dependent on the presence of MyD88, but not IPS-1, whereas induction of nuclear protein Ag-specific CD8 T cells was not impaired in the absence of either MyD88 or IPS-1. These results suggest that the adaptive immune responses to influenza A virus are governed by TLRs. Another study examined the contribution of IPS-1 and MyD88 to respiratory syncytial virus (RSV) infection in mice(104). RSV infection induced type I IFNs and inflammatory cytokines in an IPS-1-dependent and MyD88-independent manner. Nevertheless, both IPS-1 and MyD88 were important for the clearance of RSV as well as production of RSV-specific antibodies. However, mice lacking both IPS-1 and MyD88 were still capable of mounting CD8+ CTL responses to RSV infection, suggesting that RLR- and TLR-independent RNA virus recognition might be responsible for the activation of CD8+ T cells in the lung.
As described above, poly I:C is recognized by MDA5 and TLR3. The contributions of these two systems to the activation of T cell responses have been examined using mice deficient in IPS-1 or TRIF, adaptor molecules responsible for the signaling of MDA5 and TRIF, respectively. Enhancement of Ag-specific antibody responses as well as CD8 T cell expansion in response to poly I:C stimulation is impaired in IPS-1-deficient mice(105). Although the responses of TRIF-deficient mice are modestly impaired, IPS-1/TRIF doubly deficient mice are almost unresponsive to poly I:C treatment, suggesting that both MDA5 and IPS-1 contribute to mounting acquired immune responses to poly I:C stimulation.
The virus infection models tested to date support roles for TLRs, rather than RLHs, in instructing the adaptive immune system. However, further studies are required since these two PRR systems contribute differently depending on the viruses involved, and their contributions may also depend on the route of infection. Though production of type I IFNs and proinflammatory cytokines depends on the presence of TLR- and RLR-dependent signaling, activation of CD8+ T cell responses does not depend on either singling pathway. It is intriguing to explore how CTL responses are activated in response to the virus infection.
Conclusions
The innate PRRs differentially recognize viral components in cell type-specific manners. As described in this review, RIG-I and MDA5 discriminate short and long dsRNAs, respectively. However, the molecular mechanisms for how RIG-I and MDA5 distinguish the lengths of dsRNA remain to be determined. Structural analyses of MDA5 will clarify the mechanism of MDA5-mediated recognition of long dsRNA. Another issue is the role of RIG-I helicase activity in the recognition of viral RNAs. Although several reports have shown that RIG-I protein can unwind short dsRNA, it is apparent that this “helicase” activity is not required for the recognition of 5′ triphosphate ssRNA. Given that the RIG-I helicase domain catalyzes ATP, it is assumed that the helicase domain is critical for the conformational change required to expose the CARDs and trigger intracellular signaling.
In pDCs, TLR9 is essential for type I IFN production in response to DNA virus infection by recognizing viral genomic DNA. However, the presence of dsDNA detectors in the cytoplasm has been predicted(106). TLR9 activates type I IFN responses by a signaling pathway through TBK1/IKK-i. Indeed, DNA viruses, HSV and mouse cytomegalovirus, produce type I IFNs independently of the TLR system in non-pDCs. Recognition of intracellular bacteria such as Listeria and Legionella is potentially through their dsDNA (107). In addition, loss of exonuclease 1 (Trex1) resulted in accumulation of single-stranded DNA derived from endogenous retroelements leading to the development of autoimmune diseases such as Aicardi-Goutieres syndrome (AGS) via production of type I IFNs (108). Thus, accumulating evidence indicates that recognition of cytoplasmic DNA is critical for innate immune responses as well as prevention of autoimmune diseases. Although a protein named DAI/ZBP1 was proposed as a candidate for the dsDNA sensor(109), DAI/ZBP1-/- mouse cells do not show any defects in the induction of IFN-β and IFN-inducible genes(110). The identification of the dsDNA detector will open the door toward understanding the immune responses to DNA virus infection. It has been shown that STING is essential for type I IFN production to cytoplasmic dsDNA stimulation and infection with Listeria and HSV1 (61). Although STING itself is unlikely to be a DNA receptor, further analysis of the function of this protein or identification of STING binding partners might be a clue for solving the DNA recognition pathways.
Antiviral immune responses in vivo are mediated not only by DCs, macrophages, T cells and B cells, but also by many other cell types, such as NK cells and NK T cells. Thus, it is important to understand dynamic interaction between the immune cells by monitoring immune cell behavior, interaction and activation in vivo. The understanding of mechanisms for the activation of antiviral immunity will leads to development of novel immunotherapy and vaccines for infectious diseases, immune diseases and cancer.
Acknowledgments
We thank E. Kamada for excellent secretarial assistance. This work was supported in part by grants from the Special Coordination Funds of the Japanese Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labour and Welfare in Japan, the 21st Century Center of Excellence Program of Japan, and the NIH (P01 AI070167).
Contributor Information
Osamu Takeuchi, Department of Host Defense, Immunology Frontier Research Center, Osaka University, 3-1 Yamada-oka, Suita, Osaka 565-0871, Japan, Tel: +81-6-6879-8303; Fax: +81-6-6879-8305.
Shizuo Akira, Department of Host Defense, Immunology Frontier Research Center, Osaka University, 3-1 Yamada-oka, Suita, Osaka 565-0871, Japan, Tel: +81-6-6879-8303; Fax: +81-6-6879-8305.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, et al. Genetic analysis of resistance to viral infection. Nat Rev Immunol. 2007;7:753–66. doi: 10.1038/nri2174. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 4.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–22. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–59. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–7. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 7.Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci U S A. 2002;99:637–42. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacsovics M, Martinon F, Micheau O, Bodmer JL, Hofmann K, Tschopp J. Overexpression of Helicard, a CARD-containing helicase cleaved during apoptosis, accelerates DNA degradation. Curr Biol. 2002;12:838–43. doi: 10.1016/s0960-9822(02)00842-4. [DOI] [PubMed] [Google Scholar]
- 9.Yoneyama M, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–8. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 10.Rothenfusser S, et al. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol. 2005;175:5260–8. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 11.Ma E, MacRae IJ, Kirsch JF, Doudna JA. Autoinhibition of human dicer by its internal helicase domain. J Mol Biol. 2008;380:237–43. doi: 10.1016/j.jmb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito T, et al. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci U S A. 2007;104:582–7. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkataraman T, et al. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J Immunol. 2007;178:6444–55. doi: 10.4049/jimmunol.178.10.6444. [DOI] [PubMed] [Google Scholar]
- 14.Kato H, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Kato H, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–5. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 16.Melchjorsen J, et al. Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. J Virol. 2005;79:12944–51. doi: 10.1128/JVI.79.20.12944-12951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gitlin L, et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103:8459–64. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumpter R, Jr, et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689–99. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, et al. GB virus B disrupts RIG-I signaling by NS3/4A-mediated cleavage of the adaptor protein MAVS. J Virol. 2007;81:964–76. doi: 10.1128/JVI.02076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M., Jr Establishment and Maintenance of the Innate Antiviral Response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J Virol. 2007 doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loo YM, et al. Distinct Rig-I and Mda5 Signaling by Rna Viruses in Innate Immunity. J Virol. 2007 doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shingai M, et al. Differential type I IFN-inducing abilities of wild-type versus vaccine strains of measles virus. J Immunol. 2007;179:6123–33. doi: 10.4049/jimmunol.179.9.6123. [DOI] [PubMed] [Google Scholar]
- 23.Barral PM, et al. MDA-5 is cleaved in poliovirus-infected cells. J Virol. 2007;81:3677–84. doi: 10.1128/JVI.01360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato H, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–10. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samanta M, Iwakiri D, Kanda T, Imaizumi T, Takada K. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. Embo J. 2006;25:4207–14. doi: 10.1038/sj.emboj.7601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samanta M, Iwakiri D, Takada K. Epstein-Barr virus-encoded small RNA induces IL-10 through RIG-I-mediated IRF-3 signaling. Oncogene. 2008;27:4150–60. doi: 10.1038/onc.2008.75. [DOI] [PubMed] [Google Scholar]
- 27.Hornung V, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–7. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 28.Pichlmair A, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 29.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M., Jr Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–7. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grunberg-Manago M, Oritz PJ, Ochoa S. Enzymatic synthesis of nucleic acidlike polynucleotides. Science. 1955;122:907–10. doi: 10.1126/science.122.3176.907. [DOI] [PubMed] [Google Scholar]
- 31.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol. 2006;80:5059–64. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agol VI, Paul AV, Wimmer E. Paradoxes of the replication of picornaviral genomes. Virus Res. 1999;62:129–47. doi: 10.1016/s0168-1702(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 33.Pattnaik AK, Ball LA, LeGrone A, Wertz GW. The termini of VSV DI particle RNAs are sufficient to signal RNA encapsidation, replication, and budding to generate infectious particles. Virology. 1995;206:760–4. doi: 10.1016/S0042-6822(95)80005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui S, et al. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol Cell. 2008;29:169–79. doi: 10.1016/j.molcel.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 35.Takahasi K, et al. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol Cell. 2008;29:428–40. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Gack MU, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–20. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 37.Arimoto K, Takahashi H, Hishiki T, Konishi H, Fujita T, Shimotohno K. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci U S A. 2007;104:7500–5. doi: 10.1073/pnas.0611551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malathi K, Dong B, Gale M, Jr, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–9. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawai T, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–8. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 40.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–82. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Meylan E, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–72. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 42.Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–40. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Potter JA, Randall RE, Taylor GL. Crystal structure of human IPS-1/MAVS/VISA/Cardif caspase activation recruitment domain. BMC Struct Biol. 2008;8:11. doi: 10.1186/1472-6807-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar H, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Q, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–42. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci U S A. 2005;102:17717–22. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore CB, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008 doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 48.Michallet MC, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28:651–61. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Hacker H, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–7. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 50.Oganesyan G, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–11. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 51.Kayagaki N, et al. DUBA: A Deubiquitinase That Regulates Type I Interferon Production. Science. 2007 doi: 10.1126/science.1145918. [DOI] [PubMed] [Google Scholar]
- 52.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–6. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 53.Perry AK, Chow EK, Goodnough JB, Yeh WC, Cheng G. Differential requirement for TANK-binding kinase-1 in type I interferon responses to toll-like receptor activation and viral infection. J Exp Med. 2004;199:1651–8. doi: 10.1084/jem.20040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hemmi H, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–50. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Honda K, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–7. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 56.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–60. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Sasai M, et al. NAK-associated protein 1 participates in both the TLR3 and the cytoplasmic pathways in type I IFN induction. J Immunol. 2006;177:8676–83. doi: 10.4049/jimmunol.177.12.8676. [DOI] [PubMed] [Google Scholar]
- 58.Guo B, Cheng G. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J Biol Chem. 2007;282:11817–26. doi: 10.1074/jbc.M700017200. [DOI] [PubMed] [Google Scholar]
- 59.Ryzhakov G, Randow F. SINTBAD, a novel component of innate antiviral immunity, shares a TBK1-binding domain with NAP1 and TANK. Embo J. 2007 doi: 10.1038/sj.emboj.7601743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi K, Kawai T, Kumar H, Sato S, Yonehara S, Akira S. Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J Immunol. 2006;176:4520–4. doi: 10.4049/jimmunol.176.8.4520. [DOI] [PubMed] [Google Scholar]
- 61.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008 doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bell JK, Askins J, Hall PR, Davies DR, Segal DM. The dsRNA binding site of human Toll-like receptor 3. Proc Natl Acad Sci U S A. 2006;103:8792–7. doi: 10.1073/pnas.0603245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choe J, Kelker MS, Wilson IA. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science. 2005;309:581–5. doi: 10.1126/science.1115253. [DOI] [PubMed] [Google Scholar]
- 64.Liu L, et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–81. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki N, et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–6. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 66.Kawagoe T, et al. Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor-mediated immune responses but not in TCR signaling. J Exp Med. 2007;204:1013–24. doi: 10.1084/jem.20061523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawagoe T, et al. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol. 2008;9:684–91. doi: 10.1038/ni.1606. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 69.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–65. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sato S, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–95. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto M, et al. Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat Immunol. 2006;7:962–70. doi: 10.1038/ni1367. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto M, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–72. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 74.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–7. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 75.Sato S, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–10. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 76.Meylan E, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–7. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 77.Ermolaeva MA, et al. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol. 2008 doi: 10.1038/ni.1638. [DOI] [PubMed] [Google Scholar]
- 78.Pobezinskaya YL, et al. The function of TRADD in signaling through tumor necrosis factor receptor 1 and TRIF-dependent Toll-like receptors. Nat Immunol. 2008 doi: 10.1038/ni.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Honda K, et al. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc Natl Acad Sci U S A. 2004;101:15416–21. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawai T, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–8. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 81.Hoshino K, et al. IkappaB kinase-alpha is critical for interferon-alpha production induced by Toll-like receptors 7 and 9. Nature. 2006;440:949–53. doi: 10.1038/nature04641. [DOI] [PubMed] [Google Scholar]
- 82.Uematsu S, et al. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J Exp Med. 2005;201:915–23. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–64. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 84.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–8. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 85.Casrouge A, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–12. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi K, et al. A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J Exp Med. 2007;204:2963–76. doi: 10.1084/jem.20071132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wakabayashi Y, et al. A protein associated with toll-like receptor 4 (PRAT4A) regulates cell surface expression of TLR4. J Immunol. 2006;177:1772–9. doi: 10.4049/jimmunol.177.3.1772. [DOI] [PubMed] [Google Scholar]
- 88.Asagiri M, et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science. 2008;319:624–7. doi: 10.1126/science.1150110. [DOI] [PubMed] [Google Scholar]
- 89.Matsumoto F, et al. Cathepsins are required for Toll-like receptor 9 responses. Biochem Biophys Res Commun. 2008;367:693–9. doi: 10.1016/j.bbrc.2007.12.130. [DOI] [PubMed] [Google Scholar]
- 90.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 91.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 92.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 93.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–7. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–6. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–56. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–8. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 97.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–82. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 98.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–75. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 99.Kanneganti TD, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–8. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 100.Muruve DA, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–7. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 101.Kumagai Y, et al. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–52. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 102.Jung A, et al. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces cytotoxic T cell response via MyD88. J Virol. 2007 doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koyama S, et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–20. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 104.Bhoj VG, et al. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2008;105:14046–51. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar H, Koyama S, Ishii KJ, Kawai T, Akira S. Cutting Edge: Cooperation of IPS-1- and TRIF-Dependent Pathways in Poly IC-Enhanced Antibody Production and Cytotoxic T Cell Responses. J Immunol. 2008;180:683–7. doi: 10.4049/jimmunol.180.2.683. [DOI] [PubMed] [Google Scholar]
- 106.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–8. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 107.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 108.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–98. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–5. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 110.Ishii KJ, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–9. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]