Abstract
IGF-I is a pivotal hormone in pediatric musculoskeletal development. Though recent data suggest that the role of IGF-I in total body lean mass and total body bone mass accrual may be compromised in children with insulin resistance, cortical bone geometric outcomes have not been studied in this context. Therefore, we explored the influence of insulin resistance on the relationship between IGF-I and cortical bone in children. A secondary aim was to examine the influence of insulin resistance on the lean mass-dependent relationship between IGF-I and cortical bone. Children were otherwise healthy, early adolescent black and white boys and girls (ages 9–13 years) and were classified as having high (n=147) or normal (n=168) insulin resistance based on the homeostasis model assessment of insulin resistance (HOMA-IR). Cortical bone at the tibia diaphysis (66% site) and total body fat-free soft tissue mass (FFST) were measured by pQCT and DXA, respectively. IGF-I, insulin and glucose were measured in fasting sera and HOMA-IR was calculated. Children with high HOMA-IR had greater unadjusted IGF-I (p<0.001). HOMA-IR was a negative predictor of cortical bone mineral content, cortical bone area (Ct.Ar) and polar strength strain index (pSSI; all p≤0.01) after adjusting for race, sex, age, maturation, fat mass, and FFST. IGF-I was a positive predictor of most musculoskeletal endpoints (all p<0.05) after adjusting for race, sex, age, and maturation. However, these relationships were moderated by HOMA-IR (pInteraction<0.05). FFST positively correlated with most cortical bone outcomes (all p<0.05). Path analyses demonstrated a positive relationship between IGF-I and Ct.Ar via FFST in the total cohort (βIndirect Effect=0.321, p<0.001). However, this relationship was moderated in the children with high (βIndirect Effect=0.200, p<0.001) versus normal (βIndirect Effect=0.408, p<0.001) HOMA-IR. These data implicate insulin resistance as a potential suppressor of IGF-I-dependent cortical bone development, though prospective studies are needed.
Keywords: GH/IGF-I, skeletal muscle, bone QCT/μCT, pQCT
INTRODUCTION
Nearly one third of US children have a body mass index (BMI) ≥ 85th percentile (for sex and age), and are thus considered overweight or obese.(1) Of the various adverse health characteristics that have been linked to childhood overweight and obesity, musculoskeletal health has received little attention, and data relating to the fat-bone relationship in children are mixed. For instance, greater tibia cortical bone strength has been shown in obese children versus their non-obese peers.(2) However, others have identified fat mass as a negative predictor of radius cortical bone strength during childhood,(3) lending an explanation for the greater propensity for skeletal fractures in overweight and obese youth.(4,5) Whereas transient fluctuations in insulin resistance accompany pubertal maturation,(6) obesity-related insulin resistance may underpin the fat-bone connection.(7,8) For instance, in the English boys and girls who participated in the Avon Longitudinal Study of Parents and Children, fasting insulin, an indicator of insulin resistance, was a negative predictor of mid-tibia cortical bone volumetric density, size, and estimated bending strength. Therefore, these data suggest that processes involved in cortical bone areal expansion may be affected.(8)
Of the various hormones involved in pediatric skeletal development, insulin-like growth factor I (IGF-I) plays a pivotal role.(9–11) Indeed, IGF-I promotes bone mineral accrual and cortical bone areal expansion by acting directly upon the bone-forming osteoblasts; preferentially those located toward the periosteum.(12–14) In addition, the trophic effect of IGF-I on lean body mass is suspected to precede skeletal changes.(11,15–17) Therefore, IGF-I promotes cortical bone growth through both direct and lean mass-dependent processes. Moreover, IGF-I is similar to the pancreatic β-cell-derived insulin in terms of structure, downstream signaling processes, and cellular target tissues (e.g., muscle and bone).(18,19) Skeletal muscle is most prone to developing insulin resistance and, as noted above, is an integral link between IGF-I and bone. Therefore, recent cross-sectional data showing a suppressed total body lean mass-dependent relationship between IGF-I and total body bone mass in girls with high insulin resistance may be attributed to suboptimal IGF-I action.(10) Cortical bone outcomes have yet to be studied in the context of insulin resistance, IGF-I, and pediatric bone; thus representing a key gap in the current body of evidence. In this study, we explored the influence of insulin resistance on the relationship between IGF-I and cortical bone in children. Considering the role of IGF-I in promoting cortical bone areal growth, we hypothesized that insulin resistance would moderate the relationship between IGF-I and cortical bone size, and consequently estimated bending strength. As a secondary aim, we examined the influence of insulin resistance on the lean mass-dependent relationship between IGF-I and cortical bone.
METHODS
Study participants
This is a cross-sectional, ancillary study using baseline data from children who participated in the GAPI study (The University of Georgia [UGA], Purdue University [PU], and Indiana University [IU] multi-site, double blinded, randomized placebo-controlled vitamin D supplementation trial).(20,21) This secondary data analysis considers all participants with available data on the homeostasis model assessment of insulin resistance (HOMA-IR), and includes black and white males and females, ages 9 to 13 years, who were in the early stages of pubertal development (N=315). All children were recruited at sexual maturation rating stage 2 or 3 based on self-reported breast or genital development.(22–24) Potential participants were excluded from this study if they already commenced menarche (females), had a prior diagnosis of any chronic disease or growth disorder, or were using any medications and/or dietary/herbal supplements known to influence musculoskeletal metabolism. “High” and “normal” HOMA-IR groups were determined using a HOMA-IR cutoff of 4.0.(25) Those designated as having normal HOMA-IR (i.e., HOMA-IR < 4.0) represent the group with “normal” insulin sensitivity, and those designated as having high HOMA-IR (i.e., HOMA-IR ≥ 4.0) represent the group with the greatest insulin resistance. The Institutional Review Board for Human Subjects at UGA, PU, and IU approved all study protocols and procedures. All participants and parents/guardians provided written informed assent and permission, respectively.
Anthropometric measurements
Weight was measured using an electronic scale, height was measured using a wall-mounted stadiometer, and BMI percentiles (for sex and age) were calculated.(26) Single-measure intraclass correlation coefficients (ICCs) and test-retest coefficients of variation (CV) for height (0.99% and 0.4%) and weight (0.99% and 1.4%) were determined previously in our lab in 6 to 10-year-old girls (N=10) who were measured by the same researcher twice over a 2-week period.(20)
Dual energy X-ray absorptiometry
Fat mass (kg), fat-free soft tissue mass (FFST; kg) and percent body fat (%) were measured via DXA at each study site (Delphi-A, Hologic Inc [UGA]; Lunar iDXA, GE Medical Instruments [PU]; and Discovery-W, Hologic Inc [IU]). The same researcher at each site performed and analyzed all DXA scans through instrument-specific software and procedures. At the UGA study site, ICCs were calculated from ten females ages 5–8 years who were scanned twice over a 7-day period (all ≥ 0.98). As reported previously,(20,21,27) DXA scanners at each testing site were cross-calibrated and regression formulae were derived and used to adjust data from UGA and IU to PU values.
Peripheral quantitative computed tomography
As reported previously,(21) peripheral quantitative computed tomography (pQCT) scans were performed using Stratec XCT 2000 scanners (Stratec Medizintechnik GmbH, Pforzheim, Germany). To ensure comparability of machines between each testing site, a cortical bone phantom with known properties was scanned a minimum of 20 times on each scanner. The variation in phantom measures differed by < 1%. Scans were performed on the non-dominant lower leg, as determined by self-report. Tibia length (cm) was measured using the medial tibial plateau and the distal edge of the medial malleolus as points of reference. Relative to the total leg length and measured from the distal region, a pen mark was placed upon the 66% site of the tibia diaphysis. The lower leg was centered within the gantry while the subject was sitting upright and facing the instrument. The scan beam was placed upon the pen mark and a single tomographic slice was taken using a slice thickness of 2.3 mm, voxel size of 400 μm and a scan speed of 20 mm/s.
Using a threshold of 710 mg/cm3, cort mode 1 was used to determine cortical volumetric bone mineral density (Ct.vBMD, mg/cm3), cortical bone mineral content (Ct.BMC, mg/mm) and cortical bone area (Ct.Ar, cm2). Using this same threshold, contour mode 1 was used to define the outermost edge of the bone and peel mode 2, using a threshold of 400 mg/cm3, was used to separate the cancellous and cortical bone compartments. Total bone area (Tt.Ar, mm2), cortical thickness (Ct.Th, mm), periosteal circumference (Peri.Circ, mm) and endosteal circumference (Endo.Circ, mm) were measured. Cort mode 2 (threshold of 400 mg/cm3) was used to determine polar strength strain index (pSSI), which uses Ct.vBMD, section modulus, and normal physiological bone density that is estimated at 12,000 mg/mm3.(21,28,29) Muscle cross-sectional area (MCSA) was measured using a F03F05 filer (contour mode 3 [threshold of −100 mg/cm3] and peel mode 2). At the UGA study site, test-retest reliability was performed by scanning five healthy females (ages 18 to 24 years).(30) One-way random effects model, single measure ICCs for all pQCT measurements were R ≥ 0.97.
Serum biochemistries
Blood samples were collected in the morning following an overnight fast and were stored in a −80 °C freezer until the time of analyses. Serum glucose was measured in triplicate using a microtiter modification of the enzymatic Autokit Glucose method (Wako Chemicals). The mean intra-assay CV for this analysis was 1.8% and the mean inter-assay CV was 2.2%. Serum insulin was assayed in duplicate using the Human Insulin Specific RIA (HI-14K, Millipore). The mean intra-assay CV for this analysis was 3.5% and the mean inter-assay CV was 5.3%. The homoeostasis model assessment of insulin resistance (HOMA-IR) was calculated (fasting insulin [uU/mL] x fasting glucose [mg/dL]/405).(31) As described previously,(10) serum IGF-1 (ng/mL) was measured in duplicate using a quantitative sandwich immunoassay technique with recombinant human IGF-1 (R&D Systems). Mean interassay CVs ranged from 5.6 to 8.7%.
Statistical analyses
Histograms of all variables were inspected for outliers and non-normal distributions. Non-normal distributions were corrected by performing log (insulin, HOMA-IR, IGF-I, FFST, fat mass, Tt.Ar, Ct.Th and pSSI) or square root (tibia length) transformations. The results of the descriptive comparisons using the transformed and untransformed values were similar. Thus, the untransformed data are presented in Table 1 for ease of interpretation. Unadjusted, between-group differences in participant characteristics were determined using independent samples t-tests and X-square tests.
Table 1.
Participant characteristics
| Total Cohort N=315 |
High HOMA-IR n=147 |
Normal HOMA-IR n=168 |
pa | |
|---|---|---|---|---|
| Demographics | ||||
| Race (n; black)b | 159 | 95 | 64 | <0.001 |
| Sex (n, female)b | 154 | 86 | 68 | 0.001 |
| Age (years) | 11.3 ± 1.2 | 11.2 ± 1.3 | 11.5 ± 1.2 | 0.067 |
| Sexual maturation stage | 2.4 ± 0.5 | 2.4 ± 0.5 | 2.3 ± 0.5 | 0.022 |
| Anthropometrics | ||||
| Height (cm) | 150.7 ± 9.3 | 151.5 ± 9.3 | 150.1 ± 9.3 | 0.175 |
| Weight (kg) | 47.4 ± 12.1 | 52.1 ± 12.4 | 43.3 ± 10.3 | <0.001 |
| BMI-for-age (percentile) | 68.2 ± 29.3 | 81.0 ± 21.5 | 57.0 ± 30.7 | <0.001 |
| Tibia length (cm) | 35.0 ± 3.5 | 35.7 ± 3.2 | 34.3 ± 3.7 | <0.001 |
| Body Composition | ||||
| FFST (kg) | 30.5 ± 6.9 | 31.7 ± 6.4 | 29.3 ± 7.1 | 0.002 |
| Fat mass (kg) | 14.7 ± 7.3 | 17.9 ± 8.0 | 11.9 ± 5.3 | <0.001 |
| Percent body fat (%) | 31.0 ± 9.4 | 34.6 ± 9.5 | 27.9 ± 8.1 | <0.001 |
| MCSA (mm2) | 4848.8 ± 1050.0 | 5071.4 ± 998.6 | 4655.7 ± 1058.1 | <0.001 |
| Serum Biochemistries | ||||
| Insulin (uU/mL) | 19.9 ± 10.1 | 27.3 ± 10.2 | 13.4 ± 3.3 | <0.001 |
| Glucose (mg/dL) | 89.0 ± 7.1 | 91.3 ± 7.2 | 87.0 ± 6.5 | <0.001 |
| HOMA-IR | 4.4 ± 2.4 | 6.2 ± 2.4 | 2.9 ± 0.7 | <0.001 |
| IGF-I (ng/mL) | 232.4 ± 97.9 | 256.3 ± 107.3 | 211.5 ± 83.7 | <0.001 |
| Cortical Bone | ||||
| Ct.vBMD (mg/cm3) | 1062.6 ± 35.0 | 1066.7 ± 35.0 | 1059.1 ± 34.8 | 0.060 |
| Ct.BMC (mg/mm) | 252.2 ± 47.8 | 262.0 ± 48.0 | 243.7 ± 46.1 | 0.001 |
| Tt.Ar (mm2) | 446.6 ± 86.5 | 464.8 ± 87.2 | 430.8 ± 83.0 | 0.001 |
| Ct.Ar (mm2) | 237.4 ± 44.5 | 245.4 ± 43.1 | 230.4 ± 44.5 | 0.003 |
| Ct.Th (mm) | 3.8 ± 0.5 | 3.8 ± 0.5 | 3.7 ± 0.5 | 0.116 |
| Peri.Circ (mm) | 74.6 ± 7.2 | 76.1 ± 7.2 | 73.3 ± 6.9 | 0.001 |
| Endo.Circ (mm) | 50.9 ± 6.7 | 52.1 ± 6.9 | 49.8 ± 6.3 | 0.003 |
| pSSI (mm3) | 1684.2 ± 467.0 | 1786.7 ± 475.9 | 1594.9 ± 441.5 | <0.001 |
Data are presented as mean ± standard deviation unless otherwise indicated
Test of between-group significance based on independent samples t-test
Test of between-group significance based on X-square test
The relationship between HOMA-IR and musculoskeletal endpoints was examined using liner regression while including race, sex, age, sexual maturation rating stage, and total body fat mass as covariates. Analyses involving cortical bone measures included FFST and tibia length as additional covariates. However, tibia length was not included as a covariate in the final analyses, as it did not alter the relationship between HOMA-IR and any cortical bone outcome.
Linear regression analyses predicting musculoskeletal outcomes from IGF-I and FFST were performed. All analyses included race, sex, age, and sexual maturation rating stage as covariates. A two-step linear regression procedure was used to assess whether HOMA-IR moderated the relationship between IGF-I/FFST and musculoskeletal endpoints. In the first step of this procedure, the covariates, moderator variable (i.e., HOMA-IR), and independent variable were entered into the regression model. Second, the HOMA-IR by independent variable interaction was entered into the model, and the F change statistic was evaluated (i.e., pInteraction). This procedure was also performed while using group and sex as moderator variables. The above-mentioned statistical analyses were performed using SPSS version 23.
The SPSS PROCESS program was used to perform a Model 58 moderated mediation to determine whether the indirect relation between IGF-I and Ct.Ar via FFST differed between HOMA-IR groups.(32) The index of moderated mediation, standard error, and the bias corrected 95% confidence interval (10,000 bootstrap samples) were calculated. The index of moderated mediation was statistically significant (Supplemental Figure 1), indicating that 1) the IGF-I-FFST-Ct.Ar relationship was moderated in those with high HOMA-IR and 2) that this difference was attributed to the suppressed path from IGF-I to FFST. Therefore, justifying the comparison of path analyses between the two groups. Using Mplus software (version 7.31), path analysis was performed to examine the FFST-mediated relationship between IGF-I and Ct.Ar. Indirect effects tests were conducted using the product coefficient method.(33) Each of the above-mentioned path models were just-identified and included race, sex, and age as covariates. All significant p-values within each path analysis remained significant after adjusting for multiple comparisons through the Holm-Bonferroni technique. A p-value < 0.05 was considered statistically significant for all analyses.
RESULTS
Descriptive participant characteristics are presented in Table 1. The high versus normal HOMA-IR group had a greater number of black and female participants, were on average more sexually mature, and had a greater body weight, BMI-for-age percentile, tibia length, FFST, fat mass, percent body fat, MCSA, insulin, glucose, HOMA-IR, and IGF-I (all p<0.05). With the exception of Ct.vBMD and Ct.Th, the unadjusted cortical bone outcomes were higher in the children with high HOMA-IR versus normal HOMA-IR (all p<0.01).
After controlling for race, sex, age, sexual maturation rating stage, and fat mass, HOMA-IR was a positive predictor of FFST and MCSA (both p<0.01; Table 2). However, HOMA-IR was a negative predictor of Ct.BMC, Ct.Ar, and pSSI after adjustment for race, sex, age, sexual maturation rating stage, fat mass, and FFST (all p≤0.01).
Table 2.
Relationships between HOMA-IR and musculoskeletal outcomes while adjusting for covariates
| β | p | |
|---|---|---|
| FFST | 0.185 | <0.001 |
| MCSA | 0.149 | 0.007 |
| Ct.vBMD | −0.094 | 0.133 |
| Ct.BMC | −0.128 | <0.001 |
| Tt.Ar | −0.065 | 0.111 |
| Ct.Ar | −0.116 | 0.001 |
| Ct.Th | −0.103 | 0.075 |
| Peri.Circ | −0.074 | 0.068 |
| Endo.Circ | −0.033 | 0.562 |
| pSSI | −0.090 | 0.010 |
Each analysis includes, race, sex, age, sexual maturation rating stage and total body fat mass as covariates
Analyses involving cortical bone outcomes also include FFST as an additional covariate
IGF-I was a significant positive predictor of FFST, MCSA, Ct.BMC, Tt.Ar, Ct.Ar, Ct.Th, Peri.Circ, and pSSI in each of our analyses after adjusting for race, sex, age, and sexual maturation rating stage (Table 3). However, IGF-I was a negative predictor of Ct.vBMD and a positive predictor of Endo.Circ in our total cohort and normal HOMA-IR group only (all p<0.05). The relationship between IGF-I and FFST, MCSA, Ct.vBMD, Tt.Ar, Ct.Ar, Peri.Circ, Endo.Circ, and pSSI was moderated by HOMA-IR (all pInteraction<0.05). After additional adjustment for FFST, IGF-I did not correlate with any of the cortical bone outcomes (data not shown).
Table 3.
Relationships between IGF-I and musculoskeletal outcomes while adjusting for covariates
| Total Cohort | High HOMA-IR | Normal HOMA-IR | pInteractiona | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| β | p | β | p | β | p | ||
| FFST | 0.431 | <0.001 | 0.286 | <0.001 | 0.510 | <0.001 | <0.001b |
| MCSA | 0.372 | <0.001 | 0.238 | 0.012 | 0.424 | <0.001 | <0.001b |
| Ct.vBMD | −0.136 | 0.025 | −0.048 | 0.581 | −0.251 | 0.002 | <0.001b |
| Ct.BMC | 0.301 | <0.001 | 0.255 | 0.001 | 0.326 | <0.001 | 0.097 |
| Tt.Ar | 0.292 | <0.001 | 0.211 | 0.020 | 0.337 | <0.001 | 0.001 |
| Ct.Ar | 0.332 | <0.001 | 0.279 | 0.001 | 0.367 | <0.001 | 0.019 |
| Ct.Th | 0.277 | <0.001 | 0.260 | 0.004 | 0.301 | <0.001 | 0.435 |
| Peri.Circ | 0.286 | <0.001 | 0.212 | 0.019 | 0.327 | <0.001 | 0.003 |
| Endo.Circ | 0.176 | 0.005 | 0.110 | 0.274 | 0.206 | 0.012 | 0.012 |
| pSSI | 0.322 | <0.001 | 0.248 | 0.003 | 0.362 | <0.001 | 0.002 |
Race, sex, age, and sexual maturation rating stage were included as covariates for all analyses
Represents the IGF-I x HOMA-IR interaction
IGF-I x group interaction p-value < 0.05
After adjusting for race, sex, age, and sexual maturation rating stage, FFST was a positive predictor of Ct.BMC, Tt.Ar, Ct.Ar, Ct.Th, Peri.Circ, Endo.Circ, and pSSI in each of our analyses (all p≤0.001, Table 4), but a negative predictor of Ct.vBMD in our total cohort and normal HOMA-IR group only (both p<0.005; pInteraction<0.005).
Table 4.
Relationships between FFST and cortical bone outcomes while adjusting for covariates
| Total Cohort | High HOMA-IR | Normal HOMA-IR | pInteractiona | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| β | p | β | p | β | p | ||
| Ct.vBMD | −0.195 | 0.002 | −0.061 | 0.496 | −0.280 | 0.002 | 0.001b |
| Ct.BMC | 0.690 | <0.001 | 0.639 | <0.001 | 0.771 | <0.001 | 0.838 |
| Tt.Ar | 0.747 | <0.001 | 0.783 | <0.001 | 0.747 | <0.001 | 0.727 |
| Ct.Ar | 0.736 | <0.001 | 0.675 | <0.001 | 0.811 | <0.001 | 0.219 |
| Ct.Th | 0.444 | <0.001 | 0.300 | 0.001 | 0.569 | <0.001 | 0.102 |
| Peri.Circ | 0.748 | <0.001 | 0.786 | <0.001 | 0.750 | <0.001 | 0.841 |
| Endo.Circ | 0.586 | <0.001 | 0.686 | <0.001 | 0.521 | <0.001 | 0.267b |
| pSSI | 0.750 | <0.001 | 0.758 | <0.001 | 0.785 | <0.001 | 0.552 |
Race, sex, age, and sexual maturation rating stage were included as covariates for all analyses
Represents the IGF-I x HOMA-IR interaction
IGF-I x group interaction p-value < 0.05
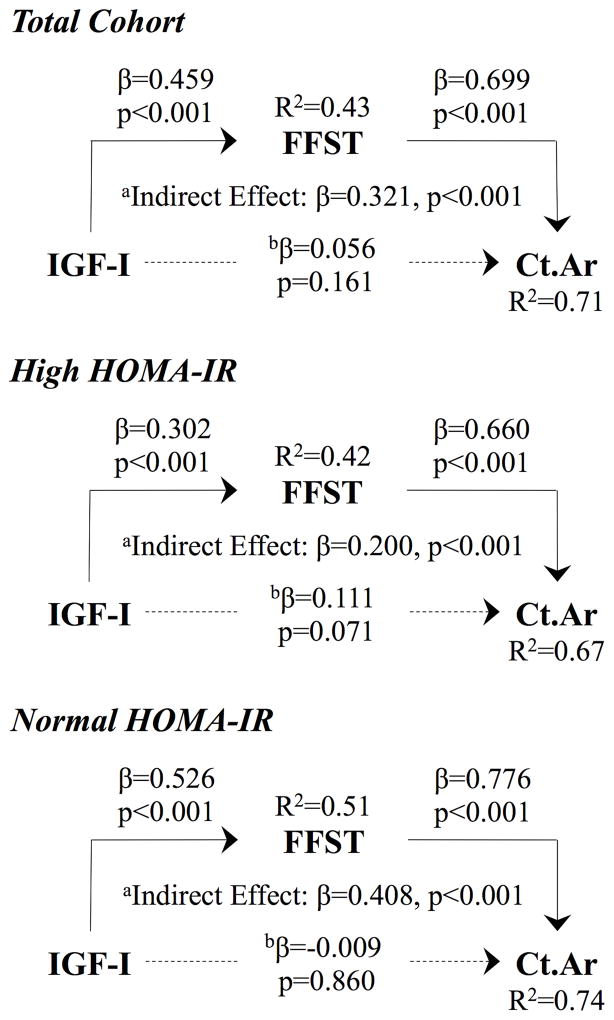
The path models presented in Figure 1 represent the FFST-dependent relationship between IGF-I and Ct.Ar while controlling for race, sex, and age. In each of our analyses, IGF-I was a positive predictor of FFST and FFST was a positive predictor of Ct.Ar (all p<0.001). IGF-I did not predict Ct.Ar in any of the path models after controlling for the mediator, FFST. The test for an indirect effect was significant in the total cohort, high HOMA-IR group, and normal HOMA-IR group (all p<0.001). However, this relationship was moderated in the children with high HOMA-IR. The explained variability of Ct.Ar was 7% greater in those with normal versus high HOMA-IR.
Figure 1.
IGF-I predicts Ct.Ar via FFST in the total cohort, high HOMA-IR group, and normal HOMA-IR group. However, this FFST-dependent relationship is moderated in the children with high HOMA-IR. aRelationship between IGF-I and Ct.Ar through FFST. bRelationship between IGF-I and Ct.Ar while controlling for the mediator (i.e., FFST). Broken lines represent nonsignificant relationships.
Relationships between HOMA-IR, IGF-I, and FFST with musculoskeletal outcomes while adjusting for covariates were tested in males versus females (Supplemental Table 1). HOMA-IR was positively associated with FFST (females and males) and MCSA (males), but negatively associated with Ct.BMC (females and males), Ct.Ar (males), and pSSI (males; all p<0.05). In both females and males, IGF-I was positively associated with FFST, MCSA, Ct.BMC, Tt.Ar, Ct.Ar, Ct.Th, Peri.Circ, and pSSI (all p<0.05). However, IGF-I correlated negatively with Ct.vBMD (p<0.05) and positively with Endo.Circ (p<0.005) in males only. The relationship between IGF-I and FFST, Ct.vBMD, Ct.BMC, and Ct.Ar was moderated in females (all pSex diff.<0.05). Whereas FFST was positively correlated with most cortical bone outcomes in females and males (all p<0.05), FFST was negatively correlated with Ct.vBMD in males only (p<0.05; pSex diff.<0.05).
DISCUSSION
The primary aim of this study was to examine the influence of insulin resistance, as measured by HOMA-IR, on the relationship between IGF-I and cortical bone in children. These data show that the relationship between IGF-I and cortical bone are moderated in children with higher insulin resistance. In addition, insulin resistance suppressed the prediction of FFST and MCSA from IGF-I. Consequently, the lean body mass-dependent relationship between IGF-I and cortical bone was moderated in the children with higher insulin resistance. Considering the role of IGF-I in promoting cortical bone areal expansion, insulin resistance-related cortical bone size and strength deficits may be attributed to mechanisms involving IGF-I.
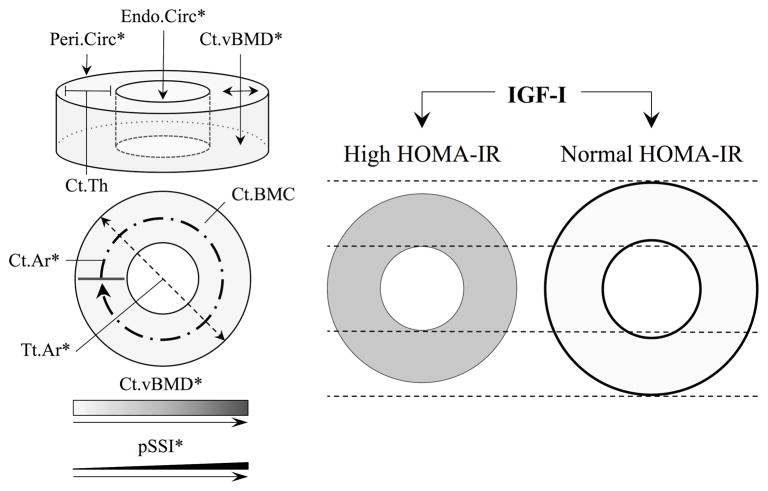
To date, this is the first study to examine the IGF-I-cortical bone relationship within the context of insulin resistance. The role of IGF-I in musculoskeletal development during adolescence has been well characterized.(9,11,34) With respect to cortical bone, Xu and colleagues(11) showed in a cohort of Finnish girls that IGF-I was an important determinant of skeletal development, specifically in relation to periosteal expansion and cortical bone mass accrual, over a period of seven years. Likewise, in the current study IGF-I was a positive predictor of various cortical bone size and strength outcomes. However, we also identified inconsistencies in these relationships between the children with high versus normal HOMA-IR. Specifically, insulin resistance blunted the strength of the relationship between IGF-I and Tt.Ar, Peri.Circ, and Ct.Ar. In a liver-specific IGF-I-deficient mouse model (i.e., the LID mouse), Yakar et al(14) showed reductions in femoral cortical bone area and strength compared to wild-type controls, yet tissue mineral density did not differ between the two. Indirectly, these data in the murine model help clarify the negative relationship between IGF-I and Ct.vBMD. One explanation is that these inverse associations are attributed to the IGF-I-related preferential deposition of bone mineral toward the periosteum, therefore occurring at the expense of the inner-cortex. Taken together, the suppressed relationship between IGF-I and pSSI in the children with higher insulin resistance was attributed to IGF-I-related deviations in cortical bone size, rather than volumetric density (Figure 2). Moreover, it is plausible that the lower Ct.BMC, Ct.Ar, and pSSI in those with higher HOMA-IR involve IGF-I-related mechanisms. These results are of concern given that cortical bone bending strength is highly dependent upon areal dimensions(35) and that the majority of skeletal fractures sustained by children and adolescents,(36–38) particularly those with excess adiposity,(4,39) occur at long-bone sites of predominantly cortical bone.
Figure 2.
Schematic depicting the differences in the IGF-I-cortical bone relationship in the children with high versus normal HOMA-IR. The strength of the relationship between IGF-I and Tt.Ar, Ct.Ar, and Peri.Circ, was suppressed in the children with high HOMA-IR. However, IGF-I was a negative predictor of Ct.vBMD (depicted by shading of the cortical compartment) and a positive predictor of Endo.Circ in the children with normal but not high HOMA-IR. Consequently, IGF-I was a stronger positive predictor of pSSI in those with normal versus high HOMA-IR (depicted by line thickness). *Significant IGF-I by HOMA-IR interaction (pInteraction<0.05) for the corresponding cortical bone outcome.
Accompanying the moderated IGF-I-cortical bone relationship, the children with high HOMA-IR also had lower FFST and MCSA relative to IGF-I. Lean body mass and MCSA are strong predictors of cortical bone areal measures(40–43) and are an integral link between IGF-I and bone.(10,11,42) Mouse(42) and human(10,11) studies have provided evidence supporting the facilitative role of lean body mass in the link between IGF-I and bone. In a previous cross-sectional study of premenarcheal girls,(10) our group showed an indirect relationship between IGF-I and total body bone mass via lean body mass. However, we also demonstrated that the IGF-I-lean body mass relationship was attenuated in the girls with higher insulin resistance (i.e., HOMA-IR > 4.0).(25) Likewise, in the current study, the relationship between IGF-I and Ct.Ar was FFST-dependent and was suppressed in the children with high versus normal HOMA-IR due to differences in the path from IGF-I to FFST. Between the two groups, we found an approximate 9% difference in explained variability of FFST in favor of those with normal HOMA-IR. If in fact IGF-I-dependent lean mass and skeletal muscle accrual is hampered in children with insulin resistance, this may, in turn, have a downstream influence on skeletal development considering that muscle and lean mass growth precede and contribute to bone accretion.(40,44) We have speculated previously that the insulin resistance-related suppression of the IGF-I-FFST-total body bone mass relationship was accompanied by corresponding deficits in cortical bone geometry.(10) The results of the current study are in support of this hypothesis.
Whereas the inevitable question, “Are children who are insulin resistant also IGF-I resistant?” remains unanswered, previous studies provide indirect evidence in support of this position. Insulin and IGF-I are similar in terms of structure, cellular target tissues (e.g., muscle and bone), and downstream signaling processes, specifically through the AKT/mTOR pathway.(18,19,45) As implied in the current study, lean body mass is a facilitator of the relationship between IGF-I and bone and is the primary site of insulin-mediated glucose uptake,(46) thus being most prone to fluctuations in insulin sensitivity. The bone-forming osteoblasts are also insulin-dependent and susceptible to impaired downstream signaling.(47) Factors that contribute to insulin resistance, such as chronic low-grade inflammation, compromise the myogenic and osteogenic effect of IGF-I.(48,49) Therefore, it is reasonable to suspect that the role of IGF-I in pediatric musculoskeletal development is altered in individuals with impaired glucose handling. Despite being tightly regulated throughout maturation, fasting serum glucose was higher in those with higher HOMA-IR. Hyperglycemia may lead to the non-enzymatic glycation of bone collagenous proteins and consequently the accumulation of advanced glycation end products.(50) In addition to being directly implicated in skeletal fragility,(51–53) advanced glycation end products may modulate osteoblast IGF-I function.(54,55) Further, insulin promotes hepatic IGF-I production,(56) likely contributing to the ~20% greater total IGF-I in the high HOMA-IR group. However, the majority of systemic IGF-I is bound to a variety of regulatory binding proteins. Due to alterations in IGF binding proteins, obese and/or hyperinsulinemic individuals may have a greater proportion of bioavailable relative to total IGF-I versus their healthier counterparts.(57) Therefore, we do not suspect that the insulin resistance-related musculoskeletal inadequacies reported in the current study were attributed to differences in total and/or bioavailable IGF-I.
When interpreting our findings, certain aspects of this study warrant consideration. First, making causal inferences based on our data would be inappropriate given the cross-sectional design. In addition, there are various unique attributes of pubertal maturation that are difficult to capture through cross-sectional analyses. For instance, cortical bone areal expansion and mineral acquisition lag behind longitudinal growth,(40,44) while fluctuations in insulin resistance occur normally during pubertal maturation.(6) Collecting prospective data throughout the adolescent years is an important next step in this line of inquiry. Moreover, consideration of sensitive measures of skeletal maturation will also enhance the understanding of whether or not excessive insulin resistance uncouples the coordinated process of musculoskeletal development. Second, we measured only total circulatory IGF-I concentrations and did not have data available on IGF binding proteins, so we can only speculate on differences in IGF-I bioavailability. Third, although HOMA-IR performs well against the oral glucose tolerance test in children,(58) including more dynamic measures of glucose metabolism would strengthen our methodological approach. Finally, whereas our sample size was sufficient to explore the intended research question, we were unable to perform analyses in groups stratified by race and sex. In accordance with one previous study,(8) relationships between insulin resistance and cortical bone did not differ between sexes. However, with respect to IGF-I, relationships with most musculoskeletal outcomes were stronger in the males versus females while adjusting for covariates including race, age, and maturation. Data pertaining to the sex-dependency of the IGF-I-bone relationship in humans are scarce, yet animal studies indicate that the growth hormone/IGF-I axis, along with sex steroids, contributes to the cortical bone sexual dimorphism.(15) Given that insulin resistance is greater in females versus males during maturation,(6) it is plausible that the influence of insulin resistance on IGF-I-dependent musculoskeletal development differs by sex. Data from our previous study indicate that insulin resistance moderates the relationship between IGF-I and musculoskeletal outcomes in females who were at the early stages of sexual maturation.(10) However, additional work is needed to corroborate these findings in males. Furthermore, we were not adequately powered to include additional control variables into our path models. The influence of insulin resistance on the relationships between IGF-I and musculoskeletal endpoints was evident whether or not sexual maturation was included as a covariate. Therefore, we do not suspect this omission to be problematic.
The unique strengths of this study include our utilization of path analysis statistical techniques for the testing of FFST as a mediator in the IGF-I-cortical bone relationship. Additionally, we included pQCT-derived measures of appendicular cortical bone geometry and strength, which addresses the most evident limitation of our previous work.(10)
Conclusions
This cross-sectional study corroborates the positive relationship between IGF-I and cortical bone size and strength outcomes in children, and we show for the first time that insulin resistance moderated these relationships. Given that lean body mass is an integral intermediary in the IGF-I-bone relationship and is prone to fluctuations in insulin resistance, our results may have been attributed to the suppressed lean body mass-dependent link between IGF-I and cortical bone. Future studies examining the role of IGF-I in pediatric musculoskeletal development within the context of insulin resistance should include measures of IGF-I bioavailability and prospective data collected throughout the adolescent years, specifically in children with obesity-related chronic health conditions. IGF-I is suspected to contribute to the sexual dimorphism observed in skeletal development.(15) Thus, whether insulin resistance influences the IGF-I-bone relationship differently in boys versus girls warrants exploration. Furthermore, biological factors associated with insulin resistance and hyperglycemia, for instance, biomarkers of inflammation and advanced glycation end products, also warrant consideration in subsequent studies. Since nearly one in four US children and adolescents is at risk of developing type-2 diabetes, a condition characterized by insulin resistance, it is a viable concern that IGF-I-dependent skeletal development is hampered in a relatively large subset of American youth.(59)
Supplementary Material
Supplemental Figure 1. The attenuated IGF-I-FFST-Ct.Ar relationship in the children with high HOMA-IR was attributed to the moderated path from IGF-I to FFST. Normal HOMA-IR and high HOMA-IR groups were coded as 0 and 1, respectively. aPrediction of FFST from the IGF-I by group interaction. bPrediction of Ct.Ar from the FFST by group interaction. cRelationship between IGF-I and Ct.Ar while controlling for the mediator (i.e., FFST). Broken lines represent nonsignificant relationships. Values are path coefficient, p-value.
Supplemental Table 1. Relationships between HOMA-IR, IGF-I, and FFST with musculoskeletal endpoints in males versus females while adjusting for covariates
Acknowledgments
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant RO1HD057126, by the Allen Foundation grant 2.008.319, and by the University of Georgia Agricultural Experiment Station, HATCH projects GEO00797 and GEO00700. ClinicalTrials.gov Identifier: NCT00931580.
We thank Ms. Jessica Smith, the staff and students at the University of Georgia, Purdue University, and Indiana University who were involved in this study, as well as the participants and their families for their commitment to this research.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Authors’ roles: Study design: JMK, EML, NKP, AO, RDL. Study conduct: All authors. Data collection: JMK, EML, RDL, CMI, KHD. Data interpretation: All authors. Drafting manuscript: JMK. Revising manuscript content: All authors. Approving final version of manuscript: All authors. JMK and RDL take responsibility for the integrity of the data analysis.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leonard MB, Zemel BS, Wrotniak BH, et al. Tibia and radius bone geometry and volumetric density in obese compared to non-obese adolescents. Bone. 2015;73:69–76. doi: 10.1016/j.bone.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wey HE, Binkley TL, Beare TM, Wey CL, Specker BL. Cross-sectional versus longitudinal associations of lean and fat mass with pQCT bone outcomes in children. J Clin Endocrinol Metab. 2011;96(1):106–14. doi: 10.1210/jc.2010-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler J, Koebnick C, Smith N, Adams A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin Orthop Relat Res. 2013;471(4):1199–207. doi: 10.1007/s11999-012-2621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor ED, Theim KR, Mirch MC, et al. Orthopedic complications of overweight in children and adolescents. Pediatrics. 2006;117(6):2167–74. doi: 10.1542/peds.2005-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–50. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 7.Afghani A, Cruz ML, Goran MI. Impaired glucose tolerance and bone mineral content in overweight latino children with a family history of type 2 diabetes. Diabetes Care. 2005;28(2):372–8. doi: 10.2337/diacare.28.2.372. [DOI] [PubMed] [Google Scholar]
- 8.Sayers A, Lawlor DA, Sattar N, Tobias JH. The association between insulin levels and cortical bone: findings from a cross-sectional analysis of pQCT parameters in adolescents. J Bone Miner Res. 2012;27(3):610–8. doi: 10.1002/jbmr.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breen ME, Laing EM, Hall DB, et al. 25-hydroxyvitamin D, insulin-like growth factor-I, and bone mineral accrual during growth. J Clin Endocrinol Metab. 2011;96(1):E89–98. doi: 10.1210/jc.2010-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kindler JM, Pollock NK, Laing EM, et al. Insulin Resistance Negatively Influences the Muscle-Dependent IGF-1-Bone Mass Relationship in Premenarcheal Girls. J Clin Endocrinol Metab. 2016;101(1):199–205. doi: 10.1210/jc.2015-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Wang Q, Wang Q, et al. Concerted actions of insulin-like growth factor 1, testosterone, and estradiol on peripubertal bone growth: a 7-year longitudinal study. J Bone Miner Res. 2011;26(9):2204–11. doi: 10.1002/jbmr.422. [DOI] [PubMed] [Google Scholar]
- 12.Cao JJ, Kurimoto P, Boudignon B, Rosen C, Lima F, Halloran BP. Aging impairs IGF-I receptor activation and induces skeletal resistance to IGF-I. J Bone Miner Res. 2007;22(8):1271–9. doi: 10.1359/jbmr.070506. [DOI] [PubMed] [Google Scholar]
- 13.Sakata T, Wang Y, Halloran BP, Elalieh HZ, Cao J, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J Bone Miner Res. 2004;19(3):436–46. doi: 10.1359/JBMR.0301241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yakar S, Canalis E, Sun H, et al. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J Bone Miner Res. 2009;24(8):1481–92. doi: 10.1359/JBMR.090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callewaert F, Venken K, Kopchick JJ, et al. Sexual dimorphism in cortical bone size and strength but not density is determined by independent and time-specific actions of sex steroids and IGF-1: evidence from pubertal mouse models. J Bone Miner Res. 2010;25(3):617–26. doi: 10.1359/jbmr.090828. [DOI] [PubMed] [Google Scholar]
- 16.Courtland HW, Elis S, Wu Y, et al. Serum IGF-1 affects skeletal acquisition in a temporal and compartment-specific manner. PLoS One. 2011;6(3):e14762. doi: 10.1371/journal.pone.0014762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Shen X, Wan C, et al. Effects of insulin and insulin-like growth factor 1 on osteoblast proliferation and differentiation: differential signalling via Akt and ERK. Cell Biochem Funct. 2012;30(4):297–302. doi: 10.1002/cbf.2801. [DOI] [PubMed] [Google Scholar]
- 18.Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol. 2010;167(3):344–51. doi: 10.1016/j.ygcen.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Entingh-Pearsall A, Kahn CR. Differential roles of the insulin and insulin-like growth factor-I (IGF-I) receptors in response to insulin and IGF-I. J Biol Chem. 2004;279(36):38016–24. doi: 10.1074/jbc.M313201200. [DOI] [PubMed] [Google Scholar]
- 20.Lewis RD, Laing EM, Hill Gallant KM, et al. A randomized trial of vitamin D(3) supplementation in children: dose-response effects on vitamin D metabolites and calcium absorption. J Clin Endocrinol Metab. 2013;98(12):4816–25. doi: 10.1210/jc.2013-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warden SJ, Hill KM, Ferira AJ, et al. Racial differences in cortical bone and their relationship to biochemical variables in Black and White children in the early stages of puberty. Osteoporos Int. 2013;24(6):1869–79. doi: 10.1007/s00198-012-2174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanner J. Growth and adolescence. 2. Oxford, UK: Blackwell Scientific Publications; 1962. [Google Scholar]
- 23.Laing EM, Wilson AR, Modlesky CM, O’Connor PJ, Hall DB, Lewis RD. Initial years of recreational artistic gymnastics training improves lumbar spine bone mineral accrual in 4- to 8-year-old females. J Bone Miner Res. 2005;20(3):509–19. doi: 10.1359/JBMR.041127. [DOI] [PubMed] [Google Scholar]
- 24.Neinstein LS. Adolescent self-assessment of sexual maturation: reassessment and evaluation in a mixed ethnic urban population. Clin Pediatr (Phila) 1982;21(8):482–4. doi: 10.1177/000992288202100806. [DOI] [PubMed] [Google Scholar]
- 25.Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89(5):419–22. doi: 10.1136/adc.2003.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 27.Ferira AJ, Laing EM, Hausman DB, et al. Vitamin D Supplementation Does Not Impact Insulin Resistance in Black and White Children. J Clin Endocrinol Metab. 2016;101(4):1710–8. doi: 10.1210/jc.2015-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdonald H, Kontulainen S, Petit M, Janssen P, McKay H. Bone strength and its determinants in pre- and early pubertal boys and girls. Bone. 2006;39(3):598–608. doi: 10.1016/j.bone.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 29.Rauch F, Schoenau E. Peripheral quantitative computed tomography of the proximal radius in young subjects--new reference data and interpretation of results. J Musculoskelet Neuronal Interact. 2008;8(3):217–26. [PubMed] [Google Scholar]
- 30.Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr. 2007;86(5):1530–8. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Hayes AF. An Index and Test of Linear Moderated Mediation. Multivariate Behav Res. 2015;50(1):1–22. doi: 10.1080/00273171.2014.962683. [DOI] [PubMed] [Google Scholar]
- 33.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 34.Kirmani S, Christen D, van Lenthe GH, et al. Bone structure at the distal radius during adolescent growth. J Bone Miner Res. 2009;24(6):1033–42. doi: 10.1359/JBMR.081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonseca H, Moreira-Goncalves D, Coriolano HJ, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44(1):37–53. doi: 10.1007/s40279-013-0100-7. [DOI] [PubMed] [Google Scholar]
- 36.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP. Epidemiology of childhood fractures in Britain: a study using the general practice research database. J Bone Miner Res. 2004;19(12):1976–81. doi: 10.1359/JBMR.040902. [DOI] [PubMed] [Google Scholar]
- 37.Lyons RA, Delahunty AM, Kraus D, et al. Children’s fractures: a population based study. Inj Prev. 1999;5(2):129–32. doi: 10.1136/ip.5.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayranpaa MK, Makitie O, Kallio PE. Decreasing incidence and changing pattern of childhood fractures: A population-based study. J Bone Miner Res. 2010;25(12):2752–9. doi: 10.1002/jbmr.155. [DOI] [PubMed] [Google Scholar]
- 39.Adams AL, Kessler JI, Deramerian K, et al. Associations between childhood obesity and upper and lower extremity injuries. Inj Prev. 2013;19(3):191–7. doi: 10.1136/injuryprev-2012-040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu L, Nicholson P, Wang Q, Alen M, Cheng S. Bone and muscle development during puberty in girls: a seven-year longitudinal study. J Bone Miner Res. 2009;24(10):1693–8. doi: 10.1359/jbmr.090405. [DOI] [PubMed] [Google Scholar]
- 41.Kindler JM, Lewis RD, Hamrick MW. Skeletal muscle and pediatric bone development. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):467–74. doi: 10.1097/MED.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 42.Banu J, Wang L, Kalu DN. Effects of increased muscle mass on bone in male mice overexpressing IGF-I in skeletal muscles. Calcif Tissue Int. 2003;73(2):196–201. doi: 10.1007/s00223-002-1072-z. [DOI] [PubMed] [Google Scholar]
- 43.Garnett SP, Hogler W, Blades B, et al. Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr. 2004;80(4):966–72. doi: 10.1093/ajcn/80.4.966. [DOI] [PubMed] [Google Scholar]
- 44.Jackowski SA, Faulkner RA, Farthing JP, Kontulainen SA, Beck TJ, Baxter-Jones AD. Peak lean tissue mass accrual precedes changes in bone strength indices at the proximal femur during the pubertal growth spurt. Bone. 2009;44(6):1186–90. doi: 10.1016/j.bone.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253(8):2769–76. [PubMed] [Google Scholar]
- 46.O’Neill BT, Lauritzen HP, Hirshman MF, Smyth G, Goodyear LJ, Kahn CR. Differential Role of Insulin/IGF-1 Receptor Signaling in Muscle Growth and Glucose Homeostasis. Cell Rep. 2015;11(8):1220–35. doi: 10.1016/j.celrep.2015.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pramojanee SN, Phimphilai M, Chattipakorn N, Chattipakorn SC. Possible roles of insulin signaling in osteoblasts. Endocr Res. 2014;39(4):144–51. doi: 10.3109/07435800.2013.879168. [DOI] [PubMed] [Google Scholar]
- 48.Choukair D, Hugel U, Sander A, Uhlmann L, Tonshoff B. Inhibition of IGF-I-related intracellular signaling pathways by proinflammatory cytokines in growth plate chondrocytes. Pediatr Res. 2014;76(3):245–51. doi: 10.1038/pr.2014.84. [DOI] [PubMed] [Google Scholar]
- 49.Broussard SR, McCusker RH, Novakofski JE, et al. IL-1beta impairs insulin-like growth factor i-induced differentiation and downstream activation signals of the insulin-like growth factor i receptor in myoblasts. J Immunol. 2004;172(12):7713–20. doi: 10.4049/jimmunol.172.12.7713. [DOI] [PubMed] [Google Scholar]
- 50.Vashishth D. The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep. 2007;5(2):62–6. doi: 10.1007/s11914-007-0004-2. [DOI] [PubMed] [Google Scholar]
- 51.Viguet-Carrin S, Roux JP, Arlot ME, et al. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006;39(5):1073–9. doi: 10.1016/j.bone.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 52.Saito M, Kida Y, Kato S, Marumo K. Diabetes, collagen, and bone quality. Curr Osteoporos Rep. 2014;12(2):181–8. doi: 10.1007/s11914-014-0202-7. [DOI] [PubMed] [Google Scholar]
- 53.Furst JR, Bandeira LC, Fan WW, et al. Advanced Glycation Endproducts and Bone Material Strength in Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101(6):2502–10. doi: 10.1210/jc.2016-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCarthy AD, Etcheverry SB, Cortizo AM. Effect of advanced glycation endproducts on the secretion of insulin-like growth factor-I and its binding proteins: role in osteoblast development. Acta Diabetol. 2001;38(3):113–22. doi: 10.1007/s005920170007. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi T. Bone fragility in type 2 diabetes mellitus. World J Orthop. 2010;1(1):3–9. doi: 10.5312/wjo.v1.i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frystyk J, Hussain M, Skjaerbaek C, et al. Serum free IGF-I during a hyperinsulinemic clamp following 3 days of administration of IGF-I vs. saline. Am J Physiol. 1997;273(3 Pt 1):E507–13. doi: 10.1152/ajpendo.1997.273.3.E507. [DOI] [PubMed] [Google Scholar]
- 57.Nam SY, Lee EJ, Kim KR, et al. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21(5):355–9. doi: 10.1038/sj.ijo.0800412. [DOI] [PubMed] [Google Scholar]
- 58.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):e500–3. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 59.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129(6):1035–41. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The attenuated IGF-I-FFST-Ct.Ar relationship in the children with high HOMA-IR was attributed to the moderated path from IGF-I to FFST. Normal HOMA-IR and high HOMA-IR groups were coded as 0 and 1, respectively. aPrediction of FFST from the IGF-I by group interaction. bPrediction of Ct.Ar from the FFST by group interaction. cRelationship between IGF-I and Ct.Ar while controlling for the mediator (i.e., FFST). Broken lines represent nonsignificant relationships. Values are path coefficient, p-value.
Supplemental Table 1. Relationships between HOMA-IR, IGF-I, and FFST with musculoskeletal endpoints in males versus females while adjusting for covariates