Abstract
In our studies of life-supporting GalT-KO pig-to-baboon kidneys, we have found that some recipients developed increased serum creatinine with growth of the grafts, without histologic or immunologic evidence of rejection. We hypothesized that the rapid growth of orthotopic pig grafts in smaller baboon recipients may have led to deterioration of organ function. To test this hypothesis for both kidneys and lungs, we have assessed whether the growth of outbred (Yorkshire) organ transplants in miniature swine is regulated by intrinsic (graft) or extrinsic (host environment) factors. Yorkshire kidneys exhibited persistent growth in miniature swine, reaching 3.7x their initial volume over 3 months vs. 1.2x for miniature swine kidneys over the same time period. Similar rapid early growth of lung allografts was observed and, in this case, led to organ dysfunction. For xenograft kidneys, a review of our results suggests that there is a threshold of 25cm3/kg for kidney graft volume/recipient body weight that induces cortical ischemia in transplanted GalT-KO kidneys in baboons. These results suggest that intrinsic factors are responsible, at least in part, for growth of donor organs and that this property should be taken into consideration for growth-curve mismatched transplants, especially for life-supporting organs transplanted into a limited recipient space.
INTRODUCTION
Organ shortage remains one of the primary obstacles in clinical transplantation (Tx) (1). As the incidence of end-stage organ disease increases, the consequent need for transplantable organs also increases, and there is a vast discrepancy between the number of patients on waiting lists and the number of available donor organs. Given this shortage, xenotransplantation (XenoTx), offers the potential benefit of an inexhaustible supply of organs. Pigs will likely be the ideal donors for xenoTx, as they are physiologically and anatomically similar to humans (2) and amenable to genetic modification. Recent improvements in genetic engineering using CRISPR/CAS9 technology have markedly increased the efficiency of multiple gene manipulations, making the development of new lines of genetically modified (GM) swine far easier than previously thought (3, 4). The use of new multi-transgenic GM pigs has led to marked prolongation of porcine xenograft survival, including the recently reported survival of CD46/hTBM GalT-KO heterotopic pig-to-baboon heart transplant for over 2 years (5).
In an effort to bring xenoTx to clinical feasibility, our lab has been testing a strategy to induce tolerance in a pig-to-baboon model, through co-transplantation of vascularized porcine thymus. With this strategy we have previously reported 83-day survival of a GalT-KO pig thymokidney (TK) transplanted into a baboon (6). The recipient baboon maintained stable renal function, as evidenced by a normal creatinine (Cre) level, and without histological evidence of rejection. More recently, with a modified immunosuppressive regimen (Yamada et al., manuscript in preparation), we have improved survival greater than 6 months and have increased the average survival to >125 days. However, on analyzing the clinical course and histological findings in recipient baboons, we have found that some recipients that had maintained a normal Cre for the first 2 months post-transplant, gradually experienced an increase in Cre without exhibiting evidence of rejection as measured either by elicited anti-pig antibodies or T-cell sensitization. Histologic examination of their grafts demonstrated cortical ischemia without vasculitis, despite all anastomoses being patent. These data led us to hypothesize that the rapid growth of orthotopic pig grafts might have led to deterioration of organ function due to compartment syndrome, causing insufficient intra-graft blood flow and cortical necrosis.
To further test this hypothesis, we have now examined: 1) the apparent threshold volume that induces cortical ischemia in transplanted life-supporting GalT-KO kidneys in baboons; and 2) the growth of outbred, Yorkshire swine-to-miniature swine kidney and lung transplants to determine whether growth of these organs is regulated by intrinsic (graft) or extrinsic (host environment) factors. Although pig organs grow at a much faster rate those of primates (7), the latter experiment attempted to mimic the size and growth situation for pig-to-primate xenografts by using domestic, Yorkshire swine, which reach body weights of over 100kg by 6 months (8) as donors, and miniature swine, which reach only 40kg at 6 months, as recipients.
MATERIALS AND METHODS
Details are described in Supplemental document
Animals
MHC-defined inbred miniature swine (9, 10) were used as recipients of allogeneic kidney (n=5) or lung (n=2) Tx. Donor organs were taken from two miniature swine and five Yorkshire swine. For xenoTx studies, inbred GalT-KO miniature swine without other gene modification were used as donors (6, 11) and baboons (Papio hamadryas. Mannheimer Foundation, Homestead, FL) were used as recipients.
Allogeneic Kidney Transplantation
Five miniature swine received allogeneic kidneys; three were from size-matched Yorkshire pig donors (experimental) and two were from size-matched miniature swine (control). In order to minimize potential pharmacological effects of immunosuppressive drugs in experimental animals, a tolerance-inducing regimen consisting of 12 or 28 days of continuous intravenous FK506 was used, as described previously for induction of tolerance to allogeneic kidneys and hearts in miniature swine (12, 13). Surgical methods for allogeneic pig kidney transplantation have been described in detail previously (14).
Xenogeneic Thymokidney (TK) Transplantation
Surgical methods for pig-to-baboon kidney xenoTx have also been described in detail previously (6). Two baboons were treated with an anti-CD154 plus Rituximab based regimen, as described previously (15). The other three animals received a modified regimen that included CTLA4-Ig (20mg/kg on Day 7 or 9 and weekly thereafter at 10mg/kg) and conversion from MMF to rapamycin IM (0.1mg/kg) (16) that began 3–5 weeks after Tx (Yamada et al., manuscript in preparation).
Allogeneic Lung Transplantation
Three miniature swine received size-matched lungs from Yorkshire pigs. Orthotopic left lung Tx was performed as previously described (17, 18).
Histology
Kidney and lung biopsy specimens were examined and graded by an experienced pathologist.
Measurement of Kidney Size following Transplantation
Biweekly renal ultrasound examinations were performed to monitor allograft volume changes. Kidney volumes were determined from the maximum measured length, width and depth, using the formula:(length x width x depth x π/6) - the volume for an ellipsoid (19).
Lung Allograft Assessment
Lung allografts were assessed by physical examination, including auscultation of respiratory sounds and by chest X-ray. Following euthanasia, the grafts were explanted and the size and weight were obtained.
ELISPOT assay
Baboon cytokine ELISPOT assays were performed to detect interferon gamma (IFN-γ) after Tx as previously reported (20).
Mixed Lymphocyte Reaction (MLR)
MLR assays were performed as previously reported (21, 22) with minor modifications.
RESULTS
1. Effects of renal growth following life-supporting GalT-KO pig-to-baboon kidney transplantation
We analyzed six baboons that received life-supporting porcine TKs using an anti-CD154 mAb induction regimen (15) in this study (Table 1). TK volumes were measured at day of TK Tx and every 2–3 weeks post Tx in baboon #1 and baboon #2. These recipients received TKs from a swine donor that had two TKs of very disparate size into recipient baboons of similar size (male, 6.5kg and 7.2kg, respectively). The first animal (baboon #1) received a 100cm3 TK, while the second recipient (baboon #2) received a 31cm3 TK. Both baboons maintained stable renal function during the first 6 weeks (both 1.4mg/dL at POD40). These size differences in the donors are likely congenital, but both the smaller and larger kidneys functioned normally in the first 6 weeks, indicating that both kidneys were functionally normal. However, Cre levels in the first recipient began to increase gradually at seven weeks following TKTx, and were 3.5mg/dL at 8 weeks and 8.4mg/dL at 12 weeks, at which time the animal was euthanized due to uremia. In contrast, the second recipient maintained stable renal function throughout the post-operative period (Cre 1.9mg/dL at POD89), until it was euthanized on POD93, due to common iliac arterial and venous thrombosis related to the indwelling central line. Histology did not demonstrate any evidence of rejection or interstitial edema (Fig. 1A).
Table 1.
Experimental animals in a pig-to-baboon thymokidney model
| Combination | Recipient | Body Weight (kg) | Donor | Body Weight (kg) | Age (weeks) |
|
|---|---|---|---|---|---|---|
| # 1 | GalKO pig to Baboon | B393 | 7.2 | 22814 | 13.8 | 16 |
| # 2 | GalKO pig to Baboon | B394 | 6.5 | 22814 | 13.8 | 16 |
| # 3 | GalKO pig to Baboon | 14P5 | 6.5 | 23542 | 9.0 | 12 |
| # 4 | GalKO pig to Baboon | 13P28 | 5.8 | 23320 | 15.3 | 17 |
| # 5 | GalKO pig to Baboon | B374 | 6.4 | 22468 | 10.0 | 17 |
| # 6 | GalKO pig to Baboon | 11P80 | 9.8 | 23340 | 15.3 | 17 |
Figure 1.
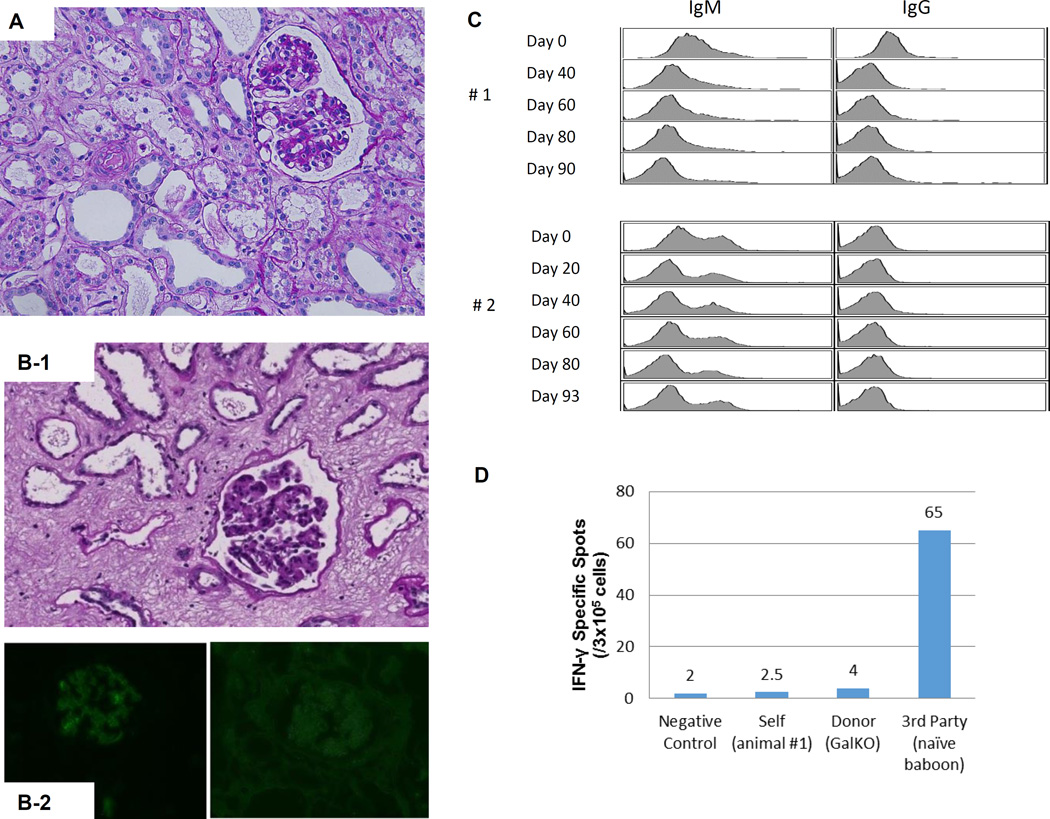
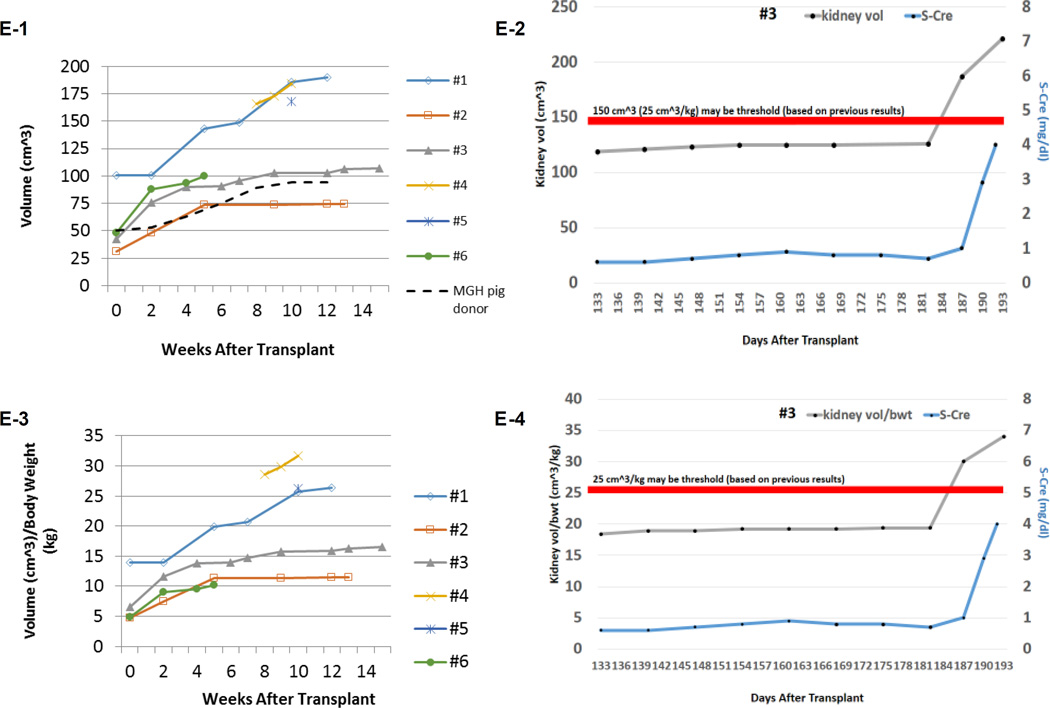
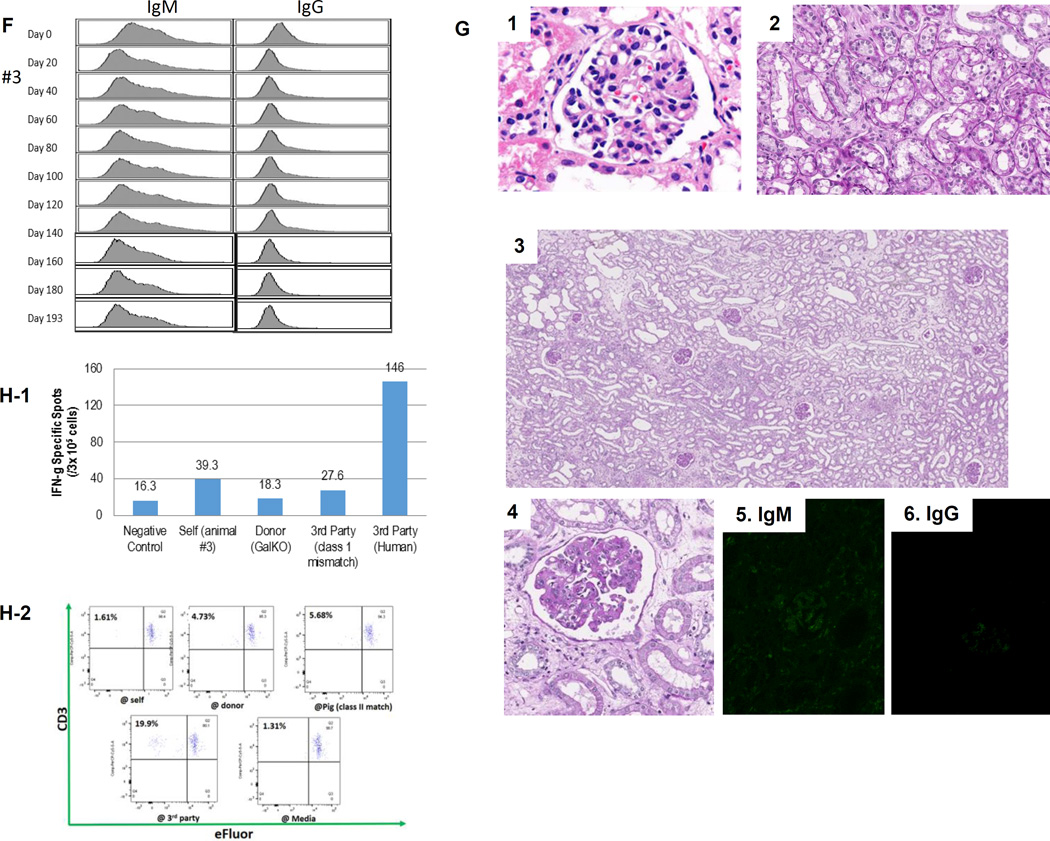
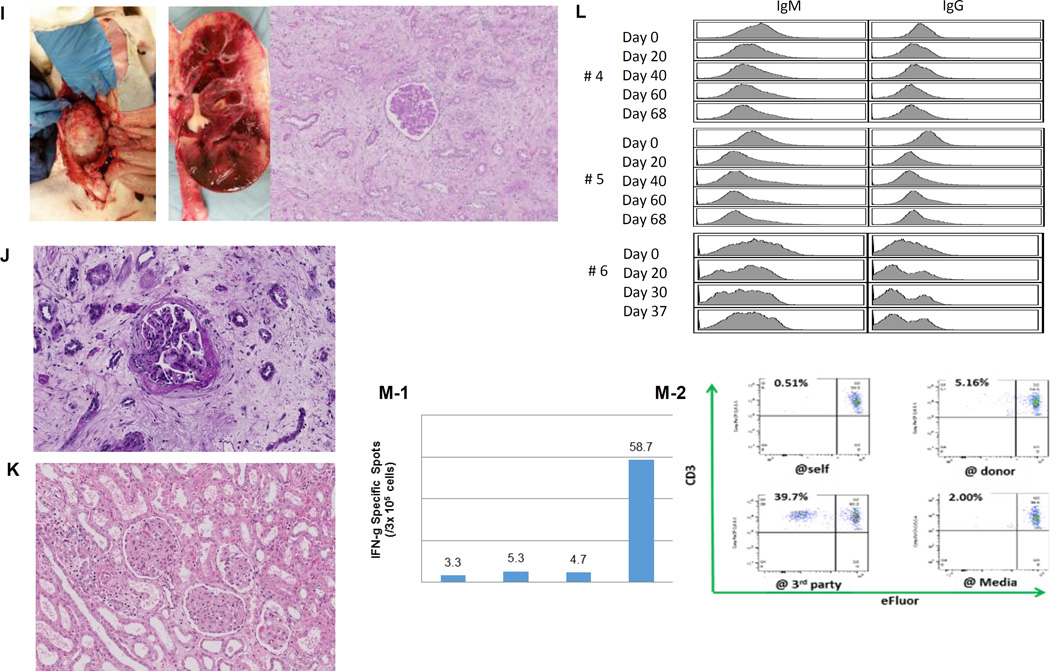
(A) Periodic acid-Schiff (PAS) staining of a kidney graft for baboon #2 on day 93. No sign of rejection or interstitial fibrosis. (B-1) PAS staining of a kidney graft for baboon #1 on day 90 showed interstitial fibrosis and tubular atrophy but no evidence of glomerulitis, peritubular capillaritis or a mononuclear cell infiltration. (B-2) Immunofluorescence staining for baboon #1 on POD 90. IgM staining showed mild mesangial deposition (left) while IgG deposition wasn’t seen (right). (C) Anti-non-Gal natural antibody for baboons #1 and #2 assessed by flow-cytometry demonstrating neither elicit anti-pig IgM nor IgG developed.(D) ELISPOT assay for IFN-γ at the time of euthanasia (POD 90) for baboon #1. Many spots could be seen in the wells stimulated by 3rd party while only a small number of spots against GalTKO pig cells similar to self were seen. (E-1) Kidney volume (cm3) of all xenoTK Tx recipients against weeks after Tx (solid lines). The growth of the TKs (solid lines) was similar to what was observed in a remaining kidney of a miniature swine donor who was kept alive after kidney donation in order to measure autologous kidney growth (dashed line).(E-2) Kidney volume and serum Cre levels between 19 and 27 weeks after Tx for baboon #3. (E-3) Ratio of kidney volume (cm3) / recipient’s body weight (kg) against weeks after Tx. (E-4) Ratio of kidney volume (cm3) / recipient’s body weight (kg) and serum Cre levels for baboon #3 between 19 and 27 weeks after Tx.(F) Anti-non-Gal natural antibody for baboon #3 assessed by flow-cytometry demonstrating neither elicit anti-pig IgM nor IgG developed.(G-1 and 2) The graft biopsy at POD 121 from baboon #3 showed no interstitial edema and normal appearance of glomeruli. (G-3 and 4) Biopsy at POD 193 from baboon #3 showed well preserved glomeruli with no glomerulitis, no endoarteritis, and no apparent interstitial cell infiltration. However, widening of interstitial area was noted with interstitial edema. (G-5 and 6) Neither IgM nor IgG deposition was observed in the excised grafts from baboon #3. (H-1 and 2) ELISPOT assay for IFN-γ and MLR at the time of euthanasia for baboon #3 demonstrated that animal was anti-pig specific unresponsive. (I) Necropsy sample of kidney graft (left) and PAS staining of kidney graft for baboon #4 on POD 68 (right). Glomerular capillaries were relatively preserved with diffuse tubular necrosis and absence of thrombi in the small interlobular arteries. (J) PAS staining of a kidney graft for baboon #5 on day 68 showed interstitial fibrosis and tubular atrophy but glomerular capillaries were relatively preserved. (K) Kidney graft for baboon #6 on day 37 showed no sign of rejection. (L) Anti-non-Gal natural antibody for baboon #4, #5 and #6 assessed by FACS demonstrating neither elicit anti-pig IgM nor IgG developed. (M-1) INF-γ ELISPOT assay for baboon #4 at the end of study (POD68). (M-2) MLR for baboon #4 on day 59.
Although Cre levels in the first recipient began to rise seven weeks post-transplant, there were no histologic or immunologic changes to suggest cellular or humoral sensitization. Histological findings revealed interstitial edema with tubular atrophy but without evidence of endothelialitis or a mononuclear cell infiltration (Fig. 1B-1). Immunofluorescence staining for IgM showed mild mesangial deposition while IgG deposition was not seen (Fig. 1B-2). No anti-pig elicited antibodies developed after TK Tx (Fig 1C). An IFN-γ ELISPOT assay at the time of euthanasia demonstrated strong positivity to allogeneic cells and only minimal reactivity to GalT-KO cells, similar to the anti-self response (Fig. 1D).
The most striking finding was the growth of the thymokidney grafts at 12 weeks following TK Tx, with volume increasing from 100 cm3 to 190 cm3 in the first recipient and from 31cm3 to 74cm3 in the second (Fig. 1E-1. blue and red lines). Both porcine TKs grew about two-fold (1.9x in #1 and 2.4x in #2) over the first 3 months in baboons. The growth of the TKs was similar to what was observed in a remaining kidney of a miniature swine donor who was kept alive after kidney donation in order to measure autologous kidney growth (1.9x) (Fig. 1-E. dashed line). Since animal #2 received a smaller TK from the donor, the right abdominal space was not fully occupied by the graft. In contrast, we found that the TK graft in animal #1 was packed into the lateral side of the right abdominal space.
More recently, we repeated this experiment with the same induction regimen. The recipient (#3) was 6.5kg on Day 0 which is the same size as #1 and #2 baboons, but it received a smaller TK graft (42cm3) than TK for animal #1, similar to TK for animal #2. Cre levels were stable without any small increase in Cre for 6 months. However, Cre slowly increased after POD 180 (0.7mg/dL at POD 182 and 1.0 mg\dL at POD 187) and it reached 4 mg/dL at POD 193. Since data on animals #1 and #2 suggested that its graft size exceeded the limiting size, determined from the excised graft of animal #1 (Fig. 1E-1) since flow-cytometric analysis of IgM and IgG anti-pig antibodies indicated no development of circulating anti-pig elicited Ab (Fig. 1F), we euthanized animal #3 at POD 193, despite the fact that its urinary output remained approximately 1mL/kg/hour (150mL/day). The kidney biopsy at POD 121 showed no interstitial edema and normal appearance of glomeruli and tubules (Fig. 1G-1 and 2). Histology of the excised TK at POD 193 showed well preserved glomeruli and small arteries with no glomerulitis, endothelialitis or vasculopathy (Fig. 1G-3). However, ischemic tubular injuries were seen with dilatation of some renal tubules. Widening of the interstitial areas was noted with interstitial edema where minimal mononuclear and neutrophil infiltrate was seen. (Fig. 1G-4). I. Taken together, these tubular and interstitial alterations suggested a pathologic diagnosis of cortical ischemia. The glomerular capillaries and vessels had no evidence of rejection (Fig. 1G-3 and -4) and neither IgM nor IgG deposition was observed in the excised graft (Fig. 1G-5 and 6). Furthermore, cellular assays at POD 193 demonstrated that animal #3 was specifically unresponsive to pig in MLR, a likely measure of the anti-pig Class II response, and Elispot IFN-γ, assessing memory CD8 response against pig Ag (Fig. 1H-1 and -2).
The graft volume curve was similar to the graft volume curve for animal #2, 42cm3 (Day 0), 75.7cm3 (2 weeks), 90.0cm3 (4 weeks), 96.4cm3 (8 weeks), 103.0cm3 (12 weeks) and 117.6cm3 (18 weeks) (Fig, 1E1 #3). As Figure 1E-2 demonstrates, the graft volume for animal #3 slowly increased until 26 weeks after TK transplantation and eventually reached close to 25cm3/kg. Similar to #1, the graft size increased more obviously thereafter without any evidence of immunologic sensitization, as described above.
Given the findings in these three cases, we performed a retrospective analysis of 3 previous cases, to determine whether there was a threshold volume beyond which additional growth of the kidney graft might compromise renal function. TK graft volumes had been measured at 8, 9 and 10 weeks following TKTx in baboon #4, at the time of euthanasia at 10 weeks in baboon #5, and at the time of TKTx and biweekly thereafter in baboon #6. These 3 recipients had also maintained stable renal function for 6 weeks following Tx (Cre level <2.0mg/dL). The 4th recipient experienced an increase in Cre level starting on POD 60 (2.1mg/dL) and was euthanized due to renal failure on POD 68 (8.3mg/dL). Histology of the TK graft in this recipient demonstrated diffuse cortical necrosis without arterial or venous thrombosis and glomerular capillaries were relatively preserved (Fig, 1I, right panel). Cre levels in animal #5 began to increase on POD 45 and plateau at 10.3mg/dL on POD 68. Histology revealed interstitial fibrosis and tubular atrophy but glomerular capillaries were relatively preserved, suggesting cortical ischemic change (Fig. 1J). In contrast, animal #6 maintained stable renal function throughout the experimental period (1.4 mg/dL) and histology did not demonstrate any evidence of rejection (Fig. 1K). None of these three recipients developed an IgM or IgG anti-pig antibody response (Fig. 1L). Figure 1-M-1 shows an interferon IFN-γ ELISPOT assay at the time of euthanasia of baboon #4 on POD 68, demonstrating only minimal reactivity with GalT-KO pig cells, similar to the response to self, while strong reactivity (i.e. multiple spots) was observed to allogeneic cells. The MLR also demonstrated anti-pig specific hyporesponsiveness (Fig. 1M-2). Notably, kidney volumes were increased at the time of euthanasia in both recipients with elevated Cre (baboons #4 and #5) when compared to those on the day of transplantation. Baboon #4 had a graft that was 184cm3, while baboon #5’s graft was 167cm3. In comparison, the kidney volume for baboon #6 who maintained stable renal function, was only 100.2cm3. During necropsy, xenografts in baboons #4 and #5 were occupied almost the entire right abdomen (Fig. 1I left panel).
Such graft dysfunction has been observed in compartment syndrome (23, 24). Therefore, in order to compare graft growth versus host growth, we took growth curves of hosts into account for this analysis (Growth Index, cm3/kg) for all six recipients of GalT-KO TK grafts. Notably, recipients maintained stable Cre (baboon #1, #2, #3, #6) while the Growth Indices were less than 25cm3/kg, whereas the Growth Indices were >25cm3/kg for all recipients who succumbed due to an elevated Cre and histologic evidence of cortical ischemia (baboons #4, #5, and #1 - after week 10) (Fig. 1E-3). The Growth Index for animal #3 increased slightly between 18 weeks and 26 weeks after transplant. After the Growth Index reached 25 cm3 at 26 weeks, the Cr increased to 4 mg/dl (Fig. 1E-4).
2. Examine whether growth of outbred organs is host dependent (natural growth) or recipient dependent
Given our experience with the xenogeneic Tx as described above, and in order to confirm whether intrinsic factors within an organ promote growth, we established an allogeneic model of both kidney and lung transplantation using donor organs from outbred Yorkshire swine into miniature swine recipients, the rationale for which relied on the different growth patterns of the two breeds.
A. Kidneys
Yorkshire kidney grafts grew rapidly in miniature swine, reaching >12 times their original size by 8 months
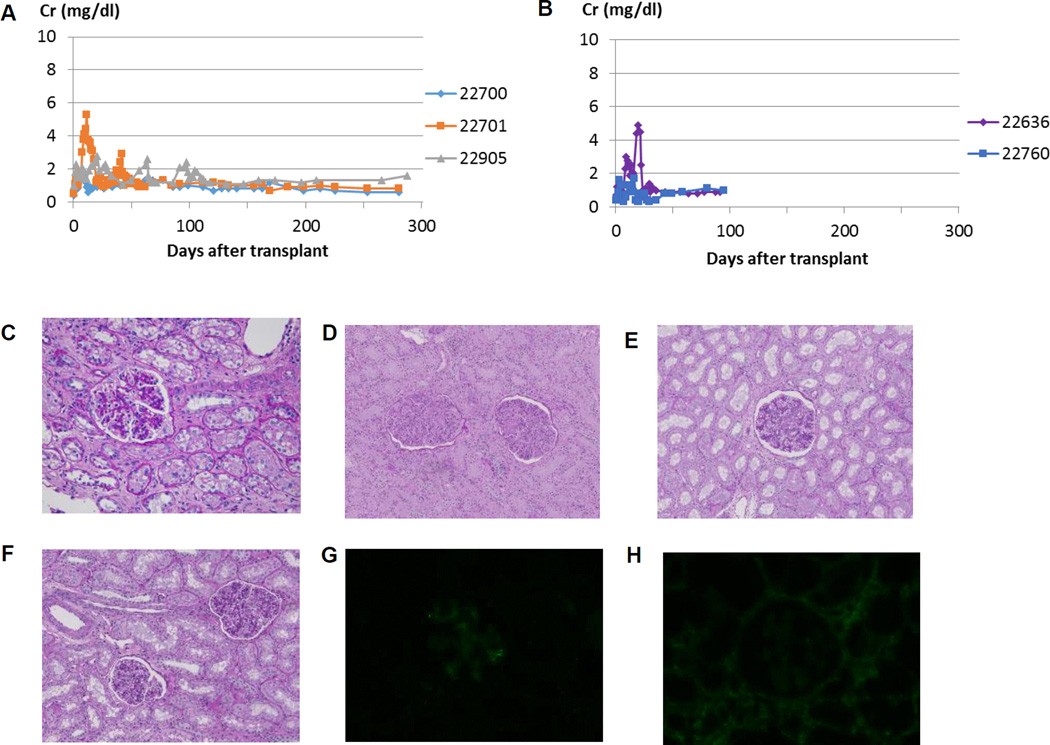
Fig. 2A demonstrates Cre levels in miniature swine recipients of Yorkshire kidneys, while Fig. 2B demonstrates Cre levels in miniature swine recipients of miniature swine kidneys. No recipient in either group demonstrated evidence of graft rejection throughout the experimental period. Kidney graft histology is provided in Figs. 2C-H. One recipient of a Yorkshire kidney graft required a 14-day course of low dose of FK506 as rescue therapy between POD41 and 54 (red line, Fig. 2A). Importantly, there was no histological evidence of rejection at 3 months (Fig. 2C) or at the end of the study on excised kidney grafts (Figs. 2D, 2E and 2F), as well as no evidence of antibody deposition in recipients of Yorkshire kidneys (Figs. 2G and 2H). These results suggest that the recipients were tolerant of the Yorkshire kidneys. Results in the control animals (miniature swine kidneys transplanted into miniature swine recipients) were similar to the findings in the experimental (Yorkshire kidney to miniature swine) animals, well as to historical controls (12). The induction of tolerance was important as it allowed evaluation of the kidney graft size without having to take into consideration inflammatory changes that could have caused the grafts to enlarge during rejection crises or the effects of chronic immunosuppression.
Figure 2.
Creatinine (Cre) levels following Tx of (A) Yorkshire kidneys and (B) miniature swine kidneys in miniature swine recipients (x-axis represents days after kidney Tx). Stable Cre levels were maintained until euthanasia in both groups. Histologic findings in the Yorkshire kidney graft for (C) 22905(POD 91), (D) 22700, (E) 22701(POD 350) and (F) animal 22905(POD 306). Minimal interstitial cell infiltration was seen on PAS staining (C), and neither IgM (G) nor IgG (H) deposition was observed within the graft. No evidence of rejection was observed in York-to-mini kidney Tx models 300 days following kidney Tx (Figs. D, E and F).
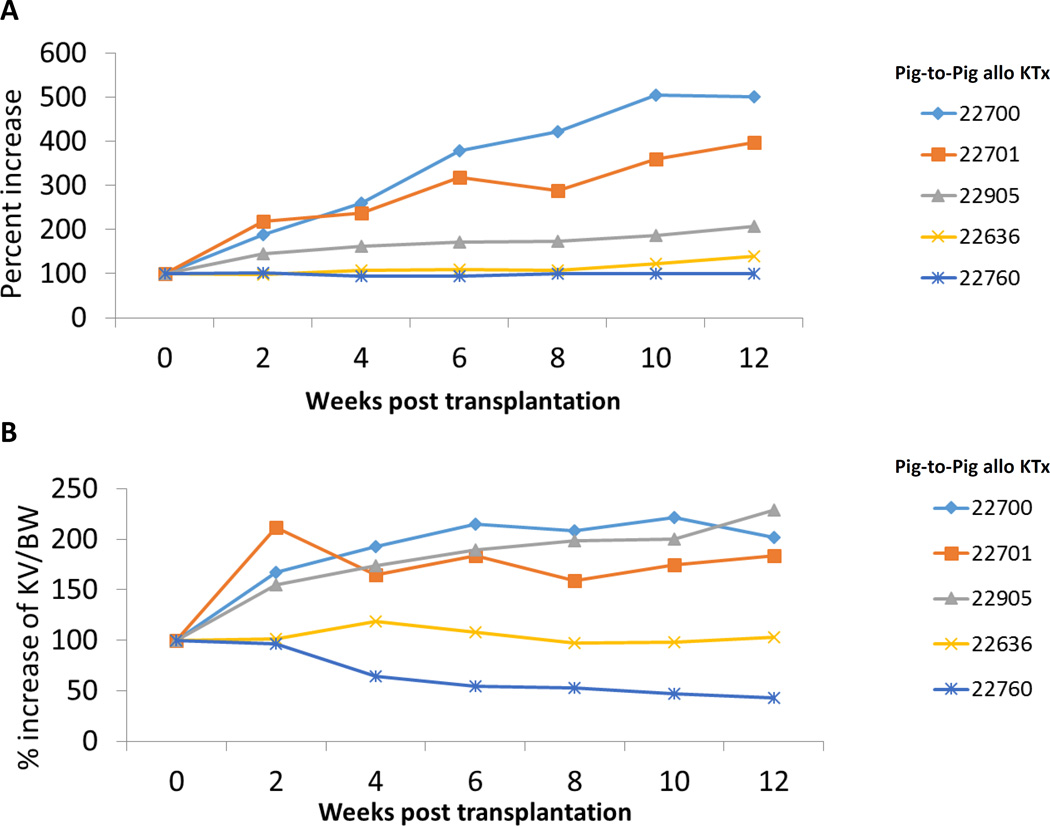
Kidney graft growth in the Yorkshire swine-to-miniature swine model was compared with that of the miniature swine-to-miniature swine model for 3 months, as this was the relevant survival period of the life-supporting GalT-KO kidneys in our pig-to-baboon model, to which these results were being compared (see above). Clear differences between Yorkshire and miniature swine kidney grafts were observed (Figs. 3A and 3B). Yorkshire kidney grafts grew rapidly for 2–4 weeks following Tx while miniature swine kidney grafts demonstrated much slower growth (Fig 3A). When body weight is taken into account (Graft Index), differences in overall growth percentages between the two groups were clear (Fig.3B). The growth of the Yorkshire kidney grafts in miniature swine recipients far exceeded that of the overall body growth of miniature swine (Fig.3B). These findings support marked regulation of growth of kidney grafts by factors intrinsic to the kidney graft.
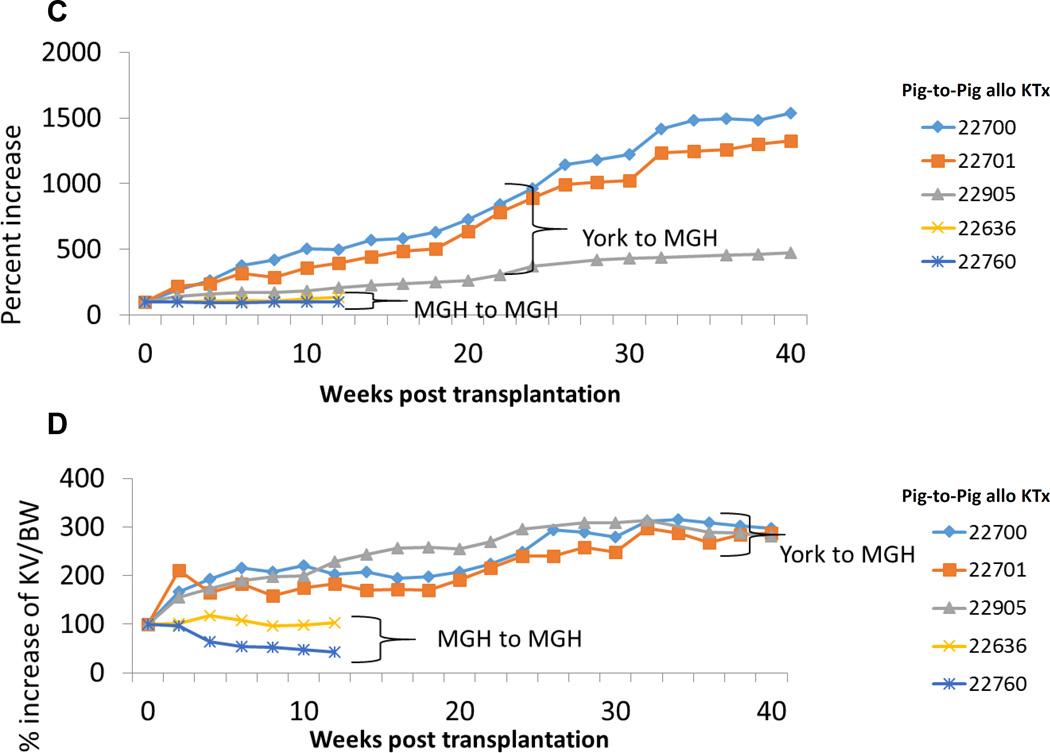
Figure 3.
(A) % increase in kidney graft volume after fully MHC mismatched kidney Tx in first 12 weeks. (B) % increase of kidney volume (cm3) / recipent’s body weight (kg) in first 12 weeks. (C) % increase in kidney graft volume after fully MHC mismatched kidney Tx 40 weeks. (D) % increase of kidney volume (cm3) / recipient’s body weight (kg) 40 weeks.
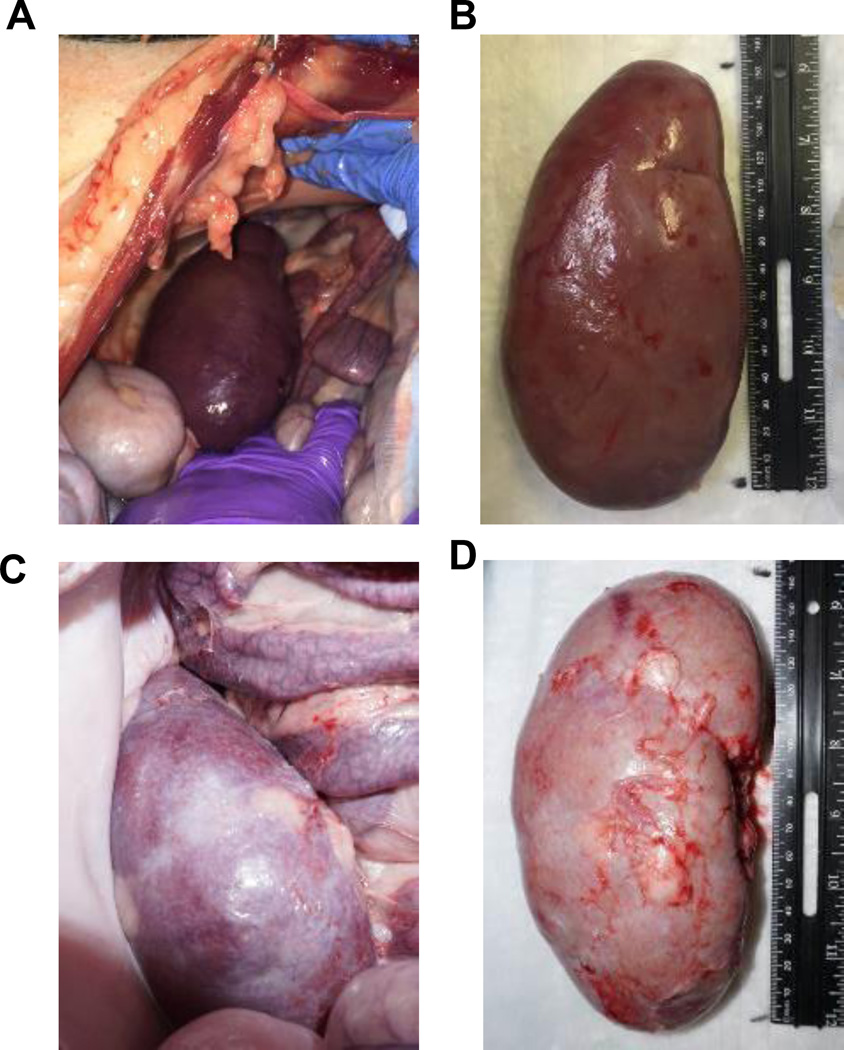
During an extended follow-up period of 8 months, Yorkshire kidney grafts continued to exhibit growth, reaching >12x their initial size (Fig. 3C). Indeed, Yorkshire kidney grafts continued to grow more rapidly than miniature swine kidney grafts with respect to the recipient overall body growth for up to 10 months following kidney Tx (Fig. 3D). However, growth did eventually plateau at 10 months, suggesting regulation of kidney growth by recipient factors as well, although by that time, the Yorkshire kidneys occupied the entire right abdomen (Figs. 4A-D).
Figure 4.
(A) Representative image of a kidney graft at the time of euthanasia and (B) explanted kidney graft for 22700. (C) Picture at time of euthanasia and (D) explanted kidney graft for 22701.
B. Lungs
Rapid growth of Allogeneic Yorkshire lungs following transplantation
The first recipient (pig ID #23056) received an outbred Yorkshire lung and was treated with a 28-day course of FK506 monotherapy. Unlike the kidney allografts, lung grafts were lost due to pneumonia and acute cellular rejection on POD 10 (Figs. 5A and 5B). In order to avoid these complications, in subsequent experiments both the donor and the recipient (23148 and 23145) were treated with macrolide antibiotics preoperatively. In addition, low dose oral rapamycin (0.5–2mg/day) was added to the immunosuppressive regimen instead of using higher doses of FK506, which has the potential to cause cardiac toxicity in swine (12).
Figure 5.
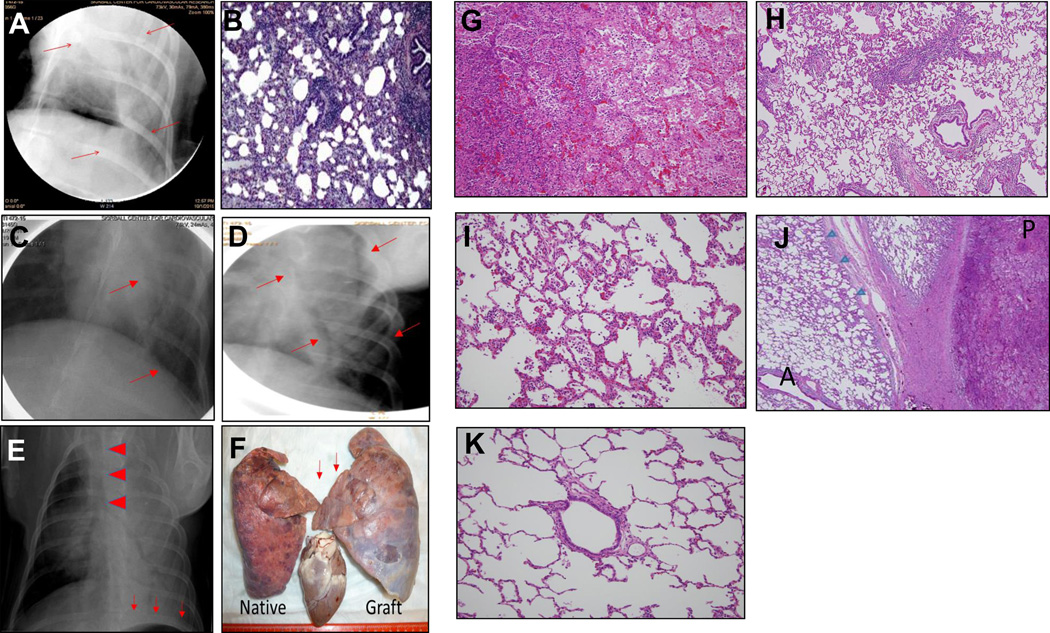
(A) POD 8 chest X-ray (1st recipient) following allogeneic lung Tx. The arrows demonstrate areas of consolidation within the graft. (B) Pathological examination revealed infiltration of the graft with inflammatory cells and lymphocytes, suggesting pneumonia and cellular rejection. (C,D): POD 15 chest X-ray (C, 2nd recipient) and POD 16 chest x-ray (D, 3rd recipient) demonstrating clear aeration of the lung allografts. (E) POD 29 chest x-ray (2nd recipient). Arrows depict caudal displacement of the left hemi-diaphragm, suggesting enlargement of the graft. Arrowheads demonstrate displacement of the trachea to the right. Consolidation was found diffusely, especially in the left upper lung, suggesting atelectasis and subsequent pneumonia. (F) Necropsy findings of the native (right) and lung graft (left). The graft enlarged more than that of native lung, especially caudally. Arrows indicate the compressed upper lobe of the graft, which enlarged toward the mediastinum. (G-K) Representative pathological findings of the native and grafted lung: (G) Bleeding, neutrophil infiltration and cellular debris were seen, indicating pneumonia. (H) Mild bronchiolitis in the lower lobe of the graft. (I) Mild pneumonia in the upper lobe of the native lung. (J) There were well-aerated regions (A) and lobular pneumonia regions (P) in the upper lobe of the graft. Evidence of pneumonia extended from the pleural region (arrow heads). (K) No evidence of cellular rejection was found in the graft.
Both the second and third recipients demonstrated a similar clinical course, with lung grafts clearly aerated as assessed by chest X-ray on POD15 and 16. Neither exhibited early evidence of pneumonia or acute rejection (Figs. 5C and 5D). On POD29, however, the chest X-ray in the second recipient revealed enlargement of the graft, compared to the native right lung, as well as tracheal deviation to the right, depression of the left hemidiaphragm and the development of pneumonia (Fig. 5E). The animal was subsequently euthanized and necropsy revealed compression of the upper lobe of the graft, with extension of the lower lobe in to the mediastinum (Fig.5F). Histopathology revealed severe pneumonia in the upper lobe of the graft and evidence of early pneumonia in both the lower lobe of the graft and in the upper lobe of the native right lung (Figs. 5G-K). The severity of the pneumonia in the transplanted upper lobe (Fig. 5G), suggested that the pneumonia could have originated there, before spreading to both the lower as well as the contralateral (native) lobe (Figs. 5H and 5I). Interestingly, pneumonia was also observed along the left pleura, suggesting that the etiology resulted from compression of the lung against the thoracic wall (Fig. 5J). It should be noted that there was no evidence of endothelialitis, suggesting absence of rejection and that graft loss was directly related to compression atelectasis and subsequent infection (Fig. 5K). Results in the third case were similar to the second recipient, with loss of the lung graft on POD28 due to pneumonia in the upper lobe of the transplanted graft and without any histologic evidence of acute cellular rejection.
DISCUSSION
Recent improvements in survival following pig-to-nonhuman primate (NHP) Tx suggest that xenoTx may be feasible for clinical trials. However, growth of porcine organs in NHP following Tx is poorly understood. Previous reports by Soin et al., in which survival to 48-days following pig-to-NHP xenoTx was achieved, have demonstrated that porcine kidney xenografts in NHP grew in the first two weeks following transplantation, at which point further growth ceased and growth plateaued in three of six cases (7). In the remaining three pigs, graft size increased due to acute vascular rejection, however, two animals in which graft size plateaued, acute cellular rejection was also present.
In this study, with long follow up periods, our data suggest that intrinsic factors within the transplanted organ are largely involved in graft growth following Tx, in both allogeneic and xenogeneic transplant models. We demonstrated that (i) baboon recipients of GalTKO TK without additional gene modification enjoyed prolonged graft survival without immunologic sensitization up to 193 days, (ii) however, if the ratio of the pig kidney volume to the recipient animal's body weight was greater than 25cm3/kg there appeared to be a deleterious effect on renal function, (iii) allogeneic kidney graft size and percentage increase of kidney volume/body weight from outbred Yorkshire swine in miniature swine increased three times more than those of grafts from miniature swine to miniature swine at 12 weeks post-Tx, and (iv) rapid growth of Yorkshire lungs caused compression, presumably due to the rigid thorax, and led to the development of atelectasis and subsequent pneumonia in the upper lobes of the allografts.
The three TK grafts for which kidney volumes were measured, grew 1.9x, 2.4x and 2.5x over the three months following transplantation. This growth pattern is a similar to that seen in a miniature swine that was size-matched to #1–#3’s donor miniature swine, after kidney donation (1.9x). These included two (baboons #1 and #3) that eventually lost function with no evidence of sensitization/rejection by in vitro immunological assessment. Most importantly, four grafts including a retrospective study showed that Growth Indices were >25cm3/kg for all recipients (baboons #1, #3, #4 and #5) who succumbed due to an elevated Cre with histologic evidence of cortical ischemia. Therefore, since baboons do not grow as fast as pigs, the growth index, which takes growth curves of hosts into account (kidney volume/recipient body weight) is a more appropriate assessment of non-immunologic graft dysfunction due to size mismatch than a simple measurement of width, depth and height of the xenografts or % increase in volume of the xenografts.
Close follow-up measurements of graft volumes in baboon recipient #1, #2 and #3 showed that baboon recipients #1 and #3 increased graft volume without any signs of immunologic sensitization until the Growth Index approached 25cm3. Histology of the TK graft at this time showed minimal cell infiltrate in the interstitial edema without tubulitis, vasculitis or endothelialitis. It is well known that the reduction in renal blood flow because of an imbalance between vasoactive mediators and endothelial dysfunction leads to interstitial edema and leukocyte trafficking in ischemic re-perfusion injury models (25–28). Since no histological and immunological evidence of sensitization was observed in all of TK recipients in this study, these changes are likely due to cytokine release associated with hypoxia of grafts which was accelerated, especially after the Graft Index exceed the threshold. The possibility of immunologic responses or xenogeneic effects not captured by the assays may be considered. However, our immunologic data in recipients of TK showed immunologic pig specific unresponsiveness, absence of anti-donor IFN-γ Elispot, anti-donor MLR and ant-pig elicited antibodies.
Cellular factors, including the function of organ progenitor cells, molecular and genetic pathways involving growth hormones (e.g. the insulin receptor-phosphoinositide 3 kinase (PI3K) or telomerase pathways), as well as environmental factors, such as hypoxia, are involved in organ growth (29, 30). Among these factors, growth hormone might be expected to be a major factor for organ growth following transplantation. Behncken et al. have reported that porcine growth hormone did not activate human hormone receptor due to amino acid differences of the growth hormone structure between species (31). However, previous studies have demonstrated that human growth hormone could have biological effects in pigs (32). Still another study reported that human growth hormone did not affect bone and mineral metabolism in juvenile pigs (33). Considering these conflicting results, further studies should be performed to determine whether extrinsic factors growth of transplanted organs. On the other hand, our results, showing rapid growth of Yorkshire kidneys and lungs in miniature swine as well as growth of pig kidneys in baboons indicate that factors intrinsic to the organ may play an important role in transplanted grafts.
In our kidney Tx of both pig-to-baboon and pig-to-pig models, porcine kidney grafts were transplanted in an orthotopic, intra-abdominal fashion, into the abdominal cavity. A major differences between xeno KTx and allo KTx is that pig recipients have large abdominal space and are able to accommodate growth both laterally and craniocaudally. These allogeneic kidneys grew without compression until the graft reached the right liver or iliac bones and maintained stable renal function over the course of 280-days. However, xenogeneic kidney grafts demonstrated decreased graft function, possibly explained by either: 1) insufficient cortical blood flow due to continuous daily growth of pig kidneys, in comparison to the much more limited growth of the recipient baboons; or 2) an anatomical difference between the porcine and NHP renal artery. Additionally, a baboon’s abdominal cavity is small and lateral extension is limited due to bipedalism, an effect reminiscent of that seen in renal enlargement due to polycystic kidney disease, where intra-renal ischemia is seen (34). Although we did not find any evidence of technical failures, such as an anastomotic stricture or kinking of vessels, the small diameter of the porcine renal artery may not allow for expansion and absence of increased compensatory blood flow to the large graft. Similar to xenoTx kidneys, Yorkshire lung grafts experienced deterioration in their graft function with prominent atelectasis in the upper lobe due to lack of accommodation for growth in the mediastinum, where the thoracic cavity is circumferentially limited by a bony framework.
The latest results in heterotopic heart xenoTx by Mohiuddin’s group (6) raise an interesting concern over the difference in growth curves of heart grafts transplanted either heterotopically or orthotopically. Since the left ventricular system of a heterotopically transplanted heart is dysfunctional, heterotopically transplanted hearts do not grow normally. In addition, heterotopically transplanted hearts in the abdomen have more space in the abdomen as opposed to orthotopic heart transplantation into the mediastinum, where accommodation for growth is limited. Although other factors may be involved, McGregor’s group reported that heterotopically transplanted hearts maintained prolonged graft survival for up to 146-days, while hearts orthotopically transplanted, and therefore transplanted into the limited space of the chest, survived only up to 57 days (8, 9).
In summary, our results, from both allogeneic and xenogeneic Tx studies, indicate that (i) above a threshold of 25cm3/kg (kidney volume/recipient body weight) cortical ischemia occurs in a pig-to-baboon model; (ii) organ growth is predominantly regulated by intrinsic factors; and (iii) deterioration of organ function is observed earlier for orthotopic allo-lung transplants compared to allo-kidneys, presumably due to more limited space in the mediastinum than in the abdomen. These results suggest that intrinsic factors are responsible, at least in part, for growth of donor organs and that this property should be taken into consideration for growth-curve mismatched transplants, especially for life-supporting organs transplanted into a limited recipient space.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Masayuki Tasaki for his helpful advice and review of this manuscript. We thank Bristol-Myers Squibb for providing CTLA4-Ig, Genzyme for rabbit ATG and Genentech for Rituximab. This research was supported by NIH grant 2P01AI45897-11A1 (Project 1).
Abbreviations
- Cre
creatinine
- CRISPR/CAS9
clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9
- GalT-KO
alpha-1,3 galactocyl transferase knock out
- hTBM
human thrombomodulin
- IFN-γ
interferon gamma
- MLR
Mixed Lymphocyte Reaction
- MMF
Mycophenolate Mofetil
- mTOR
mammalian target of rapamycin
- NHP
nonhuman primate
- PI3K
phosphoinositide 3
- POD
postoperative day
- TK
thymokidney
- Tx
transplantation
- XenoTx
xenotransplantation
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Materials and Methods
References
- 1.Klassen DK, Edwards LB, Stewart DE, Glazier AK, Orlowski JP, Berg CL. The OPTN Deceased Donor Potential Study: Implications for Policy and Practice. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(6):1707–1714. doi: 10.1111/ajt.13731. [DOI] [PubMed] [Google Scholar]
- 2.Sachs DH. The pig as a xenograft donor. Pathologie-biologie. 1994;42(3):217–219. [PubMed] [Google Scholar]
- 3.Sato M, Kagoshima A, Saitoh I, Inada E, Miyoshi K, Ohtsuka M, et al. Generation of alpha-1,3-Galactosyltransferase-Deficient Porcine Embryonic Fibroblasts by CRISPR/Cas9-Mediated Knock-in of a Small Mutated Sequence and a Targeted Toxin-Based Selection System. Reproduction in domestic animals = Zuchthygiene. 2015;50(5):872–880. doi: 10.1111/rda.12565. [DOI] [PubMed] [Google Scholar]
- 4.Hai T, Teng F, Guo R, Li W, Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell research. 2014;24(3):372–375. doi: 10.1038/cr.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohiuddin MM, Singh AK, Corcoran PC, Thomas Iii ML, Clark T, Lewis BG, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nature communications. 2016;7:11138. doi: 10.1038/ncomms11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nature medicine. 2005;11(1):32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 7.Soin B, Ostlie D, Cozzi E, Smith KG, Bradley JR, Vial C, et al. Growth of porcine kidneys in their native and xenograft environment. Xenotransplantation. 2000;7(2):96–100. doi: 10.1034/j.1399-3089.2000.00046.x. [DOI] [PubMed] [Google Scholar]
- 8.Cai W, Kaiser MS, Dekkers JC. Bayesian analysis of the effect of selection for residual feed intake on growth and feed intake curves in Yorkshire swine. Journal of animal science. 2012;90(1):127–141. doi: 10.2527/jas.2011-4293. [DOI] [PubMed] [Google Scholar]
- 9.Sachs DH, Leight G, Cone J, Schwarz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22(6):559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Pennington LR, Lunney JK, Sachs DH. Transplantation in miniature swine. VIII. Recombination within the major histocompatibility complex of miniature swine. Transplantation. 1981;31(1):66–71. [PubMed] [Google Scholar]
- 11.Griesemer AD, Hirakata A, Shimizu A, Moran S, Tena A, Iwaki H, et al. Results of gal-knockout porcine thymokidney xenografts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(12):2669–2678. doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Utsugi R, Barth RN, Kitamura H, Ambroz J, Sachs DH, Yamada K. Tolerance across a two-haplotype, fully MHC-mismatched barrier induced in miniature swine renal allografts treated with a 12-day course of tacrolimus. Transplantation proceedings. 2001;33(1–2):101. doi: 10.1016/s0041-1345(00)01925-4. [DOI] [PubMed] [Google Scholar]
- 13.Nobori S, Samelson-Jones E, Shimizu A, Hisashi Y, Yamamoto S, Kamano C, et al. Long-term acceptance of fully allogeneic cardiac grafts by cotransplantation of vascularized thymus in miniature swine. Transplantation. 2006;81(1):26–35. doi: 10.1097/01.tp.0000200368.03991.e0. [DOI] [PubMed] [Google Scholar]
- 14.Yamada K, Gianello PR, Ierino FL, Lorf T, Shimizu A, Meehan S, et al. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. The Journal of experimental medicine. 1997;186(4):497–506. doi: 10.1084/jem.186.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasaki M, Shimizu A, Hanekamp I, Torabi R, Villani V, Yamada K. Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation. Journal of the American Society of Nephrology : JASN. 2014;25(4):737–744. doi: 10.1681/ASN.2013040363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paoloni MC, Mazcko C, Fox E, Fan T, Lana S, Kisseberth W, et al. Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs. PloS one. 2010;5(6):e11013. doi: 10.1371/journal.pone.0011013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahara H, Shimizu A, Setoyama K, Oku M, Okumi M, Nishimura H, et al. Beneficial effects of perioperative low-dose inhaled carbon monoxide on pulmonary allograft survival in MHC-inbred CLAWN miniature swine. Transplantation. 2010;90(12):1336–1343. doi: 10.1097/TP.0b013e3181ff8730. [DOI] [PubMed] [Google Scholar]
- 18.Madariaga ML, Michel SG, La Muraglia GM, 2nd, Sihag S, Leonard DA, Farkash EA, et al. Recipient-matching of Passenger Leukocytes Prolongs Survival of Donor Lung Allografts in Miniature Swine. Transplantation. 2015;99(7):1372–1378. doi: 10.1097/TP.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones TB, Riddick LR, Harpen MD, Dubuisson RL, Samuels D. Ultrasonographic determination of renal mass and renal volume. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1983;2(4):151–154. doi: 10.7863/jum.1983.2.4.151. [DOI] [PubMed] [Google Scholar]
- 20.Tasaki M, Wamala I, Tena A, Villani V, Sekijima M, Pathiraja V, et al. High incidence of xenogenic bone marrow engraftment in pig-to-baboon intra-bone bone marrow transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(4):974–983. doi: 10.1111/ajt.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinger JG, Weist BM, Plaisted WC, Klaus SM, Walsh CM, Lane TE. MHC mismatch results in neural progenitor cell rejection following spinal cord transplantation in a model of viral-induced demyelination. Stem cells. 2012;30(11):2584–2595. doi: 10.1002/stem.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nature protocols. 2007;2(9):2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 23.Ball CG, Kirkpatrick AW, Yilmaz S, Monroy M, Nicolaou S, Salazar A. Renal allograft compartment syndrome: an underappreciated postoperative complication. American journal of surgery. 2006;191(5):619–624. doi: 10.1016/j.amjsurg.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Fontana I, Bertocchi M, Centanaro M, Varotti G, Santori G, Mondello R, et al. Abdominal compartment syndrome: an underrated complication in pediatric kidney transplantation. Transplantation proceedings. 2014;46(7):2251–2253. doi: 10.1016/j.transproceed.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 25.Rao J, Lu L, Zhai Y. T cells in organ ischemia reperfusion injury. Current opinion in organ transplantation. 2014;19(2):115–120. doi: 10.1097/MOT.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legrand M, Mik EG, Johannes T, Payen D, Ince C. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Molecular medicine. 2008;14(7–8):502–516. doi: 10.2119/2008-00006.Legrand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yap SC, Lee HT. Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology. 2012;116(5):1139–1148. doi: 10.1097/ALN.0b013e31824f951b. [DOI] [PubMed] [Google Scholar]
- 28.Karim AS, Reese SR, Wilson NA, Jacobson LM, Zhong W, Djamali A. Nox2 is a mediator of ischemia reperfusion injury. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(11):2888–2899. doi: 10.1111/ajt.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, et al. Akt/protein kinase B promotes organ growth in transgenic mice. Molecular and cellular biology. 2002;22(8):2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paisley D, Bevan L, Choy KJ, Gross C. The pneumonectomy model of compensatory lung growth: insights into lung regeneration. Pharmacology & therapeutics. 2014;142(2):196–205. doi: 10.1016/j.pharmthera.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Behncken SN, Rowlinson SW, Rowland JE, Conway-Campbell BL, Monks TA, Waters MJ. Aspartate 171 is the major primate-specific determinant of human growth hormone. Engineering porcine growth hormone to activate the human receptor. The Journal of biological chemistry. 1997;272(43):27077–27083. doi: 10.1074/jbc.272.43.27077. [DOI] [PubMed] [Google Scholar]
- 32.Pursel VG, Rexroad CE, Jr, Bolt DJ, Miller KF, Wall RJ, Hammer RE, et al. Progress on gene transfer in farm animals. Veterinary immunology and immunopathology. 1987;17(1–4):303–312. doi: 10.1016/0165-2427(87)90149-8. [DOI] [PubMed] [Google Scholar]
- 33.Pointillart A, Bonneau M, Kann G. Effect of human growth hormone-releasing factor on bone and mineral metabolism in growing pigs. Journal of animal science. 1991;69(4):1454–1460. doi: 10.2527/1991.6941454x. [DOI] [PubMed] [Google Scholar]
- 34.Rahbari-Oskoui F, Williams O, Chapman A. Mechanisms and management of hypertension in autosomal dominant polycystic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(12):2194–2201. doi: 10.1093/ndt/gft513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.