Abstract
Treatment of secondary pediatric osteoporosis—particularly that due to chronic diseases, immobilization, and necessary medical treatments—is currently limited by a poor understanding of the long-term efficacy and safety of skeletal metabolism modifying drugs. This study aimed to characterize longitudinal effects of representative anabolic (parathyroid hormone, PTH) and anti-catabolic (zoledronic acid, ZA) drugs on skeletal morphology, mechanical strength, and growth in juvenile mice. BALB/cJ mice aged four weeks were given PTH(1–34) or vehicle (control) daily for eight weeks, or four weekly doses of ZA, and evaluated at time points 0–26 weeks after treatment initiation. There were no enduring differences in body length or mass between treatment groups. ZA increased femur size as early as week 0, including increased distal femur bone volume and diaphyseal cross-sectional area, persisting through week 26. PTH treatment only transiently increased bone size, including distal femur volume at weeks 4–12. ZA decreased diaphyseal cortical tissue mineral density (TMD) at 12–26 weeks vs. controls; PTH decreased TMD only at 2 weeks (vs. controls). ZA increased bending strength at 0–12 weeks and flexural strength at week 4 (vs. controls), but decreased flexural strength and modulus at week 26. PTH treatment increased bending strength only at 4 weeks, and did not affect flexural strength. Overall, ZA rapidly and persistently increased femur strength and size, but compromised bone material quality long-term. In healthy juvenile mice, limited-duration PTH treatment did not exert a strong anabolic effect, and had no adverse effects on femur strength, morphology, or growth.
KEY WORDS/PHRASES: parathyroid hormone (PTH), zoledronic acid, juvenile mice, bone strength
INTRODUCTION
Reduced bone mass in childhood may be precipitated by a variety of factors, including genetic conditions, chronic illness and diseases (including leukemia, lymphoma, and sarcomas), chronic immobilization, eating disorders, endocrine disorders, and idiopathic conditions such as medications and other treatments (including glucocorticoids, chemotherapeutic agents, and radiotherapy).1 Although clinical data is limited, meta-analyses have suggested an association between low bone density and increased fracture risk in pediatric patients.2 For many of these causes, specific treatments are established. However, for others—particularly those associated with the long-term effects of chronic diseases, necessary medications for medical conditions, and childhood malignancy—standard treatment protocols have not been established due to a limited understanding of the long-term effects of standard osteoporosis drugs in this young patient population.
There is limited clinical data available addressing the use of these drugs in pediatric patients. While bisphosphonate therapy is a standard treatment for osteogenesis imperfecta (with the exception of type VI),3 this drug class is not widely applied to other cases of low bone mass, in part due to concerns about the safety of inhibiting bone remodeling long-term. Results from clinical studies of bisphosphonate treatment in pediatric cases of secondary osteoporosis have not consistently demonstrated treatment efficacy.4 Even less is known about the use of anabolic agents such as PTH. Collectively, the clinical data indicate a need for more focused, in-depth investigations into the efficacy of drug treatments such as anabolic and anti-catabolic agents for restoring or preventing loss of bone mass in pediatric populations.
Investigation of these two classes of drugs in animal models has been largely constrained to disease models in mature animals. Several studies have investigated the effects of bone metabolism modifying drugs in animal models of dysfunctional mineralization,5 Paget’s disease,6 or chronic inflammatory conditions.7 These models, however, use genetically modified (knockout, point mutation) animals and did not study drug effects on wild-type animals. An exception is delivery of an anti-sclerostin antibody, which has been shown to increase cortical and trabecular bone density and thickness, as well as bending strength in juvenile wild-type mice.8 Tissue level properties determined by nanoindentation were, however, not found to change in response to anti-sclerostin antibody treatment.9
To date, the two most frequently used skeletal metabolism modifying drug classes have been assessed primarily in mature animals. Safety profiles, long-term skeletal effects, and efficacy in non-transgenic juvenile animals are not known for these drugs. The impact of rapid bone modeling and the grossly pro-anabolic environment of immature skeletal tissues on the efficacy and safety of anti-resorptive and anabolic drugs remain unknown. Before testing PTH or a bisphosphonate as representative agents for the anabolic and anti-catabolic classes of osteoporotic drugs in a juvenile animal model of secondary osteoporosis, the efficacy and long-term effects of these drugs in normal growing mice should be established.
Two classes of drugs (anabolic and anti-catabolic) were tested in this study with the expectation that the magnitude and duration of their effects on bone morphology and strength would vary in a rapidly growing juvenile animal model due to different mechanisms of action. PTH, a key regulator in calcium and phosphate homeostasis, was selected as a proof-of-concept anabolic agent. Pulsatile administration of PTH(1-34) stimulates net anabolic bone remodeling, and in adult animal models results in an increased bone mass, structural strength, stiffness, and energy absorption.10–12 In a pro-inflammatory juvenile mouse model of osteoporosis, osteoprotegerin and PTH combination prevented bone loss.7 While there is currently a black box warning against pediatric use of PTH (teriparatide) in patients with open epiphyses because of increased incidence osteosarcoma in rat studies,13; 14 clinical data casts doubt on the connection between PTH and increased neoplasm risk in humans.15; 16 Zoledronic acid (ZA), a pyrophosphate analog bisphosphonate, was chosen as the proof-of-concept anti-catabolic drug.17 In adult rats and humans, bisphosphonate attenuation of catabolic osteoclast activity consistently results in increased bone mass, strength, and bone mineral density.18–20 In growing male mice, alendronate, zoledronic acid, pamidronate, and clodronate treatment resulted in substantial increases in cortical and trabecular bone mass, suggesting that bone strength could be improved, but changes in mechanical strength were not measured.21
This study aimed to characterize longitudinal effects of limited-duration PTH(1-34) and zoledronic acid delivery on skeletal morphology, mechanical bone strength, and animal growth in a juvenile mouse model. We hypothesized that PTH and ZA would increase bone volume and bone strength without adversely affecting the normal growth (mass, length, body composition) of juvenile mice.
METHODS
Animal Model
All procedures were approved by the SUNY Upstate Institutional Animal Care and Use Committee. Female BALB/cJ mice (Jackson Labs, Bar Harbor, ME) were randomly assigned to one of three drug treatment groups, with drug delivery commencing at four weeks of age. Animals were maintained in community housing (≤ 5 mice/cage, 22°C) on a 12 hour light/dark cycle with ad libitum access to food and water, daily welfare observations, and biweekly cage and bedding changes. The first group received daily injections of PTH (1-34) (5 days/week, 40 μg/kg SC in saline with 0.1% bovine serum albumin, Sigma-Aldrich, St. Louis, MO) for eight weeks. The second group received four consecutive weekly injections of zoledronic acid (once weekly, 100 μg/kg SC in saline). The control group received injections of vehicle (0.1% bovine serum albumin in saline) paralleling the frequency and volume of PTH delivery. At end points of 0, 1, 2, 4, 8, 12, and 26 weeks (9 mice/group/time point, 189 mice total), mice were euthanized. Mice were weighed weekly to track growth, with drug dosages adjusted accordingly. Body length (rostrum to base of tail) was measured at each time point. While no adverse events or unexpected deaths observed in any treatment group, one animal from the vehicle group at week 8 was excluded from femur-specific analyses due to grossly anomalous bone morphology.
Imaging and Morphological Assessment by DXA and μCT
Euthanized intact mice were imaged with dual-energy X-ray absorptiometery (DXA, PIXImus 2, GE Lunar, Madison, WI) to quantify whole body composition and regional areal bone mineral density (aBMD) of the distal 50% of the right femur and over the whole body (excluding the cranium). Following this, femurs were disarticulated, cleaned of soft tissue, wrapped in saline moistened gauze, and frozen at −80°C. Right femurs were imaged by micro-computed tomography (μCT) using a 12 μm voxel resolution (225 kV, 144 mA, 200 s integration time using a micro-CT 40, Scanco Medical AG, Brüttisellen, Switzerland) (Figure 1). Four volumes of interest (VOIs) were analyzed, applying a global lower threshold of 654 mg HA/cm3. The volumetric tissue mineral density (TMD) was quantified at the middle 7% of the diaphyseal cortex (VOI shown in Figure 2A). Mid-diaphyseal morphology outcomes included cortical bone, marrow, and total cross-sectional areas as well as mean cortical thickness and minimum outer caliper (Feret) diameter.22 The TMD and bone volume (BV) were quantified over the distal 33% of the femur (VOI shown in Figure 3A). The trabecular TMD, bone volume fraction (BV/TV), trabecular number, trabecular thickness and other parameters were quantified in the epiphyseal and metaphyseal VOIs (VOIs shown in Figure 4A & C). The distal metaphysis VOI was 3% of the length starting proximal to the growth plate, and the distal epiphysis VOI was 2.8% of the length proximal to the intracondylar groove, but distal to the growth plate. To account for the substantial growth occurring in juvenile animals over the six month long study, VOIs were defined relative to the total femur length rather than as an absolute position.
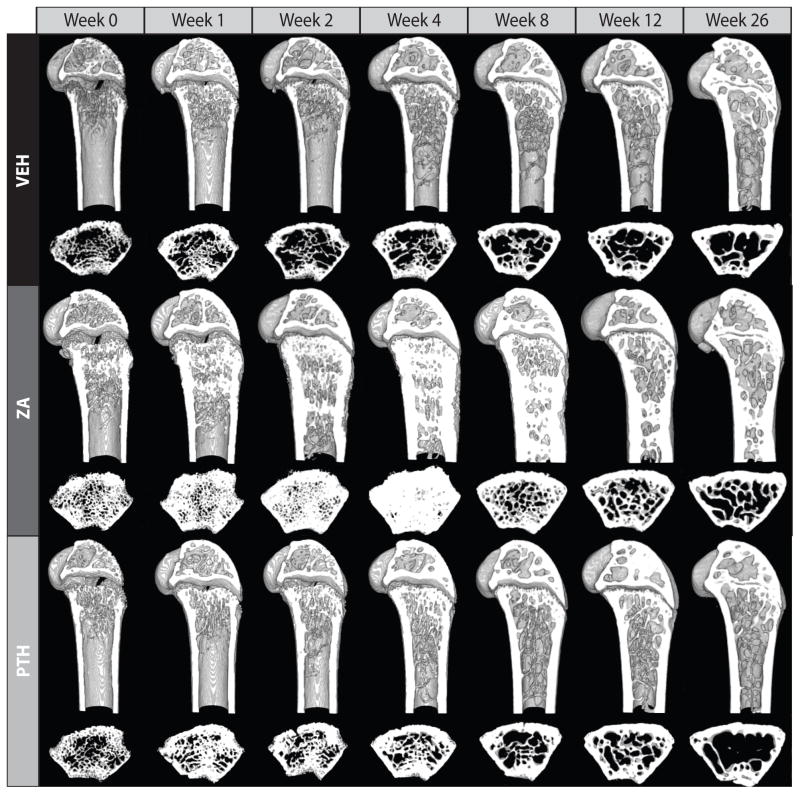
Figure 1.
Reconstructed μCT images of representative distal femurs from juvenile mice for each time point for vehicle (VEH), parathyroid hormone (PTH), and zoledronic acid (ZA) treatment groups. The distal 5 mm of each femur (digitally sectioned in the sagittal plane), and a 120 μm thick transverse section of the metaphysis are shown. In the ZA treated group, sclerotic trabeculae can be seen filling the marrow canal, however, the PTH group appears very similar to the control group.
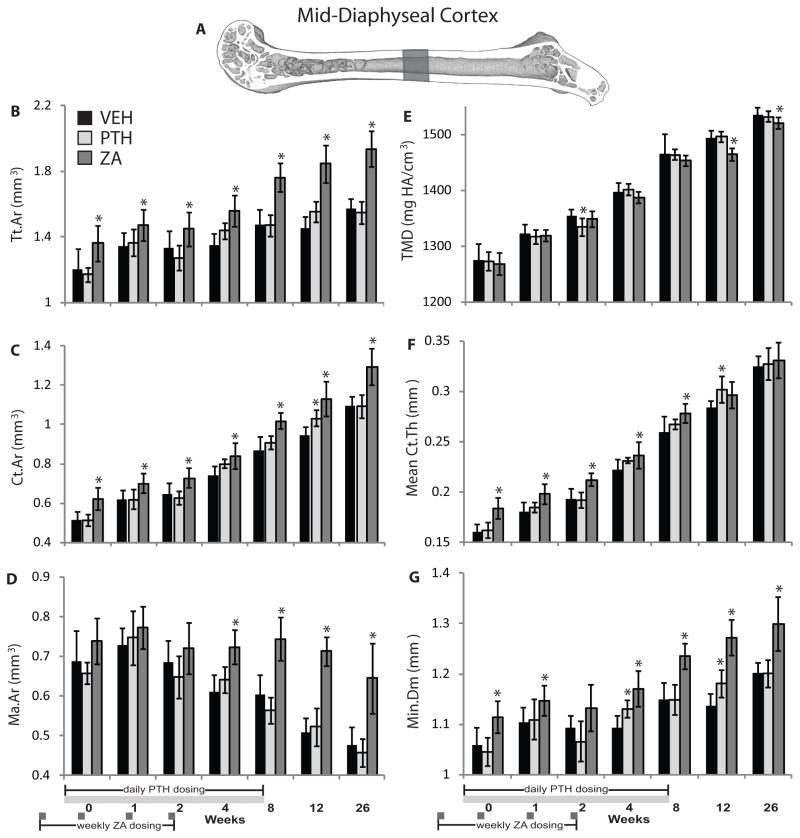
Figure 2.
(A) Mid-diaphyseal cortical morphology volume of interest; (B) total cross-sectional area (Tt.Ar); (C) cortical bone cross-sectional area (Ct.Ar); (D) marrow cross-sectional area (Ma.Ar); (E) tissue mineral density (TMD); (F) mean thickness of the cortex (Ct.Th); and (G) minimum caliper diameter of femurs (Min.Dm) from growing mice treated with vehicle (VEH), parathyroid hormone (PTH), or zoledronic acid (ZA). Data is presented as mean ± standard deviation. * denotes p < 0.05 vs. vehicle group within time point.
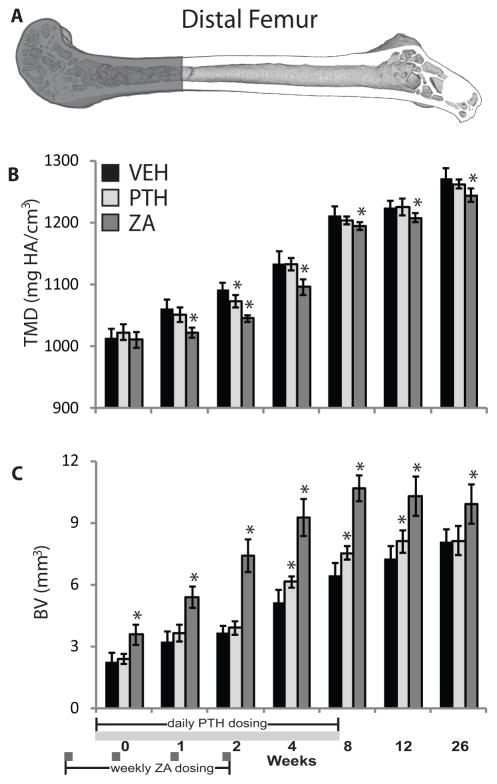
Figure 3.
(A) Distal femur volume of interest; (B) tissue mineral density (TMD); and bone volume (BV) of growing juvenile mice treated with vehicle (VEH), parathyroid hormone (PTH), or zoledronic acid (ZA). Data is presented as mean ± standard deviation. * denotes p < 0.05 vs. vehicle group within time point.
Figure 4.
(A) Epiphyseal trabecular bone volume of interest; (B) epiphyseal bone volume fraction (BV/TV); (C) metaphyseal trabecular bone volume of interest; and (D) metaphyseal BV/TV of growing juvenile mice treated with vehicle (VEH), parathyroid hormone (PTH), or zoledronic acid (ZA). Data is presented as mean ± standard deviation. * denotes p < 0.05 vs. vehicle group within time point.
Mechanical testing in three-point bending
Whole femur mechanical properties (structural strength/stiffness) and material properties (flexural strength/modulus) were quantified using a three-point bending test (Q-test, MTS, Eden Prairie, MN). Femurs were centered over two supports (8 mm span) with a 1 N preload before loading to failure at a rate of 1 mm/minute with the anterior surface in tension (Figure 5A). Outcome measures included whole femur bending strength, bending stiffness, post-peak displacement, cortical bone flexural strength, and flexural modulus. The latter was calculated using standard bending equations for asymmetrical beams with geometric parameters (e.g. section modulii) obtained from BoneJ. Briefly, the μCT image stacks were imported into ImageJ (NIH, Bethesda, MD), aligned digitally as they were in the mechanical test rig, the loading point was identified, and sectional parameters at that point determined using the BoneJ plugin.
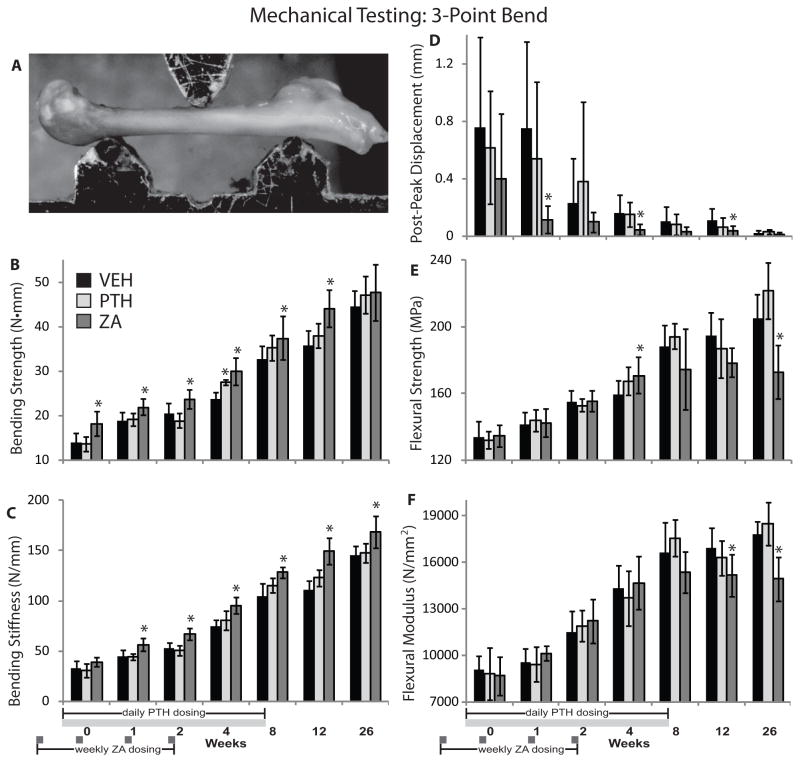
Figure 5.
(A) Three-point bending mechanical testing setup; (B) bending strength; (C) bending stiffness; (D) post-peak displacement; (E) flexural strength; and (F) flexural modulus of growing juvenile mice treated with vehicle (VEH), parathyroid hormone (PTH), or zoledronic acid (ZA). Data is graphed as mean ± standard deviation. * denotes p < 0.05 vs. vehicle within time point.
Statistical Analyses
Data were analyzed using one-way analysis of variance (ANOVA) with Tukey’s post-hoc tests to compare the drug treated groups (PTH, ZA) to vehicle controls (VEH) at each time point. The percentage differences in outcome measures (OM) for PTH versus VEH and ZA versus VEH were calculated ((treatment − VEH)/VEH) and are indicated as ΔOM with positive (+) indicating an increase and negative (−) indicating a decrease relative to the VEH group. Analysis of covariance (ANCOVA) with the covariate time was used to compare rates of change between treatment groups for independent variables over weeks 0–26. A log transformation of time was used to achieve normality in the linear regression model errors. Statistical significance was defined at p < 0.05. One animal from the ZA group at week 12 was excluded from bone strength analyses because the bending and flexural strength fell at least 4 standard deviations below the group mean, and was determined to be an outlier based on an extreme Studentized deviate test.
RESULTS
Femoral Morphology
Qualitatively, the effect of ZA on gross bone morphology was substantial (Figure 1). Administration of ZA was associated with an early increase in endosteal and intramedullary bone quantity, which started to occlude the marrow space at weeks 2–8. By week 12, however, the metaphyses of ZA-treated femurs appeared more similar to controls, although a band of sclerotic bone was retained more proximally in the diaphysis. PTH increased the size and number of trabeculae in the epiphysis compared to the control but appeared similar to the control in the metaphysis and diaphysis (Figure 1).
Mid-Diaphyseal Cortical Morphology
Mid-diaphyseal cross-sectional total area (Tt.Ar) and cortical bone area (Ct.Ar) were significantly increased by ZA compared to VEH at all time points (ΔTt.Ar: ranging from +9.3 to +27%, p ≤ 0.043; ΔCt.Ar: +13 to +21%, p<0.004). Tt.Ar was unaffected by PTH, and Ct.Ar increased only at 12 weeks in the PTH group (+9.1%, p = 0.014) (Figure 2B and C). Mid-diaphyseal cross-sectional marrow area (Ma.Ar) was significantly increased in ZA-treated mice at 4–26 weeks (ΔMa.Ar: +19 to +40%, p ≤ 0.001), and unaffected by PTH (Figure 2D). The cortical volumetric tissue mineral density (TMD) at the femoral mid-diaphysis was significantly decreased compared to vehicle controls at 12–26 weeks in the ZA group (ΔTMD: −0.9 to −1.9%, p ≤ 0.021), and at 2 weeks in the PTH group (ΔTMD: −1.6%, p = 0.009) (Figure 2E). Mean cortical thickness (Ct.Th) was increased from weeks 0 through 8 by ZA treatment (ΔCt.Th: +6.6 to +14%, p ≤ 0.015), and by PTH treatment only at 12 weeks (ΔCt.Th: 6.3%, p = 0.008) (Figure 2F). Minimum mid-diaphyseal diameter (Min.Dm) was significantly increased by ZA at all time points except 2 weeks (ΔMin.Dm: +3.9 to +12%, p ≤ 0.031), and was significantly increased by PTH at 4 and 12 weeks (ΔMin.Dm: +3.4 and +3.9%, respectively, p ≤ 0.011) (Figure 2G). The rate of increase in Tt.Ar was significantly elevated by ZA treatment (p = 0.001 by ANCOVA), but not PTH compared to the vehicle control group. The Ct.Ar increased more rapidly in the ZA group (p = 0.002 vs. VEH by ANCOVA), but rate of increase did not differ between PTH and VEH groups (p = 0.121). Ma.Ar decreased less rapidly in the ZA treatment group (p = 0.001 by ANCOVA) compared to the vehicle control group, and was unaffected by PTH treatment. The Min.Dm responded similarly, increasing more rapidly in ZA-treated femurs (p = 0.001 vs. VEH), and remaining unaffected by PTH (p = 0.136 vs. VEH). The rate of change in TMD and cortical thickness was not significantly different between vehicle and drug treated groups.
Distal Femur Morphology
In the distal femur, ZA treatment induced a significant decrease in TMD from weeks 1 through 26 compared to vehicle controls (ΔTMD: −1.4 to −4.4%, p ≤ 0.010). PTH treatment was associated with significantly decreased TMD only at 2 weeks (ΔTMD: −1.7%, p = 0.001 vs. VEH) (Figure 3B). Distal femur BV was significantly increased at all time points by ZA (ΔBV: +23 to +103%, p ≤ 0.001 vs. VEH), and by PTH at 4–12 weeks (ΔBV: +11 to +19%, p ≤ 0.040 vs. VEH) (Figure 3C).
Epiphyseal and Metaphyseal Trabecular Morphology
Epiphyseal trabecular bone volume fraction (BV/TV) (Figure 4A) was significantly increased compared to vehicle controls at 2 and 26 weeks by ZA treatment (ΔBV/TV: +27 and +17%, respectively, p ≤ 0.012) (Figure 4B), and at 1 and 4–12 weeks by PTH treatment (ΔBV/TV: +9.7 to +17%, p ≤ 0.024). Compared to vehicle controls, metaphyseal BV/TV (Figure 4C) was significantly increased by ZA treatment at 0–4 and 12 weeks (ΔBV/TV: +45 to +332%, p ≤ 0.001) (Figure 4D), and by PTH at 1–4 weeks (ΔBV/TV: +81 to +127%, p ≤ 0.018). Additional epiphyseal and metaphyseal trabecular bone measures including trabecular number, thickness, spacing, structure model index, and degree of anisotropy are reported in the Supplementary Table 1.
Mechanical Testing
Three point bending strength (Figure 5B) was significantly increased in ZA-treated femurs from weeks 0–12 (ΔBendStrength: +14 to +29%, p ≤ 0.045 vs. VEH). Bone bending stiffness (Figure 5C) was significantly increased in the ZA group at 1–26 weeks (ΔStiffness: +16 to +35%, p ≤ 0.001 vs. VEH), and was unaffected by PTH. Post-peak displacement (Figure 5D) was decreased by ZA treatment at 1, 4, and 12 weeks (ΔPostPeakDispl: −72 to −85%, p ≤ 0.037), and was unaffected by PTH treatment. Flexural strength (Figure 5E) was increased only at week 4 (ΔFlexStrength: +7%, p = 0.036 vs. VEH). By week 26, femoral flexural strength in the ZA group was significantly decreased (ΔFlexStrength: −16%, p = 0.001 vs. VEH) and there was lower flexural modulus (ΔFlexModulus: −16%, p = 0.001) (Figure 5F). PTH significantly increased bending strength only at 4 weeks (ΔBendStrength: +16%, p = 0.002 vs. VEH), and did not significantly affect flexural strength or modulus compared to vehicle controls. The rate of increase in bending strength was significantly greater in the PTH group (p = 0.033 vs. VEH by ANCOVA), but was not significantly affected by ZA treatment (p = 0.416 vs. VEH). The rate of increase in flexural strength did not differ between the PTH and VEH groups (p = 0.123), and was not evaluated for the ZA group due to the biphasic response. Bending stiffness increased at a more rapid rate in the ZA and PTH groups (p = 0.001, 0.038 respectively by ANCOVA) versus the control group. The post-peak displacement decreased more rapidly in the ZA group (p = 0.001 by ANCOVA vs. VEH).
Overall Growth
Overall, there were no enduring differences between the three treatment groups in body mass, body length (rostrum to tail), or DXA-derived body composition. Initial (pre-treatment) body mass of mice in the ZA group was, on average, 0.9 grams less than that of mice in the VEH group (p = 0.017). Final (end point) body mass was greater for mice in the ZA group (vs. VEH) group at 0 weeks (ΔBodyMass: +13%, p = 0.009), and decreased in the ZA group at 26 weeks (ΔBodyMass: −13%, p = 0.046 vs. VEH) (Supplementary Table 2). Body length was significantly increased 0, 1, and 8 weeks in the ZA group (ΔBodyLength: +3.9 to +11%, p ≤ 0.002 vs. VEH), but significantly decreased in the PTH group only at the 2 week time point (ΔBodyLength: −3.0%, p = 0.046 vs. VEH) (Supplementary Table 2). Body composition in the ZA group differed from the VEH group only at 0 weeks (p = 0.003) (ΔFatMass: +2.7%; ΔLeanMass: −3.6%), and did not differ at any point between the PTH and VEH groups (Supplementary Table 2).
DXA-Derived BMD
Areal BMD (aBMD) and corresponding bone area was measured by DXA over the distal 50% of each femur. The aBMD data diverged greatly from the μCT-derived volumetric TMD values. With ZA treatment, distal femur aBMD was increased at all time points (ΔaBMD: +20 to +58%, p ≤ 0.001 vs. VEH), and at 8 weeks with PTH (ΔaBMD: +12%, p ≤ 0.004 vs. VEH) (Supplementary Table 3). Whole body assessment of DXA-derived BMD was done excluding the cranium. With ZA treatment, whole body aBMD was increased at all time points (ΔaBMD: +13 to +27, p ≤ 0.001 vs. VEH), and at 8 and 12 weeks with PTH (ΔaBMD: +4.9 and +3.8%, respectively, p ≤ 0.006 vs. VEH) (Supplementary Table 2).
DISCUSSION
In this juvenile mouse model, treatment with PTH and ZA produced no enduring adverse effects on femur strength, morphology, body size, or body composition; the one exception being decreased flexural strength and modulus at 26 weeks following zoledronic acid delivery. The two mechanical strength outcomes used in this study indicate that the PTH and ZA drug treatments can differentially affect structural and material strength of growing bone. Bending strength, which represents the functional strength of the femur, is affected by both the amount of bone (size) and the material properties of cortical bone. In contrast, flexural strength and modulus reflect the intrinsic properties of the bone material rather than the whole bone as a structural unit. The increased bending strength in response to ZA treatment was driven by an increase in bone size, as evidenced by persistently increased Tt.Ar, Ct.Ar, Ma.Ar, Ct.Th, and Min.Dm. The decreased flexural strength and modulus in ZA-treated femurs at 26 weeks suggests that there is reduced bone material quality that may persist long-term. This could result from a variety of factors, including buildup of poor quality bone matrix (improperly oriented/crosslinked collagen fibrils) or accumulation of microdamage resulting from bisphosphonate attenuation of bone remodeling.23 Therefore, while femur strength for a single loading event may increase with ZA treatment, there may be other risks associated with reduced bone quality such as reduced ability to resist crack propagation (embrittlement).24; 25 The possibility of material embrittlement by ZA is further supported by the decrease in post-peak displacement at 1, 4, and 12 weeks. Material embrittlement of cortical bone is particularly concerning given recent reports of atypical long bone fractures in human patients following long-term bisphosphonate therapy.26–28 Additional studies are needed to quantify the long-term effects of zoledronic acid on resistance to crack propagation as well as the changes to matrix and mineral chemistry.
Interestingly, the PTH treatment group demonstrated minimal changes in many outcome measures, with the exception of the metaphyseal and epiphyseal trabecular compartments, both of which had modest anabolic responses. This unexpectedly mild response may be attributable to the animal model — anabolic potential and endogenous PTH-responsive systems may already be at near maximum in rapidly growing juvenile mice, particularly in the metaphysis.
In adult mice, it was previously reported that PTH treatment increased femur cortical bone strength, cortical thickness, and periosteal circumference.29 Anabolic effects of PTH treatment have also been demonstrated in the proximal tibia of juvenile rats.30 While limited changes in response to PTH treatment were seen in our study of juvenile mouse femurs, this may be because the actively growing juvenile mouse is already at peak anabolic function, resulting in a ceiling effect, essentially preventing any additional anabolic effects to be seen from the addition of exogenous agents. However, the positive effects of PTH delivery in a juvenile animal model could potentially occur if bone modeling were attenuated by metabolic bone diseases, chronic disease, immobilization, or following radiotherapy or chemotherapy.31 Anti-sclerostin antibody, an anabolic drug, has been shown to increase bone mass and strength (but not elastic modulus) in growing wild type and osteogenesis imperfecta mice.8; 9 A study in juvenile rats found that local bone loss resulting from focal irradiation was attenuated by PTH treatment,32 possibly due to improved osteoblast/osteocyte survival.33 PTH, however, did not prevent glucocorticoid-induced osteoporosis in growing mice.34
Effects from bisphosphonate treatment may be more dramatic compared to the anabolic agent PTH, because zoledronic acid functions by inhibiting osteoclastic bone resorption. Inhibiting bone resorption in an environment of robust anabolic activity (here, a growing mouse), allows newly formed matrix to persist without remodeling for long periods of time, resulting in increased bone quantity. This could also explain the robust effects of ZA in the rapidly remodeling metaphyseal compartment compared to more static epiphyseal region. In adult mice, rats, and humans, bisphosphonate treatment consistently result in increased bone mass and structural strength.18–20; 29; 35; 36 In contrast to increased whole bone strength, the tissue material strength and work to fracture were shown to decrease with bisphosphonate treatment in adult rats and dogs.19; 23; 37 Results of the juvenile mouse study presented here are consistent with the literature in demonstrating a bisphosphonate-induced increase in bone quantity leading to increased bone strength, accompanied by decreased bone tissue quality.
Tissue mineral density in the distal femur was significantly decreased in the ZA group—an unexpected finding that contrasts with previous clinical studies and work in adult mouse models that found TMD increased after bisphosphonate administration.38; 39 Differential responses shown in this study may be due to rapid bone modeling in juvenile mice combined with inhibited turnover due to the drug. In mature animals, the decreased number of active bone modeling sites may permit more complete tissue mineralization to occur with ZA treatment, resulting in a net increase in bone density. In this skeletally immature model, ZA-mediated attenuation of remodeling coupled with rapid anabolic modeling may be permitting appositional bone modeling to occur over top of incompletely mineralized osteoid, resulting in persistently decreased tissue mineral density. ZA treatment decreased TMD and flexural modulus as would be expected given that bone mineral is the primary influencing factor of bone material stiffness.40–42 However, the overall femoral bending stiffness increased for the ZA group compared to controls, despite the decrease in material stiffness, indicating that the increase in femoral stiffness by ZA was driven by the increased bone size.
While the μCT-derived volumetric TMD was decreased by ZA treatment in the distal femur, the DXA-derived areal BMD for the same region was significantly increased. This discrepancy is important to consider given the widespread clinical use of DXA-derived BMD measurements for fracture risk assessment. The difference in measured densities can be explained by the distribution of the newly modeled tissue. In the distal part of ZA-treated femurs, inhibition of osteoclastic activity prevents resorption of trabecular bone, allowing old trabecular structures to persist in the medullary space as the mice grow (Figures 1 & 4). While this trabecular bone is normally remodeled as the mice grow, attenuation of osteoclastic activity by ZA allows these structures to accumulate and effectively increase the BMD by filling in the normally hollow space without expanding the bone area measured by DXA relative to controls. Increasing the amount of bone tissue without a change in bone area results in an increase in aBMD, as reported here. Note that the changes in bone mineral content with time and treatment, as reported by DXA and μCT, are consistent. At the mid-diaphysis, bone modeling in the ZA group appears more prevalent on the periosteal surface, as evidenced by an increase in Tt.Ar and larger Min.Dm (Figure 2).
There are several limitations to the study presented here. First, only female mice were studied, and it is possible that there may be sex-associated differences in response to drug treatments. Adult female (wild-type) mice were shown to be more responsive to RANK-Fc treatment than male mice, although when genetically modified to model osteogenesis imperfecta (oim/oim), male mice were more responsive to anti-catabolic RANK-Fc treatment.43 Other studies have demonstrated substantial increases in both cortical and trabecular bone with bisphosphonate treatments in young male mice.21 Further investigation is needed into the sex-dependent effects of these drugs in pediatric models. Secondly, only one dosing strategy was evaluated in this study for each of the proof-of-concept drugs. Further optimization of drug dosage and delivery strategy would be necessary in order to advance towards clinical use. The 40 μg/kg/day dosage of PTH(1-34) employed here is consistent with that used by several other research groups,44; 45 including in studies of juvenile animals.32; 33 The zoledronic acid dose (100 μg/kg, human equivalent dose ~8 μg/kg) was selected based on prior efficacy in attenuating radiation-induced bone loss46 and tumor osteolysis47 and was utilized as a proof-of-concept to ensure a worst case scenario effect for use of bisphosphonate effects on skeletal metabolism.
In the study presented here, ZA was efficacious in increasing bone quantity (size), and therefore bending strength. However, these benefits were accompanied by a persistent decrease in bone tissue mineral density (TMD) and diminished flexural strength and modulus at 26 weeks, suggesting that ZA treatment results in decreased material properties long-term. PTH treatment had minimal effects on morphology, density, and strength. The effects of PTH treatment in a juvenile animal model may be more robust if endogenous anabolic activity is impaired, as in radiation therapy, disuse, disease, or injury models. Further evaluation of ZA and PTH treatment effects on growing bones is still required, including investigation into how these drugs affect fracture toughness and matrix quality in a juvenile animal model of limited field radiotherapy.
Supplementary Material
Trabecular number, thickness, spacing, connectivity density, structure model index, and degree of anisotropy for the metaphyseal and epiphyseal trabecular compartments (VOIs shown in Figure 4A & C) of femurs from growing juvenile mice treated with vehicle (VEH), parathyroid hormone (PTH), and zoledronic acid (ZA). Data is presented as mean ± standard deviation. Arrows (↑↓) indicate significant differences from vehicle (p < 0.05). Asterisks (*) indicate outliers for the outcome measure.
Body mass, body length (rostrum to tail), lean and fat body composition, and BMD (total body minus cranium) of growing juvenile mice treated with vehicle (VEH), parathyroid hormone (PTH), and zoledronic acid (ZA). Data is presented as mean ± standard deviation. Arrows (↑↓) indicate significant differences from vehicle (p < 0.05).
Distal femur bone mineral density (BMD) of growing juvenile mice treated with vehicle (VEH), parathyroid hormone (PTH), and zoledronic acid (ZA). Data is presented as mean ± standard deviation. Arrows (↑↓) indicate significant differences from vehicle (p < 0.05).
SIGNIFICANCE.
In healthy, growing, juvenile mice, limited-duration PTH treatment appears to have no short-term or long-term adverse effects on femur strength, morphology, body size, or body composition, and does not have a strong anabolic effect on skeletal growth. Zoledronic acid induced long-term increases in femur strength and bone quantity in growing mice, but may have late adverse effects on bone material quality.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number #AR065414, the Jim and Juli Boeheim Foundation, and by the David G. Murray Endowment (SUNY Upstate Medical University). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions: MEO, KAM, and TAD were responsible for study design and concept development. CMB, MEO, KAM, NDZ, and BBB contributed to study execution and data collection. CMB, MEO, and KAM, were responsible for data analysis and presentation. All authors were involved in manuscript preparation.
LITERATURE CITED
- 1.Golden NH, Abrams SA Committee on N. Optimizing bone health in children and adolescents. Pediatrics. 2014;134:e1229–1243. doi: 10.1542/peds.2014-2173. [DOI] [PubMed] [Google Scholar]
- 2.Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics. 2006;117:e291–297. doi: 10.1542/peds.2005-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rauch F, Travers R, Plotkin H, et al. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest. 2002;110:1293–1299. doi: 10.1172/JCI15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward L, Tricco AC, Phuong P, et al. Bisphosphonate therapy for children and adolescents with secondary osteoporosis. Cochrane Database Syst Rev. 2007:CD005324. doi: 10.1002/14651858.CD005324.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geoffroy V, Paschalis EP, Libouban H, et al. Effects of risedronate in Runx2 overexpressing mice, an animal model for evaluation of treatment effects on bone quality and fractures. Calcif Tissue Int. 2011;88:464–475. doi: 10.1007/s00223-011-9480-6. [DOI] [PubMed] [Google Scholar]
- 6.Shoji S, Tabuchi M, Miyazawa K, et al. Bisphosphonate inhibits bone turnover in OPG(−/−) mice via a depressive effect on both osteoclasts and osteoblasts. Calcif Tissue Int. 2010;87:181–192. doi: 10.1007/s00223-010-9384-x. [DOI] [PubMed] [Google Scholar]
- 7.Del Fattore A, Cappariello A, Capulli M, et al. An experimental therapy to improve skeletal growth and prevent bone loss in a mouse model overexpressing IL-6. Osteoporos Int. 2014;25:681–692. doi: 10.1007/s00198-013-2479-2. [DOI] [PubMed] [Google Scholar]
- 8.Sinder BP, Salemi JD, Ominsky MS, et al. Rapidly growing Brtl/+ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment. Bone. 2015;71:115–123. doi: 10.1016/j.bone.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinder BP, Lloyd WR, Salemi JD, et al. Effect of anti-sclerostin therapy and osteogenesis imperfecta on tissue-level properties in growing and adult mice while controlling for tissue age. Bone. 2016;84:222–229. doi: 10.1016/j.bone.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen M, Burr D. Parathyroid hormone and bone biomechanics. Clinic Rev Bone Miner Metab. 2006;4:259–268. [Google Scholar]
- 11.Stewart AF, Cain RL, Burr DB, et al. Six-Month Daily Administration of Parathyroid Hormone and Parathyroid Hormone—Related Protein Peptides to Adult Ovariectomized Rats Markedly Enhances Bone Mass and Biomechanical Properties: A Comparison of Human Parathyroid Hormone 1–34, Parathyroid Hormone-Related Protein 1–36, and SDZ-Parathyroid Hormone 893. Journal of Bone and Mineral Research. 2000;15:1517–1525. doi: 10.1359/jbmr.2000.15.8.1517. [DOI] [PubMed] [Google Scholar]
- 12.Gunness M, Hock JM. Bone Morphometry 1992 Sixth International Congress ProceedingsAnabolic effect of parathyroid hormone on cancellous and cortical bone histology. Bone. 1993;14:277–281. doi: 10.1016/8756-3282(93)90152-z. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe A, Yoneyama S, Nakajima M, et al. Osteosarcoma in Sprague-Dawley rats after long-term treatment with teriparatide (human parathyroid hormone (1-34)) J Toxicol Sci. 2012;37:617–629. doi: 10.2131/jts.37.617. [DOI] [PubMed] [Google Scholar]
- 14.Vahle JL, Long GG, Sandusky G, et al. Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol. 2004;32:426–438. doi: 10.1080/01926230490462138. [DOI] [PubMed] [Google Scholar]
- 15.Subbiah V, Madsen VS, Raymond AK, et al. Of mice and men: divergent risks of teriparatide-induced osteosarcoma. Osteoporos Int. 2010;21:1041–1045. doi: 10.1007/s00198-009-1004-0. [DOI] [PubMed] [Google Scholar]
- 16.Bang UC, Hyldstrup L, Jensen JE. The impact of recombinant parathyroid hormone on malignancies and mortality: 7 years of experience based on nationwide Danish registers. Osteoporos Int. 2014;25:639–644. doi: 10.1007/s00198-013-2470-y. [DOI] [PubMed] [Google Scholar]
- 17.Russell RG, Watts NB, Ebetino FH, et al. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 18.Krause M, Soltau M, Zimmermann EA, et al. Effects of long-term alendronate treatment on bone mineralisation, resorption parameters and biomechanics of single human vertebral trabeculae. Eur Cell Mater. 2014;28:152–163. doi: 10.22203/ecm.v028a12. discussion 163–155. [DOI] [PubMed] [Google Scholar]
- 19.Shahnazari M, Yao W, Dai W, et al. Higher doses of bisphosphonates further improve bone mass, architecture, and strength but not the tissue material properties in aged rats. Bone. 2010;46:1267–1274. doi: 10.1016/j.bone.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell GM, Bernhardt R, Scharnweber D, et al. The bone architecture is enhanced with combined PTH and alendronate treatment compared to monotherapy while maintaining the state of surface mineralization in the OVX rat. Bone. 2011;49:225–232. doi: 10.1016/j.bone.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Zhu ED, Louis L, Brooks DJ, et al. Effect of bisphosphonates on the rapidly growing male murine skeleton. Endocrinology. 2014;155:1188–1196. doi: 10.1210/en.2013-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doube M, Klosowski MM, Arganda-Carreras I, et al. BoneJ: Free and extensible bone image analysis in ImageJ. Bone. 2010;47:1076–1079. doi: 10.1016/j.bone.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang SY, Allen MR, Phipps R, et al. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20:887–894. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy GP, Wood MB, Rock MG, et al. Vascularized free fibular transfer combined with autografting for the management of fracture nonunions associated with radiation therapy. J Bone Joint Surg Am. 2000;82:544–554. doi: 10.2106/00004623-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Paulino AC. Late effects of radiotherapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys. 2004;60:265–274. doi: 10.1016/j.ijrobp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Adler RA, Fuleihan GE, Bauer DC, et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 28.Odvina CV, Zerwekh JE, Rao DS, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 29.Johnston S, Andrews S, Shen V, et al. The effects of combination of alendronate and human parathyroid hormone(1-34) on bone strength are synergistic in the lumbar vertebra and additive in the femur of C57BL/6J mice. Endocrinology. 2007;148:4466–4474. doi: 10.1210/en.2007-0229. [DOI] [PubMed] [Google Scholar]
- 30.Altman AR, Tseng WJ, de Bakker CM, et al. Quantification of skeletal growth, modeling, and remodeling by in vivo micro computed tomography. Bone. 2015;81:370–379. doi: 10.1016/j.bone.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Oronzo S, Stucci S, Tucci M, et al. Cancer treatment-induced bone loss (CTIBL): pathogenesis and clinical implications. Cancer Treat Rev. 2015;41:798–808. doi: 10.1016/j.ctrv.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Chandra A, Lan S, Zhu J, et al. PTH prevents the adverse effects of focal radiation on bone architecture in young rats. Bone. 2013;55:449–457. doi: 10.1016/j.bone.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandra A, Lin T, Tribble MB, et al. PTH1-34 alleviates radiotherapy-induced local bone loss by improving osteoblast and osteocyte survival. Bone. 2014;67:33–40. doi: 10.1016/j.bone.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Postnov A, De Schutter T, Sijbers J, et al. Glucocorticoid-induced osteoporosis in growing mice is not prevented by simultaneous intermittent PTH treatment. Calcif Tissue Int. 2009;85:530–537. doi: 10.1007/s00223-009-9301-3. [DOI] [PubMed] [Google Scholar]
- 35.Wernle JD, Damron TA, Allen MJ, et al. Local irradiation alters bone morphology and increases bone fragility in a mouse model. J Biomech. 2010;43:2738–2746. doi: 10.1016/j.jbiomech.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Ferretti JL, Cointry GR, Capozza RF, et al. Effects of bisphosphonates on the mechanical efficiency of normal and osteopenic bones. Medicina (B Aires) 1997;57(Suppl 1):83–92. [PubMed] [Google Scholar]
- 37.Allen MR, Burr DB. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don’t know. Bone. 2011;49:56–65. doi: 10.1016/j.bone.2010.10.159. [DOI] [PubMed] [Google Scholar]
- 38.Boivin G, Meunier PJ. Effects of bisphosphonates on matrix mineralization. J Musculoskelet Neuronal Interact. 2002;2:538–543. [PubMed] [Google Scholar]
- 39.Renders GA, Vermeer JA, Leung PM, et al. Implications of high-dosage bisphosphonate treatment on bone tissue in the jaw and knee joint. Calcif Tissue Int. 2014;95:436–445. doi: 10.1007/s00223-014-9912-1. [DOI] [PubMed] [Google Scholar]
- 40.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 41.Reilly DT, Burstein AH. The Mechanical Properties of Cortical Bone. The Journal of Bone & Joint Surgery. 1974;56:1001–1022. [PubMed] [Google Scholar]
- 42.John DC. Role of collagen and other organics in the mechanical properties of bone. Osteoporos Int. 2003;14(Suppl 5):S29–36. doi: 10.1007/s00198-003-1470-8. [DOI] [PubMed] [Google Scholar]
- 43.Boskey AL, Marino J, Spevak L, et al. Are Changes in Composition in Response to Treatment of a Mouse Model of Osteogenesis Imperfecta Sex-dependent? Clin Orthop Relat Res. 2015;473:2587–2598. doi: 10.1007/s11999-015-4268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motyl KJ, McCauley LK, McCabe LR. Amelioration of type I diabetes-induced osteoporosis by parathyroid hormone is associated with improved osteoblast survival. J Cell Physiol. 2012;227:1326–1334. doi: 10.1002/jcp.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheyn D, Cohn Yakubovich D, Kallai I, et al. PTH promotes allograft integration in a calvarial bone defect. Mol Pharm. 2013;10:4462–4471. doi: 10.1021/mp400292p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keenawinna L, Oest ME, Mann KA, et al. Zoledronic acid prevents loss of trabecular bone after focal irradiation in mice. Radiat Res. 2013;180:89–99. doi: 10.1667/RR3200.1. [DOI] [PubMed] [Google Scholar]
- 47.Arrington SA, Fisher ER, Willick GE, et al. Anabolic and antiresorptive drugs improve trabecular microarchitecture and reduce fracture risk following radiation therapy. Calcif Tissue Int. 2010;87:263–272. doi: 10.1007/s00223-010-9390-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trabecular number, thickness, spacing, connectivity density, structure model index, and degree of anisotropy for the metaphyseal and epiphyseal trabecular compartments (VOIs shown in Figure 4A & C) of femurs from growing juvenile mice treated with vehicle (VEH), parathyroid hormone (PTH), and zoledronic acid (ZA). Data is presented as mean ± standard deviation. Arrows (↑↓) indicate significant differences from vehicle (p < 0.05). Asterisks (*) indicate outliers for the outcome measure.
Body mass, body length (rostrum to tail), lean and fat body composition, and BMD (total body minus cranium) of growing juvenile mice treated with vehicle (VEH), parathyroid hormone (PTH), and zoledronic acid (ZA). Data is presented as mean ± standard deviation. Arrows (↑↓) indicate significant differences from vehicle (p < 0.05).
Distal femur bone mineral density (BMD) of growing juvenile mice treated with vehicle (VEH), parathyroid hormone (PTH), and zoledronic acid (ZA). Data is presented as mean ± standard deviation. Arrows (↑↓) indicate significant differences from vehicle (p < 0.05).