Abstract
Adolescents with acute lymphoblastic leukemia (ALL) develop osteopenia early in therapy, potentially exacerbated by high rates of concurrent Vitamin D deficiency. We conducted a randomized clinical trial testing a Vitamin D-based intervention to improve Vitamin D status and reduce bone density decline. Poor adherence to home supplementation necessitated a change to directly observed therapy (DOT) with intermittent, high-dose Vitamin D3 randomized versus standard of care (SOC). Compared to SOC, DOT Vitamin D3 successfully increased trough Vitamin 25(OH)D levels (p=0.026) with no residual Vitamin D deficiency, 100% adherence to DOT Vitamin D3, and without associated toxicity. However, neither Vitamin D status nor supplementation impacted bone density. Thus, this adherence-optimized intervention is feasible and effective to correct Vitamin D deficiency in adolescents during ALL therapy. Repletion of Vitamin D and calcium alone did not mitigate osteopenia, however, and new, comprehensive approaches are needed to address treatment-associated osteopenia during ALL therapy.
Keywords: Acute lymphoblastic leukemia, Vitamin D, bone mineral density, osteopenia, adolescent and young adult, medication adherence
INTRODUCTION
Treatment for pediatric acute lymphoblastic leukemia (ALL) commonly results in glucocorticoid-induced osteopenia and osteonecrosis [1]. Risk stratification of glucocorticoid delivery has decreased the prevalence of osteonecrosis [2, 3] but not therapy-related osteopenia and structural changes to bone [1, 4]. Current evidence now indicates osteopenia develops during the early phases of intensive treatment [5, 6, 7, 8], and even within the first 28 days of chemotherapy alone (i.e. the “Induction” phase) [9]. The deficits in bone remodeling that occur during ALL therapy are multifactorial. Glucocorticoids, methotrexate, and other lymphoid-directed chemotherapies directly affect osteoblasts and osteoclasts[10], while prolonged periods of decreased weight-bearing activity due to fatigue and other comorbidities [11, 12] may also contribute to osteopenia and long-term fracture risk.
Adolescents are at greater risk than younger children for osteopenia and fractures both during [13, 14] and after ALL therapy [15, 16]. We hypothesized that in this adolescent population receiving osteotoxic chemotherapy [10], Vitamin D insufficiency or deficiency potentiates early-onset osteopenia and that repletion of Vitamin D would not reverse the process but would slow its development and/or mitigate its severity. We therefore conducted a randomized clinical trial to treat Vitamin D insufficiency/deficiency in a cohort of adolescents undergoing therapy for newly-diagnosed ALL. The primary aim was to test the feasibility and efficacy of the intervention to normalize serum Vitamin D levels. Key secondary aims explored changes in body composition during ALL therapy, and compared bone mineral density and geometry between the two randomized groups. We previously reported [9] the pre-randomization observation that adolescents treated for newly-diagnosed ALL exhibit Vitamin D deficiency at baseline more frequently than expected [17] and a precipitous onset of osteopenia by end of Induction. We now report the results of the randomized intervention to correct the observed Vitamin D deficiency.
PATIENTS AND MATERIALS
Study Design
Adolescents between 10–21 years of age [18] newly diagnosed with de novo B-ALL or T-ALL were eligible for study. As described previously [19], all subjects were enrolled prior to the end of Induction and treated following contemporary, lineage-directed Children’s Oncology Group regimens. This included up to 28 days of glucocorticoid steroids during Induction, 5gm/m2 of “high-dose” methotrexate (HDMTX) during Interim Maintenance (IM), and repeat steroid pulses during Delayed Intensification (DI) [2]. The study was IRB-approved and informed consent was documented prior to enrollment. The study was registered on ClinicalTrials.gov (NCT01317940).
Due to in vitro evidence for a potential adverse influence of high-doses of Vitamin D on glucocorticoid cytotoxicity [20], a delayed randomization post-Induction was used to avoid any theoretical interaction with curative leukemia therapy. Enrolled subjects eligible for randomization if they were Vitamin D insufficient as per the Endocrine Society guidelines (25-hydroxy vitamin D (Vitamin 25(OH)D) <30 ng/ml) [21], had no underlying bone disorders, and had not previously received bone modulating agents. A randomization table was provided by the statistician and stratified on Body Mass Index percentile (BMI%) according to CDC definitions for obese, overweight, and normal. The study opened as a double-blinded, placebo-controlled prospective trial testing a daily oral combination Vitamin D3 + calcium citrate (2,000IU + 1,000mg respectively, Douglas Laboratories, Pittsburgh, PA). A planned interim analysis following the first one-third of target accrual revealed markedly poor adherence to study medication. The study was then amended to an open-label trial testing an adherence-optimized novel supplementation regimen using principles of Directly Observed Therapy (DOT) [22] randomized 2:1 versus standard of care (SOC; permuted blocks of size 3). All subjects received education regarding the risks for therapy-induced osteopenia at time of enrollment. The DOT group received oral “high dose” Vitamin D3 100,000IU (10,000IU/1ml, Douglas Laboratories, Pittsburgh, PA) administered in the clinic setting at the start of each of the three included chemotherapy phases (~2 month intervals). This interval was selected based on cholecalciferol pharmacokinetics [23] and from efficacy seen in a non-oncologic pediatric population [24]. Calcium supplementation was changed to flavored, chewable tablets to promote adherence. The calcium tablets were taken on an empty stomach to provide 400mg elemental calcium carbonate twice daily. Adherence was measured by DOT, tablet counts, and self-report; the overall duration of supplementation was recorded. The SOC group received routine encouragement from their clinical team regarding activity and ad hoc nutritional monitoring. A revised power analysis for the open-label randomization demonstrated continued 80% power to detect a 50% difference in normalization of vitamin D levels between the intervention and SOC with a Type I error of 5%. All subjects enrolled but not eligible for the open-label randomization (i.e. those Vitamin D sufficient at end of Induction, failure to meet organ function criteria, or documented non-adherence to the initial formulation) were followed as an internal “natural-history” group to further explore potential associations among serum Vitamin 25(OH)D, body fat, osteopenia, and bone structure. The study period comprised the interval between end of Induction through end of DI; the time-period for intervention was selected to target the period of intensive chemotherapy previously associated with greatest risk for osteopenia or fracture [5, 6, 8].
Study Outcomes
The study’s primary outcome measure was change in serum Vitamin 25(OH)D level as measured by liquid chromatography-tandem mass spectrometry at Quest Diagnostics Nichols Institute (San Juan Capistrano, CA). Adherence to supplementation was defined as ≥75% of the study medication by DOT, self-report, and tablet counts. Secondary aims examined the effects of the intervention on structural bone changes. Osteopenia was assessed by volumetric bone density (vBMD) in three-dimensional detail using quantitative computerized tomography (QCT). The details of the imaging protocols have been described previously [9]. Briefly, cancellous vBMD was assessed at L1-L3 of the lumbar spine (vBMDLS). Cortical vBMD and geometric properties of bone were obtained at the femur mid-shaft (vBMDFem). In the “natural history” cohort, cortical vBMD was instead obtained at the tibia mid-shaft (vBMDTib). Body composition was concurrently measured using the gold-standard whole-body dual-energy X-ray absorptiometry (DXA, fan beam densitometer in array mode [Delphi W; Hologic, Inc., Waltham, MA]). Changes in bone structure and geometry by QCT, Vitamin 1,25-dihydroxyvitamin D (Vitamin 1,25 (OH)D), and serum markers of bone metabolism were compared between randomized groups. Serial self-report using the WHO Health Behavior in School-aged Children physical activity survey (WHO HBSC) [25] recorded frequency of moderate-exertion exercise and level of sedentary behavior during therapy. Targeted toxicities of Vitamin D therapy (hypervitaminosis, hypercalcemia, nephrolithiasis, renal insufficiency, and transaminitis) were monitored.
Statistical Considerations
For the randomized cohort, repeated-measure linear regression evaluated the intervention’s effect on change in Vitamin 25(OH)D over time. During the clinical trial, the Institute of Medicine (IOM) published strict, physiologically-defined thresholds for insufficiency (<20ng/ml) and deficiency (<12 ng/ml) [26]. While the ES thresholds were preserved to determine eligibility, the IOM thresholds were instead used to categorically analyze the prevalence and physiologic impact of Vitamin D status during therapy. Linear regression models analyzed the association of body fat tertiles (<30%, 31–39%, ≥40%) on Vitamin 25(OH)D level. Metrics of adherence are reported descriptively (calculated as doses delivered/doses prescribed). For the entire enrolled cohort, we explored the effect of Vitamin 25(OH)D status and body composition on vBMD and structure. To evaluate the effect of cumulative Vitamin D3 exposure on bone density, we performed an area-under-curve (AUC) type analysis of trough Vitamin 25(OH)D levels using linear regression to model the association of AUC with vertebral and cortical vBMD. Similar regression models evaluated the effect of body fat/body composition on all pre- and post- comparisons of vBMD, structure, and serum markers. For cortical bone, we examined these effects overall and separately for vBMDFem and vBMDTib. The effects of exercise and sedentary behavior on change in body fat and vBMD were analyzed as both predictive and moderating variables. All randomized outcomes were analyzed as intent-to-treat. All analyses were two-sided with significance set at p<0.05. Statistical computations were performed using STATA (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
RESULTS
Fifty-one subjects were enrolled on the study between May 2011 and November 2014. Of these, 29 were enrolled on the open-label randomization (Supplemental Figure 1, CONSORT diagram) and 20 followed in the “natural history” group. No significant differences were detected in the demographic, body composition, and treatment characteristics among the intervention, SOC, and “natural history” groups (Table 1). No differences between groups were found between those in early versus late adolescence (10–14 and 15–21 years respectively) [18]. A higher prevalence of overweight or obesity (41%) was observed compared with the national adolescent average (34%) [27]. Consistent with institutional demographics, the cohort ethnicity was preponderantly Hispanic (84%).
Table 1.
Detailed description of cohort
| Intervention | Standard of Care | Natural History1 | p-value2 | |||||
|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |||
| Total Cohort | 19 | (100%) | 10 | (100%) | 20 | (100%) | – | |
| Age, Year | Median (range) | 15.2 | (11–19) | 14.6 | (11–18) | 13.8 | (10–19) | 0.4243 |
| <15 years | 10 | (53%) | 3 | (30%) | 7 | (35%) | 0.394 | |
| ≥15 years | 9 | (47%) | 7 | (70%) | 13 | (65%) | ||
| Sex | Male | 13 | (68%) | 6 | (60%) | 11 | (55%) | 0.688 |
| Ethnicity | Hispanic | 17 | (89%) | 8 | (80%) | 16 | (80%) | 0.688 |
| Not-Hispanic | 2 | (11%) | 2 | (20%) | 4 | (20%) | ||
| Phenotype | B-ALL | 15 | (79%) | 10 | (100%) | 15 | (75%) | 0.231 |
| T-ALL* | 4 | (21%) | 0 | (0%) | 5 | (25%) | ||
| Tanner Stage | 1 | 1 | (6%) | 1 | (10%) | 5 | (25%) | 0.198 |
| 2 | 1 | (6%) | 1 | (10%) | 2 | (25%) | ||
| 3 | 0 | (0%) | 0 | (0%) | 3 | (10%) | ||
| 4 | 5 | (28%) | 3 | (30%) | 1 | (5%) | ||
| 5 | 11 | (61%) | 5 | (50%) | 9 | (45%) | ||
| BMI stratum | <85% | 10 | (53%) | 5 | (50%) | 14 | (70%) | 0.779 |
| 85 to <95% | 4 | (21%) | 2 | (20%) | 3 | (15%) | ||
| ≥95% | 5 | (26%) | 3 | (30%) | 3 | (15%) | ||
| EOI Vitamin D4 | Sufficient | 8 | (42%) | 4 | (40%) | 10 | (50%) | 0.906 |
| Insufficient | 11 | (53%) | 6 | (60%) | 9 | (45%) | ||
| Deficient | 1 | (5%) | 0 | (0%) | 1 | (5%) | ||
Includes non-adherent subjects enrolled prior to open-label amendment (n=16) and those not eligible for randomization (n=4), excludes subjects who did not reach time of randomization (n=2).
pearson Chi-square unless otherwise specified.
Analysis of variance.
Vitamin D category at time of randomization [end of Induction] per Institute of Medicine thresholds.
One T-ALL patient was treated on the AALL0434 study without high-dose methotrexate. B-ALL = B-precursor ALL; T-ALL = T-cell ALL; BMI = Body Mass Index, EOI = End of Induction
Effect of Intervention on Vitamin D
Supplementation continued for a median of 6.7 months (range 5.5–8.7). Intermittent Vitamin D3 administered by DOT during intensive pre-maintenance chemotherapy increased trough serum Vitamin 25(OH)D levels from 19.5±4.8 at baseline to 26.5±12.4 ng/ml at study end versus no change for the SOC group (18.5±4.2 to 19.0±7.4 ng/ml) (Figure 1A). By study end, Vitamin 25(OH)D levels in the DOT Vitamin D3 group were, on average, +5.5±1.7 ng/ml higher than baseline as compared to −0.30 ± 2.2 ng/ml for the SOC arm (p=0.026). The distribution of IOM-defined Vitamin D insufficiency and deficiency during therapy by treatment arm is depicted in Figure 1B. As would be expected during effective repletion,[28] Vitamin 1,25(OH)D levels were inversely related to serum Vitamin 25(OH)D and decreased in the intervention arm but remained stable in the SOC group (Intervention 43.8±18.3 to 25.35±14.8 versus SOC 45.8±17.5 to 37.6±27.6 pg/ml, p=0.062). Serum Vitamin 25(OH)D was not related to baseline percentage body fat (p=0.460).
Figure 1. Effect of intervention on Vitamin 25(OH)D status and levels during therapy.
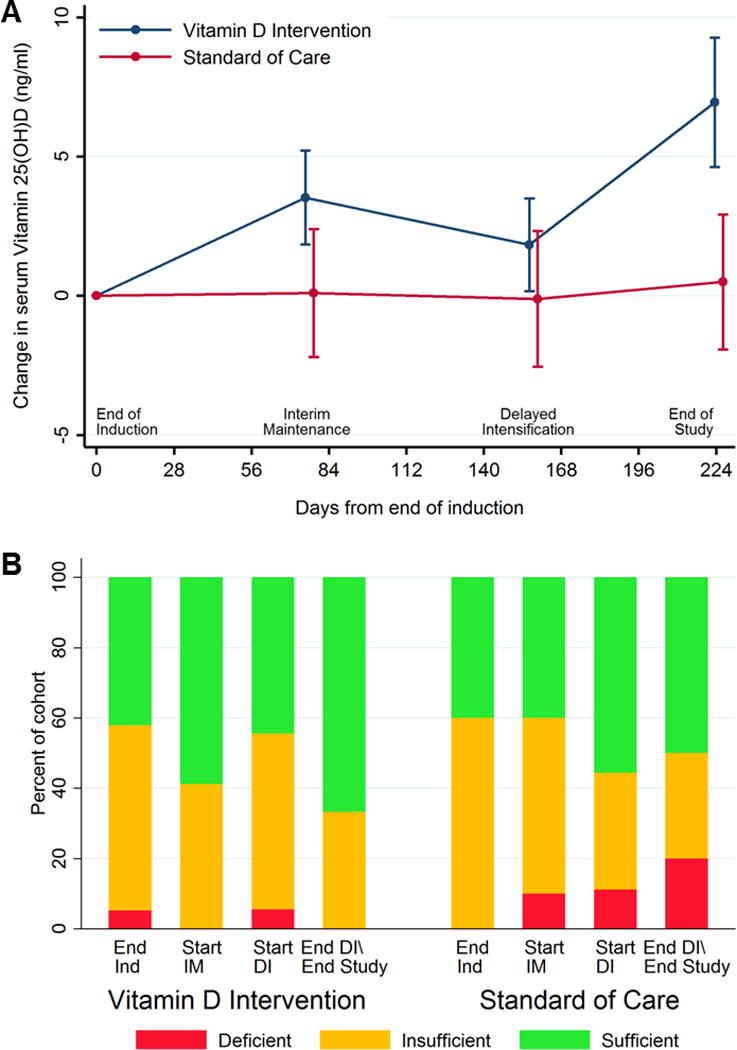
(A) Change in trough Vitamin 25(OH)D levels from the start of intervention. Depicted is mean ±S.E. of change from baseline computed within treatment arm and time-point. (B) Distribution of IOM-defined Vitamin D status for each study time-point during the intervention period.
Effects of Intervention on Bone
As shown in Table 2 and Figure 2, despite improvement in Vitamin D status, no significant difference were observed for cancellous vBMDLS, cortical vBMDFem, or bone structure and geometry between randomized groups. Similarly, no differences between randomized groups were observed for biochemical markers of bone turnover (Table 2). No clinically-evident fractures occurred during the study period. For the entire cohort, cancellous vBMDLS decreased between end of Induction and end of DI (mean change – 44.0±6.6mg/cm3, p<0.001) but no significant changes were found in cortical vBMD (mean change at tibia −3.6±4.9mg/cm3, p=0.47 and femur +8.5±10.5mg/cm3, p=0.43). Similarly irrespective of randomized group, biochemical markers of bone metabolism indicated overall “recovery” following Induction with net bone formation by end of DI. While not yet reflected in change in vBMD, the bone resorption marker C-telopeptide decreased on average −654±143pg/ml (p<0.001), while bone formation markers Bone-specific alkaline phosphatase and osteocalcin increased by 27.8±5.7mcg/L and 21.8±3.5ng/ml, respectively (both p<0.001). IOM-defined Vitamin D category was not associated with differences in vBMDLS (p=0.11); nor was cumulative Vitamin D by AUC significantly associated with cancellous or cortical vBMD (p=0.72 and 0.71 respectively, Figure 3A and 3B). Finally, while the majority of the cohort experienced significant bone loss during Induction [9], earlier sensitivity to bone loss/turnover during Induction did not impact the subsequent efficacy of the intervention.
TABLE 2.
Effect of vitamin D and calcium intervention on bone metabolism
| Characteristics of Bone1 | Intervention (Mean±SD) | Standard of Care (Mean±SD) | p-value2 | ||
|---|---|---|---|---|---|
| End of Induction | End of Delayed Intensification | End of Induction | End of Delayed Intensification | ||
| Radiographic Measures | |||||
| Cancellous vBMDLS (cm3) | 249.3±71.0 | 203.8±77.1 | 234.6±52.0 | 201.4±66.4 | 0.432 |
| Cortical vBMDFem (cm3) | 2091.4±43.5 | 2093.1±62.5 | 2081.7±66.2 | 2090.9±26.7 | 0.915 |
| Cortical Area (mm2) | 407.3±90.5 | 405.4±101.3 | 394.3±73.7 | 389.8±75.3 | 0.945 |
| Medullary Canal Area (mm2) | 119.8±27.1 | 137.3±39.9 | 130.1±36.7 | 134.4±48.6 | 0.555 |
| Average Cortical Thickness (mm) | 6.75±1.3 | 6.51±1.5 | 6.53±0.7 | 6.40±0.8 | 0.621 |
| Max Cortical Thickness (mm) | 9.25±1.8 | 8.68±2.1 | 9.47±1.0 | 8.98±1.3 | 0.627 |
| Imax (mm4)3 | 26.4±10.0 | 26.7±9.6 | 27.4±11.6 | 27.0±12.0 | 0.716 |
| Imin (mm4)3 | 180.1±63.4 | 191.0±66.0 | 168.3±56.0 | 169.5±67.2 | 0.302 |
| J (mm4)3 | 44.4±15.7 | 45.8±16.0 | 44.2±17.1 | 44.0±18.6 | 0.397 |
| Biochemical Measures | |||||
| Corrected Calcium (mg/dl)4 | 9.24±0.22 | 9.40±0.54 | 9.38±0.39 | 9.38±0.46 | 0.516 |
| Phosphorus (mg/dl) | 4.2±1.0 | 4.3±0.9 | 3.7±1.0 | 4.5±0.7 | 0.957 |
| Intact PTH (pg/ml)5 | 73.6±36.1 | 38.8±25 | 80.7±47.6 | 50.0±16.8 | 0.549 |
| Osteocalcin (ng/ml) | 10.2±8.9 | 29.5±15.8 | 5.2±4.3 | 28.7±15.6 | 0.652 |
| Bone-specific ALK (mcg/L) | 18.6±8.8 | 48.3±32.4 | 20.9±9.7 | 53.1±59.7 | 0.856 |
| C-telopeptide (pg/ml) | 1467±770 | 971±362 | 1524±603 | 833±365 | 0.461 |
Cortical measures excludes two subjects who switched radiographic assessment site from tibia to femur at time of amendment.
Differences in slopes between study arms, repeated measures linear regression.
in thousands.
Corrected for hypoalbuminemia.
Assay age-specific norms range 9–74 pg/ml.
vBMD= volumetric bone mineral density at lumbar spine (LS) or femur (Fem). I = principal moment of inertia. J = polar moment of inertia. PTH = parathyroid hormone. Bone-specific ALK = Bone-specific alkaline phosphatase
Figure 2. Effect of intervention on changes to bone density.

Change in cancellous and cortical volumetric bone mineral density according to randomized arm. Median and interquartile range are depicted.
Figure 3. Association of cumulative Vitamin 25(OH)D exposure and change in volumetric bone mineral density.

Association of cumulative exposure to Vitamin D using an area under the curve analysis of trough Vitamin 25(OH)D levels during therapy and change in vBMD for (A) cancellous bone at the lumbar spine and (B) overall cortical bone at femur or tibia.
Body fat was found to be a significant predictor of loss of vBMDLS. When examined in a cross-sectional regression model of all values, every additional 1% of body fat was associated with −4.5±0.8 mg/cm3 lower vBMDLS (Supplemental Figure 2A). A similar effect of body fat was not seen for cortical vBMD at the femur or tibia (Supplemental Figure 2B), nor for bone geometry and structure (not shown). Lean muscle mass loss showed a near-perfect converse correlation with body fat gain. As reported on the WHO HBSC survey, increased frequency of moderate exercise and less sedentary behavior were inversely associated with body fat (p=0.009). While these behaviors were also inversely associated with loss of vBMDLS over time (p<0.001), examination of body fat, exercise, and sedentary behavior together showed body fat demonstrated the strongest association with lower vBMDLS.
Feasibility of the Adherence-Optimized Regimen
Only 3/13 subjects (23%) were adherent by report and tablet count to the initial, exclusively home-based regimen of Vitamin D+calcium supplementation, and less than 10% of prescribed doses were delivered (median 7%, range 0–110%). No difference in adherence was observed between placebo and vitamin D+calcium formulations. In contrast, the DOT Vitamin D3 intervention demonstrated excellent adherence. For the 18 subjects on the intervention arm who survived until the end of DI, 100% of the intended liquid Vitamin D doses were successfully administered to 100% of the subjects. As opposed to calcium in pill form, tablet counts revealed excellent adherence to the flavored, chewable home calcium supplementation, with the majority of the cohort (83%, n=15/18) receiving calcium supplementation throughout the study period and most (61%, n=11/18) adhering to ≥75% of their prescribed doses (median 93%, range 43–123%). Three subjects (3/18) opted to continue with oral Vitamin D3 supplementation but to discontinue the home calcium due to dislike of the calcium formulation (discontinued at +23, +36, and +45 days). Tablet counts were consistent with self-report. There were no targeted toxicities or adverse events on the study attributable to Vitamin D or calcium supplementation.
DISCUSSION
We report here the results of the first randomized trial, to our knowledge, targeting Vitamin D deficiency to mitigate the development of early-onset bone resorption in a population of adolescents treated for newly-diagnosed ALL. We discovered a high prevalence of Vitamin D insufficiency and deficiency in this population beginning at diagnosis and persisting throughout the subsequent months of chemotherapy. By using principles of DOT, we achieved 100% adherence to Vitamin D supplementation and found that intermittent high-dose Vitamin D3 administered in a clinic setting concurrently with chemotherapy was feasible, safe, and effective in increasing Vitamin D stores as evidenced by serum Vitamin 25(OH)D. This approach is therefore practical for convenient supplementation of Vitamin D for adolescents receiving treatment for ALL typically necessitating frequent outpatient visits. However, while we previously showed osteopenia to develop during Induction [9], we found here that neither Vitamin D and calcium supplementation nor normalized Vitamin 25(OH)D level prevented further deterioration of bone density or structure, indicating that a multimodal intervention beyond supplementation alone is required to alter the course of this toxicity. Nonetheless, for adolescent patients requiring Vitamin D supplementation, the directly observed regimen described here offers clear benefit in its simplicity and efficacy. While supplementation alone was not beneficial to mitigate osteopenia, this regimen remains relevant for the widespread Vitamin D insufficiency we found during ALL therapy wherein repletion is strongly recommended [26, 28]. As seen on this and other bone health clinical trials [12], targeting non-adherence is a threshold issue for any adolescent-focused osteopenia intervention. The success of the current study lies not only in fulfilling the primary aim of increasing Vitamin D stores, but in developing a Vitamin D supplementation regimen specifically designed to optimize adherence in a difficult population.
Vitamin D is recognized as a crucial vitamin for bone formation [26], and the high prevalence of Vitamin D insufficiency and deficiency in our study population is striking. While reversal of osteopenia typically requires years of treatment, the objective of our study was to slow or lessen therapy-related changes to bone. Contrary to general recommendations for treatment of Vitamin D insufficiency to prevent bone loss [21, 26], in the context of intensive chemotherapy, we discovered Vitamin D insufficiency and deficiency did not exacerbate bone resorption nor did repletion alleviate changes to bone. The lack of association between serum Vitamin 25(OH)D levels and chemotherapy-related bone loss suggests our findings do not represent a dose-dependent failure of the intervention. The decrease in Vitamin 1,25(OH)D levels further supports that the intervention achieved physiological Vitamin D repletion in the sample [28]. As repletion of Vitamin D and calcium did not successfully mitigate therapy-associated osteopenia, sufficiency of these important substrates and regulators of bone metabolism is essential but unfortunately not sufficient to overcome the multifactorial etiology of bone loss during treatment. We would theorize that potential benefit from supplementation was offset from the direct impact of chemotherapy on osteoblast and osteoclast activity [10] compounded by decreased stimulus for bone formation from reduced weight-bearing activity seen on this study and others [11, 12]. Moreover, Vitamin D bioavailability may have been reduced by the large increases in adiposity during ALL therapy [19, 29] causing “resistance” to supplementation [30, 31] and further mitigating its benefit. While even higher doses of Vitamin D3 may be safe [32] to overcome sequestration in adipose tissue, new approaches beyond supplementation alone are likely necessary to impact this common toxicity.
Our novel description of obesity and vBMD may offer one such exploratory path forward. In our study population, we found a strong association between greater adiposity and lower cancellous vBMD during ALL therapy. Although historically, activity level and weight-associated mechanical stress are correlated with gains in bone density in the general population [33, 34], body fat and not sedentary behavior was surprisingly more strongly associated with lower bone density in our cohort. While both likely play a role in osteopenia, recent findings of adiposity and visceral fat adversely affecting bone density [35, 36, 37] support a potential independent impact of body fat. This is particularly relevant due to ALL therapy’s propensity to stimulate visceral fat gain [38] and increase obesity and overall adiposity [19, 29]. As seen here, cancellous bone may be more susceptible to the influence of adiposity, although a cumulative effect of adiposity on slow-changing cortical bone over years of therapy was not explored in this study. Behavioral approaches focused on reducing the impact of obesity may be optimal for some patients, but comorbidities and/or the complexity of such an approach may instead warrant pharmaceutical interventions for many others. Bisphosphonates have proven benefit to improve bone mineral density and prevent fracture risk across multiple populations [39]. Early small trials of bisphosphonates during ALL therapy suggest oral formulations may improve bone density and be better tolerated [40, 41] than intravenous administration [42], but large-scale studies in ALL have not been performed to date. Emerging new agents such as RANKL inhibitors offer additional promise to directly reduce osteoclast activity with less potential risk for adverse chemotherapy interactions [43], but will similarly require extensive testing to guide adoption into a chemotherapy platform. Prior to implementing such trials, renewed scrutiny is essential to develop predictive pediatric ALL models to delineate those at high risk for fractures to optimize a risk-stratified approach to such behavioral and/or pharmaceutical interventions.
The trial achieved its primary aim to address Vitamin D insufficiency but has some limitations. The study was conducted as a prospective, randomized trial, but we acknowledge the small sample size requires replication among a larger, multi-institution cohort. Similarly, the change in study design preserved power for the primary, randomized aim but limited our ability to detect differences in secondary outcomes. We are confident that trends observed here are consistent with those likely to be seen in a larger cohort, but cannot exclude the possibility that subtle benefits were not detectable. Moreover, we also acknowledge the difference in goals for use of Vitamin D supplementation. We would emphasize our secondary objective for the relatively short study time-frame was to alleviate or slow the development of osteopenia, not to reverse it. While mitigating exacerbation of osteopenia was a reasonable expectation in the context of prevalent Vitamin D deficiency, reversal requires prolonged treatment beyond the duration of the study intervention. However, as the regimen was effective in replenishing Vitamin D, continuation of the intervention into the Maintenance phase, a traditionally challenging period for adherence [44], might offer additional benefit not seen here. Dietary assessment was beyond the study’s purview as we instead investigated the direct measure of objective serum levels to gauge the influence of Vitamin D status on osteopenia; we therefore cannot comment on subtle differences in typical dietary intake during therapy. Exploration of a potential effect of ethnicity or race on adherence and study outcomes was also limited by our preponderantly Hispanic cohort.
We report here a new, well-tolerated, adherence-promoting, and effective regimen for integrating Vitamin D supplementation into ALL chemotherapy. While Vitamin D in addition to routine activity did not limit the development of osteopenia on our study, this regimen provides an approach upon which to build a comprehensive strategy to prevent or reverse this common adverse health outcome.
Supplementary Material
Supplemental Figure 1: CONSORT diagram for study enrollment
Supplemental Figure 2: Association between percentage body fat and bone density
Association across all time-points of body fat percentage as assessed by DXA and (A) cancellous bone density measured at the spine (B) cortical bone density measured at the tibia and at the femur.
Acknowledgments
The study team would like to thank our research coordinator, Swati Gulati, as well as Lisa Villanueva and Mercedes Lavanderia from the Department of Radiology, and the patients and families who participated on the clinical trial.
Funding: Leukemia and Lymphoma Society (LLS-6249-11), Hyundai Hope on Wheels Foundation, NIH/NCATS (UL1TR000130) via the SC-CTSI (CHLA). The content contained herein is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: AM Butturini is currently employed by Puma Biotechnology, Inc.
DISCLOSURE STATEMENT
Dr. Butturini has since taken a position at Puma Biotechnology, Inc. The authors report no other conflicts of interest. No other disclosures to report.
References
- 1.Haddy TB, Mosher RB, Reaman GH. Late effects in long-term survivors after treatment for childhood acute leukemia. Clin Pediatr (Phila) 2009;48:601–8. doi: 10.1177/0009922809332680. [DOI] [PubMed] [Google Scholar]
- 2.Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, Mattano LA, Jr, Cole C, Eicher A, Haugan M, Sorenson M, Heerema NA, Carroll AA, Gastier-Foster JM, Borowitz MJ, Wood BL, Willman CL, Winick NJ, Hunger SP, Carroll WL. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.62.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattano LA, Jr, Devidas M, Nachman JB, Sather HN, Hunger SP, Steinherz PG, Gaynon PS, Seibel NL, Children’s Oncology G Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: results from the CCG-1961 randomised cohort trial. Lancet Oncol. 2012;13:906–15. doi: 10.1016/S1470-2045(12)70274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makitie O, Heikkinen R, Toiviainen-Salo S, Henriksson M, Puukko-Viertomies LR, Jahnukainen K. Long-term skeletal consequences of childhood acute lymphoblastic leukemia in adult males: a cohort study. Eur J Endocrinol. 2013;168:281–8. doi: 10.1530/EJE-12-0702. [DOI] [PubMed] [Google Scholar]
- 5.Cummings EA, Ma J, Fernandez CV, Halton J, Alos N, Miettunen PM, Jaremko JL, Ho J, Shenouda N, Matzinger MA, Lentle B, Stephure D, Stein R, Sbrocchi AM, Rodd C, Lang B, Israels S, Grant RM, Couch R, Barr R, Hay J, Rauch F, Siminoski K, Ward LM, Canadian SC. Incident Vertebral Fractures in Children With Leukemia During the Four Years Following Diagnosis. J Clin Endocrinol Metab. 2015;100:3408–17. doi: 10.1210/JC.2015-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Sluis IM, van den Heuvel-Eibrink MM, Hählen K, Krenning EP, de Muinck Keizer-Schrama SM. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J Pediatr. 2002;141:204–10. doi: 10.1067/mpd.2002.125728. [DOI] [PubMed] [Google Scholar]
- 7.Halton J, Gaboury I, Grant R, Alos N, Cummings EA, Matzinger M, Shenouda N, Lentle B, Abish S, Atkinson S, Cairney E, Dix D, Israels S, Stephure D, Wilson B, Hay J, Moher D, Rauch F, Siminoski K, Ward LM, Canadian SC. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009;24:1326–34. doi: 10.1359/jbmr.090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alos N, Grant RM, Ramsay T, Halton J, Cummings EA, Miettunen PM, Abish S, Atkinson S, Barr R, Cabral DA, Cairney E, Couch R, Dix DB, Fernandez CV, Hay J, Israels S, Laverdiere C, Lentle B, Lewis V, Matzinger M, Rodd C, Shenouda N, Stein R, Stephure D, Taback S, Wilson B, Williams K, Rauch F, Siminoski K, Ward LM. High incidence of vertebral fractures in children with acute lymphoblastic leukemia 12 months after the initiation of therapy. J Clin Oncol. 2012;30:2760–7. doi: 10.1200/JCO.2011.40.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orgel E, Mueske NM, Wren TA, Gilsanz V, Butturini AM, Freyer DR, Mittelman SD. Early injury to cortical and cancellous bone from induction chemotherapy for adolescents and young adults treated for acute lymphoblastic leukemia. Bone. 2016;85:131–7. doi: 10.1016/j.bone.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies JH, Evans BA, Jenney ME, Gregory JW. In vitro effects of chemotherapeutic agents on human osteoblast-like cells. Calcified tissue international. 2002;70:408–15. doi: 10.1007/s002230020039. [DOI] [PubMed] [Google Scholar]
- 11.Winter C, Muller C, Brandes M, Brinkmann A, Hoffmann C, Hardes J, Gosheger G, Boos J, Rosenbaum D. Level of activity in children undergoing cancer treatment. Pediatr Blood Cancer. 2009;53:438–43. doi: 10.1002/pbc.22055. [DOI] [PubMed] [Google Scholar]
- 12.Hartman A, te Winkel ML, van Beek RD, de Muinck Keizer-Schrama SM, Kemper HC, Hop WC, van den Heuvel-Eibrink MM, Pieters R. A randomized trial investigating an exercise program to prevent reduction of bone mineral density and impairment of motor performance during treatment for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53:64–71. doi: 10.1002/pbc.21942. [DOI] [PubMed] [Google Scholar]
- 13.Rayar MS, Nayiager T, Webber CE, Barr RD, Athale UH. Predictors of bony morbidity in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59:77–82. doi: 10.1002/pbc.24040. [DOI] [PubMed] [Google Scholar]
- 14.Strauss AJ, Su JT, Dalton VM, Gelber RD, Sallan SE, Silverman LB. Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol. 2001;19:3066–72. doi: 10.1200/JCO.2001.19.12.3066. [DOI] [PubMed] [Google Scholar]
- 15.Vitanza NA, Hogan LE, Zhang G, Parker RI. The Progression of Bone Mineral Density Abnormalities After Chemotherapy for Childhood Acute Lymphoblastic Leukemia. J Pediatr Hematol Oncol. 2015;37:356–61. doi: 10.1097/MPH.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 16.Kaste SC, Rai SN, Fleming K, McCammon EA, Tylavsky FA, Danish RK, Rose SR, Sitter CD, Pui CH, Hudson MM. Changes in bone mineral density in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2006;46:77–87. doi: 10.1002/pbc.20553. [DOI] [PubMed] [Google Scholar]
- 17.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. NCHS Data Brief. US Department of Health and Human Services; 2011. Vitamin D Status: United States, 2001–2006; p. 59. Available online, http://wwwcdcgov/nchs/data/databriefs/db59pdf, Last Accessed 12-3-2015. [PubMed] [Google Scholar]
- 18.World Health Organization. Health for the world’s adolescents. 2014 http://www.who.int/maternal_child_adolescent/documents/adolescent/en/, last accessed May 16, 2016.
- 19.Orgel E, Mueske NM, Sposto R, Gilsanz V, Freyer DR, Mittelman SD. Limitations of body mass index to assess body composition due to sarcopenic obesity during leukemia therapy. Leuk Lymphoma. 2016:1–8. doi: 10.3109/10428194.2015.1136741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antony R, Sheng X, Ehsanipour EA, Ng E, Pramanik R, Klemm L, Ichihara B, Mittelman SD. Vitamin D protects acute lymphoblastic leukemia cells from dexamethasone. Leuk Res. 2012;36:591–3. doi: 10.1016/j.leukres.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 22.Baio G, Barbagallo M, D’Avola G, Di Luccio A, Di Tanna GL, Falaschi P, Iolascon G, Malavolta N, Robbiati F, Ulivieri FM. Improving adherence in osteoporosis: a new management algorithm for the patient with osteoporosis. Expert opinion on pharmacotherapy. 2011;12:257–68. doi: 10.1517/14656566.2011.537259. [DOI] [PubMed] [Google Scholar]
- 23.Ilahi M, Armas LA, Heaney RP. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr. 2008;87:688–91. doi: 10.1093/ajcn/87.3.688. [DOI] [PubMed] [Google Scholar]
- 24.Arpadi SM, McMahon D, Abrams EJ, Bamji M, Purswani M, Engelson ES, Horlick M, Shane E. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:e121–6. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rangul V, Holmen TL, Kurtze N, Cuypers K, Midthjell K. Reliability and validity of two frequently used self-administered physical activity questionnaires in adolescents. BMC Med Res Methodol. 2008;8:47. doi: 10.1186/1471-2288-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, Institute of Medicine . Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 29.Withycombe JS, Post-White JE, Meza JL, Hawks RG, Smith LM, Sacks N, Seibel NL. Weight patterns in children with higher risk ALL: A report from the Children’s Oncology Group (COG) for CCG 1961. Pediatr Blood Cancer. 2009;53:1249–54. doi: 10.1002/pbc.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tepper S, Shahar DR, Geva D, Ish-Shalom S. Predictors of serum 25(Oh)D increase following bimonthly supplementation with 100,000IU vitamin D in healthy, men aged 25–65 years. The Journal of steroid biochemistry and molecular biology. 2014;144(Pt A):163–6. doi: 10.1016/j.jsbmb.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Camozzi V, Frigo AC, Zaninotto M, Sanguin F, Plebani M, Boscaro M, Schiavon L, Luisetto G. 25-Hydroxycholecalciferol response to single oral cholecalciferol loading in the normal weight, overweight, and obese. Osteoporos Int. 2016;27:2593–602. doi: 10.1007/s00198-016-3574-y. [DOI] [PubMed] [Google Scholar]
- 32.Cipriani C, Romagnoli E, Scillitani A, Chiodini I, Clerico R, Carnevale V, Mascia ML, Battista C, Viti R, Pileri M, Eller-Vainicher C, Minisola S. Effect of a single oral dose of 600,000 IU of cholecalciferol on serum calciotropic hormones in young subjects with vitamin D deficiency: a prospective intervention study. J Clin Endocrinol Metab. 2010;95:4771–7. doi: 10.1210/jc.2010-0502. [DOI] [PubMed] [Google Scholar]
- 33.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the university of Saskatchewan bone mineral accrual study. J Bone Miner Res. 1999;14:1672–9. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 34.McCormick DP, Ponder SW, Fawcett HD, Palmer JL. Spinal bone mineral density in 335 normal and obese children and adolescents: evidence for ethnic and sex differences. J Bone Miner Res. 1991;6:507–13. doi: 10.1002/jbmr.5650060513. [DOI] [PubMed] [Google Scholar]
- 35.Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2008;23:17–29. doi: 10.1359/JBMR.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonard MB, Shults J, Wilson BA, Tershakovec AM, Zemel BS. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am J Clin Nutr. 2004;80:514–23. doi: 10.1093/ajcn/80.2.514. [DOI] [PubMed] [Google Scholar]
- 37.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94:3387–93. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. 2014;1842:473–81. doi: 10.1016/j.bbadis.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi: 10.1002/14651858.CD001347.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lethaby C, Wiernikowski J, Sala A, Naronha M, Webber C, Barr RD. Bisphosphonate therapy for reduced bone mineral density during treatment of acute lymphoblastic leukemia in childhood and adolescence: a report of preliminary experience. J Pediatr Hematol Oncol. 2007;29:613–6. doi: 10.1097/MPH.0b013e318142b7a1. [DOI] [PubMed] [Google Scholar]
- 41.Wiernikowski JT, Barr RD, Webber C, Guo CY, Wright M, Atkinson SA. Alendronate for steroid-induced osteopenia in children with acute lymphoblastic leukaemia or non-Hodgkin’s lymphoma: results of a pilot study. J Oncol Pharm Pract. 2005;11:51–6. doi: 10.1191/1078155205jp145oa. [DOI] [PubMed] [Google Scholar]
- 42.Barr RD, Guo CY, Wiernikowski J, Webber C, Wright M, Atkinson S. Osteopenia in children with acute lymphoblastic leukemia: a pilot study of amelioration with Pamidronate. Med Pediatr Oncol. 2002;39:44–6. doi: 10.1002/mpo.10057. [DOI] [PubMed] [Google Scholar]
- 43.Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann N Y Acad Sci. 2008;1143:123–50. doi: 10.1196/annals.1443.016. [DOI] [PubMed] [Google Scholar]
- 44.Bhatia S, Landier W, Hageman L, Kim H, Chen Y, Crews KR, Evans WE, Bostrom B, Casillas J, Dickens DS, Maloney KW, Neglia JP, Ravindranath Y, Ritchey AK, Wong FL, Relling MV. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124:2345–53. doi: 10.1182/blood-2014-01-552166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: CONSORT diagram for study enrollment
Supplemental Figure 2: Association between percentage body fat and bone density
Association across all time-points of body fat percentage as assessed by DXA and (A) cancellous bone density measured at the spine (B) cortical bone density measured at the tibia and at the femur.


