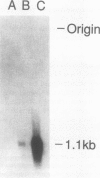
Abstract
Membrane bound and soluble forms of a high-affinity folate binding protein have been found in kidney, placenta, serum, milk, and in several cell lines. The two forms have similar binding characteristics for folates, are immunologically cross-reactive and based upon limited amino acid sequence data, are nearly identical. Based upon pulse-chase experiments, a precursor-product relationship has been suggested. The membrane form has been shown to mediate the transport of folate in cells grown in physiological concentrations of folate. A function for the soluble form has not yet been identified. We constructed a cDNA library from a human carcinoma cell line, Caco-2, which expresses the membrane form abundantly. The library was screened and a near full-length cDNA for the folate binder was isolated. Transfection of COS cells with the cDNA inserted in an expression vector resulted in marked overexpression of a membrane-associated folate binder as assessed by direct binding of radiolabeled folate and by indirect immunofluorescence. The deduced amino acid sequence is not consistent with a typical membrane spanning domain but rather with a signal for anchoring via a glycosyl-phosphatidylinositol linkage. Release of the binder with a phosphatidylinositol-specific phospholipase C strongly supports this hypothesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G. Methods for visualization of the LDL pathway in cultured human fibroblasts. Methods Enzymol. 1986;129:201–216. doi: 10.1016/0076-6879(86)29070-9. [DOI] [PubMed] [Google Scholar]
- Antony A. C., Kane M. A., Portillo R. M., Elwood P. C., Kolhouse J. F. Studies of the role of a particulate folate-binding protein in the uptake of 5-methyltetrahydrofolate by cultured human KB cells. J Biol Chem. 1985 Dec 5;260(28):14911–14917. [PubMed] [Google Scholar]
- Antony A. C., Utley C. S., Marcell P. D., Kolhouse J. F. Isolation, characterization, and comparison of the solubilized particulate and soluble folate binding proteins from human milk. J Biol Chem. 1982 Sep 10;257(17):10081–10089. [PubMed] [Google Scholar]
- Antony A. C., Utley C., Van Horne K. C., Kolhouse J. F. Isolation and characterization of a folate receptor from human placenta. J Biol Chem. 1981 Sep 25;256(18):9684–9692. [PubMed] [Google Scholar]
- Bird P., Gething M. J., Sambrook J. Translocation in yeast and mammalian cells: not all signal sequences are functionally equivalent. J Cell Biol. 1987 Dec;105(6 Pt 2):2905–2914. doi: 10.1083/jcb.105.6.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davitz M. A., Hereld D., Shak S., Krakow J., Englund P. T., Nussenzweig V. A glycan-phosphatidylinositol-specific phospholipase D in human serum. Science. 1987 Oct 2;238(4823):81–84. doi: 10.1126/science.2443973. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Fischer C. D., da Costa M., Rothenberg S. P. The heterogeneity and properties of folate binding proteins from chronic myelogenous leukemia cells. Blood. 1975 Dec;46(6):855–867. [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Sterchi E. E., Bienz D., Fransen J. A., Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985 Sep;101(3):838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen B. A., Capdevila A. Receptor-mediated folate accumulation is regulated by the cellular folate content. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5983–5987. doi: 10.1073/pnas.83.16.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen B. A., Caston J. D. Purification of folate binding factor in normal umbilical cord serum. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4261–4264. doi: 10.1073/pnas.72.11.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen B. A., Wang M. T., Streckfuss A. J., Peryea X., Anderson R. G. Delivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recycles. J Biol Chem. 1988 Sep 25;263(27):13602–13609. [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Kolhouse J. F. The interrelationship of the soluble and membrane-associated folate-binding proteins in human KB cells. J Biol Chem. 1986 Nov 25;261(33):15625–15631. [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhrs C. A., Pitiranggon P., da Costa M., Rothenberg S. P., Slomiany B. L., Brink L., Tous G. I., Stein S. Purified membrane and soluble folate binding proteins from cultured KB cells have similar amino acid compositions and molecular weights but differ in fatty acid acylation. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6546–6549. doi: 10.1073/pnas.84.18.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg S. P. A macromolecular factor in some leukemic cells which binds folic acid. Proc Soc Exp Biol Med. 1970 Feb;133(2):428–432. doi: 10.3181/00379727-133-34489. [DOI] [PubMed] [Google Scholar]
- Roy-Choudhury S., Mishra V. S., Low M. G., Das M. A phospholipid is the membrane-anchoring domain of a protein growth factor of molecular mass 34 kDa in placental trophoblasts. Proc Natl Acad Sci U S A. 1988 Mar;85(6):2014–2018. doi: 10.1073/pnas.85.6.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinoff M., Abramson R., Schreiber C., Waxman S. Effect of a folate-binding protein on the plasma transport and tissue distribution of folic acid. Acta Haematol. 1981;65(3):145–152. doi: 10.1159/000207170. [DOI] [PubMed] [Google Scholar]
- Rubinoff M., Schreiber C., Waxman S. The isolation and characterization of the folate binding protein from goat milk. FEBS Lett. 1977 Mar 15;75(1):244–248. doi: 10.1016/0014-5793(77)80096-3. [DOI] [PubMed] [Google Scholar]
- Salter D. N., Ford J. E., Scott K. J., Andrews P. Isolation of the folate-binding protein from cow's milk by the use of affinity chromatography. FEBS Lett. 1972 Feb 15;20(3):302–306. doi: 10.1016/0014-5793(72)80092-9. [DOI] [PubMed] [Google Scholar]
- Salter D. N., Scott K. J., Slade H., Andrews P. The preparation and properties of folate-binding protein from cow's milk. Biochem J. 1981 Feb 1;193(2):469–476. doi: 10.1042/bj1930469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J., Franklin W. A. The folate-binding protein of rat kidney. Purification, properties, and cellular distribution. J Biol Chem. 1984 May 25;259(10):6601–6606. [PubMed] [Google Scholar]
- Shukla S. D., Coleman R., Finean J. B., Michell R. H. Selective release of plasma-membrane enzymes from rat hepatocytes by a phosphatidylinositol-specific phospholipase C. Biochem J. 1980 Apr 1;187(1):277–280. doi: 10.1042/bj1870277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani M., Fushiki T., Iwai K. Influence of folate-binding protein from bovine milk on the absorption of folate in gastrointestinal tract of rat. Biochim Biophys Acta. 1983 Jun 9;757(3):274–281. doi: 10.1016/0304-4165(83)90051-x. [DOI] [PubMed] [Google Scholar]
- Tani M., Iwai K. Some nutritional effects of folate-binding protein in bovine milk on the bioavailability of folate to rats. J Nutr. 1984 Apr;114(4):778–785. doi: 10.1093/jn/114.4.778. [DOI] [PubMed] [Google Scholar]
- Thomsen D. R., Stenberg R. M., Goins W. F., Stinski M. F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S., Schreiber C. Characteristics of folic acid-binding protein in folate-deficient serum. Blood. 1973 Aug;42(2):291–301. [PubMed] [Google Scholar]
- Waxman S., Schreiber C. The purification and characterization of the low molecular weight human folate binding protein using affinity chromatography. Biochemistry. 1975 Dec 16;14(25):5422–5428. doi: 10.1021/bi00696a007. [DOI] [PubMed] [Google Scholar]
- van den Bosch R. A., du Maine A. P., Geuze H. J., van der Ende A., Strous G. J. Recycling of 5'-nucleotidase in a rat hepatoma cell line. EMBO J. 1988 Nov;7(11):3345–3351. doi: 10.1002/j.1460-2075.1988.tb03206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]