Abstract
Histone deacetylase inhibitors (HDACi) have proven activity in hematologic malignancies, and their FDA approval in multiple myeloma (MM) and T-cell lymphoma highlights the need for further development of this drug class. We investigated AR-42, an oral pan-HDACi, in a first-in-man phase 1 dose escalation clinical trial. Overall, treatment was well tolerated, no DLTs were evident, and the MTD was defined as 40 mg dosed three times weekly for three weeks of a 28-day cycle. One patient each with MM and mantle cell lymphoma demonstrated disease control for 19 and 27 months (ongoing), respectively. Treatment was associated with reduction of serum CD44, a transmembrane glycoprotein associated with steroid and immunomodulatory drug resistance in MM. Our findings indicate that AR-42 is safe and that further investigation of AR-42 in combination regimens for the treatment of patients with lymphoma and MM is warranted. Trial registration: http://clinicaltrials.gov/ct2/show/NCT01129193
Keywords: histone deacetylase inhibitor, multiple myeloma, lymphoma, phase 1, pharmacokinetics
INTRODUCTION
Histone deacetylase inhibitors (HDACi) are an expanding and promising group of anti-cancer therapeutics that induce growth arrest, differentiation, and intrinsic and extrinsic apoptosis of malignant cells via varying degrees of activity on histone acetylation-dependent and -independent mechanisms. Numerous classes of these agents are now in development and clinical use, including the pan-HDACi hydroxamic acid (vorinostat, panobinostat, and belinostat) and short-chain aliphatic acid (phenylbutyrate) derivatives, and the more selective class I HDACi cyclic tetrapeptide (romidepsin) and benzamide (entinostat) derivates. Use of these agents has been most successful in hematologic malignancies as evidenced by the FDA approval of vorinostat[1,2], romidepsin[3,4], and belinostat[5] in T-cell lymphomas, and more recently, panobinostat in combination with bortezomib and dexamethasone for patients with relapsed multiple myeloma (MM)[6]. In MM, vorinostat and panobinostat in combination with bortezomib are associated with modest improvement in PFS, and routine clinical use of panobinostat is limited by toxicities, as highlighted by 2/3 of patients enrolled on the PANORAMA 1 study experiencing grade 3 and 4 adverse events including cytopenias, diarrhea, fatigue, and peripheral neuropathy[11]. Clearly, HDACi’s have antitumor activity in lymphoma and MM, but identifying better tolerated HDACi’s in a subpopulation most likely to respond is needed.
AR-42 (Arno Therapeutics) is an orally bioavailable, hydroxamate-tethered phenylbutyrate derived small molecule that targets and inhibits Class I and IIB HDAC enzymes[12,13], and has antitumor activity in in vitro and in vivo models of solid tumors[14–18] and numerous B-cell malignancies including chronic lymphocytic leukemia (CLL), Burkitt’s lymphoma, mantle cell lymphoma (MCL), and MM[19–21]. Similar to other pan-HDACi like vorinostat and panobinostat, AR-42 suppresses tumor cell growth via a broad spectrum of mechanisms. However, in comparison to vorinostat, AR-42 has been shown to be four- to seven-fold more potent and associated with increased cell killing of multiple MM cell lines (U266, H929, RPMI 8226, ARH-77, and IM-9) and primary MM cells via targeting of the gp130/STAT3 pathway, modulation of downstream cell survival pathways including downregulation of Akt and NF-KB signaling, and enhanced cleavage of caspase-3, 8, and 9; a distinct effect on caspase activity not observed with vorinostat treatment of myeloma or prostate cancer preclinical models[16,19,21,22]. It has also been demonstrated that AR-42 has more potent in vitro and in vivo activity than vorinostat in multiple preclinical lymphoma models, including the Raji Burkitt lymphoma, JeKo-1 mantle cell lymphoma, and Eu-Tcl1 murine models. These studies confirmed a 3–6 fold reduction in IC50 (50% growth inhibitory concentration) for AR-42 compared to vorinostat, and showed in multiple mouse models that treatment was associated with prolonged survival and/or reduced leukocyte counts and no significant toxicity[20]. It remains unclear whether this increased preclinical efficacy translates into improved clinical results, but we hypothesize that the lack of HDAC class IIA (HDAC 4, 5, 7, and 9) will enhance tolerability.
This phase 1 trial was the first to assess single agent AR-42 in patients with relapsed hematologic malignancies. The primary objectives of this study were to assess the safety of AR-42 while defining the maximum tolerated dose (MTD) and describe dose-limiting toxicities (DLTs).
METHODS
Study design
This was an open-label, single-center, dose-escalating, first-in-man phase 1 trial of single-agent AR-42 following a standard 3+3 cohort design in three different stages that was approved by The Ohio State University Cancer Institutional Review Board and written informed consent was obtained from all enrolled patients (NCT01129193 – AR-42 in Treating Patients with Advanced or Relapsed Multiple Myeloma, Chronic Lymphocytic Leukemia, or Lymphoma. Patients with relapsed or refractory chronic lymphocytic leukemia (CLL), Hodgkin and non-Hodgkin lymphoma as defined by the 2008 World Health Organization criteria[23], or MM as defined by the International Myeloma Working Group (IMWG) diagnostic criteria for symptomatic myeloma [24] previously treated with a immunomodulatory agent and proteasome inhibitor were eligible for enrollment. Patients must have received at least one prior antineoplastic therapy, must have progressed after at least one prior therapy, and have either had no standard therapy available or declined such interventions. AR-42 was administered orally three times weekly (Monday, Wednesday, and Friday) in 28-day cycles (three weeks of three-times-per-week dosing followed by a seven-day off treatment period), and patients continued treatment until unacceptable toxicity or disease progression.
Safety and Tolerability assessments
The Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v.4) was used to grade toxicities[25]. A DLT was defined as one of the following hematologic and/or non-hematologic toxicities that occurred during the first cycle of therapy and was determined to be possibly, probably and definitely related to single-agent AR-42. In CLL patients, hematologic toxicities included grade 4 thrombocytopenia, anemia, or neutropenia (as described by 1996 National Cancer Institute Working Group Criteria) that did not resolve within five days[26]. In lymphoma or MM, hematologic toxicities defined as DLTs included grade 4 neutropenia (ANC < 500/μL) lasting > five days or grade 4 thrombocytopenia (platelet count < 25,000/μL). Non-hematologic dose-limiting toxicities for all enrolled patients included: any grade 3 or 4 adverse event with the exception of asymptomatic laboratory abnormalities correctable within 24 hours that did not lead to missing greater than one dose, grade 3 or 4 nausea or vomiting that resolved within 24 hours with supportive care that did not lead to missing > one dose, and liver function abnormalities (aspartate transaminase (ALT), alanine transaminase (ALT), bilirubin, or alkaline phosphatase) that resolved to < grade 1 within five days and associated with missing greater than one dose of AR-42.
QTc measurement
At least three HDACi’s examined to date—including vorinostat[27], panobinostat[28], and romidepsin[29] have shown clinical evidence of QT prolongation in phase 1 and/or 2 studies. QT prolongation poses a risk of malignant cardiac arrhythmia with torsade de pointes and sudden cardiac death. QT corrections were not automated on the electrocardiogram (ECG) machines used in this study, so the Bazett formula (QTcB) was used. Patients were monitored with 12-lead ECGs at screening, cycle 1 days 1, 2, 5, 8, and 19, day 1 of all subsequent cycles, and then at the time of study treatment discontinuation.
Enzyme-linked immunoassorbent assay (ELISA) for CD44
Because CD44 is able to shed from the surface of malignant cells and its serum level has been correlated with the outcome in several forms of cancer, including MM[30,31], we investigated soluble CD44 (sCD44) levels in the serum of MM patients. ELISA assay for detection of human CD44 was performed in patient serum samples obtained at baseline prior to AR-42 treatment and then on cycle 1 day 15 according to the manufacturer (Abcam, Cambridge, MA); details can be found in the supplementary data.
RESULTS
Patients
A total of 27 patients with relapsed hematologic malignancies were enrolled including 17 with relapsed MM and ten with relapsed lymphoma; the accrual schedule is presented in Supplementary table 1. Of the patients with MM, the median age was 66 (range 49 – 76), ten were male, two were African American, and 15 were Caucasian. One patient was lenalidomide naïve, one patient was bortezomib naïve, 88% (15/17) of patients had been previously exposed to both agents, 11 were lenalidomide refractory, ten were bortezomib refractory, seven were double refractory, and the median prior lines of treatment was four (range 2 – 14) (Table 1).
Table 1.
Multiple myeloma patient demographics
| ID | Age | Race | Sex | ISS Stage | Revlimid exposed | Velcade exposed | Revlimid refractory | Velcade refractory | Prior lines of treatment | AR-42 dose (cohort) | Best response | Cycles of Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | C | M | 2 | Y | Y | Y | Y | 9 | 20 mg (1a) | PD | 1 |
| 4 | 64 | C | F | 2 | N | Y | N | N | 2 | 40 mg (2a) | MR | 3 |
| 5 | 60 | AA | M | NA | Y | Y | Y | N | 3 | 40 mg (2a) | MR | 18 |
| 6 | 68 | AA | F | NA | Y | Y | Y | N | 5 | 40 mg (2a) | SD | 3 |
| 7 | 76 | C | M | 1 | Y | Y | Y | Y | 2 | 40 mg (1b) | SD | 3 |
| 8 | 74 | C | M | 1 | Y | Y | N | N | 4 | 40 mg (1b) | PD | 1 |
| 10 | 59 | C | M | 2 | Y | Y | N | Y | 5 | 50 mg (2b) | SD | 1 |
| 11 | 67 | C | F | 1 | Y | Y | N | N | 14 | 50 mg (2b) | PD | 1 |
| 12 | 53 | C | M | 1 | Y | Y | Y | Y | 4 | 50 mg (2b) | PD | 1 |
| 13 | 71 | C | F | 2 | Y | Y | Y | Y | 4 | 50 mg (2b) | SD | 3 |
| 17 | 70 | C | M | NA | Y | Y | N | Y | 3 | 70 mg (3b) | MR | 5 |
| 20 | 66 | C | F | NA | Y | Y | Y | Y | 6 | 40 mg (4b) | SD | 2 |
| 21 | 71 | C | F | NA | Y | Y | Y | Y | 4 | 40 mg (4b) | SD | 1 |
| 22 | 57 | C | M | 2 | Y | Y | N | Y | 4 | 40 mg (4b) | SD | 2 |
| 25 | 65 | C | M | 1 | Y | Y | Y | N | 3 | 40 mg (4b) | SD | 2 |
| 26 | 49 | C | M | NA | Y | Y | Y | Y | 6 | 40 mg (4b) | SD | 1 |
| 27 | 73 | C | F | NA | Y | N | Y | N | 4 | 40 mg (4b) | SD | 3 |
Seventeen patients were treated in 6 cohorts. ISS (International Staging System), PD (progressive disease), MR (minimal response), SD (stable disease).
Ten patients with relapsed lymphoma were accrued and eligible for analysis (Table 2). The median age at the time of enrollment was 61 (range 33 – 79), and patients were generally heavily pretreated with a median of 4·5 (range 2 – 9) prior treatments. Nine of the patients had non-Hodgkin lymphoma, of which five had B-cell malignancies (low grade B cell lymphoma with plasmacytic differentiation, follicular lymphoma, marginal B-cell lymphoma, mantle cell lymphoma, and CLL with Richter transformation) and four had T-cell malignancies (mycosis fungoides, EBV+ angioimmunoblastic T-cell lymphoma, cutaneous T-cell lymphoma, and T-cell lymphoma). Additionally, one patient had nodular sclerosing Hodgkin lymphoma – syncytial variant.
Table 2.
Lymphoma patient demographics
| ID | Age | Race | Sex | Diagnosis (stage) | Prior lines of therapy | AR-42 dose (cohort) | Best response | Cycles of therapy received |
|---|---|---|---|---|---|---|---|---|
| 2 | 61 | AA | M | Lymphoplasmacytic lymphoma (IV) | 3 | 20 (1a) | SD | 4 |
| 3 | 33 | C | M | Nodular Sclerosing HL/syncytial variant (IIIB) | 6 | 20 (1a) | PD | 1 |
| 9 | 51 | C | M | Mycosis fungoides (IIB) | 3 | 40 (1b) | SD | 1 |
| 14 | 54 | C | F | Follicular lymphoma (IV) | 4 | 50 (2b) | SD | 2 |
| 15 | 71 | C | F | EBV+ angioimmunoblastic T-cell lymphoma (IV) | 2 | 50 (2b) | PD | 2 |
| 16 | 67 | C | M | Mycosis fungoides (NA) | 6 | 50 (2b) | SD | 2 |
| 18 | 79 | C | M | Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) (IVB) | 4 | 40 (4b) | SD | 6 |
| 19 | 52 | C | M | Mycosis fungoides (IIB) | 9 | 40 (4b) | SD | 12 |
| 23 | 61 | C | F | Mantle Cell lymphoma (NA) | 7 | 40 (4b) | SD | 27 |
| 24 | 77 | C | M | Richters transformation (NA) | 5 | 40 (4b) | PD | 1 |
Ten lymphoma patients were treated in 4 different cohorts. SD (stable disease), PD (progressive disease).
Three patients were treated at the starting dose of 20 mg (cohort 1a), 16 patients received 40 mg (cohorts 1b, 2a, and 4b), seven patients were treated with 50 mg (cohort 2b), and one patient received 70 mg (cohort 3b) (Supplementary table 1). Of those with MM, patients received 20 mg (n=1), 40 mg (n=11), 50 mg (n=4), and 70 mg (n=1) (Table 1). Lymphoma patients were treated with 20 mg (n=2), 40 mg (n=5), and 50 mg (n=3) (Table 2).
Safety and Toxicities
For all patients, the most common adverse events possibly, probably, or likely attributable to single agent AR-42 during cycle 1 included cytopenias (thrombocytopenia, neutropenia, leukopenia, lymphopenia, and anemia), QTc prolongation, and fatigue (Figure 1). Two different patients experienced grade 4 events, including lymphopenia and thrombocytopenia that occurred on days 8 and 15, respectively. Grade 3 events included thrombocytopenia (n=6), neutropenia (n=3), lymphopenia (n=3), anemia (n=1), and leukopenia (n=1), and grade 2 events other than cytopenias included fatigue (n=3), and muscle spasms (n=1).
Figure 1.
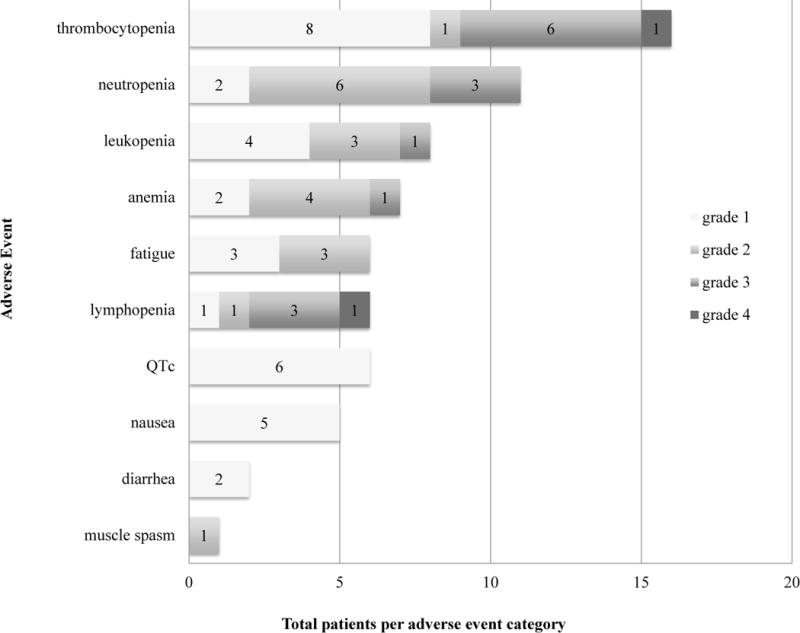
Top 10 most frequent adverse events during cycle 1 for all patients
For all patients treated at the 40 mg dose level, the most common adverse events over the entire course of treatment included grade 1 – 4 cytopenias (thrombocytopenia, leukopenia, neutropenia, lymphopenia, and anemia) (Table 3). Other than one grade 3 lung infection as previously noted, all other adverse events experienced by more than one patient were grade 1 or 2, and included fatigue (n=4), nausea (n=4), changes in taste (n=3), QTc prolongation (n=2), and diarrhea (n=3). In these patients, cytopenias were found to persist, but did not consistently worsen over the course of prolonged treatment.
Table 3.
Adverse events in all cycles for patients treated with 40 mg AR-42.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | |
|---|---|---|---|---|---|
| Thrombocytopenia | 4 | 2 | 5 | 11 | |
| Neutropenia | 2 | 5 | 2 | 1 | 10 |
| Lymphopenia | 1 | 4 | 1 | 6 | |
| Leukopenia | 3 | 3 | 6 | ||
| Anemia | 2 | 2 | 1 | 5 | |
| Fatigue | 2 | 2 | 4 | ||
| Nausea | 4 | 4 | |||
| Taste changes | 2 | 1 | 3 | ||
| QTc | 3 | 3 | |||
| Diarrhea | 3 | 3 | |||
| Lung infection | 1 | 1 | |||
| Emesis | 1 | 1 | |||
| Increased ALT | 1 | 1 | |||
| Increased AST | 1 | 1 | |||
| Dizziness | 1 | 1 | |||
| Myalgia | 1 | 1 | |||
| Increased creatinine | 1 | 1 | |||
| Reflux | 1 | 1 | |||
| Rash | 1 | 1 |
Patients treated with 40mg in 3 different cohorts most commonly experienced cytopenias (grade 1 – 4), fatigue, and nausea.
QTc was never more than grade 1 and was not associated with cardiac events or dose reductions. In total, QTc prolongation was evident during cycle 1 in six patients, and in two patients after cycle 1 (beginning of cycles two and four, respectively). In all patients, the average QTc at the time of screening was 432.6 ms (range: 399 – 489; standard deviation: 19.2), the average maximum QTc over the course of treatment was 459.6 ms (range: 435 – 539; standard deviation: 20.6), and this was associated with a mean maximum QTc change of 27.4 (range: 10 – 60; standard deviation: 14.5). In all 6 patients, QTc abnormalities spontaneously resolved without holding or discontinuing AR-42.
The median total cumulative drug exposure (milligrams of drug per week divided by number of weeks) was 450 mg (range 180 – 10,020 mg). Six total patients had dose reductions over the course of treatment, of which four were treated at the 40 mg dose levels and required dose reductions to 30 mg (n=3) and 20 mg (n=1) due to grade 4 lymphopenia, grade 3 thrombocytopenia, neutropenia, and neutropenic lung infection that occurred after three (n=3) and five (n=1) cycles. The two others were treated with 50 mg and 70 mg, and both were reduced to 40 mg following diploplia/blurry vision/memory impairment/muscle cramping in one patient, and grade 3 neutropenia in the other. Additionally, seven patients required temporary holds of study treatment for an average of 10.5 days (range 1 – 21 days) for upper respiratory infection, neutropenia, acute kidney injury, dizziness and confusion, fall, and rash.
In total, seven patients required hospitalization, one death occurred during active treatment (see Supplementary data), and two deaths related to disease progression occurred within 30 days of discontinuing study treatment. In addition to the death that occurred in the patient treated in the 50 mg cohort (cohort 2b), SAEs possibly, probably, or likely related to AR-42 occurred in five different patients. AR-42 related grade 4 thrombocytopenia and grade 2 CNS symptoms (blurred vision and memory impairment) were evident in two different patients treated with 50 mg (cohort 2b), and grade 3 lung infection (n=1, cohort 2a) and grade 4 febrile neutropenia (n=2, cohort 4b) were evident in different patients treated with 40 mg.
Discussion with the data safety monitoring committee defined the maximum tolerated dose (MTD) as 40 mg every M-W-F weekly for three weeks followed by one week off following the single grade 5 event. This determination was made based on the following: 1) death occurring at the 50 mg dose, 2) no cycle 1 dose reductions or DLTs in the 40 mg cohorts, and 3) in vitro evidence that the 40 mg dosing level achieved adequate Cmax for HDAC inhibition (Supplementary table 2).
Response Assessment
All 27 patients were eligible for response assessment. The best response in the 17 patients with MM was minimal response in three (17.6%) patients (3/17, 95% CI: 3.8 – 43.4%) for one, three, and 18 cycles (Figure 2). Two of these patients were enrolled on cohorts treated with 40 mg, and the third was initially treated with 70 mg prior to dose reduction to 40 mg; all three were eventually removed from trial following disease progression. Of the remaining patients, ten had stable disease for between 1 and 3 cycles, and four progressed during the first cycle (Table 1). In patients with relapsed lymphoma, the best response was stable disease and the longest durations of treatment were four, six, 12, and 27 cycles, and these patients had stage IV low grade B-cell lymphoma, stage IVB marginal B-cell lymphoma, stage II T-cell lymphoma, and mantle cell lymphoma/atypical chronic lymphocytic leukemia, respectively (Table 2). Those patients that completed four, six, and 12 cycles all discontinued the trial secondary to disease progression. The patient with the longest duration of stable disease remains on study. This patient was heavily pretreated prior to trial enrollment, inclusive of seven prior treatments including allogeneic transplant, numerous chemotherapy regimens, and radiation (Table 2). Analysis of numerous bone marrow biopsies from this patient revealed complex cytogenetics, including t(11;14), c-myc trisomy (84%), and IgVH somatic hypermutation positivity (91%).
Figure 2.
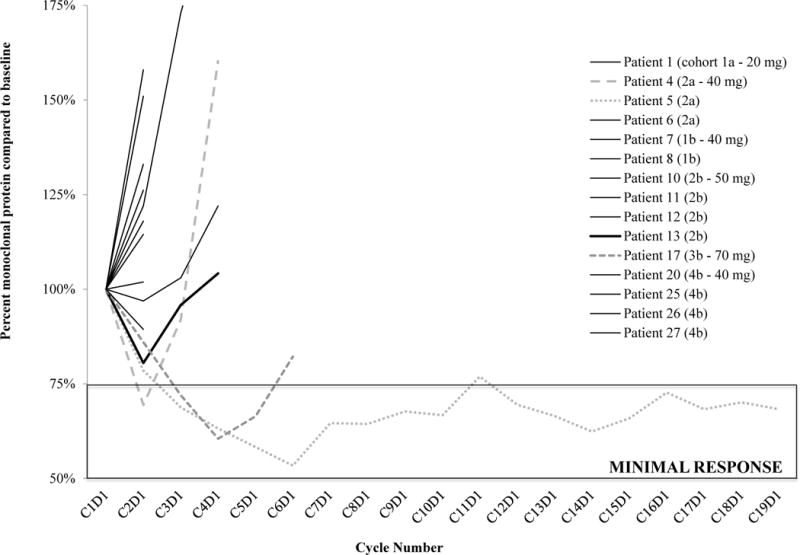
Multiple myeloma patient response
Soluble CD44
Soluble CD44 was measured in 10 MM patients pretreatment on days one and 15 of cycle 1. After 2 cycles of treatment, nine out of these ten patients had decreased sCD44 levels, and in four of these 9, CD44 decreased more than 20% by day 15. This significant reduction (mean difference: 17.95, 95% CI: 3.19–32.72, paired t-test, p=0.0022) in serum samples supports our previous in vitro findings [19], and strongly suggests that AR-42 directly affects CD44 expression in myeloma patients.
Pharmacokinetic (PK) analysis
Mean plasma concentration and time profiles of AR-42 in patients on days one and 19 after an oral administration of AR-42 are shown in Supplementary figure 1 (PK analyses were conducted according to the methods outlined in the supplementary data). The peak concentrations (geometric mean, %CV) of AR-42 administered at dose levels 20, 40 and 50 mg were 0.383 (56.2) and 0.335 (65.6), 0.794 (23.8) and 0.830 (27.9), and 1.60 (37.5) and 1·50 (45.2) μM on days one and 19, respectively (Supplementary table 2). The rate (Tmax) and extent (Cmax and AUC0-∞) of AR-42 absorption were similar between days one and 19 assessments, suggesting AR-42 plasma pharmacokinetics are not impacted by a dosing schedule of three times weekly for three weeks. However, accumulation is not expected for an agent with a 6.8 to 10.1 hour half-life when administered roughly every 48 hours (accumulation index = 1.04 to 1.01). The 2.1- and 1.9-fold increase in mean day one Cmax and AUC0-∞, respectively, between the 20 and 40 mg dose levels suggest AR-42 pharmacokinetics are dose proportional in this range, whereas proportionality is not apparent in the 2.0- and 1.7-fold increase in mean day one Cmax and AUC0-∞, respectively, between the 40 and 50 mg dose levels.
DISCUSSION
Here we report the phase 1 clinical data associated with the use of the oral pan-HDACi AR-42 in patients with multiple myeloma and lymphoma, and show that single agent treatment is safe in heavily pretreated patients. The MTD was found to be 40 mg administered orally three times weekly (Monday, Wednesday, and Friday) for three weeks of a 28-day cycle; a dosing schedule supported by our pharmacokinetic analyses.
During cycle 1 and over the course of the trial, the most common adverse events were cytopenias, most notably thrombocytopenia, leukopenia, and neutropenia. These cytopenias were dose dependent and were found to improve following cessation of treatment. There were no significant bleeding events associated with thrombocytopenia during any cycle. Neutropenia, despite being evident in 11 patients (grade 1 – 3) during cycle 1, and 12 patients (grade 1 – 4) in cycle 2 and beyond, was associated with only one clinically significant infection, a grade 3 lung infection occurring in cycle 6. Other reported toxicities, evident in a small percentage of patients, included grade 1 and/or 2 dizziness, changes in taste, nausea, and diarrhea, and did not lead to discontinuation of treatment. While fatigue is an expected side effect of HDACi therapy, no patient suffered grade 3 or 4 fatigue. During cycle 1, 22.2% (6/27) of the patients had grade 1 or 2 fatigue, which compares favorably to 47.4% treated with single agent panobinostat [32].
Based on early single center clinical experience with romidepsin, QTc prolongation and cardiac toxicity were suspected to be a class effect of HDACi[29]. However, other clinical studies investigating romidepsin failed to show similar clinically relevant drug-induced QTc changes, especially when the QTc interval was measured using Bazett’s correction formula [33,34]. Since the time of these early studies, FDA approved HDACis including romidespin [33,35,36], vorinostat [2,27,37] and panobinostat [6,11,38], have all been deemed safe and associated with minimal cardiac morbidity following relatively strict QTc recommendations. Similarly, in the 27 patients enrolled on our trial, QTc prolongation was no greater than grade 1, and did not result in dose adjustments, treatment discontinuation, or significant cardiac events in any patients, highlighting that AR-42 is also tolerated from a cardiac standpoint.
Despite a lack of grade 3 QTc prolongation and cardiac arrhythmias evident in MM patients treated with the combination of panobinostat, bortezomib, and dexamethasone, a more recent trial investigating the combination of bortezomib, dexamethasone, and quisinostat, a potent HDAC6 inhibitor, was associated with grade 3 QTc prolongation, ventricular fibrillation, and atrial fibrillation at the highest doses investigated [39]. These findings support continued close surveillance of cardiac toxicities potentially related to the use of novel HDACi, as well as further investigation regarding the possible differential cardiac effects of pan- and selective-HDACi[40].
The best clinical response in all 27 patients included three MM patients with minimal response (Table 1), though only one of these patients had a prolonged MR and most patients (20 of 27, 95% CI: 53.7 – 88.8) had stable disease for between 1 and 3 cycles. The longest duration of treatment was 18 and 27 (ongoing) cycles in a patient with myeloma and mantle cell lymphoma (MCL), respectively, and the patient with MCL had relapsed following allogeneic stem cell transplantation and treatment with ibrutinib (Table 2). This relative lack of single agent AR-42 activity is not unexpected, mirroring those of early single agent vorinostat [37] and panobinostat [32] investigations. Importantly, our response analyses highlight that 1) further work is necessary to identify those factors associated with clinical benefit, potentially, the c-myc trisomy evident in the patient with mantle cell lymphoma/atypical CLL, and 2) identification of the ideal combination regimen is needed to maximize the clinical efficacy of AR-42.
In vitro studies indicate that HDACi increase MM cell sensitivity to various therapeutic agents by interfering with cell adhesion mediated drug resistance (CAM-DR), specifically CD44[41]. CD44, a single-chain transmembrane glycoprotein, is a major cell surface receptor for hyaluronan (HA) that plays a role in the adhesion of MM cells to bone marrow stromal cells (BMSC) and induction of IL-6, and its overexpression is associated with lenalidomide and dexamethasone resistance in MM cells [41]. In fact, CD44 is highly expressed in extracellular vesicles, and has been implicated as a prognostic factor for patients with MM, such that high CD44 (> 280 ng/mL) has been associated with worse OS [30]. Until now, no agent in MM clinical trials has previously been shown to modulate CD44, and though our data is limited, nine out of ten patients had decreased sCD44 levels following treatment with AR-42. Although a 20% of sCD44 reduction in the blood of treated MM patients seems minimal, CD44 is expressed by many different types of cells, and cancer cells may represent a very minimal percentage of the CD44 positive cells in the entire body. Because of the limited number of samples, the 20% reduction in sCD44 may be associated with a greater reduction of CD44 in the myeloma cells upon AR-42 treatment, as we recently published in preclinical studies[19]. Given that the combination of AR-42 and IMiDs, including lenalidomide and pomalidomide, have been shown to result in synergistic apoptotic MM cell death in vitro[19], the clinical activity of AR-42 could overcome IMiD resistance in myeloma patients, and our correlative data justify a phase 1b combination of AR-42 and IMiD (NCT 02569320).
HDACi are therapeutic agents FDA approved for treatment of non-Hodgkin lymphoma (NHL) and MM, but their narrow therapeutic index limit their use. We report the phase 1 clinical data of AR-42, and establish its safety and tolerability in patients with multiple myeloma and lymphoma. Our data suggests that use of AR-42 in combination regimens may be an effective strategy to overcome drug resistance.
Supplementary Material
Acknowledgments
Financial support
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number U01CA076576. DWS was supported under Award Number T32CA165998. CCH, FP, ZL, and HR were supported under R21CA156222. The content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute or the National Institutes of Health. Research was also supported in part from Multiple Myeloma Opportunities for Research & Education (MMORE) and ARNO Therapeutics.
Footnotes
Authors Contributions
Clinical data analyses were performed by DWS under the mentorship of CCH. All statistical analyses were performed by EMH and XM. Clinical trial accrual, consent, and data were performed by AM, LA, RAB, BAC, DMB, JF, PP, JCB, and CCH. CCH supervised the study. DWS, CCH, CC, ZL, JW, EMH, AC, and FP wrote the manuscript. PK analyses were performed by MAP, CC, JW, WN, and MP. Correlative analyses pertaining to CD44 were performed by AC and FP. Data collection was performed by SK. All authors reviewed and approved the final manuscript prior to submission.
Declarations of Interest
The authors report no relevant conflicts of interest. Ohio State University holds the patent on the investigational drug AR-42 (US 10/597,022). The Office for Technology Licensing and Commercialization licensed AR-42 to Arno Therapeutics Inc. using the institution’s standard terms and conditions and approval process, in which no author participated.
AR-42 was generated at Ohio State University by Ching-Shih Chen PhD. Both the inventor and the university have the potential to benefit financially from AR-42 if the compound has clinical activity. None of the clinical investigators involved in this trial have personal potential to financially gain from the success of this program. To assure absence of conflict in assessment of response and attribution of toxicity, response and toxicity attribution were reviewed by CTEP prior to reporting results to mitigate institutional conflict of interest. Safety issues relative to dose increases and attribution of response were monitored by the Ohio State University Data Safety Monitoring Committee, Ohio State Phase 1 Data Safety Monitoring review committee, and the OSU Cancer Center IRB.
References
- 1.Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 3.Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–4491. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor OA, Horwitz S, Masszi T, et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol. 2015;33:2492–2499. doi: 10.1200/JCO.2014.59.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson PG, Schlossman RL, Alsina M, et al. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood. 2013;122:2331–2337. doi: 10.1182/blood-2013-01-481325. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos M, Siegel DS, Lonial S, et al. Vorinostat or placebo in combination with bortezomib in patients with multiple myeloma (VANTAGE 088): a multicentre, randomised, double-blind study. Lancet Oncol. 2013;14:1129–1140. doi: 10.1016/S1470-2045(13)70398-X. [DOI] [PubMed] [Google Scholar]
- 8.Richardson PG, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone in previously treated multiple myeloma: outcomes by prior treatment. Blood. 2016;127:713–721. doi: 10.1182/blood-2015-09-665018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson PG, Schlossman RL, Alsina M, et al. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood. 2013;122:2331–2337. doi: 10.1182/blood-2013-01-481325. [DOI] [PubMed] [Google Scholar]
- 10.San-Miguel JF, Hungria VT, Yoon S-S, et al. Final analysis of overall survival from the phase 3 Panorama 1 trial of panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2015;126:3026–3026. [Google Scholar]
- 11.San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15:1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 12.Lu Q, Wang DS, Chen CS, Hu YD, Chen CS. Structure-based optimization of phenylbutyrate-derived histone deacetylase inhibitors. J Med Chem. 2005;48:5530–5535. doi: 10.1021/jm0503749. [DOI] [PubMed] [Google Scholar]
- 13.Lu Q, Yang YT, Chen CS, et al. Zn2+-chelating motif-tethered short-chain fatty acids as a novel class of histone deacetylase inhibitors. J Med Chem. 2004;47:467–474. doi: 10.1021/jm0303655. [DOI] [PubMed] [Google Scholar]
- 14.Burns SS, Akhmametyeva EM, Oblinger JL, et al. Histone deacetylase inhibitor AR-42 differentially affects cell-cycle transit in meningeal and meningioma cells, potently inhibiting NF2-deficient meningioma growth. Cancer Res. 2013;73:792–803. doi: 10.1158/0008-5472.CAN-12-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao MW, Chu PC, Chuang HC, et al. Non-epigenetic function of HDAC8 in regulating breast cancer stem cells by maintaining Notch1 protein stability. Oncotarget. 2015 doi: 10.18632/oncotarget.6427. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Kulp SK, Chen CS, Wang DS, Chen CY, Chen CS. Antitumor effects of a novel phenylbutyrate-based histone deacetylase inhibitor, (S)-HDAC-42, in prostate cancer. Clin Cancer Res. 2006;12:5199–5206. doi: 10.1158/1078-0432.CCR-06-0429. [DOI] [PubMed] [Google Scholar]
- 17.Li DR, Zhang H, Peek E, et al. Synergy of Histone-Deacetylase Inhibitor AR-42 with Cisplatin in Bladder Cancer. J Urol. 2015;194:547–555. doi: 10.1016/j.juro.2015.02.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu W, Xu B, Yao Y, Yu X, Shen J. The novel HDAC inhibitor AR-42-induced anti-colon cancer cell activity is associated with ceramide production. Biochem Biophys Res Commun. 2015;463:545–550. doi: 10.1016/j.bbrc.2015.05.078. [DOI] [PubMed] [Google Scholar]
- 19.Canella A, Cordero Nieves H, Sborov DW, et al. HDAC inhibitor AR-42 decreases CD44 expression and sensitizes myeloma cells to lenalidomide. Oncotarget. 2015;6:31134–31150. doi: 10.18632/oncotarget.5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas DM, Alinari L, West DA, et al. The novel deacetylase inhibitor AR-42 demonstrates pre-clinical activity in B-cell malignancies in vitro and in vivo. PLoS One. 2010;5:e10941. doi: 10.1371/journal.pone.0010941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Suvannasankha A, Crean CD, White VL, Chen CS, Farag SS. The novel histone deacetylase inhibitor, AR-42, inhibits gp130/Stat3 pathway and induces apoptosis and cell cycle arrest in multiple myeloma cells. Int J Cancer. 2011;129:204–213. doi: 10.1002/ijc.25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai LY, Omar HA, Chiu CF, Chi ZP, Hu JL, Weng JR. Antitumor effects of (S)-HDAC42, a phenylbutyrate-derived histone deacetylase inhibitor, in multiple myeloma cells. Cancer Chemother Pharmacol. 2011;68:489–496. doi: 10.1007/s00280-010-1501-z. [DOI] [PubMed] [Google Scholar]
- 23.Parker A, Bain B, Devereux S. Best practice in lymphoma diagnosis and reporting. British Committee for Standards in Haematology with Royal College of Pathologists; London: 2008. [Google Scholar]
- 24.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 25.Health UDo, Services H. Common terminology criteria for adverse events (CTCAE) version 4.0. National Institutes of Health, National Cancer Institute; 2009. [Google Scholar]
- 26.Cheson B, Bennett J, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. 1996:4990–4997. [PubMed] [Google Scholar]
- 27.Badros A, Burger AM, Philip S, et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009;15:5250–5257. doi: 10.1158/1078-0432.CCR-08-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giles F, Fischer T, Cortes J, et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12:4628–4635. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- 29.Shah MH, Binkley P, Chan K, et al. Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2006;12:3997–4003. doi: 10.1158/1078-0432.CCR-05-2689. [DOI] [PubMed] [Google Scholar]
- 30.Harshman SW, Canella A, Ciarlariello PD, et al. Proteomic characterization of circulating extracellular vesicles identifies novel serum myeloma associated markers. Journal of Proteomics. 2016;136:89–98. doi: 10.1016/j.jprot.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HJ, Kang YH, Lee JS, et al. Positive expression of NANOG, mutant p53, and CD44 is directly associated with clinicopathological features and poor prognosis of oral squamous cell carcinoma. BMC Oral Health. 2015;15:153. doi: 10.1186/s12903-015-0120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf JL, Siegel D, Goldschmidt H, et al. Phase II trial of the pan-deacetylase inhibitor panobinostat as a single agent in advanced relapsed/refractory multiple myeloma. Leuk Lymphoma. 2012;53:1820–1823. doi: 10.3109/10428194.2012.661175. [DOI] [PubMed] [Google Scholar]
- 33.Molife R, Fong P, Scurr M, Judson I, Kaye S, de Bono J. HDAC inhibitors and cardiac safety. Clin Cancer Res. 2007;13:1068. doi: 10.1158/1078-0432.CCR-06-1715. author reply 1068–1069. [DOI] [PubMed] [Google Scholar]
- 34.Piekarz RL, Frye AR, Wright JJ, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res. 2006;12:3762–3773. doi: 10.1158/1078-0432.CCR-05-2095. [DOI] [PubMed] [Google Scholar]
- 35.Cabell C, Bates S, Piekarz R, et al. Systematic assessment of potential cardiac effects of the novel histone deacetylase (HDAC) inhibitor romidepsin. Blood. 2009;114:3709–3709. [Google Scholar]
- 36.Sager PT, Balser B, Wolfson J, et al. Electrocardiographic effects of class 1 selective histone deacetylase inhibitor romidepsin. Cancer Med. 2015;4:1178–1185. doi: 10.1002/cam4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson P, Mitsiades C, Colson K, et al. Phase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced multiple myeloma. Leuk Lymphoma. 2008;49:502–507. doi: 10.1080/10428190701817258. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Lebwohl D, Masson E, Laird G, Cooper MR, Prince HM. Clinically relevant QTc prolongation is not associated with current dose schedules of LBH589 (panobinostat) J Clin Oncol. 2008;26:332–333. doi: 10.1200/JCO.2007.14.7249. discussion 333–334. [DOI] [PubMed] [Google Scholar]
- 39.Moreau P, Facon T, Touzeau C, et al. Quisinostat, bortezomib, and dexamethasone combination therapy for relapsed multiple myeloma. Leuk Lymphoma. 2016:1–14. doi: 10.3109/10428194.2015.1117611. [DOI] [PubMed] [Google Scholar]
- 40.Aune SE, Herr DJ, Mani SK, Menick DR. Selective inhibition of class I but not class IIb histone deacetylases exerts cardiac protection from ischemia reperfusion. Journal of molecular and cellular cardiology. 2014;72:138–145. doi: 10.1016/j.yjmcc.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjorklund CC, Baladandayuthapani V, Lin HY, et al. Evidence of a role for CD44 and cell adhesion in mediating resistance to lenalidomide in multiple myeloma: therapeutic implications. Leukemia. 2014;28:373–383. doi: 10.1038/leu.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 43.Friberg LE, Isbister GK, Duffull SB. Pharmacokinetic-pharmacodynamic modelling of QT interval prolongation following citalopram overdoses. Br J Clin Pharmacol. 2006;61:177–190. doi: 10.1111/j.1365-2125.2005.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


