Abstract
Objective
Mindfulness meditation training has been previously shown to enhance behavioral measures of executive control (e.g. attention, working memory, cognitive control), but the neural mechanisms underlying these improvements are largely unknown. Here, we test whether mindfulness training interventions foster executive control by strengthening functional connections between dorsolateral prefrontal cortex (dlPFC) - a hub of the executive control network – and frontoparietal regions that coordinate executive function.
Methods
Thirty-five adults with elevated levels of psychological distress participated in a 3 day RCT of intensive mindfulness meditation or relaxation training. Participants completed a resting state fMRI scan before and after the intervention. We tested whether mindfulness meditation training increased resting state functional connectivity (rsFC) between dlPFC and frontoparietal control network regions.
Results
Left dlPFC showed increased connectivity to the right inferior frontal gyrus (T = 3.74), right middle frontal gyrus (T = 3.98), right supplementary eye field (T = 4.29), right parietal cortex (T = 4.44), and left middle temporal gyrus (T = 3.97; all p<0.05) following mindfulness training relative to the relaxation control. Right dlPFC showed increased connectivity to right middle frontal gyrus (T = 4.97, p < 0.05).
Conclusions
We report that mindfulness training increases rsFC between dlPFC and dorsal network (superior parietal lobule, supplementary eye field, MFG) and ventral network (right IFG, middle temporal/angular gyrus) regions. These findings extend previous work showing increased functional connectivity amongst brain regions associated with executive function during active meditation by identifying specific neural circuits in which rsFC is enhanced by a mindfulness intervention in individuals with high levels of psychological distress.
Trial Registration
Clinicaltrials.gov (#NCT01628809)
Keywords: mindfulness, resting state functional connectivity, executive control
Introduction
Mindfulness meditation interventions, which train the capacity for an open and receptive attention toward present-moment experience, produce many positive physical and psychological health effects, including increased stress resilience and greater executive control (1–3). However, the specific aspects of mindfulness training that increase executive control – and their underlying neural mechanisms – have yet to be fully elucidated. One promising candidate brain region that may underlie the enhanced executive function observed with mindfulness practice is the dorsolateral prefrontal cortex.
Dorsolateral prefrontal cortex (dlPFC), a key region in the central executive network (a neural network that activates during tasks requiring executive control), is broadly implicated in the regulation of attention, decision making, working memory, and cognitive control (4), and is the key hub of a dorsal neural pathway for the control of behavior (5). Moreover, a growing body of literature shows that dlPFC is active during meditative states, including focused attention meditation practices (6,7), open-monitoring mindfulness meditation practices (8), and in response to affective stimuli in trained meditators (9). This suggests a dlPFC-specific pathway by which mindfulness may encourage executive control, which is further supported by recent evidence that mindfulness training enhances functional coupling between dlPFC and default mode network regions (10), as well as behavioral evidence that mindfulness increases performance on various cognitive tasks, including attention and working memory (11,12), self-regulation (12), and perceptual discrimination (11) (although not all studies have shown beneficial effects of mindfulness on executive function tasks (13,14)). As mindfulness trains the capacity for focused attention as well as open monitoring – cognitive processes that recruit dlPFC as well as dorsal and ventral regions for cognitive control, e.g. parietal cortex, superior temporal cortex, ventrolateral prefrontal cortex – increased functional coupling between dlPFC and these regions may be a neural mechanism underlying the enhanced executive control outcomes observed with mindfulness training. A hypothesis not previously explored in the literature is that mindfulness fosters greater executive control (e.g. attention, working memory, emotion regulation, cognitive control) by strengthening the intrinsic functional connections between dlPFC and the dorsal and ventral frontoparietal control regions that coordinate executive control – specifically, intraparietal sulcus, frontal and supplementary eye fields, posterior parietal cortex, temporoparietal junction, ventrolateral frontal cortex, and inferior frontal gyrus.
Dorsolateral PFC has functional and anatomic connections to networked regions for attention and cognitive control, including dorsal regions (e.g., bilateral intraparietal sulcus, frontal and supplementary eye fields, and superior and posterior parietal cortex) involved in top-down directing of attention to specific inputs, and ventral regions (e.g., right-lateralized temporoparietal junction, ventral frontal cortex, superior temporal gyrus, and inferior frontal gyrus), thought to be responsible for monitoring and reorienting attention in response to salient stimuli (4,15,16). Anatomical tracing studies in primates demonstrate that dlPFC (primate brain area 9 and 46) is densely connected to these regions, with axonal tracts projecting to cingulate cortex, lateral prefrontal cortex, superior, middle and inferior frontal gyri, premotor and supplementary motor areas, orbitofrontal cortex, and insular cortex (17). Yet, no research has evaluated how mindfulness training might modulate dlPFC resting state functional connectivity to these key ventral and dorsal frontoparietal control network regions.
Resting state functional connectivity (rsFC) has proven to be a robust method of evaluating inter-regional dynamics. It has the advantage of being task-independent, reliable, and shows consistent correlations with known functional and structural topography (18,19), and is thus an ideal tool for investigating dlPFC functional connections in the context of mindfulness training, allowing us to build a functional network-based account of mindfulness effects for cognitive control. Previous studies provide evidence that dlPFC resting state functional connectivity changes with mindfulness; specifically, increased coupling is observed between dlPFC and default mode network regions (e.g. dorsal anterior cingulate, posterior cingulate cortex), consistent with decreased mind-wandering and increased capacity for attention-shifting in experienced meditators (10,20,21).
In addition to these studies of mindfulness-associated dlPFC functional connectivity changes, clinical studies have shown that dlPFC resting state functional connectivity is altered by neuropsychiatric conditions; in schizophrenia, rsFC is reduced between dlPFC and parietal cortex, posterior cingulate, thalamus, and striatum, and increased between dlPFC and paralimbic structures as well as left temporal lobe (22). In euthymic bipolar disorder patients, right dlPFC-medial PFC rsFC is increased relative to controls (23). In patients with chronic hallucinations, reduced rsFC is observed between right dlPFC and right IFG (24). In all these conditions, altered dlPFC rsFC is thought to underlie the cognitive changes associated with these disorders, including working memory deficits, emotion regulation, and somatosensory processing. Although no studies have directly tested for dlPFC alterations after mindfulness training, there are studies showing that these executive functions are enhanced by mindfulness training (11,12,25). Moreover, there is evidence that dlPFC functional connectivity changes may relate to behavioral measures of executive control; during a 2-back working memory task, decreased FC between right dlPFC and left inferior parietal cortex and increased FC between left dlPFC and the right inferior temporal lobe was observed in autistic subjects relative to a control group (26). Finally, dlPFC activity has been shown to be stress-sensitive; acute psychological stress decreases dlPFC activity during working memory tasks, indicating a shift of neural resources away from executive control network regions (27). Chronic psychosocial stress disrupts functional connectivity between dlPFC and other frontoparietal network regions associated with attentional shifts; significantly, this disrupted connectivity was shown to be reversed after 1 month of decreased stress, indicating that stress-related changes in dlPFC connectivity are highly plastic (28). Therefore, it is plausible that a mindfulness training intervention in a high-stress sample could reverse stress-related decreases in dlPFC resting state functional connectivity.
To test this possibility, we developed a well-controlled training format for evaluating mindfulness meditation training effects on the brain in a high-stress community sample by adapting 8-week mindfulness meditation and relaxation training programs (1,29,30) to a 3-day residential retreat format. We recruited high stress unemployed job-seeking adults and randomized them to either a 3-day mindfulness meditation training program or a matched 3-day relaxation training lacking a mindfulness training component, allowing us to test for effects specific to mindfulness training and not general relaxation. This approach improves study internal validity by increasing experimental control of treatment delivery (as both the meditation and relaxation programs were delivered at the same time in the same relaxing retreat setting) and fosters improved treatment compliance and reduced participant attrition in hard-to-reach-and-retain high-stress patient populations. In initial work from this study, we focused on the effects of mindfulness on resting state default mode network connectivity (10). We now expand upon this work by probing dlPFC functional connectivity to specific a priori defined brain regions of interest in intraparietal sulcus, frontal eye fields, posterior parietal cortex, temporoparietal junction, ventrolateral frontal cortex, middle and inferior frontal gyrus. To investigate how mindfulness training may modulate resting state functional connectivity of the dlPFC, we tested the hypothesis that this high-stress unemployed sample of community adults would show increased connectivity between dlPFC and regions that comprise resting state dorsal and ventral attention and executive function networks previously identified in the literature (intraparietal sulcus, frontal and supplementary eye fields, posterior parietal cortex, temporoparietal junction, ventrolateral frontal cortex, and inferior frontal gyrus) (31–33) after a mindfulness training intervention, relative to a well-matched relaxation control program.
Methods
Participants
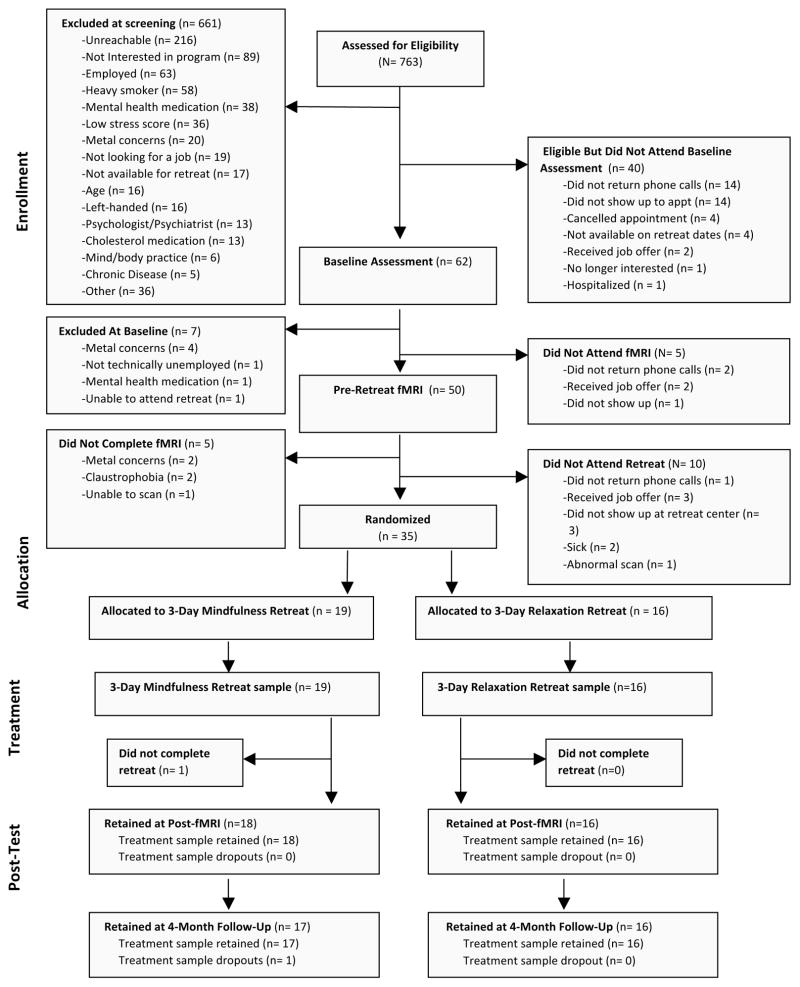
Thirty-five stressed unemployed job-seeking community adults (who indicated moderate to high levels of perceived job-seeking stress over the past month, scoring >9 on an adapted 4-item Perceived Stress Scale (PSS) for job-seeking stress; i.e., “In the last month, how often have you felt confident about your ability to handle your job-related problems?” α=.6) participated in a single-blind RCT of 3-day intensive mindfulness meditation or relaxation training (see Table 1 for participant characteristics). Participants were recruited via newspaper advertisements and through employment agencies in Pittsburgh, PA. Participants were also English-speaking, had no pre-existing health conditions, were willing and available to participate in all study assessments, and were willing to be randomly assigned to one of two study conditions. Callers who met these qualifications were invited to come to Carnegie Mellon University for an in-person screening interview and baseline assessment, where the full study procedures were explained. Interested participants provided informed consent. A more in-depth screening interview followed, including assessments of basic cognitive ability, right- or left-handedness and internal metal content (for fMRI eligibility), employment background (to probe for unemployment-related stress), medical history, and health behavior. Subjects taking psychotropic medications were excluded. Demographic information was collected as well, including age, race, education, income, and marital and family status. Qualified participants completed a baseline psychosocial assessment on their own, described below. Participants were compensated $20 for this assessment. Figure 1 depicts the flow of participants through the RCT. This study was approved by the Carnegie Mellon University Internal Review Board and all participants provided written informed consent.
Table I.
Baseline Characteristics of Randomized Controlled Trial Participants.
| Characteristic | Mindfulness Group | Relaxation Group | Difference Statistic |
|---|---|---|---|
| Age [mean years (SD)] | 37.94 | 41.00 | t(33)= −.48, p= 0.64 |
| (10.96) | (9.55) | ||
| Sex | χ2(1)=.24, p= 0.63 | ||
| Male | 11 | 9 | |
| Female | 7 | 8 | |
| Ethnicity | χ2(3)= 4.36, p= 0.23 | ||
| White | 10 | 13 | |
| African American | 6 | 2 | |
| Asian American | 1 | 0 | |
| Latino(a) | 0 | 0 | |
| Native American | 0 | 0 | |
| Other | 0 | 1 | |
| Years Unemployed | 8.17 | 10.58 | t(33)= −.43, p= 0.67 |
| (12.48) | (20.31) | ||
| Education | χ2(8)= 8.43, p= 0.39 | ||
| No high school degree | 1 | 0 | |
| GED | 1 | 0 | |
| High school degree | 1 | 2 | |
| Technical training | 3 | 2 | |
| Some college | 4 | 3 | |
| Associate degree | 2 | 0 | |
| Bachelor’s degree | 2 | 7 | |
| Master’s degree | 3 | 3 | |
| MD/PhD/JD/PharmD | 1 | 0 |
Notes: Standard deviation values are provided in parentheses. Mindfulness group refers to the 3-Day Health Enhancement thru Mindfulness (HEM) intervention. Relaxation group refers to the 3-Day Health Enhancement thru Relaxation (HER) intervention.
Figure 1.
CONSORT flowchart of participants retained at each stage of the Mindfulness Meditation Training RCT.
Procedure
We conducted this RCT between December 2010 and October 2011. Beginning four weeks before the 3-day training intervention, participants completed a baseline neuroimaging session. All participants began with a 5-minute resting state scan (where they passively viewed a fixation cross), followed by several functional tasks in counterbalanced order and an 8-minute perfusion MRI scan (the results of these tasks will be reported in separate papers). After neuroimaging, participants were invited to a nearby residential retreat center where they were randomized to either a 3-day intensive mindfulness meditation training (N=18) or matched 3-day relaxation residential retreat intervention (N=17) (described in Interventions below). Only the participant, project manager, treatment program staff members, and the treatment program instructor were aware of the participant’s study condition. Participants returned for a neuroimaging assessment within two weeks of completing the 3-day intervention and completed an identical scanning procedure as at baseline, including the same 5-minute resting state scan. At both neuroimaging sessions, participants were instructed to passively view a fixation cross during the resting state scan period and not to sleep or engage in any meditation or relaxation practices (which was verbally confirmed in all participants at the conclusion of the neuroimaging session). 97% of randomized participants were retained at the post-intervention neuroimaging assessment (3% study attrition). As part of the larger study, participants completed a comprehensive battery of psychosocial measures and provided a blood draw at baseline and at 4-month follow-up; the present report focuses on testing how mindfulness meditation training changes rsFC patterns using the 5-minute resting state BOLD scan at baseline and in the two weeks following the 3-day intensive training period (post-intervention).
Interventions
We adapted the standardized and manualized 8-week Mindfulness-Based Stress Reduction (MBSR) program (which includes a day-long retreat) (1,29) into a condensed 3-day residential retreat format, entitled Health Enhancement through Mindfulness (HEM). Delivery of the HEM program in a structured residential retreat format improves compliance with training, reduces treatment attrition, and greater experimental control is afforded by offering a parallel matched relaxation training retreat (in a separate wing of the retreat center). The HEM instructor was a doctoral level psychologist with 7 years of MBSR teaching experience. Briefly, the HEM program consists of mindfulness training through body scan awareness exercises, sitting and walking meditations, mindful eating, and mindful movement (gentle hatha yoga postures). After each formal meditation period, participants engaged in discussion of their observations about themselves and the practices. The instructor modeled and encouraged attitudes to foster mindfulness, such as letting go of judgment and expectations, cultivating self-care, patience, and friendly curiosity toward present moment experience. On the third day, formal meditation practices were extended to discussions about how participants could use mindful awareness for their unemployment and job-seeking stress.
We developed a structurally matched Health Enhancement through Relaxation (HER) program that included similar behavioral training activities (e.g., walking, stretching, and didactics) as HEM, but all trainings emphasized participation in these activities in a restful way rather than a mindful way and did not include progressive muscle relaxation. The HER program instructor was a licensed social worker with over 2 decades of clinical experience in stress management. The use of a structurally-matched active comparison group was designed to control for non-mindfulness specific factors, such as positive treatment expectancies, group support, teacher attention, physical activity, and mental engagement.
Image Acquisition
Structural and functional images were acquired on a Siemens Verio 3T scanner using a 32-channel head coil. High-resolution T1-weighted gradient-echo images were acquired at the start of the scanning session, with a slice orientation of AC-PC aligned, temporal lobes up (TR=1800ms, TE=2.22ms, flip angle= 9°, matrix size= 256×256, number of slices= 256, FOV= (205mm, 0.8mm thick slices), GRAPPA acceleration factor PE= 2, voxel size= 0.8×0.8×0.8mm). Four functional echo-planar imaging runs were acquired, including a 300 second resting state scan (TR=2000ms, TE=30ms, flip angle=79°, matrix size=64×64, number of slices=36, FOV= 205mm, 3.2mm thick slices EPI with rate 2 GRAPPA, voxel size=3.2mm × 3.2mm × 3.2mm).
Image Preprocessing
Functional BOLD data were processed using SPM8 (Welcome Department of Cognitive Neurology, London, UK; implemented by MATLAB, MathWorks, Inc., Natick, MA, USA). First, the data were realigned to the mean image of the first run and then smoothed with a 4mm FWHM Gaussian kernel to be in the preferred format for the motion correction program, ArtRepair. Data were then submitted to motion correction using the ArtRepair utility (34,35), an interpolation-based motion correction utility program. Motion correction in ArtRepair followed a two-step process. In the first step, an algorithm was applied to each run of data to suppress interpolation errors due to large motion. The algorithm applied a larger correction to edge-wise voxels than to central voxels, since the effects of motion on BOLD signal are most pronounced in these areas. In the second step, TRs with large amounts of fast motion or large global signal variation were flagged for repair. A default motion threshold of 1mm was used, so that TRs with motion greater than 1mm were flagged for repair. Repair of the data was done through linear interpolation, so that volumes flagged for repair were filled in with the average signal value from the two nearest unrepaired TRs. After motion correction the functional data was normalized to the standard Montreal Neurological Institute (MNI) T1 template using indirect normalization, in which the functional images were first coregistered to the MPRAGE, and then the MPRAGE was normalized to the T1 template. Finally, the images were smoothed a second time with a 7mm FWHM kernel, resulting in an overall FWHM smoothing of 8mm (34).
Connectivity and Data Analyses
Preprocessing of images was conducted in SPM8 (Welcome Department of Cognitive Neurology, London, UK; run on MATLAB, MathWorks, Inc., Natick, MA, USA) and rsFC analysis was conducted using the CONN toolbox (36). The CONN toolbox estimates orthogonal time series using principal component analysis of the BOLD signal in each noise ROI. At the single subject level, functional connectivity was measured by calculating the average BOLD time series across all voxels in each seed region and calculating a bivariate correlation between each seed region of interest and every other voxel. A hemodynamic response function was used to weight down the initial scans within each resting state block to minimize potential ramping effects. Seed regions were defined by creating 8mm spheres around peak coordinates of four dlPFC clusters. We defined bilateral ROIs based on a previous study of resting state functional connectivity that showed increased dlPFC connectivity in meditators versus controls, to investigate dlPFC-associated rsFC that may be mindfulness-specific (MNI coordinates = 42, 21, 14; −48, 36, 15) (20). In order to also investigate dlPFC regions classically associated with executive control, we identified two additional ROIs by searching the features “attention” and “executive control” in the Neurosynth database (MNI = 32 50 12; −28 0 54) (37–39). Using Neurosynth to identify ROIs adds the value of an automated meta-analysis (including resting state and task-based studies) to identify neural regions that have been associated with features of interest (i.e. “attention”). See Table 2 for a complete list of ROIs and MNI coordinates. Seeded resting state BOLD fMRI images were then applied in a group-level flexible factorial analysis in SPM8 with two factors specified, time (pre- and post-intervention) and group (HEM vs HER groups). We generated a time-by-group ordinal interaction contrast that tested for baseline to post-intervention decreases in rsFC in the HEM program (relative to the HER program) using contrast weights: [1(pre,HEM), 1(pre,HER), −3(post, HEM), 1(post,HER)]. Cluster-level correction for multiple comparisons was obtained using a Monte Carlo simulation implemented by AlphaSim (National Institute of Mental Health, Bethesda, Maryland). AlphaSim was implemented with an anatomical ROI mask (generated using the Wake Forest University Pickatlas, that covered middle frontal cortex, inferior frontal cortex, superior and posterior parietal lobule, and middle temporal cortex) using an 8mm smoothing kernel and 10000 iterations. Regions included in this anatomical ROI mask were selected based on previous literature identifying resting state networks associated with executive function and attention (32). Significant clusters (P < 0.05, corrected) were defined as those involving k > 22 contiguous voxels, each at P < .005.
Table 2.
ROIs generated for seed-based analyses.
| Region of Interest | MNI coordinates | Radius |
|---|---|---|
| Left dlPFC | −48, 36, 15 | 8mm |
| Left dlPFC | −28, 0, 54 | 8mm |
| Right dlPFC | 32, 50, 12 | 8mm |
| Right dlPFC | −28, 0, 54 | 8mm |
Results
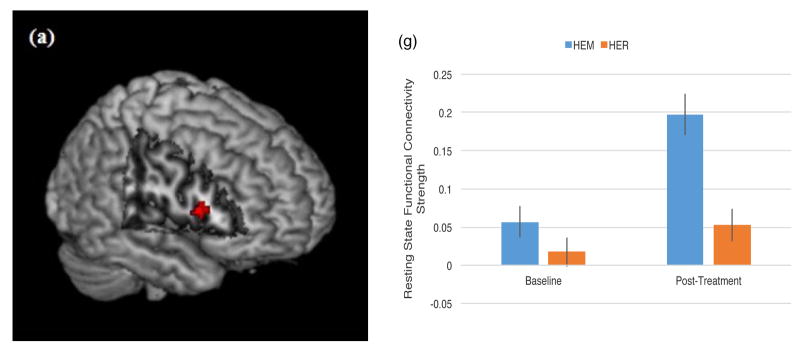
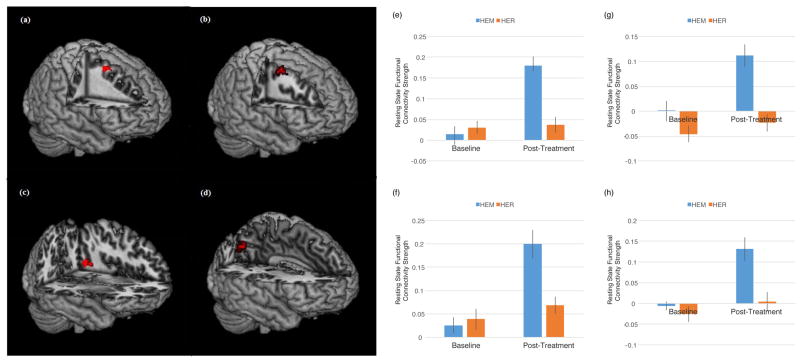
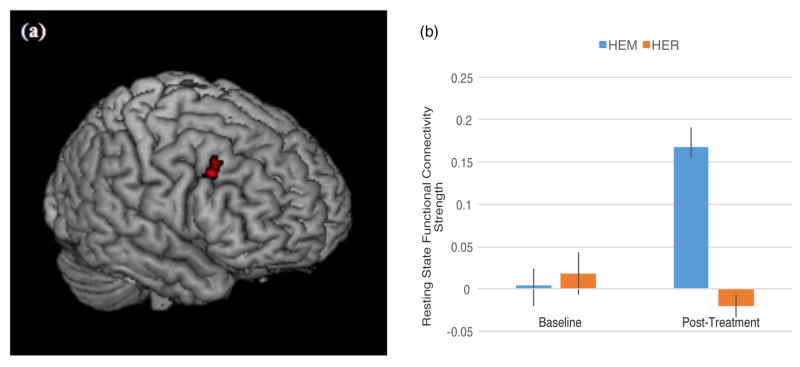
It was predicted that mindfulness meditation training (relative to a well-matched relaxation training program without a mindfulness component) would increase rsFC between dlPFC and ventral and dorsal [executive control] system regions (intraparietal sulcus, supplementary and frontal eye fields, posterior parietal cortex, temporoparietal junction, ventrolateral prefrontal cortex) in stressed, unemployed community adults. Consistent with this prediction, left dlPFC (MNI = −48, 36, 15) showed increased connectivity to the right inferior frontal gyrus (or vlPFC, a key ventral attention control region) (p<0.05, k=28, corrected for multiple comparisons; Table 3, Figure 2). Left dlPFC (MNI = −28, 0, 54) also showed increased rsFC to the right middle frontal gyrus (k=34), right supplementary eye field (k = 38) and right superior/posterior parietal cortex (k = 23) (dorsal attention network regions) (p<0.05, corrected for multiple comparisons; Table 3, Figure 3), and to the left middle temporal gyrus (p<0.05, k=52, corrected for multiple comparisons; Table 3, Figure 3) following mindfulness training relative to the relaxation control group. This pattern of mindfulness-associated increased rsFC supports the idea that mindfulness may modulate task-independent functional connectivity between executive and attentional brain regions.
Table 3.
Clusters with significantly increased rsFC to dlPFC seed regions after mindfulness training relative to a relaxation control intervention (p < 0.05, corrected for multiple comparisons).
| Seed ROI | MNI | k | T | |
|---|---|---|---|---|
| Left dlPFC (−48, 36, 15) | Right IFG | 54 16 14 | 28 | 3.74 |
| Left dlPFC (−28, 0, 54) | Right SEF (BA 6) | 22 12 58 | 38 | 4.29 |
| Right MFG | 34 2 58 | 34 | 3.98 | |
| Left Superior parietal lobule (BA 7) | −10 −78 36 | 23 | 4.44 | |
| Left Middle Temporal/Angular Gyrus | −42 −58 10 | 52 | 3.97 | |
| Right dlPFC (32, 50, 12) | Right MFG | 46 20 40 | 30 | 4.97 |
IFG = inferior frontal gyrus, SFG = superior frontal gyrus, SEF = supplementary eye fields, MFG = middle frontal gyrus.
Figure 2.
(a) Regions that showed increased resting state functional connectivity with left dlPFC (−28, 0, 54) from pre- to post-mindfulness meditation training (HEM) relative to relaxation training (HER) (p<0.05, corrected for multiple comparisons, cluster-thresholded k>21). Specifically, a condition by time spreading interaction analysis revealed a significant cluster in right inferior frontal gyrus (k = 28, peak MNI coordinates (54, 16, 14), T = 3.74). (b) Mean connectivity strength signal change for right IFG for the mindfulness (HEM) and relaxation (HER) training groups at each of the two time points (pre-intervention and post-intervention). Error bars depict +/− 1 standard error. Parameter estimates were extracted in SPM8.
Figure 3.
Regions that showed increased resting state functional connectivity with left dlPFC (−48, 36, 15) from pre- to post-mindfulness meditation training (HEM) relative to relaxation training (HER) (p<0.05, corrected for multiple comparisons, cluster-thresholded k>21). Specifically, a condition by time spreading interaction analysis revealed significant clusters in (a, e) right SEF (k = 38, peak MNI coordinates (22, 12, 58), T = 4.29), (b, f) right middle frontal gyrus (k = 34, peak MNI coordinates (34, 2, 58), T = 3.98), (c, g) left middle temporal/angular gyrus (k =52, peak MNI coordinates (−42, −58, 10), T = 3.97), and (d, h) left posterior parietal cortex (k = 23, peak MNI coordinates (−10, −78, 36), T = 4.44). (e – h) Mean connectivity strength signal change for the mindfulness (HEM) and relaxation (HER) training groups at each of the two time points (pre-intervention and post-intervention). Error bars depict +/− 1 standard error. Parameter estimates were extracted in SPM8.
Right dlPFC (MNI = 32, 50, 12) showed increased connectivity to right middle frontal gyrus (p < 0.05, k = 30, corrected for multiple comparisons; Table 3, Figure 4). Right dlPFC cluster (MNI = 42, 21, 24) showed no significant time by group differences in resting state functional connectivity. For all dlPFC seed regions, no significantly reduced pre-post rsFC with other brain regions was observed in the mindfulness training group relative to the relaxation control group (p < 0.05, k > 22, corrected for multiple comparisons).
Figure 4.
(a) Right MFG region that showed increased resting state functional connectivity with right dlPFC (32, 50, 12) from pre- to post-mindfulness meditation training (HEM) relative to relaxation training (HER) (p<0.05, corrected for multiple comparisons, cluster-thresholded k>21). Specifically, a condition by time spreading interaction analysis revealed a significant cluster in right middle frontal gyrus (k = 30, peak MNI coordinates (46, 20, 40), T = 4.97). (b) Mean connectivity strength signal change for right MFG for the mindfulness (HEM) and relaxation (HER) training groups at each of the two time points (pre-intervention and post-intervention). Error bars depict +/− 1 standard error. Parameter estimates were extracted in SPM8.
Discussion
Here we report that mindfulness training, relative to a well-matched relaxation control intervention, increases resting state functional connectivity between dlPFC and dorsal network (superior parietal lobule, supplementary eye field, MFG) and ventral network (right IFG, middle temporal/angular gyrus)-associated regions. Consistent with our hypotheses, these findings broadly suggest that brief mindfulness training increases functional connectivity between a hub in the executive control network (the dlPFC) and dorsal and ventral corticolimbic circuits involved in cognitive control. These findings build upon previous work showing that functional connectivity amongst broadly distributed brain regions associated with attention, interoception, and emotional processing increases during active meditation (40), and that dlPFC connectivity is strengthened following stress-reduction interventions (27), by identifying specific neural circuits in which resting state functional connectivity is enhanced by a mindfulness training intervention in a high-stress participant sample.
The superior parietal cortex, supplementary eye fields, and MFG are key regions functionally connected to left dlPFC and associated with a dorsal circuit for goal-directed, sustained control of behavior and attention allocation (5). Strong coactivation is particularly reported between dlPFC and parietal cortex; posterior and superior parietal regions play a role in spatial orientation and focused visuospatial attention, and the dlPFC-posterior parietal pathway is thought to be engaged when extra cognitive control is required to process incoming stimuli and select behavioral outputs (41). One of the primary skills trained by mindfulness is focused attention, and focused attention meditation has been previously associated with increased dlPFC activity (6). Increased resting state functional connectivity between dorsal stream regions and dlPFC suggests that the focused attention trained by mindfulness may be enhancing the ability of dlPFC to exert top-down control for attention and action selection via strengthening of this dorsal neural circuit.
The SEF has direct anatomical projections to dlPFC (42), and plays a functional role in planning saccadic eye movements, updating and error monitoring for movement plans, and mapping stimulus-response associations for cognitive-behavioral learning (43,44). Our finding of greater rsFC between the SEF and dlPFC may reflect greater executive control over action output (in particular, modifying behavior based on visual stimuli) reported among mindfulness-trained subjects (although we do not measure executive control in the present study). As previously discussed, SEF is considered part of a dorsal frontoparietal attention network that also includes dlPFC and posterior parietal cortex, including the superior parietal lobule (45). SEF activity is observed during both attention shifting (requiring a saccade) and peripheral attention tasks that do not require a saccadic eye movement (44), indicating a broader role for SEF in attentional processes, such as the focused attention and open monitoring trained by mindfulness. Of note, previous studies have shown that the SEF shows increased functional connectivity specifically to the left superior parietal lobule during active allocation of attention (45). Here, we similarly observe left-lateralized increased dlPFC rsFC with SEF and superior parietal lobule, supporting a lateralized, functionally connected dorsal attention system enhanced by mindfulness training.
Recently, it has been recognized that ventral corticolimbic circuitry also plays a distinct role in top-down regulation; in contrast to the dorsal control pathway, ventral circuitry is thought to link salience processing to immediate behavioral control (5). The right IFG is a key hub in this pathway, where it is responsible for active maintenance of stimulus information and integrating salient, interoceptive, and sensory inputs, creating a top-down biasing effect that leads to immediate action selection by posterior cortical regions (41). Importantly, right IFG is thought to have an orienting function in switching between internally and externally oriented control modes in response to salient stimuli (46) and coordinate further processing of salient stimuli (47). The role of IFG in salience processing and responding is corroborated by studies showing that cognitive control-related ventrolateral prefrontal cortex activity (including IFG and anterior insula) inhibits processing of emotional stimuli (48,49); such top-down control has been posited to be an important mechanism for emotion regulation and coping – that engaging right IFG suppresses retrieval of emotional memories (50) and predicts self-reported pain symptom improvements after administration of a placebo (51). Moreover, dispositional mindfulness is associated with more successful cognitive reappraisal of negative emotions (52) and increased activation in vlPFC during affect labeling (53). Together, these studies suggest that more mindful individuals may be better able to use this ventral pathway, including right IFG, for top-down regulation of emotion. Our finding of increased dlPFC-right IFG coupling supports the theory that mindfulness training strengthens a resting state ventral control pathway for salience processing and emotion regulation. We postulate that at the behavioral level, mindfulness causes this effect by training open monitoring skills, which promote active awareness and maintenance of internal and external stimuli as they arise – functions attributed to a right-lateralized ventral frontoparietal network.
We also report increased rsFC after mindfulness training from left dlPFC to left middle temporal gyrus extending to the angular gyrus, another ventral control-associated region that plays a role in attention allocation to salient stimuli (31), lending further support to the theory that mindfulness strengthens the functional connections between executive control and salience-responding ventral attentional regions. Additionally, this finding accords with previous imaging studies showing changes in the left temporal lobe with meditation practice, including increased grey matter concentration (54,55) and volume (56). Moreover, dlPFC and middle temporal regions are structurally connected by the temporal component of the superior longitudinal fasciculus (tSLF), and enhanced connectivity of the left tSLF has been previously observed in long-term meditators (57). Our finding of increased rsFC between dlPFC and left middle temporal gyrus extends these findings and suggests that functional connectivity changes from brief mindfulness training may precede these structural changes associated with long-term meditation practice.
Contrary to hypotheses, we observed no mindfulness-associated rsFC changes between dlPFC and the frontal eye fields or intraparietal sulcus. This may be due to topographical differences in frontal-posterior parietal cortical functional connectivity. For example, in a previous study of spatial attention, whereas SEF showed increased functional connectivity specifically to the superior parietal lobule (SPL) (regions in which we do observe increased rsFC to dlPFC in this study), the frontal eye fields showed greater functional connectivity to intraparietal sulcus (IPS) (45). In the same study, robust structural connections between FEF-IPS and between SEF-SPL were also shown, consistent with the observed attention-associated functional connectivity patterns (45). It has been suggested that there are thus distinct FEF-IPS and SEF-SPL pathways for spatial attention (45). Although all of these regions have anatomical connections to dlPFC, our pattern of results suggests that mindfulness training may specifically enhance left dlPFC rsFC to the SEF/SPL pathway, but not the FEF/IPS pathway. As there are also different behavioral correlates for these pathways (SEF and SPL play greater roles in task-switching (58), condition-action associations (43), and object- and gaze-centered attentional representations (45), whereas FEF and IPS respond to viewer-centered representations (45) and are thought to contain a salience map of the visual environment for focused spatial attention (59)), our positive findings with one pathway and not the other could also be due to the particular aspects of attention that were trained within our brief mindfulness intervention.
We also saw no changes in dlPFC rsFC with temporoparietal junction (TPJ) rsFC, a region implicated in responding to salient stimuli (60), theory of mind (61), and attentional orienting (60) and associated with mindfulness meditation (with greater TPJ activation observed during focused breathing and greater TPJ cortical thickness observed after an 8 week MBSR program) (55,62). Previous studies investigating the functional connectivity of TPJ have produced variable results; greater positive functional connectivity has been observed between TPJ and ventral PFC during the resting state (33) and between TPJ and anteromedial PFC on a social emotion task (63), while both positive and negative rsFC has been reported with dlPFC (64). Recent work has suggested that this may be due to topographical differences in structural and functional connectivity of TPJ subregions to prefrontal cortex (65); one explanation for our negative finding may be that our particular dlPFC seed regions do not have robust connections to TPJ.
The lateralization in dlPFC functional connectivity changes we report here (e.g. left dlPFC to right SEF, IFG, and MFG and left parietal lobule and temporal/angular gyrus; right dlPFC to right MFG) may be a product of hemispheric differences in dlPFC function. Furthermore, human and primate studies have demonstrated that lateral frontal cortex is organized axially into functionally distinct areas with different axonal projections (17), suggesting that functional connectivity will be highly seed region-dependent. Left dlPFC activation has been associated with response choice, rapid attention adjustment, neutrally valenced reasoning, and higher-level motor planning (66–69), and greater left dlPFC and posterior parietal co-activation is thought to reflect increased task-positive attention allocation and executive control (70); this emphasis on the function of left dlPFC in neutral higher cognitive functions and action output is consistent with our account of increased left dlPFC to SEF, middle frontal, and parietal connectivity. In contrast, right dlPFC is implicated in working memory for emotional stimuli (71), attentional conflict (68), and planning performance (72). Right dlPFC activations spatially similar to our seed region have been reported in association with increased neuroticism-associated functional connectivity during viewing of angry and fearful facial expressions (73), in perceptual tasks as a function of task difficulty (74), during attention shifting (44), response inhibition tasks (75), and encoding and retrieval of valenced words (76). We report stronger right dlPFC rsFC to middle frontal gyrus, a region frequently coactivated with right dlPFC on emotional and attention tasks (44,76), and previous mindfulness studies suggest that mindfulness training enhances the ability to regulate emotion (9,77–79). Increased right-lateralized dlPFC-MFG functional connectivity may potentially underlie mindfulness-associated improvements in top-down control of emotion regulation.
While we investigated rsFC changes in the present study, it will be important to probe these same functional connections in task-based cognitive control tasks, particularly given the previous literature relating meditation to greater dlPFC activity during cognitive tasks (53,80). Our present findings of enhanced rsFC indicate that individuals’ neural networks function differently at rest after mindfulness training; however, behavioral correlates will be needed in order to determine whether this in fact translates into improved executive function under stress outside the scanner. Future studies utilizing behavioral correlates for executive function can lend support to the supposition that enhanced dlPFC rsFC is, in fact, adaptive (81). Moreover, while we posit that the focused attention and open monitoring aspects of mindfulness training underlie these neural changes, behavioral experiments can directly test this theory. An additional limitation of the present seed-based rsFC analysis is that it precludes inferences about the directionality of our reported effects; in the future, effective connectivity analyses (such as dynamic causal modeling) will provide opportunities to test for causal interactions between these control-networked regions.
Acknowledgments
Funding Sources: This research was supported by funding from the Pittsburgh Life Sciences Greenhouse Opportunity Fund and NIH R01 HL-089850.
Laura Pacilio, Baldwin Way, Lei Sheu, and Shinzen Young provided advice on the design, analysis, and interpretation of results. We thank the Scientific Imaging & Brain Research (SIBR) center for neuroimaging support and the Kearns Spirituality Center for use of their retreat center.
Glossary
- rsFC
resting state functional connectivity
- dlPFC
dorsolateral prefrontal cortex
- IFG
inferior frontal gyrus
- MFG
middle frontal gyrus
- SEF
supplementary eye field
Footnotes
Conflict of Interest
Conflicts of interest: none.
References
- 1.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4(1):33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 2.Grossman P, Niemann L, Schmidt S, Walach H, et al. Mindfulness-based stress reduction and health benefits-A meta-analysis. J Psychosom Res. 2004;57(1):35–44. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 3.Roth B, Robbins D. Mindfulness-Based Stress Reduction and Health-Related Quality of Life: Findings From a Bilingual Inner-City Patient Population. Psychosom Med. 2004 Jan 1;66(1):113–23. doi: 10.1097/01.psy.0000097337.00754.09. [DOI] [PubMed] [Google Scholar]
- 4.Goldman-Rakic PS. Architecture of the Prefrontal Cortex and the Central Executive. Ann N Y Acad Sci. 1995;769(1):71–84. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- 5.Tops M, Boksem MAS. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Cognition. 2011;2:330. doi: 10.3389/fpsyg.2011.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. NeuroImage. 2012 Jan 2;59(1):750–60. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci. 2007 Jul 3;104(27):11483–8. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007 Dec 1;2(4):313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farb NAS, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one’s emotions: Mindfulness training alters the neural expression of sadness. Emotion. 2010;10(1):25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creswell JD, Taren AA, Lindsay EK, Greco CM, Gianaros PJ, Fairgrieve A, Marsland AL, Brown KW, Way BM, Rosen RK, Ferris JL. Alterations in Resting-State Functional Connectivity Link Mindfulness Meditation With Reduced Interleukin-6: A Randomized Controlled Trial. Biol Psychiatry [Internet] 2016 Jan 29; doi: 10.1016/j.biopsych.2016.01.008. [cited 2016 May 3];0(0). Available from: http://www.biologicalpsychiatryjournal.com/article/S0006322316000792/abstract. [DOI] [PubMed]
- 11.MacLean KA, Ferrer E, Aichele SR, Bridwell DA, Zanesco AP, Jacobs TL, King BG, Rosenberg EL, Sahdra BK, Shaver PR, Wallace BA, Mangun GR, Saron CD. Intensive Meditation Training Improves Perceptual Discrimination and Sustained Attention. Psychol Sci. 2010 Jun 1;21(6):829–39. doi: 10.1177/0956797610371339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y-Y, Ma Y, Wang J, Fan Y, Feng S, Lu Q, Yu Q, Sui D, Rothbart MK, Fan M, Posner MI. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci. 2007 Oct 23;104(43):17152–6. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isbel B, Mahar D. Cognitive mechanisms of mindfulness: A test of current models. Conscious Cogn. 2015 Dec 15;38:50–9. doi: 10.1016/j.concog.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Gallant SN. Mindfulness meditation practice and executive functioning: Breaking down the benefit. Conscious Cogn. 2016 Feb;40:116–30. doi: 10.1016/j.concog.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 15.He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of Functional Connectivity in Frontoparietal Networks Underlies Behavioral Deficits in Spatial Neglect. Neuron. 2007 Mar 15;53(6):905–18. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Morecraft RJ, Geula C, Mesulam MM. Architecture of connectivity within a cingulo-fronto-parietal neurocognitive network for directed attention. Arch Neurol. 1993 Mar;50(3):279–84. doi: 10.1001/archneur.1993.00540030045013. [DOI] [PubMed] [Google Scholar]
- 17.Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc B Biol Sci. 2005 Apr 29;360(1456):781–95. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008 Aug;24(4):424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 19.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007 Sep;8(9):700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 20.Brewer JA, Worhunsky PD, Gray JR, Tang Y-Y, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci. 2011 Dec 13;108(50):20254–9. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, Angstadt M, Kessler D, Welsh R, Liberzon I. Altered Default Mode Network (dmn) Resting State Functional Connectivity Following a Mindfulness-Based Exposure Therapy for Posttraumatic Stress Disorder (ptsd) in Combat Veterans of Afghanistan and Iraq. Depress Anxiety. 2016 Apr 1;33(4):289–99. doi: 10.1002/da.22481. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007 May 7;417(3):297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 23.Favre P, Baciu M, Pichat C, Bougerol T, Polosan M. fMRI evidence for abnormal resting-state functional connectivity in euthymic bipolar patients. J Affect Disord. 2014 Aug 20;165:182–9. doi: 10.1016/j.jad.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 24.Sommer IE, Clos M, Meijering AL, Diederen KMJ, Eickhoff SB. Resting State Functional Connectivity in Patients with Chronic Hallucinations. PLoS ONE. 2012 Sep 6;7(9):e43516. doi: 10.1371/journal.pone.0043516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen M, Dietz M, Blair KS, van Beek M, Rees G, Vestergaard-Poulsen P, Lutz A, Roepstorff A. Cognitive-Affective Neural Plasticity following Active-Controlled Mindfulness Intervention. J Neurosci. 2012 Oct 31;32(44):15601–10. doi: 10.1523/JNEUROSCI.2957-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005 Feb 1;24(3):810–21. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 27.Qin S, Hermans EJ, van Marle HJF, Luo J, Fernández G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009 Jul 1;66(1):25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci. 2009;106(3):912–7. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York, NY: Delta; 1990. [Google Scholar]
- 30.MacCoon DG, Imel ZE, Rosenkranz MA, Sheftel JG, Weng HY, Sullivan JC, Bonus KA, Stoney CM, Salomons TV, Davidson RJ, et al. The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR) Behav Res Ther. 2012;50:3–12. doi: 10.1016/j.brat.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vossel S, Geng JJ, Fink GR. Dorsal and Ventral Attention Systems Distinct Neural Circuits but Collaborative Roles. The Neuroscientist. 2013 Jul;8:1073858413494269. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damoiseaux JS, Rombouts SaRB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci. 2006 Sep 12;103(37):13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci. 2006 Jun 27;103(26):10046–51. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazaika P, Whitfield-Gabrieli S, Reiss A. Artifact repair for fMRI data from motion clinical subjects. Presented at the Organization for Human Brain Mapping Annual Conference; 2007. [Google Scholar]
- 35.Mazaika P, Hoeft F, Glover G, Reiss A. Methods and Software for fMRI Analysis for Clinical Subjects. San Franc CA: Hum Brain Mapp; 2009. [Google Scholar]
- 36.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–41. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 37.Herath P, Klingberg T, Young J, Amunts K, Roland P. Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cereb Cortex N Y N 1991. 2001 Sep;11(9):796–805. doi: 10.1093/cercor/11.9.796. [DOI] [PubMed] [Google Scholar]
- 38.Newman SD, Keller TA, Just MA. Volitional control of attention and brain activation in dual task performance. Hum Brain Mapp. 2007 Feb;28(2):109–17. doi: 10.1002/hbm.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sripada C, Angstadt M, Kessler D, Phan KL, Liberzon I, Evans GW, Welsh RC, Kim P, Swain JE. Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. NeuroImage. 2014 Apr 1;89:110–21. doi: 10.1016/j.neuroimage.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Froeliger B, Garland EL, Kozink RV, Modlin LA, Chen N-K, McClernon FJ, Greeson JM, Sobin P. Meditation-State Functional Connectivity (msFC): Strengthening of the Dorsal Attention Network and Beyond. Evid Based Complement Alternat Med [Internet] 2012 Mar 27; doi: 10.1155/2012/680407. [cited 2013 Aug 19];2012. Available from: http://www.hindawi.com/journals/ecam/2012/680407/abs/ [DOI] [PMC free article] [PubMed]
- 41.O’Reilly RC. The What and How of prefrontal cortical organization. Trends Neurosci. 2010 Aug;33(8):355–61. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Isoda M, Matsuzaka Y, Shima K, Tanji J. Prefrontal cortical cells projecting to the supplementary eye field and presupplementary motor area in the monkey. Neurosci Res. 2005 Sep;53(1):1–7. doi: 10.1016/j.neures.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008 Nov;9(11):856–69. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 44.Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998 Oct;21(4):761–73. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 45.Szczepanski SM, Pinsk MA, Douglas MM, Kastner S, Saalmann YB. Functional and structural architecture of the human dorsal frontoparietal attention network. Proc Natl Acad Sci U S A. 2013 Sep 24;110(39):15806–11. doi: 10.1073/pnas.1313903110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sridharan D, Levitin D, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci. 2008;105(34):12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leitman DI, Wolf DH, Ragland JD, Laukka P, Loughead J, Valdez JN, Javitt DC, Turetsky BI, Gur RC. “It’s Not What You Say, But How You Say it”: A Reciprocal Temporo-frontal Network for Affective Prosody. Front Hum Neurosci. 2010;4:19. doi: 10.3389/fnhum.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007 Jul 13;317(5835):215–9. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- 49.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006 Aug 15;60(4):402–9. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 50.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010 Apr 15;50(3):1313–9. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, Mayer EA. The neural correlates of placebo effects: a disruption account. NeuroImage. 2004 May;22(1):447–55. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 52.Modinos G, Ormel J, Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Soc Cogn Affect Neurosci. 2010;5(4):369–77. doi: 10.1093/scan/nsq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med. 2007;69(6):560–5. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- 54.Hölzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc Cogn Affect Neurosci. 2008 Mar 1;3(1):55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hölzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res Neuroimaging. 2011 Jan 30;191(1):36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leung M-K, Chan CCH, Yin J, Lee C-F, So K-F, Lee TMC. Increased gray matter volume in the right angular and posterior parahippocampal gyri in loving-kindness meditators. Soc Cogn Affect Neurosci. 2012 Jul 18;:nss076. doi: 10.1093/scan/nss076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luders E, Clark K, Narr KL, Toga AW. Enhanced brain connectivity in long-term meditation practitioners. NeuroImage. 2011 Aug 15;57(4):1308–16. doi: 10.1016/j.neuroimage.2011.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esterman M, Chiu Y-C, Tamber-Rosenau BJ, Yantis S. Decoding cognitive control in human parietal cortex. Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):17974–9. doi: 10.1073/pnas.0903593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–62. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- 60.Corbetta M, Patel G, Shulman GL. The Reorienting System of the Human Brain: From Environment to Theory of Mind. Neuron. 2008 Aug 5;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Costa Paul T, McCrae Robert R. Normal personality assessment in clinical practice: The NEO Personality Inventory. Psychol Assess. 1992;4(1):5–13. [Google Scholar]
- 62.Dickenson J, Berkman ET, Arch J, Lieberman MD. Neural correlates of focused attention during a brief mindfulness induction. Soc Cogn Affect Neurosci. 2012 Mar 1;:nss030. doi: 10.1093/scan/nss030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burnett S, Blakemore S-J. Functional connectivity during a social emotion task in adolescents and in adults. Eur J Neurosci. 2009 Mar 1;29(6):1294–301. doi: 10.1111/j.1460-9568.2009.06674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kucyi A, Hodaie M, Davis KD. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J Neurophysiol. 2012 Dec 15;108(12):3382–92. doi: 10.1152/jn.00674.2012. [DOI] [PubMed] [Google Scholar]
- 65.Mars RB, Sallet J, Schüffelgen U, Jbabdi S, Toni I, Rushworth MFS. Connectivity-Based Subdivisions of the Human Right “Temporoparietal Junction Area”: Evidence for Different Areas Participating in Different Cortical Networks. Cereb Cortex. 2012 Aug 1;22(8):1894–903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- 66.Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, Maquet P. Activation of the supplementary motor area and of attentional networks during temporal processing. Exp Brain Res. 2002 Feb 1;142(4):475–85. doi: 10.1007/s00221-001-0953-0. [DOI] [PubMed] [Google Scholar]
- 67.Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci. 2001 Mar;4(3):317–23. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- 68.Vanderhasselt M-A, De Raedt R, Baeken C. Dorsolateral prefrontal cortex and Stroop performance: tackling the lateralization. Psychon Bull Rev. 2009 Jun;16(3):609–12. doi: 10.3758/PBR.16.3.609. [DOI] [PubMed] [Google Scholar]
- 69.Goel V, Dolan RJ. Reciprocal neural response within lateral and ventral medial prefrontal cortex during hot and cold reasoning. NeuroImage. 2003 Dec;20(4):2314–21. doi: 10.1016/j.neuroimage.2003.07.027. [DOI] [PubMed] [Google Scholar]
- 70.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weigand A, Grimm S, Astalosch A, Guo JS, Briesemeister BB, Lisanby SH, Luber B, Bajbouj M. Lateralized effects of prefrontal repetitive transcranial magnetic stimulation on emotional working memory. Exp Brain Res. 2013 May;227(1):43–52. doi: 10.1007/s00221-013-3483-7. [DOI] [PubMed] [Google Scholar]
- 72.Heinze K, Ruh N, Nitschke K, Reis J, Fritsch B, Unterrainer JM, Rahm B, Weiller C, Kaller CP. Transcranial direct current stimulation over left and right DLPFC: Lateralized effects on planning performance and related eye movements. Biol Psychol. 2014 Oct;102:130–40. doi: 10.1016/j.biopsycho.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 73.Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol M-J, van der Wee NJA, Veltman DJ, Roelofs K. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. NeuroImage. 2010 Jan 1;49(1):963–70. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 74.Grady CL, Horwitz B, Pietrini P, Mentis MJ, Ungerleider LG, Rapoport SI, Haxby JV. Effect of task difficulty on cerebral blood flow during perceptual matching of faces. Hum Brain Mapp. 1996;4(4):227–39. doi: 10.1002/(SICI)1097-0193(1996)4:4<227::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 75.Wager TD, Sylvester C-YC, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. NeuroImage. 2005 Aug 15;27(2):323–40. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 76.Lewis PA, Critchley HD, Smith AP, Dolan RJ. Brain mechanisms for mood congruent memory facilitation. NeuroImage. 2005 May 1;25(4):1214–23. doi: 10.1016/j.neuroimage.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 77.Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emot Wash DC. 2010 Feb;10(1):83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chambers R, Gullone E, Allen NB. Mindful emotion regulation: An integrative review. Clin Psychol Rev. 2009 Aug;29(6):560–72. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 79.Arch JJ, Craske MG. Mechanisms of mindfulness: Emotion regulation following a focused breathing induction. Behav Res Ther. 2006 Dec;44(12):1849–58. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 80.Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31(14):5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001 Feb;54(3):287–98. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]