Abstract
Objective
Exposure to stressful events is associated both with occurrence of depression and also vascular disease. The objective of this study was to determine whether higher levels of stress exposure was related to measures of pathological brain aging, specifically white matter hyperintensity volumes, in older adults with and without depression.
Methods
The sample included 130 depressed and 110 never-depressed older adults aged 60 years or older enrolled in a longitudinal study at an academic medical center. Participants completed clinical assessments, assessment of stressful event exposure and perceived stress, and magnetic resonance imaging at baseline and after two years. Analyses examined both cross-sectional and longitudinal relationships between stress measures and white matter hyperintensity volumes.
Results
There were no statistically significant relationships observed between cross-sectional baseline stress measures and either baseline hyperintensity volume or two-year change in hyperintensity volume. However, after controlling for demographic variables and baseline measures, change in stressor exposure was associated with change in hyperintensity volumes. In this analysis, increased stressor exposure was associated with greater increases in white matter hyperintensity volume, while reductions in stressor exposure was associated with less increase in hyperintensity volume. This relationship did not significantly differ based on the presence of either depression or medical comorbidities.
Conclusions
This work adds to a growing literature associating exposure to stressful events in later life with more rapid pathological brain aging. Work is needed to understand the physiological mechanisms by which stress exposure has this effect and examine whether stress reduction techniques may modify these observed outcomes.
Keywords: Geriatrics, MRI, aging, stress, vascular, depression
INTRODUCTION
White matter hyperintensities (WMH) are common findings on brain imaging of older adults, observable on T2-weighted or fluid attenuated inversion recovery (FLAIR) MRI. WMHs are associated with advanced age (Awad et al., 1986) and cerebrovascular risk factors including diabetes, cardiac disease, and hypertension (Dufouil et al., 2001; Jokinen et al., 2009; Longstreth et al., 1996; Taylor et al., 2005; Taylor et al., 2003a). Many larger WMHs are ischemic in origin (Thomas et al., 2002a; Thomas et al., 2002b) and indicate damage to the microstructural integrity of white matter tracts (Taylor et al., 2001). WMHs are clinically relevant as they are associated with important negative outcomes including cognitive deficits, dementia, stroke, disability, and mortality (Maillard et al., 2012; Sabayan et al., 2015; Steffens et al., 2002a; Taylor et al., 2013).
WMHs also appear to play a particularly important role in the pathogenesis of late-life depression (Taylor 2014). Although not universally observed, late life depression is associated with greater WMH volumes (Taylor et al., 2005) and progression of hyperintensities is associated with a poorer depression course (Taylor et al., 2003b). Such observations support the vascular depression hypothesis, proposing that cerebrovascular disease may predispose, precipitate, or perpetuate depressive syndromes (Alexopoulos et al., 1997; Taylor et al., 2013). Although the ultimate mechanisms by which WMHs contribute to depression are unclear, the “disconnection hypothesis” (Taylor et al., 2013) proposes that WMHs may directly influence the risk for or course of depression by disrupting critical neural pathways needed to maintain normal brain function and neural connectivity, particularly in areas involved in emotion regulation or cognitive processing. This theory is supported by observations associating WMH severity with dysfunction of intrinsic neural networks implicated in depression (Aizenstein et al., 2011; Venkatraman et al., 2010). However, other theories are possible. An alternative hypothesis is that common factors may contribute both to the risk of depression and the development of hyperintensities. One potential mechanism that could underlie such a relationship is the exposure to and physiological reactivity to stressful life events.
Stressful life events (SLEs) are significant risk factors for the development of episodes of Major Depressive Disorder (MDD) (Kendler et al., 1999; Zannas et al., 2012). Increased stress sensitivity, or the tendency to experience negative emotional states in response to minor stressors in daily life, is a characteristic of depression that may be predictive of depression onset (Kendler et al., 2000; Morris et al., 2012). Identifiable stressors often precede depressive episodes and the risk of developing a new MDD episode increases with both greater severity and greater number of stressful life events (Kendler et al., 1998). Over time and with repeated episodes, less stress may be required to trigger a new depressive episode (Kendler et al., 2000). Increases or decreases in stress can respectively predict poor treatment outcomes (Zannas et al., 2012) or increase the likelihood of MDD remission (Reno and Halaris 1990). These effects of stress are likely mediated through physiological reactions to stress, as hypothalamic-pituitary-adrenal (HPA) axis dysregulation and proinflammatory processes may contribute to depression vulnerability and depressive symptomatology (Alexopoulos and Morimoto 2011; Miller and Raison 2016; Pariante and Lightman 2008).
Physiological stress responses also influence vascular risk. Exposure to stressful events is associated with increased incidence of both cardiovascular and cerebrovascular disease (Jood et al., 2009; Kornerup et al., 2010). There are multiple mechanisms by which stress could contribute to vascular risk, including stress effects on behavior that may alter smoking or dietary habits. Persistent or recurrent activation of both the sympathetic nervous system and HPA axis may contribute to hypertension, metabolic disturbances, or result in a failure to contain inflammatory activity (Lambert and Lambert 2011; Nijm and Jonasson 2009; Rosmond 2005), with elevated inflammatory markers such as c-reactive protein (CRP) and IL-6 serving as predictors of cardiovascular events (Ridker et al., 1997). It is also possible that glucocorticoid alterations in response to persistent stress may exacerbate stroke risk by sensitizing the neuroimmune response to ischemia (Stuller et al., 2012). Importantly, depressive behaviors may themselves contribute to vascular risk, with a notable example being social isolation. Small social networks and social isolation are associated with increased mortality rates (House et al., 1988) and are predictive of increased stroke incidence and poor outcomes (Rutledge et al., 2009). Such relationships may be mediated by stress’s effect on the immune system, as individuals with low social support exhibit increased levels of proinflammatory cytokines (Heffner et al., 2011).
Despite evidence supporting the disconnection hypothesis of vascular depression (Taylor et al., 2013), it is possible that increased exposure to stressors and physiological responses to stress may be common factors contributing to both depression and, by increasing vascular risk, WMH progression. The current study examined whether stressful life events are associated with severity and progression of white matter hyperintensity in older adults. Study data derived from a large longitudinal study of depressed older adults and non-depressed comparison subjects. Based on past work, we hypothesized that greater exposure to stressful life events would be associated with greater white matter hyperintensity progression. We additionally sought to determine whether the presence of a depression diagnosis or comorbid hypertension affected this relationship, with the hypotheses that stress effects on WMH volumes would be greater in the depressed population and hypertensive population.
METHODS
Participants
Participants enrolled in the Neurocognitive Outcomes of Depression in the Elderly (NCODE) longitudinal study at Duke University Medical Center. Eligible depressed subjects were aged 60 years or older and at time of enrollment met Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria for Major Depressive Disorder on the basis of the NIMH Diagnostic Interview Schedule (DIS) (Robins et al., 1981) and confirmed by clinical interview. Exclusion criteria included 1) another major psychiatric illness, 2) history of substance use disorder, 3) primary neurologic illness including dementia and 4) contraindications for magnetic resonance imaging (MRI). Participants were recruited through clinical referrals, self-referral and advertisements.
As previously reported (Potter et al., 2015), at entry depressed participants were often on antidepressant medications from prior treatment. Seventy percent of individuals were taking a selective serotonin reuptake inhibitor (SSRI) but only 18% were on SSRI alone. Other medications included tricyclic antidepressants (16%), monoamine oxidase inhibitors (1%), and other antidepressants (e.g., bupropion, venlafaxine, mirtazapine, trazodone, and duloxetine; 68%). Only 9% of individuals were not taking antidepressant medications at enrollment.
Nondepressed comparison subjects were recruited through the Center for Aging Subject Registry at Duke University. Eligible comparison subjects were age 60 years or older, had a nonfocal neurological examination, no self-report of neurologic disease or depressive disorder and no evidence of depression based on DIS.
The study was approved by the Duke University Medical Center Institutional Review Board. All study participants provided written informed consent prior to enrollment.
Clinical assessments and treatment
On initial assessment, participants provided self-reported demographic and health information including the presence of medical comorbidities such as diabetes, heart disease, or hypertension (phrased as “high blood pressure”). All participants also completed the mini-mental state examination (MMSE) (Folstein et al., 1975) at baseline, and individuals scoring below 25 were not included in this study.
Participants were followed longitudinally over two years. Clinical assessments of depression severity using the Montgomery–Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979) were performed at least every three months for depressed participants. Nondepressed comparison subjects were seen annually. Life stress was measured at baseline and at the two year assessment with a self-report questionnaire (Hays et al., 1997; Landerman et al., 1989). This 20 item questionnaire assesses stressor exposure over the last year by querying about a variety of stressful life events (SLE) common with aging. We included all events, regardless of whether the event was a positive or negative event. Examples include development of a physical illness, separation from a loved one, marriage or divorce, addition or loss of family members, work related difficulties, legal problems, financial struggles, new employment and retirement. A total score was derived from the number of items endorsed. There was an additional item on perceived stress severity (phrased as “average stress” in the questionnaire) that asked participants to rate the subjective severity of life stress over the past 6 months on a scale between one and ten, with higher scores indicated a higher perceived stress severity.
During the study period, depressed participants were treated according to the Duke somatic treatment algorithm for geriatric depression (Steffens et al., 2002b). This algorithm uses a stepwise medication treatment approach taking into account previous treatments and depression severity. Although the majority of depressed participants were prescribed sertraline on study entry, switching antidepressant medications and augmentation strategies were allowed as necessary for subjects who did not respond to initial treatment. This allowed for broad use of antidepressant treatments, including lithium, psychotherapy and, in rare cases, electroconvulsive therapy.
MRI
At study entry and two years later participants were imaged using a 1.5-Tesla whole body MRI system using the standard head radiofrequency coil. The scanner alignment light was used to adjust the head tilt and rotation so that the axial plane lights passed across the canthomeatal line and the sagittal lights were aligned with the center of the nose. A rapid sagittal localizer scan confirmed the alignment.
A dual-echo fast spin-echo acquisition was obtained in the axial plane for morphometric analysis of lesion volumes. The pulse sequence parameters are relaxation time = 4000msec, excitation time = 30, 135msec, 32kHz (+/−16KHz) full imaging bandwidth, echo train length =16, a 256×256 matrix, 3-mm section thickness, 1 excitation, and a 20-cm field of view. The images were acquired in two separate acquisitions with a 3-mm gap between sections for each acquisition. The second acquisition was offset by 3mm from the first so that the resulting data set consisted of contiguous sections with no gap.
The segmentation protocol has been previously described (Payne et al., 2002), and uses a modified version of MrX software created by GE Corporate Research and Development originally modified by Brigham and Women’s Hospital for image segmentation. This semiautomated method uses the multiple MRI contrasts to identify different tissue classifications through a “seeding” process in which a trained analyst manually selected pixels in each tissue type to be identified. WMHs were selected based on a set of explicit rules developed from neuroanatomic guidelines, consultation with a neuroradiologist, and knowledge of the neuropathology of WMHs. Periventricular and deep white matter lesions were combined to provide a measure of WMHs on the segmented image.
Statistical Analysis
All statistical tests were conducted using SAS version 9.4 (Cary, NC, USA). Demographic and clinical variables were compared between diagnostic groups at baseline, using chi-square tests for categorical variables, pooled two-sample t tests for continuous variables with equal variances, and Sattherthwaite t tests for continuous variables with unequal variances. In order to be included in study analyses, participants needed to have WMH and clinical stress measures at both baseline and after two years. Missing values were handled by excluding participants from the respective analyses.
Primary analyses included three sets of models examining relationships between 1) baseline stress measures and baseline WMH volume; 2) baseline stress measures and two year change in WMH volume; and 3) two year change in stress measures and two year change in WMH volume. Each set included two models separately examining stressor exposure and perceived stress severity.
All multivariable linear regression models examined log transformed WMH volumes or log transformed changes in volumes as the dependent variables. Log transformed WMH volumes were used as the values were not normally distributed. Stress measures were included as independent variables, while controlling for age, sex, race, diagnostic cohort (depressed/nondepressed). For examination of longitudinal variables, we additionally included the respective baseline measure as a covariate.
In subsequent analyses, we tested for different effects of stress measures on WMH volume based on depression diagnosis and self-report of hypertension, diabetes, or heart disease. For these analyses, we constructed models as above but additionally included interaction terms between stress measures and depression diagnosis, and stress measures and self-report of medical comorbidity.
RESULTS
Sample Characteristics
Study analyses included 240 subjects (130 depressed and 110 nondepressed) with longitudinal data. Compared with the nondepressed group, the depressed group exhibited a significantly higher rate of hypertension and lower baseline MMSE scores, although the between-group differences in MMSE scores were not clinically relevant (Table 1). In univariate analyses, the depressed and nondepressed groups exhibited comparable rates of WMH volume change over the two-year period. The depressed group reported higher baseline levels of stressor exposure and perceived stress severity and also exhibited a greater reduction in these measures over the two-year period.
Table 1.
Characteristics of participants by diagnostic cohort
| Demographic variable | Depressed (N= 130) | Nondepressed (N=110) | Test statistic | P value |
|---|---|---|---|---|
| Age | 69.9 (7.0) | 70.2 (5.8) | T=0.28, 238 df | 0.7759 |
| Sex, % female | 62.31% (81) | 70.00% (77) | X2= 1.57, 1df | 0.2106 |
| Race, % Caucasian | 89.23% (116) | 81.8% (90) | X2 = 2.69, 1df | 0.1008 |
| Hypertension | 43.08% (56) | 22.73% (25) | X2= 11.04, 1df | 0.0009 |
| Diabetes | 3.9% (5) | 8.25 (9) | Fisher’s exact | 0.1688 |
| Heart Disease | 16.9% (22) | 8.25 (9) | X2= 3.64, 1df | 0.0564 |
| MMSE | 28.27 (2.6) | 28.95 (1.2) | T= 2.69, 187.86 df | 0.0077 |
| Baseline MADRS | 26.92 (7.3) | --- | ||
| MRI WMH Volumes | ||||
| WMH baseline (log ml) | 1.42 (0.84) | 1.24 (0.78) | T=1.79, 238 df | 0.0745 |
| WMH baseline (ml) | 6.70 (10.67) | 5.18 (7.18) | T=1.31, 227 df | 0.1911 |
| WMH change (log ml) | 0.17 (0.30) | 0.20 (0.28) | T=0.81, 238 df | 0.4216 |
| WMH change (ml) | 1.48 (3.38) | 1.45 (3.13) | T=0.08, 238 df | 0.9394 |
| Stress measures | ||||
| Baseline stressor exposure | 2.42 (1.67) | 1.18 (1.11) | T=6.81, 225.80 df | <0.0001 |
| Change in stressor exposure | −0.64 (1.88) | −0.06 (1.63) | T=2.51, 238 df | 0.0129 |
| Baseline perceived stress severity | 6.44 (2.00) | 2.69 (1.47) | T=16.67, 231.91 df | <0.0001 |
| Change in perceived stress severity | −1.57 (2.51) | −0.07 (1.53) | T=5.62, 214.27 df | <0.0001 |
Data presented as mean (standard deviation) for continuous variables, or percent (N) for categorical variables. All comparisons of continuous measures used pooled, two-tailed t-tests with 238 degrees of freedom (df), except for when variances were unequal, in which case Satterthwaite t-tests were used. Categorical comparisons used chi-square tables, aside from comparisons of reports of diabetes, which used Fisher’s exact test due to small cell samples. Medical morbidity (hypertension, diabetes, heart disease) presented as percent (N) who endorsed having these disorders. MMSE = mini-mental state exam; MADRS = Montgomery-Asberg Depression Rating Scale; WMH = white matter hyperintensity
Stress measures as predictors of WMH volume
Initial models examined the effects of baseline stress measures (stressor exposure, perceived stress severity) on baseline WMH volume. After controlling for covariates, there was no effect of either baseline stress measure on baseline WMH volume (Table 2). We next examined the effects of baseline stress measures on two year change in WMH volume. Again, there was no effect of baseline stress measures on two year change in WMH volume. Finally, we examined the relationship between change in stress measures and change in WMH volume over two years. In these analyses, change in stress exposure was positively associated with change WMH volume: increased stress exposure over the study period was associated with greater increases in WMH volume, while decreased stress exposure was associated with slower progression of WMH volume (Figure 1). Change in perceived stress severity was not associated with change in WMH volume.
Table 2.
Cross-sectional and longitudinal effects of stress measures on WMH volume
| F value | P value | |
|---|---|---|
| Effects of baseline stress measures on baseline WMH volume | ||
| Perceived stress severity | 0.00 | 0.9772 |
| Stressor exposure | 1.24 | 0.2659 |
| Effects of baseline stress measures on change in WMH volume | ||
| Perceived stress severity | 0.18 | 0.6723 |
| Stressor exposure | 0.24 | 0.6230 |
| Effects of change in stress measures on change in WMH volume | ||
| Change in perceived stress severity | 2.72 | 0.1005 |
| Change in stressor exposure | 5.46 | 0.0203 |
Separate models examined the relationship between perceived stress severity and stressor exposure on log-transformed WMH volume. Models examining effects of cross-sectional stress measures on cross-sectional WMH volumes controlled for age, sex, race, presence of hypertension, and diagnostic cohort, with 233 degrees of freedom. Models examining effects of cross-sectional stress measures on change in WMH volumes included these same covariates along with baseline log-transformed WMH volume, with 232 degrees of freedom. Models examining the relationship between change in stress measures and change in WMH volume included those same covariates plus baseline stress measures, with 231 degrees of freedom.
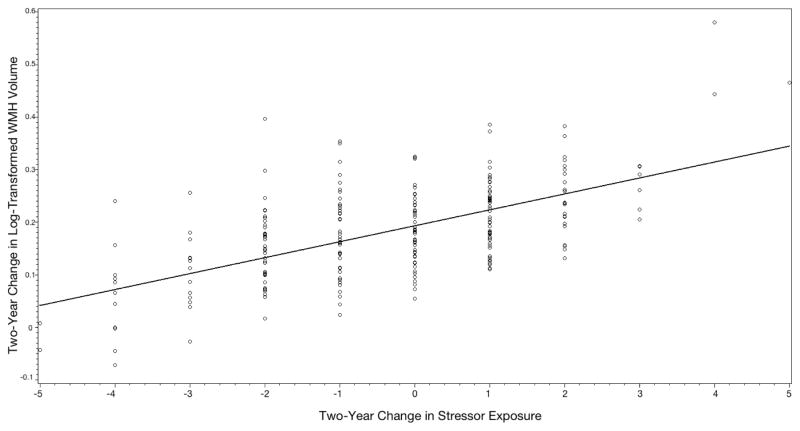
FIGURE 1. Relationship over two years between change in stress exposure and change in WMH volume.
Figure shows two-year change in log-transformed WMH volume (in milliliters) on y-axis and two-year change in stressor exposure on x-axis. Increased stressors over the study period is associated with a greater rate of WMH volume progression, while resolution of stressor over the study period is associated with a slower rate of WMH progression.
Subsequent analyses tested for interactive effects, examining the influences of depression diagnosis on the relationship between stress measures and WMH volume. We also tested for interactive effects between medical comorbidity and stress measures on WMH, specifically examining the presence or absence of hypertension, diabetes, and heart disease. These interaction terms did not achieve statistical significance in any model (data not shown).
DISCUSSION
The primary finding is that increased exposure to stressful life events is associated with an elevated rate of change of WMH volumes. Notably, the vast majority of participants exhibited an increase in WMH volume over the two-year study period. Those with a decrease in stressor exposure exhibited less change in WMH volume than those experiencing an increase in stressor exposure. To our knowledge, this is new information associating exposure to stressful events with pathological vascular brain aging. This stress-WMH relationship did not appear to differ based on the presence of either depression or vascular risk factor comorbidities.
Our findings build on past work demonstrating that exposure to stressful events has a negative effect on other measures of pathological brain aging. We previously demonstrated that increased stressor exposure is negatively associated with hippocampal volume (Zannas et al., 2013), wherein greater numbers of baseline stressful life events were significantly associated with reduction in hippocampal volume over two years. In that study, change in stressor exposure was not significantly related to change in hippocampal volume, leading us to hypothesize that stress effects on hippocampal volume lagged behind and occurred after the stress exposure. This delayed effect would be concordant with the hypothesis that atrophy was related to HPA axis dysregulation, as acute and chronic effects of HPA axis activity differ and hippocampal neurons are sensitive to persistent cortisol stimulation (Frodl and O’Keane 2013). In contrast, in the current study, change in stressor exposure was associated with concomitant changes in the rate of WMH progression. This suggests that a more acute physiological stress response, such as sympathetic nervous system activity, may underlie the relationship. This hypothesis is supported by a cross-sectional study reporting that greater changes in experimentally-induced blood pressure mediated through the sympathetic nervous system was associated with greater WMH severity (Waldstein et al., 2004).
We did not find a relationship between perceived stress severity and WMH measures. This is generally concordant with a previous study of approximately 500 elderly individuals, reporting that over a five-year period, greater perceived stress severity was significantly and independently associated with cerebral infarction and lower brain volume, but not with WMH severity (Aggarwal et al., 2014). Jointly, our data suggest that the occurrence of stressful events may be more important than the subjective perception of stress severity.
The results of this study are informative when considering the relationship between depression and WMH. When combined with past work, it does support a hypothesis that exposure to and response to stressors may contribute to both depression and WMH progression. However, this does not necessarily mean that the disconnection hypothesis is incorrect. Although stress may contribute to both depression and WMH progression, WMH may still disrupt neural circuits, contributing to deficits in neural network connectivity and function (Aizenstein et al., 2011; Venkatraman et al., 2010), creating problems for emotion regulation and cognitive performance. Thus stressor exposure may contribute to risk of both depression and vascular disease, but WMHs may also influence the presentation or course of depression by disrupting neural networks involved in cognition or emotion regulation. In the future, more complex analytic methods such as path analyses may better elucidate these potential bidirectional relationships.
Depression diagnosis did not appear to moderate the association between stress and WMHs. In other words, the effect of exposure to stressors on WMH progression did not significantly differ between individuals with and without depression. This suggests that our finding has broad implications for brain aging even in psychiatrically healthy individuals. It may be particularly relevant for populations exposed to high levels of stress or stressful environments, such as individuals living in poverty.
WMHs are associated with important negative clinical outcomes beyond depression, including cognitive deficits, dementia, stroke and disability (Steffens et al., 2002a; Taylor et al., 2013). These broad negative outcomes associated with WMHs highlight the clinical need to better understand what factors contribute to their development. Importantly, stress exposure and vulnerability to stress are also associated with declines in cognitive performance in LLD (Dickinson et al., 2011; Steffens et al., 2013). Although we cannot prevent exposure to stressors, stress reduction interventions may be an intervention that benefits brain aging and reduces cognitive decline. Preliminary evidence supports that stress-reduction techniques such as meditation may have such a benefit (Gard et al., 2014; Lavretsky et al., 2013). Clearly further work examining both the short- and long-term benefits of such interventions is needed.
Despite the study’s strengths, including a large sample and longitudinal design, it also has limitations. First, although much of our scientific model implicates the importance of physiological responses to stress, in this study we only assessed exposure to stressful life events. In a broad population we would anticipate substantial heterogeneity in the physiological responses to stressors. Stress reactivity measures such as cortisol levels, proinflammatory markers, blood pressure changes or vagal tone could be recorded in a systematic fashion in future studies. Moreover, despite a prospective study design, assessments of exposure to stressors was limited to a retrospective, self-report questionnaire that may have resulted in a memory bias. Additionally, only recent stressors were assessed. A more comprehensive evaluation should assess early life trauma exposures that may influence a person’s future response to stress. We also could not fully examine how stress exposure may interact with many vascular risk factors such as obesity or smoking. Finally, it remains unclear whether antidepressant treatment may modify these relationships, as all depressed participants were treated during the study, albeit with significant heterogeneity in the antidepressant regimen received.
CONCLUSIONS
Our findings support that stress exposure has a significant effect on the progression of WMHs, independent of depression diagnosis. Stressful life events have also been associated with other measures of accelerated brain aging such as hippocampal atrophy and also with cognitive decline. Future work in this area should pursue several paths. First, to better delineate the mechanisms by which stress exposure has this effect. With current technology, there are new methods capable of measuring stress exposures in the course of daily life, allowing us to better quantifying the duration or intensity of the exposure. These can be paired with broader physiological monitoring which may provide biological targets for intervention. Second, to identify whether a stress reactive phenotype (characterized by either neural or physiological response) may be associated with increased risks of pathological brain aging and cognitive decline. This would inform the clinical population who may benefit from interventions designed to decrease responses to stress to determine if they have benefit for depression outcomes but also delay cognitive decline.
KEY POINTS.
Increased exposures to stressful events are associated with a corresponding increase in the progression of white matter hyperintensities.
The relationship between stressful event exposure and progression of hyperintensities does not differ based on the presence of either depression or vascular medical comorbidity, two factors associated with greater white matter hyperintensity severity.
Interventions designed to modify how older adults respond to stressful events may reduce rates of pathological brain aging and potentially improve long-term cognitive outcomes.
Acknowledgments
This research was supported by NIH grants R21 MH099218, R01 MH102246, R01 MH054846, and K24 MH110598.
Footnotes
Preliminary data were presented at the 2016 Meeting of the American Association for Geriatric Psychiatry.
References
- Aggarwal NT, Clark CJ, Beck TL, Mendes de Leon CF, DeCarli C, Evans DA, Everson Rose SA. Perceived stress is associated with subclinical cerebrovascular disease in older adults. Am J Geriatr Psychiatry. 2014;22:53–62. doi: 10.1016/j.jagp.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Andreescu C, Edelman KL, Cochran JL, Price J, Butters MA, Karp J, Patel M, Reynolds CF., 3rd fMRI correlates of white matter hyperintensities in late-life depression. Am J Psychiatry. 2011;168:1075–1082. doi: 10.1176/appi.ajp.2011.10060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011 doi: 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad IA, Spetzler RF, Hodak JA, Awad CA, Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke. 1986;17:1084–1089. doi: 10.1161/01.str.17.6.1084. [DOI] [PubMed] [Google Scholar]
- Dickinson WJ, Potter GG, Hybels CF, McQuoid DR, Steffens DC. Change in stress and social support as predictors of cognitive decline in older adults with and without depression. Int J Geriatr Psychiatry. 2011;26:1267–1274. doi: 10.1002/gps.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, Alperovitch A, Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities. The EVA MRI cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frodl T, O’Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis. 2013;52:24–37. doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Gard T, Holzel BK, Lazar SW. The potential effects of meditation on age-related cognitive decline: a systematic review. Ann N Y Acad Sci. 2014;1307:89–103. doi: 10.1111/nyas.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays JC, Krishnan KR, George LK, Pieper CF, Flint EP, Blazer DG. Psychosocial and physical correlates of chronic depression. Psychiatry Res. 1997;72:149–159. doi: 10.1016/s0165-1781(97)00105-4. [DOI] [PubMed] [Google Scholar]
- Heffner KL, Waring ME, Roberts MB, Eaton CB, Gramling R. Social isolation, C-reactive protein, and coronary heart disease mortality among community-dwelling adults. Soc Sci Med. 2011;72:1482–1488. doi: 10.1016/j.socscimed.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, Gouw A, Scheltens P, Barkhof F, Visser MC, Fazekas F, et al. MRI-defined subcortical ischemic vascular disease: baseline clinical and neuropsychological findings. The LADIS Study. Cerebrovasc Dis. 2009;27:336–344. doi: 10.1159/000202010. [DOI] [PubMed] [Google Scholar]
- Jood K, Redfors P, Rosengren A, Blomstrand C, Jern C. Self-perceived psychological stress and ischemic stroke: a case-control study. BMC Med. 2009;7:53. doi: 10.1186/1741-7015-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. J Nerv Ment Dis. 1998;186:661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- Kornerup H, Osler M, Boysen G, Barefoot J, Schnohr P, Prescott E. Major life events increase the risk of stroke but not of myocardial infarction: results from the Copenhagen City Heart Study. Eur J Cardiovasc Prev Rehabil. 2010;17:113–118. doi: 10.1097/HJR.0b013e3283359c18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert EA, Lambert GW. Stress and its role in sympathetic nervous system activation in hypertension and the metabolic syndrome. Curr Hypertens Rep. 2011;13:244–248. doi: 10.1007/s11906-011-0186-y. [DOI] [PubMed] [Google Scholar]
- Landerman R, George LK, Campbell RT, Blazer DG. Alternative models of the stress buffering hypothesis. Am J Comm Psychol. 1989;17:626–642. doi: 10.1007/BF00922639. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Epel ES, Siddarth P, Nazarian N, Cyr NS, Khalsa DS, Lin J, Blackburn E, Irwin MR. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28:57–65. doi: 10.1002/gps.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth WTJ, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the cardiovascular health study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Maillard P, Carmichael O, Fletcher E, Reed B, Mungas D, DeCarli C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012;79:442–448. doi: 10.1212/WNL.0b013e3182617136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Morris MC, Rao U, Garber J. Cortisol responses to psychosocial stress predict depression trajectories: social-evaluative threat and prior depressive episodes as moderators. J Affect Disord. 2012;143:223–230. doi: 10.1016/j.jad.2012.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijm J, Jonasson L. Inflammation and cortisol response in coronary artery disease. Ann Med. 2009;41:224–233. doi: 10.1080/07853890802508934. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Payne ME, Fetzer DL, MacFall JR, Provenzale JM, Byrum CE, Krishnan KRR. Development of a semi-automated method for quantification of MRI gray and white matter lesions in geriatric subjects. Psychiatry Res. 2002;115:63–77. doi: 10.1016/s0925-4927(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Potter GG, McQuoid DR, Steffens DC. Appetite loss and neurocognitive deficits in late-life depression. Int J Geriatr Psychiatry. 2015;30:647–654. doi: 10.1002/gps.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno RM, Halaris AE. The relationship between life stress and depression in an endogenous sample. Compr Psychiatry. 1990;31:25–33. doi: 10.1016/0010-440x(90)90051-s. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Linke SE, Krantz DS, Johnson BD, Bittner V, Eastwood JA, Eteiba W, Pepine CJ, Vaccarino V, Francis J, et al. Comorbid depression and anxiety symptoms as predictors of cardiovascular events: results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Psychosom Med. 2009;71:958–964. doi: 10.1097/PSY.0b013e3181bd6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabayan B, van der Grond J, Westendorp RG, van Buchem MA, de Craen AJ. Accelerated progression of white matter hyperintensities and subsequent risk of mortality: a 12-year follow-up study. Neurobiol Aging. 2015;36:2130–2135. doi: 10.1016/j.neurobiolaging.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Bosworth HB, Provenzale JM, MacFall JR. Subcortical white matter lesions and functional impairment in geriatric depression. Depress Anxiety. 2002a;15:23–28. doi: 10.1002/da.1081. [DOI] [PubMed] [Google Scholar]
- Steffens DC, McQuoid DR, Krishnan KRR. The Duke Somatic Treatment Algorithm for Geriatric Depression (STAGED) approach. Psychopharmacol Bull. 2002b;36:58–68. [PubMed] [Google Scholar]
- Steffens DC, McQuoid DR, Smoski MJ, Potter GG. Clinical outcomes of older depressed patients with and without comorbid neuroticism. Int Psychogeriatr. 2013;25:1985–1990. doi: 10.1017/S1041610213001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuller KA, Jarrett B, DeVries AC. Stress and social isolation increase vulnerability to stroke. Exp Neurol. 2012;233:33–39. doi: 10.1016/j.expneurol.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Taylor WD. Clinical practice. Depression in the elderly. N Engl J Med. 2014;371:1228–1236. doi: 10.1056/NEJMcp1402180. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, Krishnan KR. Greater MRI lesion volumes in elderly depressed subjects than in control subjects. Psychiatry Res. 2005;139:1–7. doi: 10.1016/j.pscychresns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Provenzale JM, Payne ME, McQuoid DR, Steffens DC, Krishnan KRR. Serial MR imaging of hyperintense white matter lesion volumes in elderly subjects: correlation with vascular risk factors. Am J Roentgenol. 2003a;181:571–576. doi: 10.2214/ajr.181.2.1810571. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Payne ME, Krishnan KR, Wagner HR, Provenzale JM, Steffens DC, MacFall JR. Evidence of white matter tract disruption in MRI hyperintensities. Biol Psychiatry. 2001;50:179–183. doi: 10.1016/s0006-3223(01)01160-x. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Steffens DC, MacFall JR, McQuoid DR, Payne ME, Provenzale JM, Krishnan KRR. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003b;60:1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, O’Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, Perry RH. Ischemic basis for deep white matter hyperintensities in major depression. Arch Gen Psychiatry. 2002a;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Perry R, Barber R, Kalaria RN, O’Brien JT. Pathologies and pathological mechanisms for white matter hyperintensities in depression. Ann N Y Acad Sci. 2002b;977:333–339. doi: 10.1111/j.1749-6632.2002.tb04835.x. [DOI] [PubMed] [Google Scholar]
- Venkatraman VK, Aizenstein H, Guralnik J, Newman AB, Glynn NW, Taylor C, Studenski S, Launer L, Pahor M, Williamson J, et al. Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage. 2010;49:3436–3442. doi: 10.1016/j.neuroimage.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein SR, Siegel EL, Lefkowitz D, Maier KJ, Brown JR, Obuchowski AM, Katzel LI. Stress-induced blood pressure reactivity and silent cerebrovascular disease. Stroke. 2004;35:1294–1298. doi: 10.1161/01.STR.0000127774.43890.5b. [DOI] [PubMed] [Google Scholar]
- Zannas AS, McQuoid DR, Payne ME, Steffens DC, Macfall JR, Ashley-Koch A, Taylor WD. Negative life stress and longitudinal hippocampal volume changes in older adults with and without depression. J Psychiatr Res. 2013;47:829–834. doi: 10.1016/j.jpsychires.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, McQuoid DR, Steffens DC, Chrousos GP, Taylor WD. Stressful life events, perceived stress, and 12-month course of geriatric depression: direct effects and moderation by the 5-HTTLPR and COMT Val158Met polymorphisms. Stress. 2012;15:425–434. doi: 10.3109/10253890.2011.634263. [DOI] [PMC free article] [PubMed] [Google Scholar]