Abstract
Expression of Notch signaling molecules are increased in synovium from patients with rheumatoid arthritis (RA). However, it is not known which cell type(s) in RA synovium have Notch activation or if they play a pathogenetic role in RA. Here, we used Hes1-GFP/TNF-transgenic (TNF-Tg) mice to investigate the role of cells with active Notch signaling (GFP+) in RA. The number of GFP+ cells was significantly increased in synovium in Hes1-GFP/TNF-Tg mice and about 60% of them were F4/80+ macrophages expressing the inflammatory macrophage (M1) marker. TNF-Tg mice transplanted with Hes1-GFP/TNF-Tg bone marrow (BM) had significantly more GFP+ cells in their synovium than in BM. Intra-articular injection of Hes1-GFP/TNF-Tg or Hes1-GFP+ BM macrophages into WT and TNF-Tg mice showed highest synovial GFP+ cells in the TNF-Tg mice received Hes1-GFP/TNF-Tg cells. Thapsigargin, a Notch inhibitor, decreased TNF-induced M1 and increased M2 numbers and reduced joint lesion, synovial M1s and GFP+ cells in Hes1-GFP/TNF-Tg mice. Thapsigargin did not affect M1s from mice carrying a constitutively active Notch1. Thus, the main cells with activated Notch signaling in the inflamed synovium of TNF-Tg mice are M1s derived from BM and targeting which may represent a new therapeutic approach for patients with inflammatory arthritis.
Keywords: Hes1, Hes1-GFP, macrophage, M1/M2 polarization, Notch, Rheumatoid arthritis, TNF-Tg, TNF, Thapsigargin
INTRODUCTION
Notch signaling is highly conserved, operates in many cell types and regulates cell proliferation, fate, differentiation, and death. Notch signaling is activated by its ligands (Jagged-1, 2 [JAG1, 2] and Delta-like 1, 3, and 4) binding to Notch receptors (Notch1–Notch4), leading to γ-secretase-mediated cleavage of the Notch receptor and release of the Notch intracellular domain (NICD), which translocates into the nucleus. NICD associates with the recombination signal-binding protein jκ (RBPjκ) transcription factor on gene promoters and activates transcription of Notch target genes (Hes1, Hey1) (1). Notch signaling plays critical roles in development and many disease processes, including rheumatoid arthritis (RA).
Rheumatoid arthritis (RA) is an autoimmune inflammatory joint disorder, characterized by macrophage infiltration, lymphocyte aggregation, synoviocyte proliferation, and joint erosion (2). The expression and activation of Notch signaling components have been detected in synovial fibroblasts and vascular endothelial cells in RA tissue samples (3–5). In vivo administration of the Notch inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) ameliorated arthritic symptoms and joint damage in mice with collagen-induced arthritis (6–7), suggesting a causal relationship between Notch signaling and joint damage in RA. However, it is not known if a specific cell type is has more abundant Notch activation than others in RA joints. Here we crossed Hes1-GFP and TNF-transgenic mice to generate Hes1-GFP/TNF-Tg mice as an RA mouse model carrying the Hes1-GFP transgenic Notch reporter (8–9) and demonstrated that macrophages (macs) are the major cell type with Notch activation in inflamed synovium.
Macs play a central and critical role in RA and are abundant in the inflamed synovial tissue and at the cartilage/pannus junction (10). Macs can be divided into two main subsets according to the cytokine profile they express: M1 (pro-inflammatory) and M2 (anti-inflammatory) (11–12). M1-polarized macs produce high levels of IL-1β, TNFα, and CCL5, and are markedly pro-inflammatory, whereas M2 macs secrete IL-4 and Arginase 1 (Arg1), which can promote tissue repair and are anti-inflammatory (13–15). In arthritic joints there is an imbalance between pro-inflammatory and anti-inflammatory macs (16). Using loss of function approaches, several studies (17–19) have reported that Notch receptor (Notch1) or RBPjκ are required for M1 polarization. In the absence of Notch, macs have a M2 phenotype. However, whether activation of Notch signaling increases M1 polarization or if pharmaceutical inhibition of Notch-mediated activation of M1 macs attenuates joint disease progression has not been well studied in RA synovium. Furthermore, a recent study reported that synovial tissue resident macs are long-lived under homeostatic conditions, radiation resistant, and suppress arthritis (20), and they do not come from circulating monocytes (21). It is not known if Notch activated M1 synovial macs are derived from recruited circulating monocytes or from tissue resident macs. We hypothesize that RA synovium promotes the Notch activation in bone marrow derived macs, resulting in their M1 polarization, and Notch inhibition attenuates joint tissue damage by reducing M1 macs.
Here we used Hes1-GFP/TNF-Tg mice to examine the distribution of cell types with high Notch activation (i.e. Hes1-GFP+) in joints and demonstrated that the majority of cells with active Notch signaling are synovial M1 macs. These macs come from bone marrow, and become Notch active in response to the joint microenvironment. They are also more predisposed to M1 polarization. More importantly, we found that Thapsigargin (THAP), a Notch inhibitor, reduces TNF-induced M1 mac formation and promotes a M2 mac phenotype via Notch because its effect is lost in cells from NICD1-over-expressing mice. Thus, targeting M1 macs with activated Notch signaling may represent a new therapy for patients with inflammatory arthritis or other inflammatory disorders.
MATERIALS AND METHODS
Animals
1) TNF-Tg mice (line 3647) were originally obtained from Dr. G. Kollias and have been crossed with C57BL/6J mice for more than 10 generations. This line of TNF-Tg mice carries a modified human TNF transgene in which the 3′-region of the TNF gene was replaced with that of the human α-globin gene. Hes1-GFP transgenic mice (Hes1-GFP mice) were obtained from Dr. Ryoichiro Kageyama (Kyoto University, Japan) and were generated on a C57BL/6J background in which the 2.5-kb Hes1 promoter was inserted upstream of sequences encoding destabilized eGFP. Hes1-GFP and TNF-Tg mice were crossed to generate Hes1-GFP/TNF-Tg double-transgenic mice (23). 3- and 6-month-old male Hes1-GFP/TNF-Tg mice and their Hes1-GFP littermate controls were used. ROSA-NICD1 mice carry a constitutively active Notch1 intracellular domain, in which the Rosa26 locus is followed by a DNA fragment encoding NICD1 and preceded by aSTOP cassette flanked by loxP sites (24). Expression of NICD1 requires the excision of the STOP cassette by Cre recombination of loxP sequences. Bone marrow macrophages (BMMs) from 3-month-old ROSA-NICD1 mice were studied. All animal procedures were conducted in accordance with approved guidelines of the University of Rochester Committee for Animal Resources.
Generation of BM chimeric mice
WT and TNF-Tg mice (6-week-old, 6 or 7 mice per group) were given sulfatrim chow plus acid water two weeks prior to irradiation. Mice were lethally-irradiated with 1100Gy in two doses using a 137Cs γ-ray source of radiation. Immediately after irradiation, mice were reconstituted with 5×106 BM cells in 0.1 mL of PBS from Hes1-GFP/TNF-Tg mice (2-month-old, female) via i.v. injection. Mice were allowed to reconstitute for 8 weeks while continuing to receive sulfatrim chow plus acid water following which they were used in experimental procedures. At the same time, CD45.2 mice (6-week-old) were irradiated and transplanted with BM cells from CD45.1 mice (2-month-old) following exactly the same procedures to calculate reconstitution (Supplemental Figure 1).
BMM culture
BM cells were isolated from WT, Hes1-GFP or Hes1-GFP/TNF-Tg mice. Briefly, tibiae and femora were obtained and BM cells were flushed out using α-MEM/2% FBS. After red blood cells were lysed, BM cells were cultured with conditioned medium (1:50 dilution) from an M-CSF-producing cell line for 3 days in α-MEM/10% FBS to generate BMMs. BMMs were used for intra-articular injection or induced to M1 or M2 macs. M1 macs were induced using 20ng/ml TNFα or 100ng/ml LPS, and M2 macs using 20ng/mL IL-4. Notch inhibition was achieved by treating the cells with THAP, dose and time as specified in figure legends or DAPT (20uM, 1 or 6 hours). At the end of the culture period, cells were fixed in 4% PFA for IF, or collected for quantitative RT-PCR (qPCR) and Western blotting.
Retrovirus production and infection
MSCV-Cre and MSCV-Cre-GFP were obtained from Dr. T. Reya (UCSD, La Jolla, California, USA). Retrovirus packaging was performed by transfecting plasmids into Plat-E cells using Fugene 6 (Roche) following the manufacturer’s protocol. Supernatant containing retrovirus was collected for infection. BM cells from ROSA-NICD1 mice were infected with retrovirus for 3 days in the presence of M-CSF followed by 1μM THAP for 24 hours. Cells were fixed in 4% PFA for IF, or collected for qPCR and Western blotting.
Intra-articular injection of BMMs
1) To confirm that intra-articular injection of BMMs was successful, we used WT mice as recipients. After anaesthetizing the mice with isoflurane, 5×105 BMMs (in 5μl PBS) from mTmG mice were injected into the joint cavity of the right knee, and PBS was injected into the left knee. These mice possess loxP sites on either side of a membrane-targeted tdTomato (mT) cassette that is driven by the ROSA 26 promoter and express strong red fluorescence in all tissues and cell types. Mice were purchased from Jax (Stock Nos. 007576). 2) To confirm that the intra-articular injection procedure itself did not cause increased GFP+ cells, we used Hes1-GFP mice as recipients. After anaesthetizing the mice with isoflurane, PBS was injected into the joint cavity of the right knee, and the left knee was left without injection. The intra-articular injection was successful and injection itself did not increase GFP+ cells (Supplemental Figure 2). 3) For subsequent experiments, recipient mice were WT or TNF-Tg mice (5-month-old, male, 6 mice per group). We have reported that arthritis develops in TNF-Tg mice from 2-month-old, and it progresses with age (25). After anaesthetizing the mice with isoflurane, 5×105 BMMs from Hes1-GFP mice (5-month-old, male) were injected into the joint cavity of the right knee, whereas BMMs from Hes1-GFP/TNF-Tg mice (5-month-old, male) were into the left knee. Because M1–M2 switching can occur rapidly induced within 24 hours (26), mice were sacrificed and knees were collected for frozen sectioning 24 hours later.
Statistical analysis
Results are given as mean±SEM, or as specified in figure legends. Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA, USA). Comparisons between 2 groups were analyzed using a 2-tailed unpaired Student’s t test. Comparisons among 3 or more groups were carried using one way ANOVA followed by Dunnett’spost-hoc multiple comparisons. P values <0.05 were considered statistically significant.
RESULTS
M1 macrophages with Notch activation are increased in synovium of Hes1-GFP/TNF-Tg mice
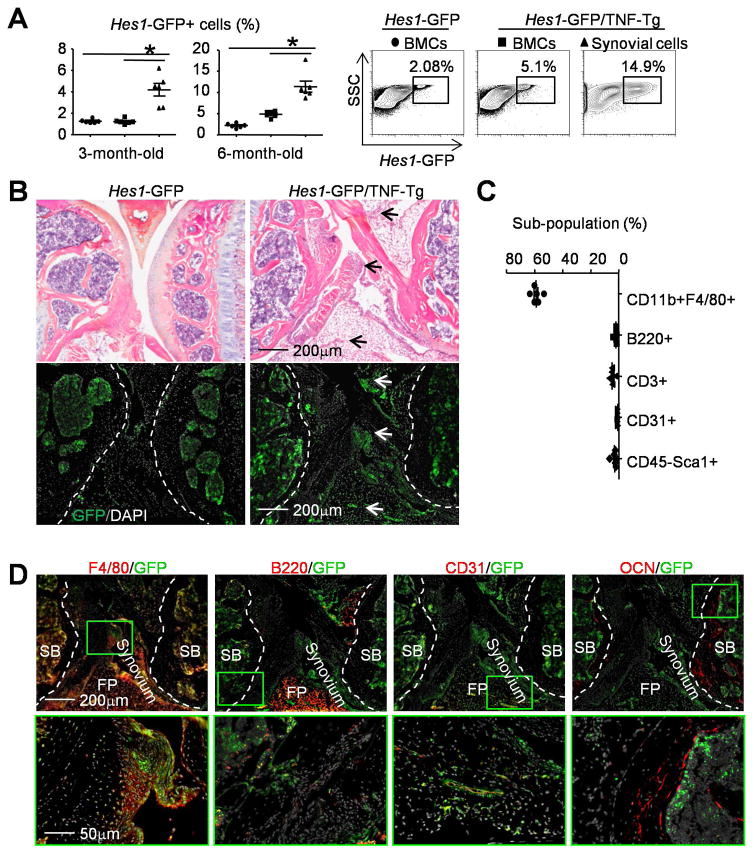
Increased expression of Notch signaling molecules has been reported in synovial samples of RA patients (3–5). However, the identity of the cell type(s) in RA joints with Notch activation is unknown. We used GFP+ cells from Hes1-GFP/TNF-Tg mice, a mouse line that we recently generated (23) to identify Notch-activated cells (those with high Hes1 expression) in the joints of these mice. We used 2 age groups of mice: 3-month-old when TNF-Tg mice develop early signs of arthritis and 6-month-old when they have severe arthritis and systemic bone loss (25,27). We used male mice in our study because female TNF-Tg mice die at 6-month-old due to severe lung inflammation (E. Schwarz, personal communication). We compared the number of GFP+ cells between synovium and BM in the same Hes1-GFP/TNF-Tg mice, as well as BM GFP+ cells between Hes1-GFP/TNF-Tg mice and their Hes1-GFP littermate controls using flow cytometric analysis. At 3-month-old, Hes1-GFP/TNF-Tg mice had a higher percentage of GFP+ cells in their synovium than in their BM (4±0.6% vs. 1±0.1%), while the percentage of GFP+ cells in their BM was similar to control mice. At 6-month-old, the percentage of GFP+ cells in synovium of Hes1-GFP/TNF-Tg mice had increased further to 11±1.3%, associated with increased BM GFP+ cells (5±0.3%) (Figure 1A). Thus, we performed subsequent experiments using 6-month-old Hes1-GFP/TNF-Tg mice because they had higher numbers of GFP+ cells. GFP+ cells were present in the subchondral BM area near the endosteal bone surface in unstained frozen sections in Hes1-GFP control mice. Few GFP+ cells were detected on the very thin synovial tissues. In Hes1-GFP/TNF-Tg mice, more GFP+ cells with stronger GFP fluorescence intensity were observed in the subchondral BM, synovium and around blood vessels (Figure 1B).
Figure 1. Hes1-GFP+/F4/80+ macrophages are markedly increased in synovium of Hes1-GFP/TNF-Tg mice.
3-month-old (A) and 6-month-old (A–D) Hes1-GFP and Hes1-GFP/TNF-Tg mice were used. (A) BM cells (BMCs) and synovial cells were analyzed by flow cytometry for Hes1-GFP+ populations at 3-month-old and 6-month-old. Representative dot-plot shows the population of Hes1-GFP+ cells at 6-month-old. N=6. (B) Frozen sections of knee (H&E stained, upper panels) and adjacent sections (un-stained, lower panels) show the distribution of GFP+ cells (white arrows) in the joint. Joint surface was outlined by a dot line. N=8. (C) Sub-populations of GFP+ cells in the synovium from Hes1-GFP/TNF-Tg mice. N=6. (D) Frozen sections of knees from Hes1-GFP/TNF-Tg mice were subjected to IF with anti-F4/80 for macrophages, anti-B220 for B cells, anti-CD31 for blood endothelial cells and anti-osteocalcin Abs for osteoblasts. Joint surface was outlined by a dot line. SB= Subchondral Bone, FP=Fat Pad. The green boxes from upper panels are shown enlarged. N=4. *, p<0.05 as indicated groups.
Synovial cells from 6-month-old Hes1-GFP/TNF-Tg mice were immune-phenotyped by flow cytometry using markers for various cell types. About 60±1.4% of total synovial GFP+ cells were CD11b+/F4/80+ macs, 2±0.4% were B220+ B cells, 5±0.6% were CD3+ T cells, 0.7±0.1% were CD31+ endothelial cells and 3±0.7% were CD45-Sca1+ mesenchymal stem cells (Figure 1C). IF staining confirmed that most of the GFP+ cells in knee joints of Hes1-GFP/TNF-Tg mice were also F4/80+ macs, few of them were B220+ B cells or CD31+ endothelial cells, and none stained for osteocalcin, which identifies mature osteoblasts (Figure 1D). These data indicate that the majority of cells with activated Notch signaling in TNF-Tg RA synovium are macs.
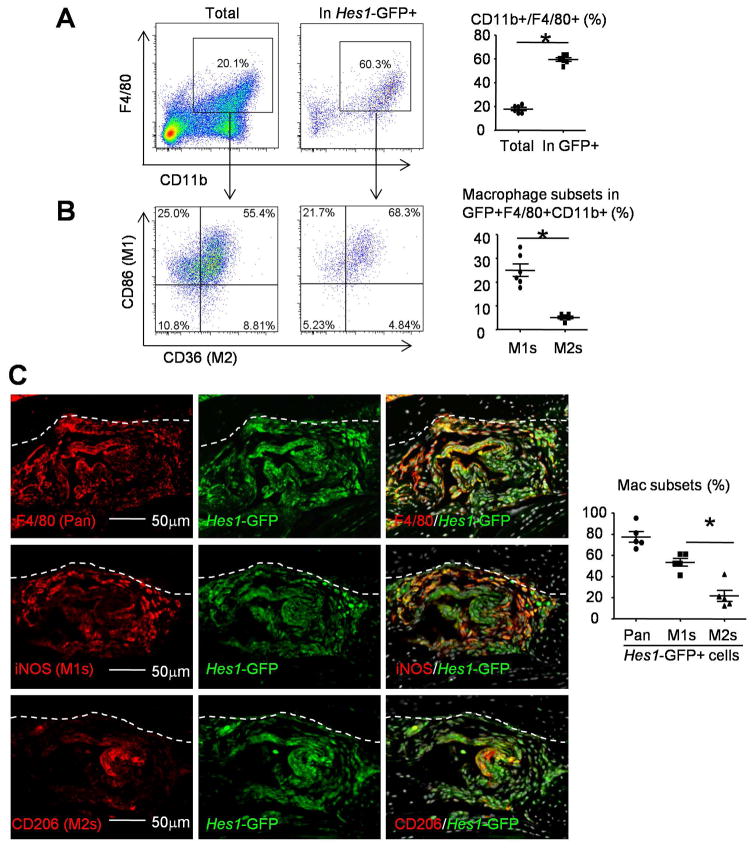
To further characterize the Hes1-GFP+ macs in TNF-Tg RA synovium, we used flow cytometry and CD86 as phenotypic marker for M1 macs and CD36 for M2 macs. CD11b+F4/80+ macs comprised 18±1.3% of the total synovial cells from Hes1-GFP/TNF-Tg mice and 59±1.6% of their Hes1-GFP+ synovial cells (Figure 2A). Within these Hes1-GFP+CD11b+F4/80+ macs, more than 60% cells expressed both M1 marker CD86+ and M2 marker CD36+ due to shared signatures between M1 and M2 macs (Figure 2B). Therefore, we defined CD86+CD36− as M1 macs and CD36+CD86− as M2 macs to investigate M1/M2 polarization of Hes1-GFP+ macs. CD86+CD36− M1 macs comprised 25±2.7% and CD36+CD86− M2 macs comprised 5±0.5% of Hes1-GFP+CD11b+F4/80+ macs (Figure 2B). To confirm these data, we performed IF using iNOS as phenotypic marker for M1 macs and CD206 for M2 macs. IF staining of synovium sections showed that 77±5.0% of GFP+ cells stained positively for F4/80, 53±3.7% stained positively for iNOS+ M1 macs and 22±5.4% stained positively for CD206+ M2 macs (Figure 2C). These data indicate that the majority of cells carrying activated Notch signaling in TNF-Tg RA synovium are M1 macs.
Figure 2. M1 macrophages are the most common Hes1-GFP+F4/80+ cells in synovium of Hes1-GFP/TNF-Tg mice.
6-month-old Hes1-GFP/TNF-Tg mice were used. (A–B) Synovial cells were stained with various Abs and subjected to flow cytometry. Dot-plot shows the % of CD11b+/F4/80+ macrophages in total or Hes1-GFP+ cells (A) and the % of CD86 (M1) and CD36 (M2) cells in F4/80+CD11b+ population (B). N=6. (C) Representative images of adjacent frozen sections from Hes1-GFP/TNF-Tg mouse synovium were subjected to IF with anti-F4/80 (pan macrophage), anti-iNOS (M1) and anti-CD206 (M2) Abs. % of F4/80, iNOS or CD206 in GFP+ cells was determined. Bone surface was outlined by a dot line. N=5. *, p<0.05 as indicated groups.
Notch activating M1 macrophages in synovium of Hes1-GFP/TNF-Tg mice are derived from bone marrow
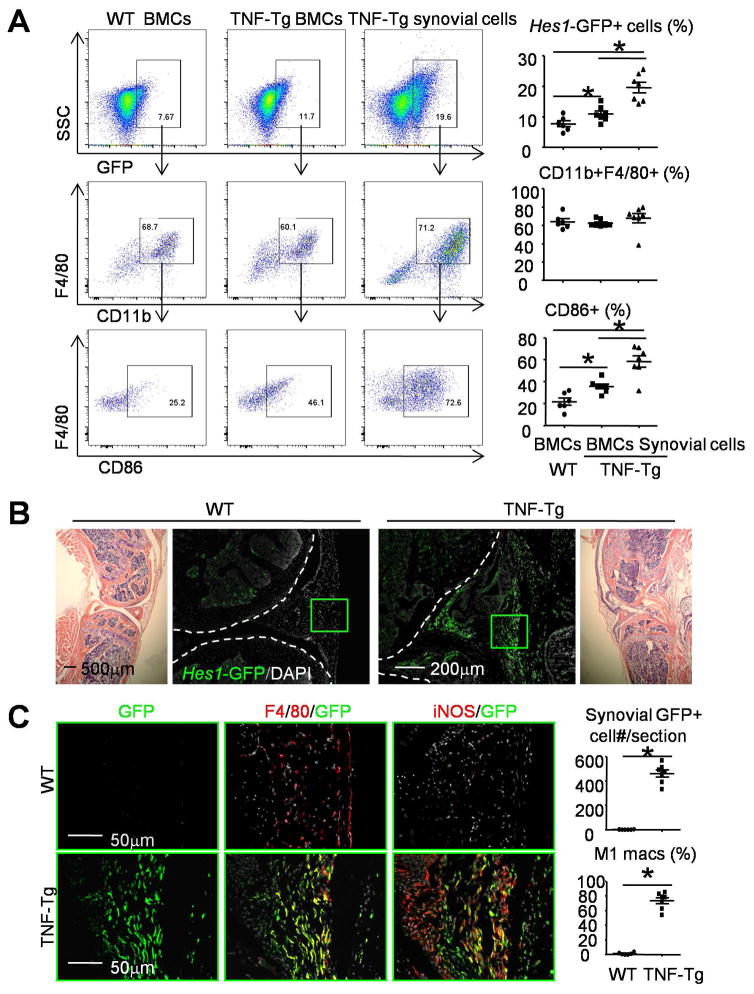
To determine the origin of Hes1-GFP+ M1 macs in the synovium of Hes1-GFP/TNF-Tg RA mice, we performed BM transplantation experiments. 6-week-old WT and TNF-Tg lethally irradiated mice were transplanted with BM cells from Hes1-GFP/TNF-Tg mice. Chimeric mice were sacrificed 8 weeks post-transplant, when they typically have had 80% reconstitution (Supplemental Figure 1). BM and synovial cells were harvested for flow cytometry with the focus on Hes1-GFP+ cells. GFP+ cells were moderately increased in BM and markedly increased in synovium of TNF-Tg mice. About 60% of GFP+ cells in WT BM, TNF-Tg BM or TNF-Tg synovium were CD11b+/F4/80+ pan macs, and of these, CD86 M1 macs comprised 22±3.3% and 36±2.2% of WT and TNF-Tg BM, respectively. In contrast, CD86 M1 macs comprised 58±5.3% of TNF-Tg synovial pan macs (Figure 3A). Similarly, IF staining of knee sections indicated numerous GFP+ cells in the synovium of TNF-Tg mice (Figure 3B), among which 73±4.3% stained positively for the M1 marker, iNOS (Figure 3C). No GFP+ cells were detected in the synovium of WT recipient mice (Figure 3C). These data indicate that the majority of Notch-activated M1 macs in the synovium of Hes1-GFP/TNF-Tg mice come from BM.
Figure 3. The majority of GFP+ M1s in the synovium of Hes1-GFP/TNF-Tg mice come from bone marrow.
(A–C) 6-week-old WT and TNF-Tg mice received lethal-dose irradiation and were transplanted with BMCs from Hes1-GFP/TNF-Tg mice. Chimeric mice were sacrificed 8 weeks post-BM transfer for analysis. (A) BMCs and synovial cells were collected and analyzed by flow cytometry. Dot-plot shows the % of GFP+ cells in total (upper panel), the % of CD11b+F4/80+ cells in the GFP+ population (middle panel), and the % of CD86+ cells in the CD11b+F4/80+ population (lower panel). N=6–7. (B) Representative images of H&E-stained sections (1st and 4th panels) and adjacent frozen sections (2nd and 3rd panels) show the distribution of Hes1-GFP+ cells in mouse joints. Bone surface was outlined by a dot line. N=6–7. (C) The green boxes from the synovium in (B) are shown enlarged. Adjacent frozen sections of knee were IF stained with anti-F4/80 and anti-iNOS Abs. The numbers of GFP+ cells per section and the % of F4/80+/iNOS+ M1 macrophages were counted. N=6–7. *, p<0.05 as indicated groups.
Both the microenvironment and macrophages themselves contribute to increased GFP+ macrophages in Hes1-GFP/TNF-Tg mouse synovium
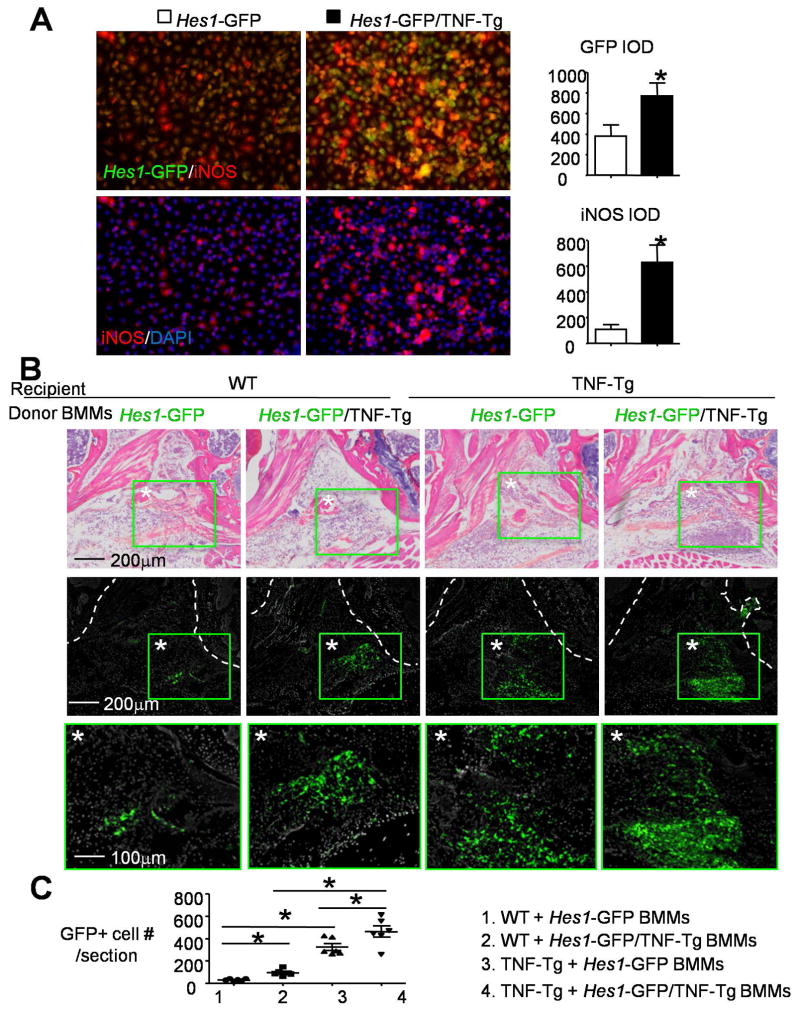
Increased numbers of GFP+ M1 cells in Hes1-GFP/TNF-Tg mouse synovium could be due to their macs being more sensitive to M1 inducers, the synovial inflammatory microenvironment, or both. To test this, we first examined if Hes1-GFP/TNF-Tg macs are more responsive to the M1 inducer, TNF, and if they have higher Notch activity, e.g. Hes1-GFP expression, than cells from Hes1-GFP control mice. When Hes1-GFP/TNF-Tg macs were cultured with a suboptimal dose of TNFα (5ng/ml) to induce M1 mac polarization (20ng/ml is optimal), they formed significantly more GFP+/iNOS+ M1 macs with higher fluorescence intensity than Hes1-GFP cells (Figure 4A). To examine if the joint microenvironment of TNF-Tg RA triggers Notch activation in M1 macs, we performed an intra-articular (IA) BMM injection. We first confirmed that injected BMMs survived 24 hours after injection using BMMs from mTmG mice, and verified that the injection procedure itself did not cause Notch activation by assessing the number of GFP+ cells (Supplemental Figure 2). Hes1-GFP/TNF-Tg or Hes1-GFP mouse BMMs were injected into the knee joints of WT or TNF-Tg mice (5-month-old, male) and the numbers of GFP+ cells were examined 24 hours later in un-stained frozen sections. We observed that GFP+ cells were mainly located within or surrounding the knee fat pad. WT mice given Hes1-GFP/TNF-Tg BMMs had more GFP+ cells than those given Hes1-GFP BMMs. TNF-Tg mice had more GFP+ cells than WT mice, and TNF-Tg mice given Hes1-GFP/TNF-Tg BMMs had the most GFP+ cells (Figure 4B&C). These results suggest that Hes1-GFP/TNF-Tg BMMs have a higher potential to become GFP+ M1 macs, and the joint microenvironment of TNF-Tg induces GFP expression in macs.
Figure 4. The synovial microenvironment and macrophages contribute to the increase in GFP+ macrophages in Hes1-GFP/TNF-Tg mouse synovium.
(A) BMCs from 6-month-old Hes1-GFP/TNF-Tg and Hes1-GFP control mice were cultured with M-CSF for 3 days to generate BMMs. BMMs were treated with 5ng/ml TNFα to induce M1 formation. Cells were subjected to IF with anti-iNOS Ab. Integrated optical density (IOD=area×intensity of fluorescence) of GFP and iNOS were measured. *, p<0.05 vs. Hes1-GFP, N=4. (B) BMMs from Hes1-GFP or Hes1-GFP/TNF-Tg mice were injected into the knee joint cavities of WT or TNF-Tg mice. Mice were sacrificed one day later. Representative images of H&E stained frozen sections of knee (upper panels) and adjacent unstained sections under fluorescence microscopy (middle panels and the green boxes from middle panels are shown enlarged in the lower panels) from Hes1-GFP+ cells in the joint. Joint surface was outlined by a dot line. (C) The number of GFP+ cells per section was assessed. N=6. *, p<0.05 as indicated groups.
TNF promotes the formation of M1 macrophages with Notch activation
To further determine that TNF over-expression mediates increased synovial GFP+ M1 macs in Hes1-GFP/TNF-Tg mice, we examined the expression of M1/M2 effectors and Notch-associated genes in vitro under TNF induction conditions, which mimics high TNF levels in RA joints. Hes1-GFP BMMs were cultured with TNF to induce M1 and IL-4 to induce M2 polarization. LPS was included as a positive control for M1 formation. TNF, but not IL-4, induced Hes1-GFP expression. IF showed that all cells treated with TNF or IL-4 stained positively for the pan mac marker, F4/80. TNF-treated cells stained positively for the M1 mac marker, iNOS, but negatively for the M2 mac marker, CD206. As a negative control, IL-4-treated cells stained positively for CD206, but not for iNOS (Supplemental Figure 3A). Consistent with this, TNF-treated cells expressed higher levels of Notch-associated genes (Hes1, Hey1 and Notch1) and M1 effector genes (TNFα, IL-1β and CCL5), but lower levels of M2 effector genes (PPARγ, IL-4, and Arg1) than control cells (Supplemental Figure 3B&C). These data indicate that TNF promotes the formation of M1 macs with Notch activation.
Thapsigargin inhibits TNF-induced M1 macrophage formation by switching them to a M2 phenotype via Notch inhibition
To determine if Notch inhibition could block M1 mac polarization, thereby attenuating joint tissue damage in RA, we used Thapsigargin (THAP), a Notch signal inhibitor that affects the maturation and activity of Notch receptors (28). THAP dose-dependently decreased the expression levels of Notch-associated genes (Notch1, Hes1, Hey1) and the M1 effector gene (CCL5), and increased M2 effector genes (IL-4, Arg1) in TNF-induced M1 macs (Figure 5A). Using Hes1 expression as an outcome measure for Notch activation, we found that THAP reduced Hes1 protein levels by IF and Western blotting (Figure 5B&C). Furthermore, THAP markedly increased CD206+ M2 formation (Figure 5B). Because THAP is also used as an endoplasmic reticulum (ER) stress inducer and ER stress is a key regulator of macrophage differentiation (29), we wanted to determine if THAP mediates mac polarization via the Notch inhibition. To test this, we infected BMMs from ROSA-NICD1 mice with Cre-GFP or GFP retrovirus to obtain Notch-active and control cells (Figure 5D). The reason we used NICD1 over-expressing cells is that Notch1 is shown to play critical roles in regulating the effector function of macrophages and to be involved in autoimmune disease (30–31). We anticipated that THAP would not affect these NICD1 over-expressing cells because it requires the full length Notch receptor for its inhibitory effect (28). As we expected, in GFP-infected control BMMs, THAP reduced the expression levels of Hes1 and IL-1β, and increased Arg1. Cre-GFP-infected Notch-active cells had elevated levels of Hes1 and IL-1β and decreased Arg1 expression, which was not affected by THAP (Figure 5E). IF confirmed that Cre-GFP retrovirus-infected BMMs had high expression levels of NICD1 and iNOS, and low levels of CD206, while THAP had no effect on them (Figure 5F). To further confirm that Notch inhibition affects mac polarization, we treated BMMs with the γ-secretase inhibitor, DAPT, to inhibit Notch signaling. Similar to THAP, DAPT reduced expression of Hes1 and TNFα, but increased IL-4 and Arg1 gene levels (Figure 5G). These data indicate that Notch inhibition reduces TNF-induced M1 macs by switching them to a M2 phenotype.
Figure 5. Thapsigargin inhibits Notch activation and promotes M2 macrophage polarization in vitro.
(A–C) BMMs from WT mice were generated as in Fig.4. BMMs were cultured with 20ng/ml TNFα to induce M1 macs, and THAP was then added to the culture medium with TNFα for 24 hours. (A) The expression levels of IL-4, Arg1, CCL5, Notch1, Hes1 and Hey1 were measured by qPCR. Values are mean±S.D. of 3 wells. *, p<0.05 vs. 0mM THAP. N=4. (B) Cells were subjected to IF with anti-Hes1, anti-F4/80 or anti-CD206 Abs after THAP treatment (1mM). N=3. (C) The effect of THAP (1mM) on Hes1 protein expression in TNF induced M1s was determined by Western blot analysis. N=3. (D–F) BMMs were generated from ROSA-NICD1 mice and infected with MSCV-GFP virus or MSCV-Cre-GFP virus. BMMs were treated with or without 1mM THAP for 24 hours. (D) NICD1 protein expression was determined by Western blot analysis. N=3. (E) Expression levels of Hes1, IL-1β and Arg1 were measured by qPCR. Values are mean±S.D. of 3 wells. *, p<0.05 in indicated groups. N=3. (F) Cells were subjected to IF with anti-NICD1, anti-iNOS and anti-CD206 Abs. N=3. (G) WT BMMs were treated with 20ng/ml TNFα overnight to induce M1 macrophages, and then treated with 20mM DAPT or Vehicle (Veh.) for 0 and 6 hours. Expression levels of Hes1, TNFα, Arg1 and IL-4 were measured by qPCR. Values are mean±S.D. of 3 wells. *, p<0.05 vs. 6h Veh. N=3.
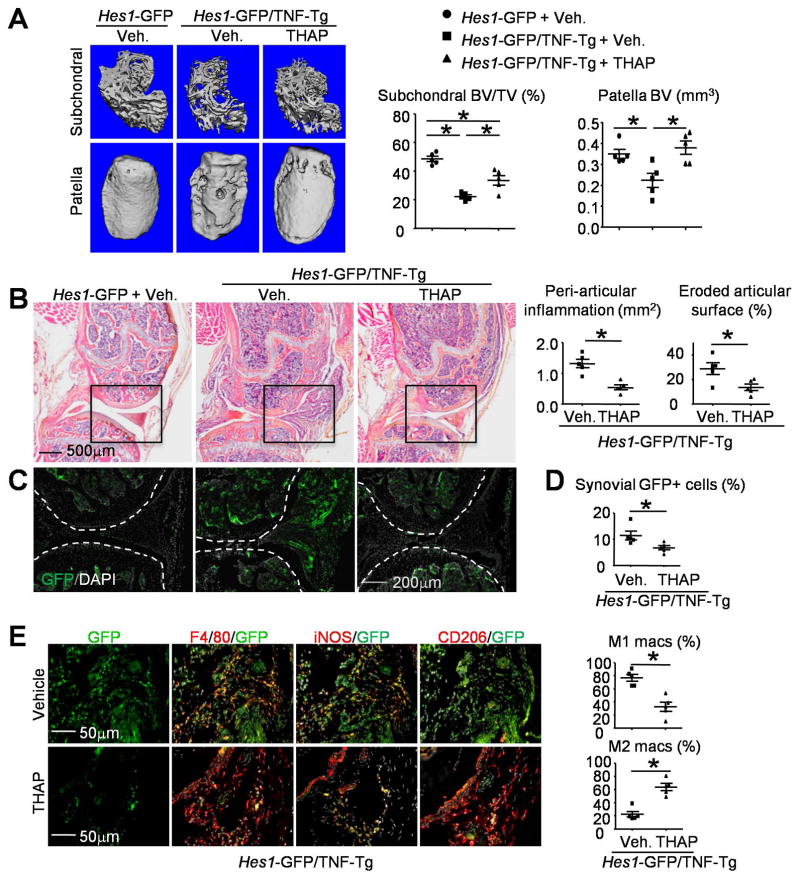
Thapsigargin inhibits Notch activation, promotes M2 macrophage polarization, and reduces joint bone loss in Hes1-GFP/TNF-Tg mice
We treated 2.5-month-old Hes1-GFP/TNF-Tg mice with THAP or vehicle (3 times/week for 8 weeks. i.p), and Hes1-GFP littermate control mice with vehicle. μCT of knee joints showed that vehicle-treated Hes1-GFP/TNF-Tg mice had significantly more subchondral and patellar bone loss than Hes1-GFP control mice, and this was prevented by THAP (Figure 6A). Histomorphometric analysis of H&E-stained sections showed significantly decreased peri-articular inflammation area and eroded articular surface in knee joints of THAP-treated Hes1-GFP/TNF-Tg mice (Figure 6B), associated with markedly reduced Hes1-GFP+ cells (Figure 6C). Decreased synovial Hes1-GFP+ cell numbers in THAP-treated mice were confirmed by flow cytometry analysis (Figure 6D).
Figure 6. Thapsigargin inhibits Notch activation, promotes M2 macrophage polarization, and reduces joint bone loss in Hes1-GFP/TNF-Tg mice.
(A–D) 2.5-month-old Hes1-GFP/TNF-Tg mice and Hes1-GFP littermates were given THAP, 0.4 mg/kg/injection i.p., or vehicle 3x/week for 8 weeks. (A) Representative μCT images of femoral subchondral bone and patella and morphometric data of femoral subchondral and patellar bone volume. N=5. (B) Representative images of H&E-stained frozen sections. Peri-articular inflammation area (mm2) and eroded articular surface (%) in the joints from Hes1-GFP/TNF-Tg mice were determined. N=5. (C) Adjacent frozen sections of B (the black boxes from B are shown enlarged) shows the distribution of Hes1-GFP+ cells. Joint surface was outlined by a dot line. N=5. (D) Synovial cells were analyzed by flow cytometry for Hes1-GFP+ populations. N=5. (E) Adjacent frozen sections of knees were IF stained with anti-F480, anti-iNOS and anti-CD206 Abs. The % of F4/80+/iNOS+ M1 macrophages and F4/80+/CD206+ M2 macrophages were counted. N=5. *, p<0.05 as indicated groups.
To investigate if THAP treatment reduces the number of M1 macs by promoting M2 mac polarization in the synovium of Hes1-GFP/TNF-Tg mice, serial sections of the knee joints from Hes1-GFP/TNF-Tg mice were subjected to IF staining with anti-iNOS and anti-CD206 antibodies. In vehicle-treated mice, we observed a high frequency of Hes1-GFP+/F4/80+ cells, most of which also expressed iNOS, but not CD206. In contrast, THAP treatment markedly reduced Hes1-GFP expression, had no obvious effect on total F4/80+ cells, but it significantly decreased iNOS+ M1 and increased CD206+ M2 macs (Figure 6E). These data suggest that THAP attenuates joint tissue damage by inhibiting Notch activation in synovial macs and promoting M2 mac polarization in TNF-Tg RA mice.
DISCUSSION
Using combinations of in vitro cell cultures and TNF-Tg arthritic mice carrying an Hes1-GFP transgene as an indicator of Notch activation, we demonstrated that Notch signaling has a close relationship with M1 macrophage (mac) polarization and that inhibition of Notch signaling reduces joint tissue damage by switching M1 to M2 macs. Thus, targeting Notch-activated M1 macs may represent a new therapy for RA. Our study indicates 3 important points: 1) among the many cell types that contribute to the pathogenesis of TNF-Tg RA, M1 macs are the most abundant cells with Notch activation; 2) most Notch-activated M1 macs are derived from bone marrow (BM); and 3) the Notch signaling modulator, Thapsigargin, attenuates joint tissue damage by switching M1 to M2 macs.
Recent research revealed that tissue mac renewal occurs independent of BM (32–33). Fate-mapping studies showed that major tissue-resident macs are seeded into the tissues before birth and maintain themselves subsequently during adulthood independent of blood monocytes (34). Our BM transplantation experiments by transferring Hes1-GFP/TNF-Tg mouse BM cells to irradiated TNF-Tg mice revealed numerous Hes1-GFP+ M1 macs in the joints of TNF-Tg recipient mice (Figure 3). These data indicate that donor-derived BM cells are recruited from the blood to inflamed synovium where they become Notch activated and undergo M1 polarization because the original Hes1-GFP/TNF-Tg mouse BM cells contained few Hes1-GFP+ cells before they were transferred. Our finding is consistent with an early publication describing ‘embryonic’ macs being replaced by circulating monocytes under severe inflammatory conditions (35). This raises an important question, e.g. if Hes1-GFP/TNF-Tg macs become more sensitive to Notch activators in TNF-Tg RA synovium. Our in vivo data in Figure 4B clearly demonstrate that macs themselves and the joint micro-environment contribute synergistically to the Notch activation in M1 macs in the TNF-Tg RA synovium.
Multiple cell types in the RA synovium produce Notch ligands including Delta1, Jagged1 and Jagged2 (4,36–37). These Notch ligands bind to Notch receptors expressed by synovial macs to activate Notch signaling. Notch activation is also involved in synovial T cell and B cell immune responses (38). However, we found that the majority of Notch activated cells in TNF-Tg RA synovium are macs (Figure 1C). This may be due to the total mac number or the expression levels of Notch receptors on macs are higher than other cell types. Another reason is that TNF-Tg mice have high levels of TNF (39) which could activate Notch signaling via a number of mechanisms. For instance, it stimulates expression of Notch ligands and receptors (4,40–41) and Notch target genes (42). We reported that TNF activates Notch signaling by promoting the nuclear translocation of NICD via non-canonical NF-κB (27). Thus, elevated TNF-environment in inflamed joints also contributes to increased numbers of Notch active M1 macs.
We found that Thapsigargin reduced TNF-induced Notch-active Hes1-GFP+ M1 mac polarization by inducing a switch to Notch-inactive Hes1-GFP- M2 macs. We examined several potential molecular mechanisms mediating this effect. First, Notch-RBPjκ signaling promotes M1 mac polarization through the transcription factor, IRF8. IRF5 (44) and IRF9 (45) are also involved in M1 macrophage formation. However, Thapsigargin does not affect their expression levels (Supplemental Figure 4). Secondly, defective autophagy promotes M1 mac polarization (46–47). DAPT promotes adipogenesis of human BM-derived MSCs by regulating autophagy via the PTEN-PI3K/Akt/mTOR pathway(48). We found that Thapsigargin induces autophagy formation by increasing expression of the autophagy/lysosome markers, LC3 II and LAMP2, in a dose- and time-dependent manner (Supplemental Figure 5), which may explain how Thapsigargin promotes the M2 phenotype. Finally, Thapsigargin regulates intracellular Ca2+ via inhibition of the ER and prevents processing of the Notch receptor in the ER, leading to an accumulation of misfolded receptor, which inhibits Notch signaling (28). Besides being a Notch inhibitor, Thapsigargin increases ER stress to generate a M2 phenotype (29) and functions as an ER stress inducer (49). We found that the effect of Thapsigargin on mac polarization is abolished in cells from mice with Notch1 conditional activation in myeloid cells, indicating the requirement of a functional Notch signaling.
We previously reported that DAPT did not affect the severity of inflammation or bone erosion in joints of TNF-Tg mice (27). However, it ameliorated murine collagen-induced arthritis (CIA) (6–7). This may be due to differences in the RA mouse models and the timing of administration. For example, DAPT was given to 2.5-month-old TNF-Tg mice, which had already developed ankle arthritis (25), while DAPT was administered to CIA mice on the day before the first immunization (6) or 21 days after primary immunization (7). DAPT treatment was given before the onset CIA in both studies. Here,we used Thapsigargin instead of DAPT to treat arthritis in TNF-Tg mice because Thapsigargin is a much more potent inhibitor of Notch signaling than DAPT, requiring 0.4 mg/kg/injections 3 times per week compared to 5 mg/kg/injection of DAPT daily (27). Our data for the first time demonstrated that Thapsigargin reduces inflammation and joint bone loss in TNF-Tg RA mice by promoting M2 mac polarization.
In the current study, we used expression of the Hes1-GFP transgene as an indicator of Notch activation because Hes1 is one of the critical Notch target genes; but there are limitations to this approach. For instance, Hes1-GFP+ cells may not absolutely represent endogenous Hes1-expressing cells and Hes1 could be activated by signals other than Notch, such as TGFβ (50), sonic hedgehog (51), and Wnt (52). However, since the expression pattern of Hes1 mRNA is similar to that of Hes1-GFP+ cells in the brain of Hes1-GFP mice by in situ hybridization, it is very likely that Hes1-GFP expression mimics endogenous Hes1 expression (8). Another concern is that we only used in vitro BMM culture to show a role of NICD1 in M1/M2 polarization. Because BMMs may not resemble macs in vivo, we can generate myeloid specific NICD-over-expression or knockout mice by crossing NICD1-Tg mice or Notch1 flox mice with LysM-cre or CX3CR1-cre mice and then performing bone marrow chimeras with the TNF-Tg mice in the absence and presence of Notch inhibitor. These studies would definitively show a role of Notch1 in M1/M2 polarization of macs and its effect on arthritis development. Finally, we used mac M1 and M2 activation states to characterize Notch active macs. However, it has been recognized for many years, these two states are insufficient to describe the complexity of mac responses within in vivo physiological or pathological conditions (11,53). Thus, more experiments, such as using transcriptomic and systems biology tools, are needed to further delineate the involvement of Notch-activate macs in the pathogenesis and treatment of RA.
In summary, using Hes1-GFP/TNF-Tg mice as an RA mouse model carrying a Notch reporter transgene, we have demonstrated markedly increased Hes1-GFP+ M1 macs in synovium of Hes1-GFP/TNF-Tg mice. These macs are derived from BM and become Notch-activate M1 macs via both microenvironment and cell autonomous mechanisms. Thapsigargin decreases TNF-induced M1 mac formation and joint tissue damage by inhibiting Notch signaling and promoting a M2 mac phenotype. Targeting Notch-activate M1 macs may represent a new therapy for patients with inflammatory arthritis or other inflammatory disorders.
Supplementary Material
Acknowledgments
The authors thank Martin Chang for technical assistance with the whole slide-scanner. Research was supported by grants from National Institute of Health USA PHS awards (AR48697, AR63650, AR069789, AR43510), NYSTEM USA N13G-084 (C029548), and the National Natural Science Foundation of China (81670965). Some experiments were performed by the CMSR cores (μCT) or using CMSR core equipment (frozen sectioning, microscopes, and whole slide imaging), which are supported by grants from National Institute of Health USA PHS awards (1S10RR027340-01, AR061307 and AR054041).
Footnotes
Disclosure: The authors have declared that no conflicts of interest exist.
Authors’ roles: Study design: WS, HZ, HW, YGC, CTR, AK, BFB, and LX. Study conduct: WS, HZ, HW, YGC, and LX. Data collection: WS and LX. Data analysis: WS and LX. Data interpretation: WS, HZ, HW, YGC, CTR, AK, BFB, and LX. Drafting manuscript: WS and LX. Revising manuscript content: WS, BFB, and LX. Approving final version of manuscript: WS, HZ, HW, YGC, CTR, AK, BFB, and LX. LX takes responsibility for the integrity of the data analysis.
References
- 1.Kim WK, Meliton V, Tetradis S, Weinmaster G, Hahn TJ, Carlson M, et al. Osteogenic oxysterol, 20(S)-hydroxycholesterol, induces notch target gene expression in bone marrow stromal cells. J Bone Miner Res. 2010;25(4):782–95. doi: 10.1359/jbmr.091024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma Y, Pope RM. The role of macrophages in rheumatoid arthritis. Curr Pharm Des. 2005;11(5):569–80. doi: 10.2174/1381612053381927. [DOI] [PubMed] [Google Scholar]
- 3.Nakazawa M, Ishii H, Aono H, Takai M, Honda T, Aratani S, et al. Role of Notch-1 intracellular domain in activation of rheumatoid synoviocytes. Arthritis Rheum. 2001;44(7):1545–54. doi: 10.1002/1529-0131(200107)44:7<1545::AID-ART278>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Ando K, Kanazawa S, Tetsuka T, Ohta S, Jiang X, Tada T, et al. Induction of Notch signaling by tumor necrosis factor in rheumatoid synovial fibroblasts. Oncogene. 2003;22(49):7796–803. doi: 10.1038/sj.onc.1206965. [DOI] [PubMed] [Google Scholar]
- 5.Gao W, Sweeney C, Walsh C, Rooney P, McCormick J, Veale DJ, et al. Notch signalling pathways mediate synovial angiogenesis in response to vascular endothelial growth factor and angiopoietin 2. Ann Rheum Dis. 2013;72(6):1080–8. doi: 10.1136/annrheumdis-2012-201978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiao Z, Wang W, Hua S, Liu M, Wang H, Wang X, et al. Blockade of Notch signaling ameliorates murine collagen-induced arthritis via suppressing Th1 and Th17 cell responses. Am J Pathol. 2014;184(4):1085–93. doi: 10.1016/j.ajpath.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Park JS, Kim SH, Kim K, Jin CH, Choi KY, Jang J, et al. Inhibition of notch signalling ameliorates experimental inflammatory arthritis. Ann Rheum Dis. 2015;74(1):267–74. doi: 10.1136/annrheumdis-2013-203467. [DOI] [PubMed] [Google Scholar]
- 8.Ohtsuka T, Imayoshi I, Shimojo H, Nishi E, Kageyama R, McConnell SK. Visualization of embryonic neural stem cells using Hes promoters in transgenic mice. Mol Cell Neurosci. 2006;31(1):109–22. doi: 10.1016/j.mcn.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Oh P, Lobry C, Gao J, Tikhonova A, Loizou E, Manent J, et al. In vivo mapping of notch pathway activity in normal and stress hematopoiesis. Cell Stem Cell. 2013;13(2):190–204. doi: 10.1016/j.stem.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2(3):189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He H, Xu J, Warren CM, Duan D, Li X, Wu L, et al. Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages. Blood. 2012;120(15):3152–62. doi: 10.1182/blood-2012-04-422758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin J, Xiao Y, Hu H, Zou Q, Li Y, Gao Y, et al. Proinflammatory TLR signalling is regulated by a TRAF2-dependent proteolysis mechanism in macrophages. Nat Commun. 2015;6:5930. doi: 10.1038/ncomms6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Yu W, Lee S, Xu Q, Naji A, Le AD. Bisphosphonate Induces Osteonecrosis of the Jaw in Diabetic Mice via NLRP3/Caspase-1-Dependent IL-1beta Mechanism. J Bone Miner Res. 2015;30(12):2300–12. doi: 10.1002/jbmr.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinne RW, Stuhlmuller B, Burmester GR. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res Ther. 2007;9(6):224. doi: 10.1186/ar2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70(12):4840–9. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Chi F, Guo T, Punj V, Lee WN, French SW, et al. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J Clin Invest. 2015;125(4):1579–90. doi: 10.1172/JCI76468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Outtz HH, Wu JK, Wang X, Kitajewski J. Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in macrophages. J Immunol. 2010;185(7):4363–73. doi: 10.4049/jimmunol.1000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misharin AV, Cuda CM, Saber R, Turner JD, Gierut AK, Haines GK, 3rd, et al. Nonclassical Ly6C(−) monocytes drive the development of inflammatory arthritis in mice. Cell Rep. 2014;9(2):591–604. doi: 10.1016/j.celrep.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheng J, Ruedl C, Karjalainen K. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity. 2015;43(2):382–93. doi: 10.1016/j.immuni.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Schwarz EM, O’Keefe RJ, Ma L, Boyce BF, Xing L. RANK signaling is not required for TNFalpha-mediated increase in CD11(hi) osteoclast precursors but is essential for mature osteoclast formation in TNFalpha-mediated inflammatory arthritis. J Bone Miner Res. 2004;19(2):207–13. doi: 10.1359/JBMR.0301233. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Sun W, Li X, Wang M, Boyce BF, Hilton MJ, et al. Use of Hes1-GFP reporter mice to assess activity of the Hes1 promoter in bone cells under chronic inflammation. Bone. 2016;90:80–9. doi: 10.1016/j.bone.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100(25):14920–5. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneki H, Guo R, Chen D, Yao Z, Schwarz EM, Zhang YE, et al. Tumor necrosis factor promotes Runx2 degradation through up-regulation of Smurf1 and Smurf2 in osteoblasts. J Biol Chem. 2006;281(7):4326–33. doi: 10.1074/jbc.M509430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, et al. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4(3):e00264–13. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Hilton MJ, Anolik JH, Welle SL, Zhao C, Yao Z, et al. NOTCH inhibits osteoblast formation in inflammatory arthritis via noncanonical NF-kappaB. J Clin Invest. 2014;124(7):3200–14. doi: 10.1172/JCI68901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roti G, Carlton A, Ross KN, Markstein M, Pajcini K, Su AH, et al. Complementary genomic screens identify SERCA as a therapeutic target in NOTCH1 mutated cancer. Cancer Cell. 2013;23(3):390–405. doi: 10.1016/j.ccr.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh J, Riek AE, Weng S, Petty M, Kim D, Colonna M, et al. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem. 2012;287(15):11629–41. doi: 10.1074/jbc.M111.338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Xu W, Xiong S. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. J Immunol. 2010;184(11):6465–78. doi: 10.4049/jimmunol.0904016. [DOI] [PubMed] [Google Scholar]
- 31.Wongchana W, Lawlor RG, Osborne BA, Palaga T. Impact of Notch1 Deletion in Macrophages on Proinflammatory Cytokine Production and the Outcome of Experimental Autoimmune Encephalomyelitis. J Immunol. 2015;195(11):5337–46. doi: 10.4049/jimmunol.1401770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–95. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7(3):265–73. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yabe Y, Matsumoto T, Tsurumoto T, Shindo H. Immunohistological localization of Notch receptors and their ligands Delta and Jagged in synovial tissues of rheumatoid arthritis. J Orthop Sci. 2005;10(6):589–94. doi: 10.1007/s00776-005-0943-3. [DOI] [PubMed] [Google Scholar]
- 37.Sekine C, Nanki T, Yagita H. Macrophage-derived delta-like protein 1 enhances interleukin-6 and matrix metalloproteinase 3 production by fibroblast-like synoviocytes in mice with collagen-induced arthritis. Arthritis Rheumatol. 2014;66(10):2751–61. doi: 10.1002/art.38743. [DOI] [PubMed] [Google Scholar]
- 38.Magee CN, Riella LV. Notch and its ligands in alloimmunity and rejection. Curr Opin Organ Transplant. 2016;21(1):15–21. doi: 10.1097/MOT.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 39.Li P, Schwarz EM, O’Keefe RJ, Ma L, Looney RJ, Ritchlin CT, et al. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50(1):265–76. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez L, Rodriguez S, Huang H, Chora A, Fernandes J, Mumaw C, et al. Tumor necrosis factor-alpha and endothelial cells modulate Notch signaling in the bone marrow microenvironment during inflammation. Exp Hematol. 2008;36(5):545–58. doi: 10.1016/j.exphem.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acharyya S, Sharma SM, Cheng AS, Ladner KJ, He W, Kline W, et al. TNF inhibits Notch-1 in skeletal muscle cells by Ezh2 and DNA methylation mediated repression: implications in duchenne muscular dystrophy. PLoS One. 2010;5(8):e12479. doi: 10.1371/journal.pone.0012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguilera C, Hoya-Arias R, Haegeman G, Espinosa L, Bigas A. Recruitment of IkappaBalpha to the hes1 promoter is associated with transcriptional repression. Proc Natl Acad Sci U S A. 2004;101(47):16537–42. doi: 10.1073/pnas.0404429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H, Zhu J, Smith S, Foldi J, Zhao B, Chung AY, et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol. 2012;13(7):642–50. doi: 10.1038/ni.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12(3):231–8. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 45.Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol. 2013;33(6):1135–44. doi: 10.1161/ATVBAHA.113.301453. [DOI] [PubMed] [Google Scholar]
- 46.Chen P, Cescon M, Bonaldo P. Autophagy-mediated regulation of macrophages and its applications for cancer. Autophagy. 2014;10(2):192–200. doi: 10.4161/auto.26927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu K, Zhao E, Ilyas G, Lalazar G, Lin Y, Haseeb M, et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy. 2015;11(2):271–84. doi: 10.1080/15548627.2015.1009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song BQ, Chi Y, Li X, Du WJ, Han ZB, Tian JJ, et al. Inhibition of Notch Signaling Promotes the Adipogenic Differentiation of Mesenchymal Stem Cells Through Autophagy Activation and PTEN-PI3K/AKT/mTOR Pathway. Cell Physiol Biochem. 2015;36(5):1991–2002. doi: 10.1159/000430167. [DOI] [PubMed] [Google Scholar]
- 49.Samali A, Fitzgerald U, Deegan S, Gupta S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int J Cell Biol. 2010;2010:830307. doi: 10.1155/2010/830307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xing Y, Li C, Li A, Sridurongrit S, Tiozzo C, Bellusci S, et al. Signaling via Alk5 controls the ontogeny of lung Clara cells. Development. 2010;137(5):825–33. doi: 10.1242/dev.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ingram WJ, McCue KI, Tran TH, Hallahan AR, Wainwright BJ. Sonic Hedgehog regulates Hes1 through a novel mechanism that is independent of canonical Notch pathway signalling. Oncogene. 2008;27(10):1489–500. doi: 10.1038/sj.onc.1210767. [DOI] [PubMed] [Google Scholar]
- 52.Aoyama K, Delaney C, Varnum-Finney B, Kohn AD, Moon RT, Bernstein ID. The interaction of the Wnt and Notch pathways modulates natural killer versus T cell differentiation. Stem Cells. 2007;25(10):2488–97. doi: 10.1634/stemcells.2007-0102. [DOI] [PubMed] [Google Scholar]
- 53.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40(2):274–88. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.