Abstract
Objective
Exercise is commonly recommended for patients with osteoarthritis (OA) pain. However, whether exercise is beneficial in ameliorating persistent NSAID-resistant ongoing pain associated with advanced OA is unknown.
Methods
Rats treated with intra-articular monosodium iodoacetate (MIA) or saline underwent treadmill exercise or remained sedentary starting 10 days post-injection. Tactile sensory thresholds and weight bearing were assessed followed by radiographs at weekly intervals. After 4 weeks of exercise, ongoing pain was assessed using conditioned place preference (CPP) to intra-articular or rostral ventromedial medulla (RVM) lidocaine. The possible role of endogenous opioids in exercise-induced pain relief was examined by systemic administration of naloxone. Knee joints were collected for μCT analysis to examine pathological changes to subchondral bone and metaphysis of the tibia.
Results
Treadmill exercise for 4 weeks reversed MIA-induced tactile hypersensitivity and weight asymmetry. Both intra-articular and RVM lidocaine D35 post-MIA induced CPP in sedentary but not exercised MIA-treated rats, indicating that exercise blocks MIA-induced ongoing pain. Naloxone re-established weight asymmetry in MIA-treated rats undergoing exercise and induced conditioned place aversion (CPA), indicating exercise-induced pain relief is dependent on endogenous opioids. Exercise did not alter radiographic evidence of OA. However, μCT analysis indicates that exercise blocked MIA-induced medial, but not lateral subchondral bone loss and trabecular bone loss in the metaphysis.
Conclusion
These findings support the conclusion that exercise induces pain relief in advanced, NSAID resistant OA, likely through increased endogenous opioid signaling. In addition, treadmill exercise blocked MIA-induced bone loss in this model, indicating a potential bone stabilizing effect of exercise on the OA joint.
Keywords: Osteoarthritis, Advanced Osteoarthritis, Opioid, Exercise, Bone Joint Pathology
Osteoarthritis (OA) is the most prevalent chronic joint disease worldwide with incidence predicted to rise due to the aging population and the impact of obesity (1–5). OA is characterized by cartilage degradation and bone remodeling observable as diminished joint space and bony growths within the joint on radiographs (6). μCT analysis of OA joints indicates development of subchondral bone remodeling that corresponds to cartilage loss (7). Notably, the degree of joint pain does not correlate with radiographic evidence of pathology, although other imaging modalities such as MRI demonstrate that development of synovitis correlates with the presence of knee pain even in the absence of radiographic signs of OA (3, 8, 9).
OA can be a debilitating condition, with pain being the most common reason for patients to seek out primary care physicians (10). OA pain is typically characterized by predictable sharp pain associated with movement or use of the joint (10). Some patients develop advanced OA characterized by NSAID-resistant constant dull and aching pain interspersed by short unpredictable episodes of intense pain (10, 11). Current treatments focus on alleviating pain and stiffness (12). Oral NSAIDs or duloxetine are the most common pharmacological treatments (13, 14). Surgery consists primarily of joint replacement options that are successful for most patients (15). However, the prevalence of OA is increasing in younger populations for whom total knee replacement may not be optimal as this procedure was originally designed for patients over 70 years old (15).
Exercise is the most commonly recommended non-pharmacological intervention (14). Clinical studies have demonstrated that aerobic and strengthening exercise improves joint function and pain in OA patients (16). Preclinical studies have demonstrated that exercise across a period of weeks alleviated hypersensitivity in a variety of rodent models of chronic pain (17–24). Blockade of opioid signaling has been shown to reverse exercise-induced pain relief in models of nerve injury (17). The present study examines the hypothesis that treadmill exercise blocks weight asymmetry and ongoing pain through enhanced opioidergic signaling in a rat model of advanced knee OA pain previously shown to produce persistent NSAID resistant ongoing pain (25).
Materials and methods
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, Indiana) weighing 175–200g were housed with a 12 hr light/dark cycle with food and water available ad libitum. Rats were group housed except for rats with cannulation of the rostroventromedial medulla (RVM) that were individually housed starting immediately post-RVM cannulation surgery. All experiments were performed in accordance with policies and recommendations of the International Association for the Study of Pain (IASP) and the National Institutes of Health (NIH) guidelines for the handling and use of laboratory animals. All experimental protocols received approval from the Institutional Animal Care and Use Committee (IACUC) of the University of New England.
Study design
Tactile hypersensitivity and weight asymmetry
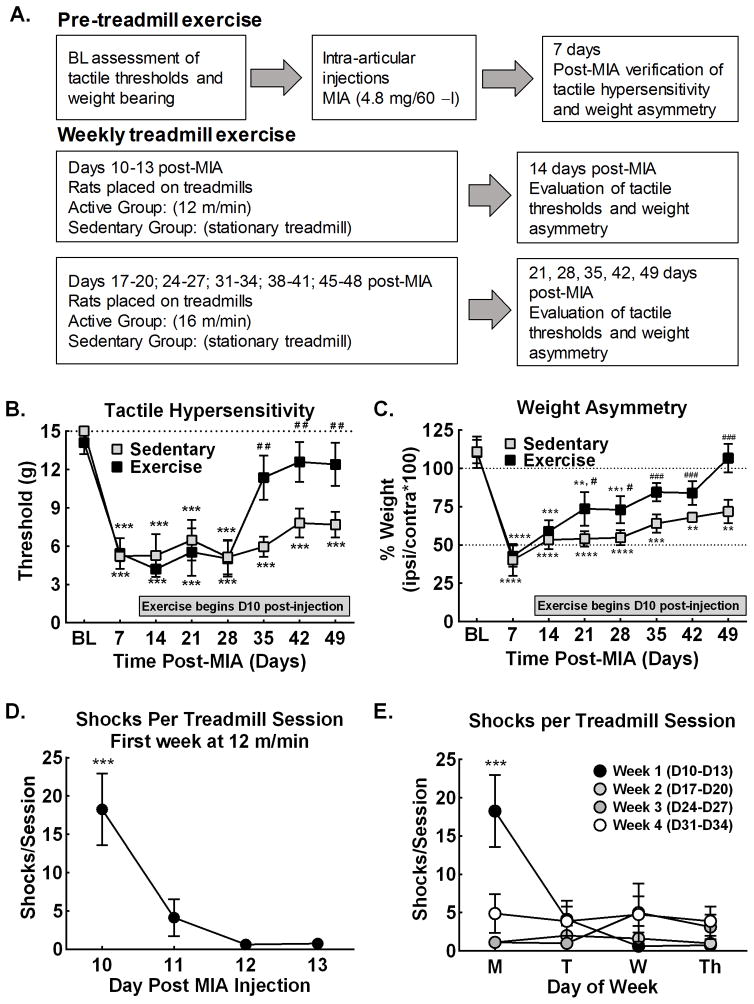
Figure 1A diagrams the protocol used to determine the impact of exercise on MIA-induced tactile hypersensitivity and weight asymmetry. Pre-MIA treatment (BL) assessment of tactile sensory thresholds followed by hindlimb weight bearing was performed. Rats received intra-articular injection of MIA (4.8 mg/60 μl) through the infra-patella ligament of the left knee under isofluorane (2% in O2) as described (25–27). Tactile sensory thresholds followed by weight asymmetry were assessed 7-days post-injection to verify induction of MIA-induced tactile hypersensitivity and weight asymmetry. Starting 10-days after intra-articular injection of MIA or saline, rats were randomly assigned to the exercise or sedentary treatment groups. Treadmill sessions were performed using a 3-lane treadmill (Columbus Instruments International Corporation) for 30 min daily across 4 sequential days as described (17). Treadmill speed was 12 m/min the first week of exercise (post-injection days 10–13) and increased to 16 m/min post-injection starting the second week (days 17–20) and continued through the study. Sedentary controls were placed on the stationary treadmill for 30 min. The treadmill was cleaned with 70% ethanol between every group of rats. Weight bearing followed by tactile sensory thresholds were determined the fifth day of each week, 20–24 hrs following the last treadmill session, and no exercise or behavioral testing occurred days 6–7 each week. This experiment included 6 exercise-MIA and 6 sedentary-MIA treated rats for tactile sensory testing and 11 exercise-MIA and 10 sedentary-MIA treated rats for weight bearing analysis (33 rats total). The experimenters were aware of treatment condition during behavioral testing. Three rats were removed as they failed to show MIA-induced weight asymmetry defined as weight distribution (L/R*100) of 85% or higher and tactile hypersensitivity defined as failure to respond at cut-off von Frey filament of 15 g (3/36 rats).
Figure 1. Treadmill exercise alleviates MIA-induced weight asymmetry and tactile hypersensitivity.
A. Diagram of experimental procedure to examine effects of treadmill exercise on MIA-induced tactile hypersensitivity and weight asymmetry. B. Treadmill exercise starting 10 days post-MIA injection reversed tactile hypersensitivity selectively in exercise treated rats by D35 post-MIA, corresponding to 4 weeks of exercise. *p<0.05, **p<0.01, ***p<0.001 vs BL; ##p<0.01 vs Week 1. C. Treadmill exercise starting 10 days post-MIA injection into the knee joint reversed weight asymmetry in exercise, but not sedentary rats with improvement observed by D21 post-MIA, corresponding to 2 weeks of exercise, and continued improvement D35 through D49 post-MIA. *p<0.05, **p<0.01, ***p<0.001 vs BL, #p<0.05, ##p<0.01, ###p<0.001 vs Wk 1. D. Average number of shocks per treadmill session of MIA treated rats across the first week of treadmill exercise demonstrates that rats quickly adapt to running on the treadmill. Analysis or running across the 4-week treatment, ***p<0.05 vs D11, D12, and D13. E. Average number of shocks per daily treadmill session of MIA treated rats across the 4-week treatment demonstrates that the number of shocks were only elevated the first day of the first week of treadmill training. All graphs represent mean ± SEM.
Conditioned place preference (CPP) measure of ongoing pain
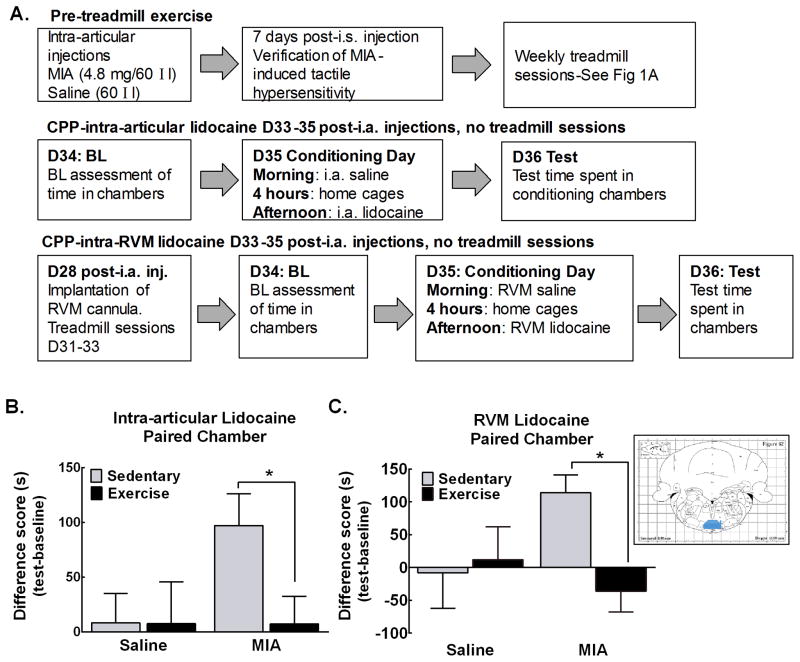
Figure 2A diagrams the protocol used to determine the impact of exercise on MIA-induced ongoing pain. Rats received intra-articular injections of MIA (4.8 mg/60 μl) or equivolume saline followed 7 days later by verification of development of weight asymmetry. Rats that failed to show weight asymmetry were removed from the study (7/75 rats for intra-articular lidocaine; 0/65 rats for RVM lidocaine). Saline and MIA treated rats were randomly divided into the treadmill exercise or sedentary treatment groups resulting in a 2 (saline x MIA) x 2 (exercise x sedentary) experimental design. CPP to pain relief induced by intra-articular or RVM lidocaine was performed using a single trial conditioning protocol days 34–36 post intra-articular injection, corresponding to week 4 of treadmill exercise.
Figure 2. Treadmill exercise alleviates MIA-induced ongoing pain.
A. Diagram of experimental procedure to examine effects of treadmill exercise on MIA-induced ongoing pain using CPP to intra-articular or RVM lidocaine. B. Sedentary MIA treated rats significantly increased time spent in the intra-articular lidocaine paired chamber. Exercised rats treated with MIA failed to show an increase in time spent in the intra-articular lidocaine paired chamber (*p<0.05 vs sedentary MIA treated rats). Rats treated with intra-articular saline did not alter time spent in the intra-articular lidocaine paired chamber. C. Sedentary MIA treated significantly increased time spent in the chamber paired with RVM lidocaine. Exercised MIA treated rats failed to increase time spent in the RVM lidocaine paired chamber (*p<0.05 vs sedentary MIA treated rats). Intra-articular saline treated rats failed to alter time spent in the RVM lidocaine paired chamber irrespective of whether they were sedentary or exercised. All graphs represent mean ± SEM. Inset is a diagram illustrating verification of bilateral cannulation of the RVM. Map from The Rat Brain in Stereotaxic Coordinates, Paxinos and Watson, 1998. Highlighted area (gray) indicates region considered a hit. Ink from one or both needles outside this area were considered misses.
Day 34 (pre-conditioning) - rats were placed into the CPP apparatus with access to all 3 chambers and baseline time spent in each chamber measured across 15 min using webcams and video analysis performed with computer program ANY-maze (Stoelting Co; Wood Dale, Il, USA). Rats spending less than 180 or more than 720 seconds in a pairing chamber during baseline testing were removed from the study (1/55 rats in the intra-articular lidocaine experiment, 9/65 rats in the intra-RVM lidocaine experiment). Drug-chamber pairings were counterbalanced across subjects.
Day 35, single trial conditioning was performed for either the intra-articular lidocaine or the RVM lidocaine experiment as follows:
Intra-articular lidocaine
Rats received intra-articular saline (200 μl) into the ipsilateral knee joint under isoflurane anesthesia and were immediately (within 2 min) confined to the pre-assigned pairing chamber for 30 min. They were then returned to their home cages. Four hours later, rats received intra-articular administration of lidocaine (200 μl of 4% w/v) under isoflurane anesthesia and were immediately confined to the opposite pairing chamber for 30 min. All rats awoke prior to placement into the pairing chambers. A total of 10 saline-sedentary, 9 saline-exercise, 18 MIA-sedentary and 18 MIA-exercise rats were tested.
RVM lidocaine
Rats received RVM microinjection of sterile saline (0.5 μl /min) with the injector remaining in place for 1 min post-injection to prevent backflow. Rats were immediately confined to the pre-assigned pairing chamber for 30 min. They were then returned to their home cages. Four hours later, rats received microinjection of RVM lidocaine (4%w/v /0.5 μl/min) and were immediately confined to the opposite pairing chamber for 30 min. A total of 9 saline-sedentary, 10 saline-exercise, 11 MIA-sedentary and 9 MIA-exercise rats were included in the data analysis following verification of cannula placement. There were 17/56 rats were eliminated from the data analysis due to missed cannula placement).
Day 36, rats were placed in the 3-chamber apparatus with access to all chambers for 15 min and time in each chamber was recorded.
Systemic administration of naloxone on place preference and weight bearing
Following pre-injection assessment of weight bearing, rats received intra-articular injection of MIA and were tested for weight asymmetry weekly throughout the treadmill exercise as described above. On D35, rats underwent pre-naloxone testing followed by injection of naloxone (3 mg/kg, i.p.). Weight bearing measurements were then performed again 30 min post naloxone administration. A total of 6 MIA sedentary and 6 MIA exercise were tested. The experimenter was blinded to the treatment condition during behavioral testing.
A single trial conditioning protocol was performed 34–36 days post intra-articular injection with BL (day 34) and test (day 36) days performed as described above. On day 35 (conditioning day), rats received systemic administration of saline (i.p.) and were immediately confined to the pre-assigned pairing chamber for 30 min. They were returned to their home cages and 4 hours later received systemic administration of naloxone (3 mg/kg, i.p.) followed immediately by confinement to the opposite pairing chamber for 30 min. A total of 12 saline-sedentary, 16 saline-exercise, 20 MIA-sedentary and 15 MIA-exercise rats were tested.
Experimental Procedures
Tactile hypersensitivity
Tactile sensory thresholds were assessed using calibrated von Frey filaments ranging from 0.41–15 g using the up-down method and analyzed using a Dixon nonparametric test and expressed as the mean withdrawal threshold on the ipsilateral hind paw as described (28, 29).
Weight asymmetry
Baseline testing was performed prior to intra-articular MIA injections and at weekly intervals through 49-days post-injection. Changes in hind paw weight distribution between the left (MIA) and right (contralateral) limbs were measured using an incapacitance tester (Stoelting Co; Wood Dale, IL, USA) and used as an index of joint discomfort in the MIA-treated knee (26). Rats were placed in an angled plexiglass chamber positioned so that each hind paw rested on a separate force plate and the force exerted by each hind limb was determined over a 5-s period. Each data point is the mean of 3 readings. As previously described (26), data are normalized as percent injured/non-injured weight bearing, such that sensitivity on the injured side is indicated by values <100%; equal weight distribution is indicated by 100%.
Rostral ventromedial medulla (RVM) cannula surgeries
Rats received RVM cannula surgeries under anesthesia (ketamine–xylazine (100 mg/kg ketamine, 10 mg/kg xylazine, i.p.)) 28 days post-injection using stereotaxic techniques as described (26). Bilateral 26-gauge guide cannulas, separated by 1.2 mm, were directed toward the lateral portions of the RVM (anteroposterior, 11.0 mm from bregma; lateral, ±0.6 mm; dorsoventral, 8.5 mm from the skull according to Paxinos and Watson (30)). Guide cannulas were secured to the skull by small stainless steel machine screws and cemented in place. Animals were allowed to recover 3 days post-surgery before returning to the treadmill for the fourth week of exercise, days 31–33 post-injection. RVM microinjections (0.5 μl) were performed over a period of 1 min through a 33-gauge injector that protruded 1 mm beyond the end of the guide cannula and into fresh tissue to prevent backflow. Cannula placement was verified at the end of the study by microinjection (0.5 μl/1 min) of Evans blue dye (50 mg/ml, Sigma Aldrich; Saint Louis, MO, USA).
Radiograph imaging
Radiograph images were taken weekly following behavioral testing through day 28 post intra-articular injection. Radiographs were not performed during CPP to avoid disruption of the learning process. Rats were lightly anesthetized with a 2% isofluorane/O2 mixture to limit movement during the x-ray imaging and radiographic images of the knee joint were captured using a digital x-ray system (Fujifilm Global).
Microcomputed tomography (μCT)
Following behavioral analyses for the naloxone-induced weight asymmetry experiment (day 42 post-MIA injection), rats were euthanized and knee joints dissected and fixed in 10% neutral buffered formalin for 3 days. They were then transferred to 70% EtOH. To characterize exercise-induced changes in joint micro-architecture, the proximal tibias were analyzed with a SCANCO VivaCT-40 scanner (Scanco Medical AG, Bassersdorf, Switzerland). Joints were loaded into 12.3 mm-diameter scanning tubes. Scans were integrated into three-dimensional voxel images (2048 X 2048 pixel matrices for trabecular and 1024 X 1024 pixel matrices for all other individual planar stacks). Rat tibias were scanned at low resolution, energy level of 55kVp, and intensity of 145 μA. Trabecular bone volume fraction and microarchitecture of the proximal metaphyseal region were evaluated below the growth plate. Approximately 375 consecutive slices were made at 10.5 μm interval at the distal end of the growth plate and extending in a proximal direction, and 250 contiguous slices were selected for analysis. Subchondral trabecular bones were scanned at low resolution, energy level of 55kVp, and intensity of 145 μA at 10.5μm. Subchondral trabecular bone for the medial and lateral tibial plateau were analyzed over 50 cross sections. The VOI included the subchondral trabecular bone starting below the subchondral plate, extending distally towards the growth plate. The images were segmented using a threshold of 260. The following three-dimensional morphometric parameters were calculated for the medial, the lateral and the total of subchondral trabecular bone. A total of 6 joints per group was analyzed for the subchondral bones. For the metaphysis, some samples were not analyzed due to insufficient area of analysis. Sample sizes were 6 MIA-sedentary, 5 saline sedentary, 6 MIA-sedentary and 4 MIA-exercise.
Statistical analysis
All statistical analyses were run using Graphpad Prism. Sample sizes for all experiments were based on those typically reported in the literature. MIA-induced changes in weight bearing and tactile hypersensitivity were assessed using a repeated measures analysis of variance ANOVA (exercise treatment x time), followed by post hoc analysis of exercise effects at each time-point compared to D7 (pre-exercise) using Bonferroni’s multiple comparisons test. Shock data were analyzed using two-way repeated measures ANOVA followed by post hoc analysis using Tukey’s multiple comparisons test.
For the CPP and CPA experiments, the effects of exercise and conditioning chamber were analyzed using the Holm-Sidak method, with alpha=5%. Computations were made using the assumption that all rows are sample from populations with the same scatter (SD). Differences between groups were assessed using difference scores that were calculated as post-conditioning (test) – pre-conditioning (BL) time spent in the drug-paired chamber. Statistical analyses for group comparisons were performed using one-way ANOVA followed by post-hoc analysis using Sidak’s multiple comparisons tests. Analysis of μCT data was performed using two-way ANOVA followed by post hoc analysis using Fisher’s Least Significant Difference tests.
3. Results
3.1 Tactile hypersensitivity and weight asymmetry
MIA-induced tactile hypersensitivity was present 7 days post-MIA and persisted throughout 7-weeks in sedentary rats (Fig. 1B). Exercise reversed the MIA-induced tactile hypersensitivity with withdrawal thresholds returning to pre-MIA values 35-days post-injection, corresponding to 4 weeks of exercise (Fig. 1B). MIA-induced weight asymmetry was present 7 days post-MIA injection and persisted throughout 7 weeks in sedentary rats (Fig. 1C). Exercise reversed MIA-induced weight asymmetry with significant improvement by day 21 post-MIA and continued improvement observed between days 35–49 post-MIA. MIA treated rats show greatly diminished exposure to the shock pad after the first day on the treadmill (Fig. 1D). Analysis of number of shocks per weekday across 4 weeks shows elevated number of shocks on the first day week 1 only of treadmill exercise (Fig. 1E).
3.2 Exercise blocks MIA-induced ongoing pain
Intra-articular lidocaine produced CPP selectively in the sedentary MIA treated rats but not in exercised MIA treated rats (Fig. 2B). Intra-articular lidocaine did not alter time spent in the lidocaine paired chamber in sedentary or exercised rats that had received intra-articular saline. RVM lidocaine also produced CPP in the sedentary MIA treated rats but not in MIA exercise rats (Fig. 2C). RVM lidocaine failed to alter time spent in the lidocaine paired chambers in the intra-articular saline treated rats. Any rats with missed injections as defined by ink marks outside the RVM (Fig. 2C-inset) were excluded from data analysis.
3.3 Naloxone induces CPA and unmasks weight asymmetry
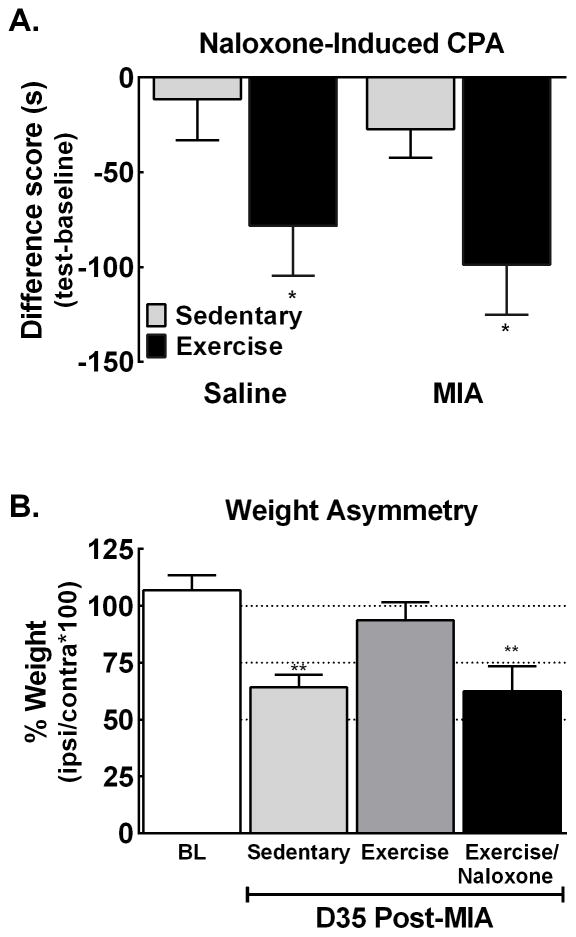
MIA induced weight asymmetry was present in sedentary rats 35 days post-MIA. MIA-treated rats that had undergone exercise for 4 weeks demonstrated normal weight bearing with equivalent weight bearing ratios to pre-MIA baseline values (p>0.05). Systemic naloxone (3 mg/kg, i.p.) unmasked weight asymmetry in MIA-treated exercised rats, with values returning to sedentary controls (Fig. 3A). To examine whether blocking endogenous opioid signaling also unmasks ongoing pain, we determined whether exercised rats that had received intra-articular MIA showed CPA to a context paired with naloxone (3 mg/kg, i.p.). Naloxone produced CPA in rats that had undergone 4 weeks of exercise but not in sedentary rats (Fig. 3B). Of interest, the naloxone-induced CPA was observed in exercised rats irrespective of whether they had received intra-articular saline or MIA (Fig. 3B).
Figure 3. Treadmill exercise across 4 weeks starting 10-days post-injection increases opioid tone.
A. MIA-induced weight asymmetry that persisted across 35 days in sedentary rats. Exercise starting 10 days post-MIA injection blocked the MIA-induced weight asymmetry. Naloxone (3 mg/kg i.p.) re-established the weight asymmetry at 30 min post-naloxone in the exercised rats. **p<0.01 vs BL, n=6 MIA sedentary, 5 MIA exercise. B. Analysis of difference scores confirms that exercised rats demonstrate equivalent decreases in time spent in the naloxone-paired chamber whereas sedentary rats failed to show significant CPA irrespective of whether they received intra-articular saline or intra-articular MIA. *p<0.05 vs sedentary rats. All graphs represent mean ± SEM.
3.4 Radiograph images indicate no effects of exercise on MIA-induced joint pathology
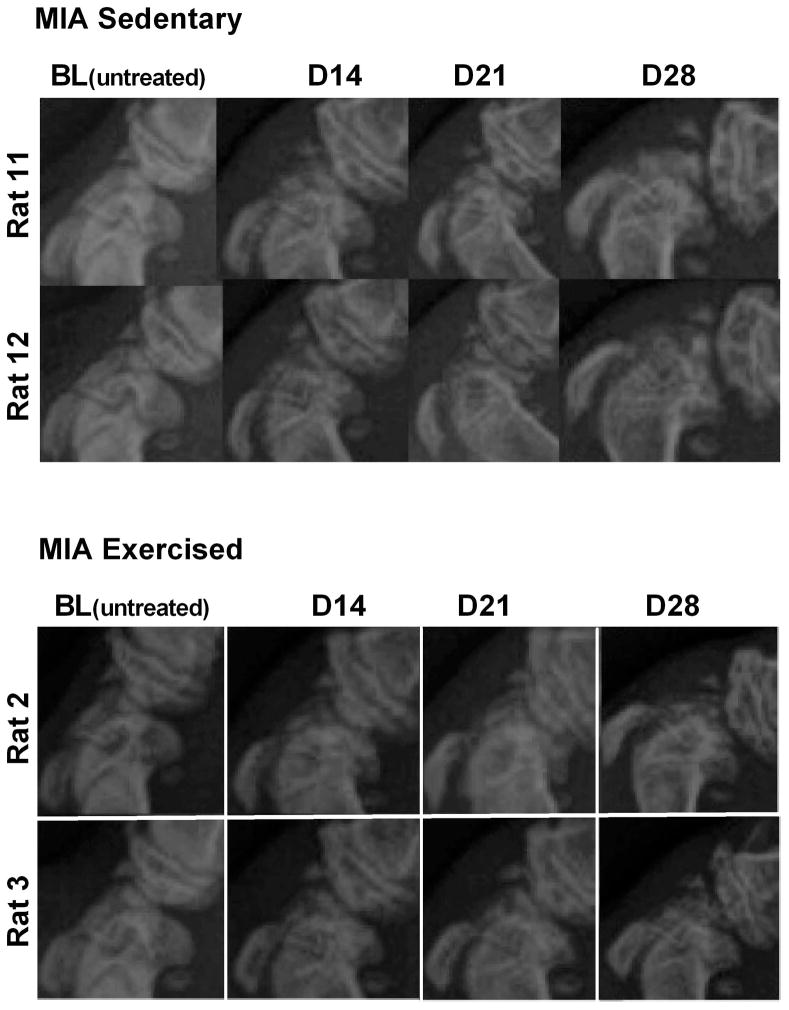
Radiographs of the femorotibial (knee) joint at weekly intervals demonstrate a time-dependent progression of MIA-induced joint pathology in both sedentary and exercise treated groups (Fig. 4). There were no apparent differences between exercise and sedentary rats.
Figure 4. Representative radiographs of sedentary or exercised MIA treated rats.
Joint pathology was evident within 14 days post-MIA injection in both treatment groups. Radiographs indicate equivalent levels of MIA-induced joint pathology irrespective of whether the rats remained sedentary or underwent exercise.
3.5 μCT analysis demonstrates exercise-induced blockade of MIA-induced bone remodeling
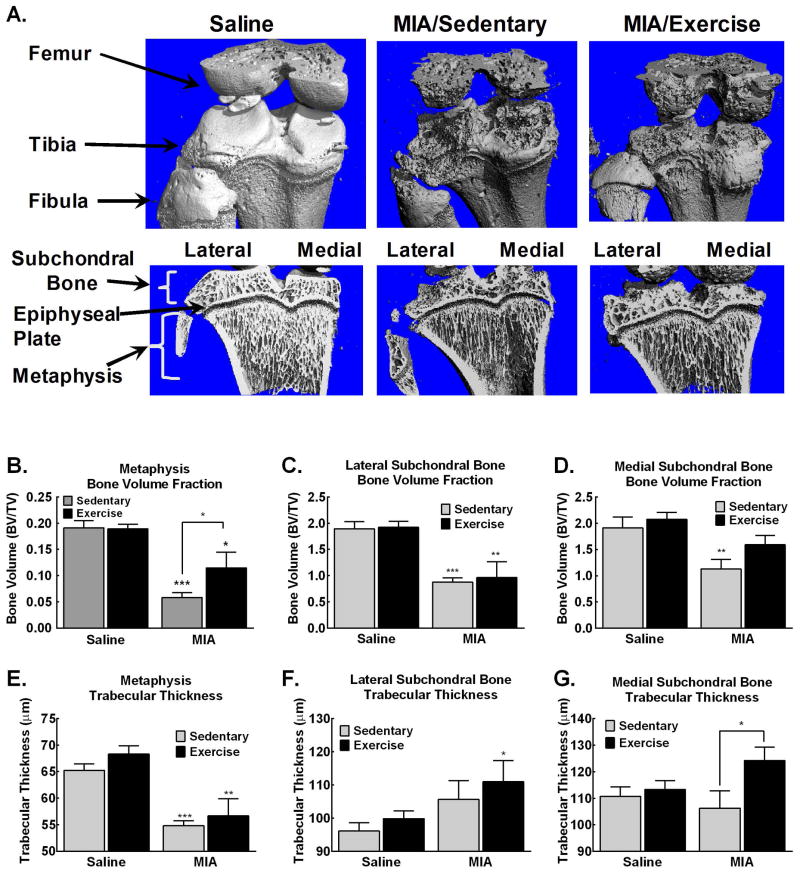
Three-dimensional images from μCT scans reveal extensive bone remodeling within the femorotibial joint, with clear development of osteophytes (bone spurs) as well as apparent pitting and lesions of the cortical bone (Fig. 5A, top panel). Reconstructed images showing trabecular bone within the subchondral and metaphysis reveals diminished trabecular bone, particularly in the metaphysis in sedentary MIA treated rats (Fig. 5A, bottom panel). Exercise appears to ameliorate the MIA-induced trabecular bone loss within the metaphysis (Fig. 5A, bottom panel).
Figure 5. MIA-induced pathological changes in the subchondral and metaphysis are blocked by treadmill exercise.
A. Representative images demonstrate MIA-induced bone remodeling of the exterior bone, with development of osteophytes and bone deformities. Trabecular bone loss is observed within subchondral bone and metaphysis. B. Significant reduction in bone volume is observed in the medial subchondral bone of MIA treated sedentary, but not MIA treated exercise rats. C. Significant reduction in bone volume is observed in the lateral subchondral bone of MIA treated sedentary and exercise treated rats. D. Diminished bone volume is observed in the trabecular region of the metaphysis in the MIA treated sedentary rats that is attenuated in the MIA treated rats that underwent treadmill exercise. E. Significant reduction of trabecular bone thickness is observed in the metaphysis of in MIA treated rats. F. Increased trabecular thickness is observed in the lateral subchondral bone of MIA treated rats. G. Increased trabecular thickness within the medial subchondral bone is observed in MIA treated rats that underwent exercise across 4 weeks. *p<0.05, **p<0.01, ***p<0.001 vs saline sedentary.
Quantitative analysis of bone volume demonstrates that MIA induced pathological changes in sedentary rats both in the metaphysis just below the epiphyseal plate and both the lateral and medial subchondral bone (Fig. 5B–D). Within the metaphysis, MIA diminished total trabecular bone volume in sedentary rats that was attenuated in exercised MIA treated rats (Fig. 5B). MIA treated rats demonstrated a significant decrease in bone volume of the lateral subchondral bone measured 35 days post-MIA that is not altered by exercise (Fig. 5C). Sedentary MIA treated rats also had decreased trabecular bone volume in the medial subchondral bone (Fig 5D). This MIA-induced bone loss was blocked by exercise (Fig. 5D). MIA diminished trabecular thickness within the metaphasis of sedentary and exercised rats (Fig 5E). In the medial subchondral bone, MIA-induced increased trabecular thickness in exercised rats compared to saline treated rats (Fig 5F). Similarly, a significant increase in trabecular thickness was observed in the lateral subchondral bone of exercised MIA treated rats compared to sedentary saline control rats (Fig 5G).
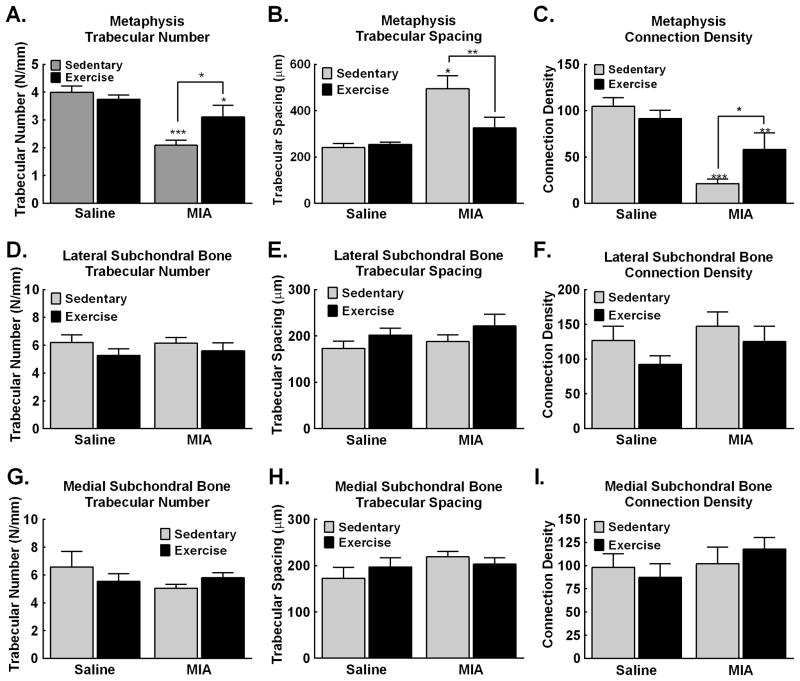
Within the metaphysis, MIA reduced the number of trabecular bone and increased trabecular spacing in sedentary rats that was blocked by 4 weeks of exercise (Fig. 6A,B respectively). MIA reduced connectivity density of trabecular bone in sedentary rats that was also blocked by the 4 weeks of exercise (Fig. 6C). Within the lateral and medial subchondral bone, no changes in trabecular number (Fig. 6D,G), trabecular spacing (Fig. 6E,H) or of connection density (Fig. 6F,I) was observed in sedentary or exercised MIA treated rats.
Figure 6. MicroCT analysis of the tibia demonstrates that MIA-induced pathological changes in the subchondral and metaphysis are blocked by treadmill exercise.
A. Analysis of the number of trabeculae within the metaphysis demonstrates decreased trabeculae in MIA treated sedentary rats. Exercise blocked the MIA-induced decrease in trabecular number, but the number was still significantly lower compared to saline sedentary control rats. B. Analysis of trabecular spacing demonstrates increased space between trabecular bone within the metaphysis in the sedentary MIA treated rats. Exercise blocked the MIA-induced increase in trabecular spacing, with trabecular spacing similar to saline sedentary control rats. C. MIA reduced connection density of trabecular bone within the metaphysis. Exercise attenuated the MIA induced reduction in connection density of trabecular bone. D–F. MIA and exercise both failed to alter the number (D), spacing (E) or connection density (F) of the trabecular bone within the lateral subchondral bone. G–I. MIA and exercise both failed to alter the number (G), spacing (H) or connection density (I) of the trabecular bone within the medial subchondral bone. Graphs represent mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 vs saline sedentary.
Discussion
We demonstrate that treadmill exercise for 4 weeks alleviates ongoing pain, restores weight bearing on the osteoarthritic knee, and alleviates hindpaw tactile hypersensitivity in this rat model of advanced OA pain. The exercise-induced pain relief is dependent on endogenous opioids, illustrated by the ability of systemic administration of the opioid receptor antagonist naloxone to unmask weight asymmetry and produce CPA in exercised rats that had OA. Naloxone also produced CPA in control rats that had received intra-articular saline, indicating that exercise increases endogenous opioid signaling in the absence of joint pathology and that blocking this signal is aversive. Exercise also attenuated MIA-induced bone remodeling within the subchondral bone and metaphysis, indicating a potentially stabilizing effect of exercise. Notably, our observations that naloxone unmasked pain behaviors in the exercise treated rats indicates that these protective effects of exercise on the bone were likely independent of the pain alleviating effects of exercise.
Our observation that 4 weeks of treadmill exercise reversed OA-induced tactile hypersensitivity and weight asymmetry are consistent with clinical studies demonstrating that exercise can improve joint function and diminish pain in OA patients (16). The reversal of weight asymmetry indicates an increased willingness of MIA treated rats that had undergone exercise to load the joint, potentially reflecting improved use and function. Analysis of joint use and weight bearing while running warrants further study. We further demonstrated that exercise reversed MIA-induced ongoing pain. Intra-articular lidocaine produced CPP in sedentary MIA treated rats, consistent with our previous observations that this concentration of MIA produces ongoing pain dependent on peripheral input from the joint (25). In contrast, lidocaine failed to induce CPP in exercised rats indicating that the exercise blocked ongoing pain following MIA pain. This conclusion is further supported by the observation that RVM lidocaine produces CPP in sedentary, but not exercised MIA treated rats. RVM lidocaine induced CPP in sedentary rats is consistent with our previous observations that blockade of descending, presumably facilitatory, output from the RVM induces CPP and supports the conclusion that advanced OA pain is dependent on descending pain facilitatory pathways (9, 26). Together, these data indicate that MIA-induced knee joint pain is reversed by 4 weeks of treadmill exercise. An important aspect of these observations is that lidocaine administration to the knee joint or RVM failed to alter the time spent in the lidocaine paired chamber in sham rats irrespective of treatment group. This suggests that lidocaine-induced CPP is specific to relief of OA-induced ongoing pain.
These findings suggest that exercise-induced pain relief may be observed in patients that develop NSAID resistant persistent ongoing joint pain in this model of OA. Development of moderate to severe pain in advanced OA has been suggested to be due to central sensitization and to neuropathic pain likely arising from damage to nerves (9, 31–33). Several studies have demonstrated signs of central sensitization in preclinical studies of MIA-induced joint pain (9, 26, 34–36). Within the spinal cord, electrophysiological studies have demonstrated enhanced evoked responses of dorsal horn neurons in response to pinch, and noxious mechanical stimuli (34). Others report that rats with MIA-induced knee joint osteoarthritis show glial activation in the spinal cord (35), a marker of spinal sensitization associated with release of pro-inflammatory cytokines and BDNF (37). Whether exercise-induced pain relief may be due in part to suppression of pro-inflammatory factors released by spinal glia remains to be determined. Supporting this possibility, several studies have reported that exercise reduced pro-inflammatory factors including BDNF, glial activation, macrophage infiltration and pro-inflammatory cytokines within the spinal cord in rat models of neuropathic pain (38–40). Further, exercise might be beneficial in that it diminishes pro-inflammatory cytokines within the DRG and the brain (39). Future studies are needed to determine whether exercise decreases central microglial activation and associated pro-inflammatory factors that promote chronic pain.
Clinical observations indicate elevated levels of the endogenous opioid β-endorphin in runners (41). Animal studies have demonstrated elevated of endogenous opioids in the serum (42) and brain regions (43) of rats including elevated β-endorphin and met-enkephalin within the RVM and PAG (17), key components of the descending pain modulatory pathways (44). Endogenous opioids may block pain through multiple mechanisms including actions on opioid mu receptors in the cortex as well as in subcortical regions, the spinal cord and the periphery (44). Moreover, spinal naloxone was demonstrated to block analgesia in Nav1.7 mutant mice, suggesting tonic opioid signaling can maintain analgesia at the level of the spinal cord (45). The release of endogenous opioids in the anterior cingulate cortex has been suggested as a general mechanism of pain relief, consistent with the present observations (46). Endogenous opioids may also improve both pain and bone remodeling through indirect actions by blocking pro-inflammatory responses by glia or peripheral immune cells (39, 47). Our observations that naloxone blocked the exercise-induced weight asymmetry is consistent with other studies demonstrating that naloxone blocked exercise-induced reversal of thermal and tactile hypersensitivity in models of nerve injury and muscle pain (17, 20). Previous studies have indicated that this is likely through descending pain inhibitory pathways from the RVM as administration of a peripherally restricted antagonist failed to block the exercise induced blockade of evoked hypersensitivity and RVM naloxone re-established the nerve-injury induced thermal and tactile hypersensitivity (17). These observations suggest that exercise normalizes the balance of descending pain modulation overcoming OA-induced net descending pain facilitatory drive. RVM lidocaine in the MIA exercised rats failed to induce CPA, likely because lidocaine inactivates both descending pain inhibitory and facilitatory pathways within the RVM (44).
In addition to re-establishing weight asymmetry, naloxone produced CPA in MIA exercised rats. This is consistent with the hypothesis that blockade of the opioid signaling unmasks ongoing pain thereby creating an aversion to the naloxone paired chamber. However, naloxone also produced CPA in the exercised control rats that did not have knee OA. These observations indicate that treadmill exercise produces a tonic endogenous opioid signaling that, when blocked, creates a contrasting aversive state. This is consistent with observations that exercise stimulates the release of endogenous opioid peptides and increases nociceptive thresholds in human and animal studies (48). In addition, chronic long-term exercise has been demonstrated to decrease sensitivity to morphine and other MOR agonists suggesting that exercise may lead to cross-tolerance to exogenously administered opioid agonists and dependence (48). Supporting this, naloxone has been reported to precipitate a mild withdrawal syndrome in exercise treated, but not sedentary rats (48). The CPA observed in MIA treated rats may result from unmasking knee joint pain a conclusion supported by the observation that naloxone also produces weight asymmetry. However, we acknowledge that other, non-pain associated factors may contribute to the naloxone induced CPA.
Preclinical studies have demonstrated that MIA causes time- and concentration-dependent cartilage loss (25, 49) and progressive changes to subchondral bone in a time-dependent manner (50–52). μCT analysis of tibias indicates that treadmill exercise blocked MIA-induced trabecular bone loss. Thus, exercise has protective effects on trabecular bone degradation in this model of advanced OA pain. Our data are consistent with reports that interval-training on a treadmill prevented MIA-induced decreased bone mineral density in the proximal tibia (52). Moreover, these data are consistent with other observations that slight to moderate, but not intense exercise has a protective effect joint pathology in a surgical model of OA (53). The potential protective effects of exercise on bone remodeling and joint pathology supports the conclusion of a recent meta-analysis indicating that running had protective effects against knee joint surgery for OA (54).
Our data demonstrate that naloxone re-established pain behaviors characteristic of OA pain indicating that the protective effects of exercise on bone remodeling within the tibia are likely to be independent of the pain alleviating effects of exercise. The mechanism by which exercise ameliorates degeneration of trabecular bone deserves further study. Potential contributing factors may include anti-inflammatory effects of endogenous opioids as indicated by the anti-inflammatory effects of met-enkephalin demonstrated in a model of inflammation associated arthritis (47). In relation to exercise, both clinical and preclinical studies have demonstrated that regular exercise diminishes pro-inflammatory markers in peripheral tissue (39). Whether endogenous opioids mediate potential exercise associated anti-inflammatory effects that diminishes advanced OA-induced bone remodeling and joint pain deserves further exploration. It should be noted that other factors, such as potential increased weight bearing on the joint during exercise, may also contribute to the protective effects observed in the treadmill exercised rats. In addition, future studies examining other aspects of joint function and use, such as joint stiffness and range of motion, are warranted to further explore potential protective effects of exercise on the joint.
In conclusion, our studies indicate that exercise may improve pain in patients with advanced OA, resulting in an overall improvement in the quality of life of these patients. In addition, the protective effects of exercise on OA associated bone remodeling may be able to prevent or delay joint replacement therapy for OA patients. Together these studies indicate potential protective effects of exercise in advanced OA joint pathology.
Acknowledgments
Statement of funding: This work supported by a COBRE grant (P20GM103643: Project PI: T. King; COBRE PI: I. Meng). μCT was performed within the Transgenic and In Vivo Imaging Core Facility was supported by NIH award 5P30GM103392 COBRE in Vascular Biology (PI: R. E. Friesel) to the Maine Medical Center Research Institute.
Footnotes
Conflict of interest: The authors have no conflict of interest
References
- 1.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33(11):2271–9. [PubMed] [Google Scholar]
- 2.Hugle T, Geurts J, Nuesch C, Muller-Gerbl M, Valderrabano V. Aging and Osteoarthritis: An Inevitable Encounter? Journal of Aging Research. 2012;2012:7. doi: 10.1155/2012/950192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of OA and the genesis of pain. Rheumatic diseases clinics of North America. 2008;34(3):623–43. doi: 10.1016/j.rdc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Annals of Physical and Rehabilitation Medicine. 2016;59(5–6):333–9. doi: 10.1016/j.rehab.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Neogi T, Zhang Y. Epidemiology of OA. Rheumatic diseases clinics of North America. 2013;39(1):1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane NE, Brandt K, Hawker G, Peeva E, Schreyer E, Tsuji W, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19(5):478–82. doi: 10.1016/j.joca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Finnila MA, Thevenot J, Aho OM, Tiitu V, Rautiainen J, Kauppinen S, et al. Association between subchondral bone structure and osteoarthritis histopathological grade. J Orthop Res. 2016 doi: 10.1002/jor.23312. epub prior to print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66(12):1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nature reviews Rheumatology. 2014;10(6):374–80. doi: 10.1038/nrrheum.2014.47. [DOI] [PubMed] [Google Scholar]
- 10.Hawker GA, Stewart L, French MR, Cibere J, Jordan JM, March L, et al. Understanding the pain experience in hip and knee osteoarthritis--an OARSI/OMERACT initiative. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16(4):415–22. doi: 10.1016/j.joca.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Liu A, Kendzerska T, Stanaitis I, Hawker G. The relationship between knee pain characteristics and symptom state acceptability in people with knee osteoarthritis. Osteoarthritis and Cartilage. 2014;22(2):178–83. doi: 10.1016/j.joca.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–26. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 13.Maserejian NN, Fischer MA, Trachtenberg FL, Yu J, Marceau LD, McKinlay JB, et al. Variations Among Primary Care Physicians in Exercise Advice, Imaging, and Analgesics for Musculoskeletal Pain: Results From a Factorial Experiment. Arthritis Care & Research. 2014;66(1):147–56. doi: 10.1002/acr.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2014;22(3):363–88. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Practice & Research Clinical Rheumatology. 2012;26(5):637–47. doi: 10.1016/j.berh.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Semanik PA, Chang RW, Dunlop DD. Aerobic activity in prevention and symptom control of osteoarthritis. PM & R : the journal of injury, function, and rehabilitation. 2012;4(5 Suppl):S37–44. doi: 10.1016/j.pmrj.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, et al. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114(4):940–8. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung A, Gregory NS, Allen LA, Sluka KA. Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain. 2016;157(1):70–9. doi: 10.1097/j.pain.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabharwal R, Rasmussen L, Sluka KA, Chapleau MW. Exercise prevents development of autonomic dysregulation and hyperalgesia in a mouse model of chronic muscle pain. Pain. 2016;157(2):387–98. doi: 10.1097/j.pain.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bement MK, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Archives of physical medicine and rehabilitation. 2005;86(9):1736–40. doi: 10.1016/j.apmr.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Sluka KA, Danielson J, Rasmussen L, DaSilva LF. Exercise-induced pain requires NMDA receptor activation in the medullary raphe nuclei. Med Sci Sports Exerc. 2012;44(3):420–7. doi: 10.1249/MSS.0b013e31822f490e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YW, Hsieh PL, Chen YC, Hung CH, Cheng JT. Physical exercise induces excess hsp72 expression and delays the development of hyperalgesia and allodynia in painful diabetic neuropathy rats. Anesth Analg. 2013;116(2):482–90. doi: 10.1213/ANE.0b013e318274e4a0. [DOI] [PubMed] [Google Scholar]
- 23.Shankarappa SA, Piedras-Renteria ES, Stubbs EB., Jr Forced-exercise delays neuropathic pain in experimental diabetes: effects on voltage-activated calcium channels. J Neurochem. 2011;118(2):224–36. doi: 10.1111/j.1471-4159.2011.07302.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. The journal of pain : official journal of the American Pain Society. 2007;8(12):989–97. doi: 10.1016/j.jpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, et al. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153(4):924–33. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havelin J, Imbert I, Cormier J, Allen J, Porreca F, King T. Central Sensitization and Neuropathic Features of Ongoing Pain in a Rat Model of Advanced Osteoarthritis. The journal of pain : official journal of the American Pain Society. 2016;17(3):374–82. doi: 10.1016/j.jpain.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, et al. Ongoing pain in the MIA model of osteoarthritis. Neuroscience letters. 2011;493(3):72–5. doi: 10.1016/j.neulet.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 29.Xie JY, Qu C, Patwardhan A, Ossipov MH, Navratilova E, Becerra L, et al. Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: a potential biomarker of efficacy. Pain. 2014;155(8):1659–66. doi: 10.1016/j.pain.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 31.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–81. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Schaible H-G. Mechanisms of Chronic Pain in Osteoarthritis. Curr Rheumatol Rep. 2012;14(6):549–56. doi: 10.1007/s11926-012-0279-x. [DOI] [PubMed] [Google Scholar]
- 33.Thakur M, Rahman W, Hobbs C, Dickenson AH, Bennett DLH. Characterisation of a Peripheral Neuropathic Component of the Rat Monoiodoacetate Model of Osteoarthritis. PLoS ONE. 2012;7(3):e33730. doi: 10.1371/journal.pone.0033730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey VL, Dickenson AH. Behavioural and Electrophysiological Characterisation of Experimentally Induced Osteoarthritis and Neuropathy in C57Bl/6 Mice. Mol Pain. 2009:5. doi: 10.1186/1744-8069-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sagar DR, Burston JJ, Hathway GJ, Woodhams SG, Pearson RG, Bennett AJ, et al. The Contribution of Spinal Glial Cells to Chronic Pain Behaviour in the Monosodium Iodoacetate Model of Osteoarthritic Pain. Mol Pain. 2011:7. doi: 10.1186/1744-8069-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman W, Bauer CS, Bannister K, Vonsy JL, Dolphin AC, Dickenson AH. Descending serotonergic facilitation and the antinociceptive effects of pregabalin in a rat model of osteoarthritic pain. Mol Pain. 2009;5:45. doi: 10.1186/1744-8069-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuda M, Beggs S, Salter MW, Inoue K. Microglia and intractable chronic pain. Glia. 2013;61(1):55–61. doi: 10.1002/glia.22379. [DOI] [PubMed] [Google Scholar]
- 38.Almeida C, DeMaman A, Kusuda R, Cadetti F, Ravanelli MI, Queiroz AL, et al. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain. 2015;156(3):504–13. doi: 10.1097/01.j.pain.0000460339.23976.12. [DOI] [PubMed] [Google Scholar]
- 39.Cooper MA, Kluding PM, Wright DE. Emerging Relationships between Exercise, Sensory Nerves, and Neuropathic Pain. Front Neurosci. 2016;10:372. doi: 10.3389/fnins.2016.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grace PM, Fabisiak TJ, Green-Fulgham SM, Anderson ND, Strand KA, Kwilasz AJ, et al. Prior voluntary wheel running attenuates neuropathic pain. Pain. 2016;157(9):2012–23. doi: 10.1097/j.pain.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colt EWD, Wardlaw SL, Frantz AG. The effect of running on plasma β-endorphin. Life Sciences. 1981;28(14):1637–40. doi: 10.1016/0024-3205(81)90319-2. [DOI] [PubMed] [Google Scholar]
- 42.Debruille C, Luyckx M, Ballester L, Brunet C, Odou P, Dine T, et al. Serum opioid activity after physical exercise in rats. Physiological research. 1999;48(2):129–33. [PubMed] [Google Scholar]
- 43.Blake MJ, Stein EA, Vomachka AJ. Effects of exercise training on brain opioid peptides and serum LH in female rats. Peptides. 1984;5(5):953–8. doi: 10.1016/0196-9781(84)90122-0. [DOI] [PubMed] [Google Scholar]
- 44.Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Current opinion in supportive and palliative care. 2014;8(2):143–51. doi: 10.1097/SPC.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minett MS, Pereira V, Sikandar S, Matsuyama A, Lolignier S, Kanellopoulos AH, et al. Endogenous opioids contribute to insensitivity to pain in humans and mice lacking sodium channel Nav1.7. Nat Commun. 2015;6:8967. doi: 10.1038/ncomms9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, et al. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(18):7264–71. doi: 10.1523/JNEUROSCI.3862-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Y, McNearney TA, Wilson SP, Yeomans DC, Westlund KN. Joint capsule treatment with enkephalin-encoding HSV-1 recombinant vector reduces inflammatory damage and behavioural sequelae in rat CFA monoarthritis. The European journal of neuroscience. 2008;27(5):1153–65. doi: 10.1111/j.1460-9568.2008.06076.x. [DOI] [PubMed] [Google Scholar]
- 48.Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu-opioid tolerance and physical dependence. Psychopharmacology (Berl) 2003;168(4):426–34. doi: 10.1007/s00213-003-1471-5. [DOI] [PubMed] [Google Scholar]
- 49.Pomonis JD, Boulet JM, Gottshall SL, Phillips S, Sellers R, Bunton T, et al. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain. 2005;114(3):339–46. doi: 10.1016/j.pain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Morenko BJ, Bove SE, Chen L, Guzman RE, Juneau P, Bocan TM, et al. In vivo micro computed tomography of subchondral bone in the rat after intra-articular administration of monosodium iodoacetate. Contemp Top Lab Anim Sci. 2004;43(1):39–43. [PubMed] [Google Scholar]
- 51.Xie L, Lin AS, Kundu K, Levenston ME, Murthy N, Guldberg RE. Quantitative imaging of cartilage and bone morphology, reactive oxygen species, and vascularization in a rodent model of osteoarthritis. Arthritis and rheumatism. 2012;64(6):1899–908. doi: 10.1002/art.34370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boudenot A, Presle N, Uzbekov R, Toumi H, Pallu S, Lespessailles E. Effect of interval-training exercise on subchondral bone in a chemically-induced osteoarthritis model. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2014;22(8):1176–85. doi: 10.1016/j.joca.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 53.Galois L, Etienne S, Grossin L, Watrin-Pinzano A, Cournil-Henrionnet C, Loeuille D, et al. Dose-response relationship for exercise on severity of experimental osteoarthritis in rats: a pilot study. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2004;12(10):779–86. doi: 10.1016/j.joca.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Timmins KA, Leech RD, Batt ME, Edwards KL. Running and Knee Osteoarthritis: A Systematic Review and Meta-analysis. The American Journal of Sports Medicine. 2016 doi: 10.1177/0363546516657531. epub prior to print. [DOI] [PubMed] [Google Scholar]