Abstract
Background
Nitroglycerin (also known as glyceryl trinitrate (GTN)), a vasodilator best known for treatment of ischemic heart disease, has also been investigated for its potential therapeutic benefit in ischemic stroke. The completed Efficacy of Nitric Oxide in Stroke trial suggested that GTN has therapeutic benefit with acute (within 6 hours) transdermal systemic sustained release therapy.
Objective
To examine an alternative use of GTN as an acute therapy for ischemic stroke following successful recanalization.
Methods
We administered GTN IA following transient middle cerebral artery occlusion in mice. Because no standard dose of GTN is available following emergent large vessel occlusion, we performed a dose–response (3.12, 6.25, 12.5, and 25 μg/μL) analysis. Next, we looked at blood perfusion (flow) through the middle cerebral artery using laser Doppler flowmetry. Functional outcomes, including forced motor movement rotor rod, were assessed in the 3.12, 6.25, and 12.5 μg/μL groups. Histological analysis was performed using cresyl violet for infarct volume, and glial fibrillary activating protein (GFAP) and NeuN immunohistochemistry for astrocyte activation and mature neuron survival, respectively.
Results
Overall, we found that acute post-stroke IA GTN had little effect on vessel dilatation after 15 min. Functional analysis showed a significant difference between GTN (3.12 and 6.25 μg/μL) and control at post-stroke day 1. Histological measures showed a significant reduction in infarct volume and GFAP immunoreactivity and a significant increase in NeuN.
Conclusions
These results demonstrate that acute IA GTN is neuroprotective in experimental ischemic stroke and warrants further study as a potentially new stroke therapy.
INTRODUCTION
Stroke, disruption of blood flow to the brain due to vascular occlusion (ischemic) or bleeding (hemorrhagic), is the second leading cause of death worldwide and a leading cause of long-term disability.1 Emergent large vessel occlusion (ELVO) is the most life-threatening and disabling type of ischemic stroke. Treatment options for ELVO consist of endovascular thrombectomy (ET) and/or IV tissue plasminogen activator (t-PA), but not all patients are eligible owing to exclusion criteria.2–4 Furthermore, while ET and IV t-PA have improved patient survival, there is a need for adjunctive therapies to be used in tandem with or as a standalone treatment following successful vessel recanalization.5 Any potential pharmacotherapy administration may be most successful and cause fewer systemic side effects if it is targeted at the site of ischemia. We hypothesize that improved treatment for ELVO might include rapid recanalization of the occluded vessel via ET combined with directed acute pharmacotherapy. To test such a targeted therapeutic approach, our laboratory uses an IA experimental model of pharmacotherapy administration in combination with transient tandem ipsilateral common carotid artery (CCA) and middle cerebral artery (MCA) occlusion, hereafter referred to as MCA occlusion (MCAo).6
Using the already exposed vasculature of the MCAo, we can selectively administer our preferred drug IA, further mimicking the human clinical condition of pharmacotherapy delivery. Combining the two methods allows us to mimic an acute ischemic stroke but also guarantee successful recanalization followed by drug delivery in a timely manner similar to ET. Thus far, we have successfully applied this experimental therapeutic approach to the administration of verapamil, demonstrating an 86% reduction in infarct volume and significant increase in functional outcome.5 These results led us to conclude that selectively administering verapamil IA was safe and neuroprotective.
In this study, we chose to investigate the therapeutic potential of acute IA administration of nitroglycerin (glyceryl trinitrate (GTN)) in our experimental stroke model. GTN is a Food and Drug Administration approved vasodilator (angina pectoris, high blood pressure) that was recently studied in the Efficacy of Nitric Oxide in Stroke (ENOS) ischemic stroke clinical trial.7–9 That study investigated the potential of GTN to reduce blood pressure and improve functional outcome following acute ischemic stroke with treatment through a systemic sustained release transdermal patch. Results showed a reduction in blood pressure on post-stroke days (PSDs) 1 and 7 but no improvement in functional outcome at day 90.7,9,10 Individuals with a stroke are at an increased risk of increased intracranial pressure; a reduction in intracranial pressure either through direct (IA) or indirect (patch) administration to the cerebrovasculature could improve outcome.11 The ENOS trial acutely reduced blood pressure and by doing so it was hoped that it would have a neuroprotective effect but it failed to show improvement beyond blood pressure reduction. These results could be directly tied to GTN’s short half-life and inability to reach the cerebrovasculature if applied as a patch on the arm or chest.
MATERIAL AND METHODS
Experiments were performed in accordance with the Institutional Animal Care and Use Committee at the University of Kentucky and ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.12 In short, 16-week-old C57/Bl6 (25–30 g) male mice ( Jackson Laboratories) were given GTN (American Reagents) diluted in vehicle (water 39.5%, ethyl alcohol 30%, propylene glycol 30%) or vehicle only IA 5 min following transient MCAo. Animals were randomly grouped into naïve (age-matched littermate controls), control (MCAo surgery with IA injection of vehicle through the internal carotid artery (ICA)), and treated (MCAo surgery with IA injection of 3.12, 6.25, 12.5, 25 and 50 μg/μL GTN through the ICA). Increasing doses of GTN were administered to determine safety and efficacy, as there is no standard dose of IA GTN. Previous flow rate and injection volume studies determined the optimal flow rate of 2.5 μL/min and injection volume of 10 μL per mouse.6 All subjects were treated in a blinded fashion and exclusion criteria were as follows: rupture of the MCA/CCA during stroke surgery, inability to place tubing in the external carotid artery (ECA), leakage of tubing around the ECA upon pumping, failure of the animal to recover from surgery resulting in death, death of the animal during the study, and signs of hemorrhage upon brain extraction (five animals were excluded based on these criteria). Our MCAo and IA model of pharmacotherapy administration has a mortality rate of <5%.6
Animal number
The experiment was conducted using 50 animals (five groups); naïve animals were excluded from laser Doppler and MouseOx studies. For laser Doppler and MouseOx (n=5/group), infarct volume measurements (n=10/group), functional outcome assessment (n=6/group), blood draws (control n=5, treated n=2), and brain immunohistochemistry (n=5/group).
Vital sign measurements
Physiological measurements (heart rate (bpm)) and blood pressure (pulse distention (μm)) were taken on animals undergoing only the IA surgery (no MCAo surgery) over a 20 min period, beginning at the IA injection and continuous through 20 min. Measurements were taken using the MouseOx Plus (Starr Life Science), where a thigh sensor was placed on the shaved left thigh of an anesthetized mouse.
Perfusion
Blood flow through the MCA was measured using the laser Doppler Periflux System 5000 with a 2 mm tip (Perimed). Blood flow measurements were taken before and after occlusion and 15 min after IA injection (IA injection occurs following reperfusion).
Middle cerebral artery occlusion and intra-arterial injection
Animals were anesthetized using a ketamine/xylazine mixture for the MCAo and IA injection procedure. Transient focal cerebral ischemia was induced using our previously described method.5,6,13,14 MCAo occlusion was for 1 hour with pre-/post-occlusion and recanalization confirmed by laser Doppler flowmetry. Body temperature was measured using the MouseOx Plus (rectal thermometer) and maintained with a heating pad.
GTN IA injection followed our previously published protocol.5,6 In brief, a midline incision was made from mandible to sternum exposing and isolating the CCA, ECA, and ICA. The ECA was permanently occluded with suture at its distal end and the ICA was temporarily occluded. Using a pair of micro-scissors, a small nick was made in the ECA midway between the CCA bifurcation and the proximal suture. Micro-angio tubing was inserted into the ECA through the nick and placed at the bifurcation and suture was used to secure the tubing into the ECA. After removing occlusion of the MCA, CCA and ICA flow was restored and the vessel was allowed to recanalize for 5 min. At that time, GTN or vehicle was injected via the IA route.5,6 Once the injection was complete the ECA was permanently occluded at its proximal end with a third suture and the animal was sutured closed.
Behavioral testing
Animals were subjected to rotor rod to determine functional ability following surgery and pharmacotherapy administration. Animals were trained for 3 days before stroke surgery and tested 3 days following stroke surgery. Rotor rod was used to determine the percentage change from baseline over a 5 min interval with an accelerating rod from 0 to 40 rpm for three trials.
Infarct volume
On PSD 7 mice were euthanized via cervical dislocation, the whole brain was removed and flash frozen, sectioned on a cryostat (20 μm), and stained using cresyl violet. Infarct volume was analyzed from scanned cresyl violet sections with Image J software (NIH).5
Blood draws
To measure nitric oxide (NOx) levels in the blood, submandibular blood draws were performed 1 day before stroke (baseline), 15 min after IA injection, and PSD 1. In short, animals were placed in the dorsal position with their head tilted exposing the submandibular vein region.15 A 5 mm Goldenrod animal lancet was used to pierce the submandibular vein, blood (10% of the total animal blood volume) was collected into K2-EDTA tube, and centrifuged at 14 000 rpm for 15 min. Plasma was collected and analyzed for NOx levels.
NOx analysis
Nitrate reductase was used to reduce nitrate to nitrite in blood plasma samples. A volume of 5 μL nitrate reductase and 5 μL of co-factor (Nitrite/Nitrate Assay Kit, Sigma-Aldrich, UK) was added to 40 μL plasma and incubated for 2 hours at 25°C. Nitrate/nitrite (NOx) concentrations were then determined using Sievers Nitric Oxide Analyzer (NOA 280i; GE Instruments, UK). During this procedure the nitrite was reduced to nitric oxide by acetic acid. Nitric oxide was then quantified using ozone-chemiluminescence technology.
Immunohistochemistry
Whole brains flash frozen were sectioned on a cryostat (Leica) at 20 μm and mounted on slides. Glial fibrillary activating protein (GFAP 1:500 antibody dilution, Sigma) immunohistochemistry was used to evaluate astrogliosis, and NeuN (1:500 antibody dilution, Abcam) immunohistochemistry was used to analyze mature neuron survival in the stroke-affected region (core and penumbra). Infarct and peri-infarct regions (defined as a 500 μm boundary extending from the edge of the infarct core, medial and lateral to the infarct) of the cortex were identified histologically after cryostat sectioning. Stains were imaged with a Nikon Eclipse Ti microscope and images collected with a charge coupled device (CCD) camera and Nikon NIS Element BR software. Photoshop software was used to analyze positive pixel density of sectioned brains from naïve, control, and treated groups.
Statistical analysis
Measured variables are shown as mean±SEM. Analysis of results for comparison between treatment groups (infarct volume, immunohistochemistry) was performed using Student’s t test. Time course comparisons (behavior) and NOx were analyzed using a two-way repeated measures analysis of variance. We defined significance as *p≤0.05, **p≤0.01, and ***p≤0.001.
Compliance with STAIR criteria
To maximize the applicability of our results, we designed the study with reference to the Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for preclinical neuroprotection research.16 We also performed a dose–response study to determine if there was a deleterious dose, and found that all animals survived IA GTN injections.
RESULTS
Vital sign measurements
Physiological measurements for heart rate (IA injection only) demonstrated no difference from baseline for all groups except the 6.25 μg/μL GTN group, which showed a significant reduction in beats per minute from immediately after IA to 15 min after IA, though these animals recovered without incident despite a lowered heart rate. Pulse distention showed no difference between groups from baseline with all animals recovering without incident. Data not shown.
Perfusion
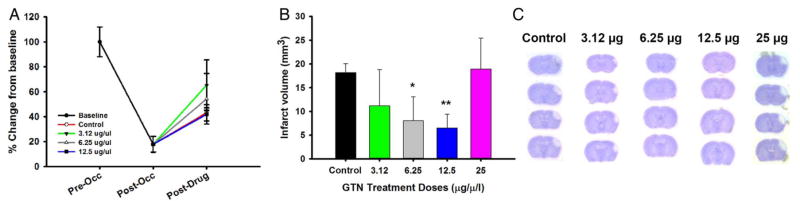
Perfusion measurements using the laser Doppler were made at baseline, occlusion, and 15 min after IA to determine whether IA GTN had any effect on the MCA perfusion. We demonstrated an 83% reduction in cortical blood flow through the MCA after occlusion. After removal of the occlusion and 5 min recanalization, GTN and vehicle were administered IA and blood flow was measured 15 min after IA. While all groups increased perfusion through the MCA there was no significant difference between GTN doses and vehicle over the time period analyzed (figure 1A).
Figure 1.
(A) Blood perfusion measurements as percentage change from baseline for combined groups (black dot), control (red line), 3.12 μg/μL (green line), 6.25 μg/μL (gray line), and 12.5 μg/μL (blue line) at baseline (0–5 min), post-occlusion (5 min after occlusion), and post-IA injection (15 min after IA injection). Combined (N=30), control (n=5), 3.12 μg/μL (n=5), 6.25 μg/μL (n=5), and 12.5 μg/μL (n=5). (B) Infarct volume analysis of control versus GTN doses (3.12, 6.25, 12.5, and 25 μg/μL). (C) Cresyl violet image for control, 3.12 μg/μL, 6.25 μg/μL, 12.5 μg/μL, and 25 μg/μL group (n=10 per group). *p<0.05; **p<0.001. GTN, glyceryl trinitrate.
Infarct volume
Infarct volume (figure 1B) was assessed using cresyl violet (figure 1C) and analyzed on PSD 7 using Image J. Mean infarct volumes (mm3) demonstrated a dose–response effect compared with control, with 12.5 μg/μL GTN showing a significant decrease in infarct volume. However, the 25 μg/μL dose showed signs of hemorrhage and an infarct volume similar to control.
Behavioral testing
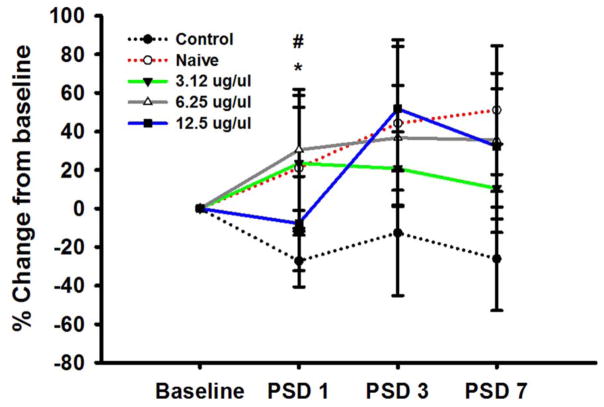
In addition to infarct volume assessment, we performed rotor rod functional analysis on all of the groups except the less well-tolerated 25 μg/μL dose. Functional assessment of IA GTN on the rotor rod was measured as the percentage change from baseline on PSDs 1, 3, and 7 (figure 2). On PSD 1 the control stroke group showed a decrease from baseline (−27.25±13.42) as expected with injury, while the naïve group without any brain injury continued to improve on the task (21.10±31.43) as expected. GTN doses of 3.12 and 6.25 μg/μL showed a significant improvement (23.48±35.30 and 30.51±25.06, respectively) compared with control, while the 12.5 μg/μL GTN dose was only slightly less than baseline (−7.36±24.52). On PSD 3, the control group was still reduced from baseline (−12.65 ±32.51) and the GTN dose of 3.12 μg/μL (20.75±19.04) remained above baseline but was slightly less than PSD 1 measurements. GTN group 6.25 μg/μL (36.73±27.2) continued to improve from baseline and PSD 1 measurements, and 12.5 μg/μL (51.77±32.29) improved significantly from PSD 1 measurements. On PSD 7, the control group remained below baseline levels (−26.11±26.88). However, the GTN dose of 3.12 μg/μL (10.51±22.91) remained above baseline measurements but was slightly less than its PSD 1 and 3 measurements and the 6.25 μg/μL (35.59±26.63) and 12.5 μg/μL (32.34 ±37.79) GTN doses continued to improve from baseline and their PSD 1 values. Nevertheless, none of the GTN dose measurements were significantly greater than the control by PSD 7.
Figure 2.
Rotor rod behavioral measurements: control (black), naïve (red), 3.12 μg/μL (green), 6.25 μg/μL (gray), and 12.5 μg/μL (blue). Rotor rod forced motor movement percentage change from baseline; groups were trained and combined for baseline measurements. Groups were separated after stroke surgery into control, naïve, and GTN treated and were tested on PSD 1, 3, and 7 (n=6 per group). */#p<0.05. GTN, glyceryl trinitrate; PSD, post-stroke day.
Blood nitric oxide measurements
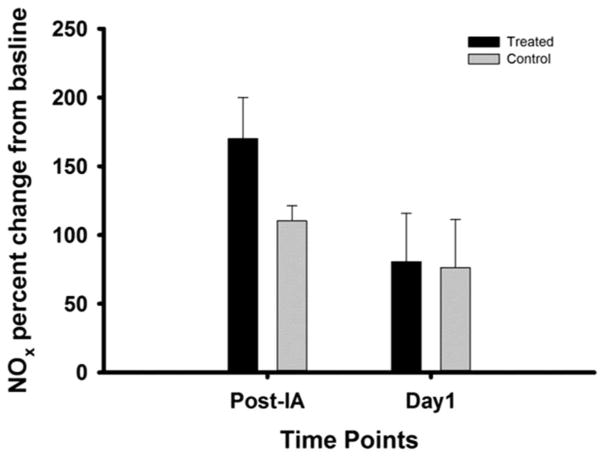
As one of the goals of the IA administration of GTN after stroke was to limit systemic effects, we investigated whether such IA GTN administration had any effect on blood NOx levels. Compared with baseline, blood NOx levels were elevated 15 min after either IA injection or GTN treatment, the latter being slightly, but not significantly, more elevated. By PSD 1, blood NOx remained similarly elevated from baseline levels in both groups (figure 3).
Figure 3.
Nitric oxide (NOx) blood concentrations for control versus GTN (12.5 μg/μL) treated on post-IA and day 1. Treated (n=2), control (n=5). GTN, glyceryl trinitrate.
Immunohistochemistry
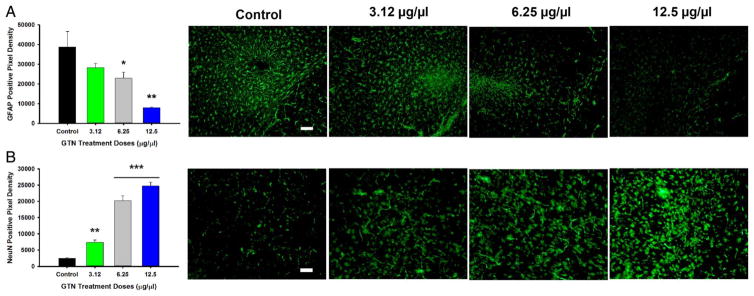
Antibodies specific to proteins associated with mature neuron survival (NeuN) and astrocytic activation (GFAP) were used (figure 4). Compared with control, GTN treated animals showed a dose–response stair step effect with a significant increase in NeuN positive pixels at doses 3.12, 6.25, and 12.5 μg/μL. Likewise, a similar stair step dose–response was seen in GFAP, with control having a significantly increased number of GFAP positive pixels compared with GTN doses 6.25 and 12.5 μg/μL.
Figure 4.
Graphs and images for immunohistochemistry, magnification at 20× with quantification of positive pixel density. (A) Graph depicting positive pixel for GFAP control versus GTN-treated doses in infarcted region with representative images. (B) Graph depicting positive pixels for NeuN stain for control versus GTN treated doses in infarcted region with representative images (n=5 per group). Scale bar=100 μm. *p<0.05; **p<0.001; ***p<0.0001. GFAP, glial fibrillary activating protein; GTN, glyceryl trinitrate.
DISCUSSION
Currently, IV t-PA is the only approved pharmacotherapy available but extensive exclusion criteria limit its use to less than 16% of those affected by stroke. With the recently completed and successful ET trials for ELVO, ET can be used to rapidly recanalize stroke through IA access. This provides a clinical/translational opportunity to co-administer potentially neuroprotective compounds. Using a clinically relevant mouse model of stroke (MCAo), we have developed a new method of IA (similar to ET) pharmacotherapy delivery that allows us to deliver a selected compound directly to the site of ischemia while mitigating systemic side effects.5
GTN was used in the recently completed ENOS trial and shown to be safe when administered in patch form following stroke. The outcome of the trial was an acute reduction in blood pressure, but no improvement in functional outcome.9–11 GTN is a known vasodilator and is commonly used to treat ischemic heart disease, but we and others believe it may also have significant neuroprotective properties as discussed below. Therefore, our goal in this study was to target GTN directly at the site of the stroke via our IA model following MCAo to evaluate these potentially neuroprotective properties.
Since no standard IA dose of GTN is available we started with a dose–response evaluation (3.12, 6.25, 12.5, and 25 μg/μL) to determine if IA GTN was safe (no bleeding into the parenchyma) and had an effect on infarct volume. Our study showed that the mice tolerated and survived IA GTN with no overt deleterious effects, and there was a positive dose-dependent effect on infarct volume with a significant reduction noted with the 12.5 μg/μL dose. However, the 25 μg/μL dose resulted in bleeding into the ventricles and an infarct volume similar to control. Therefore, we conclude that IA GTN is a potentially safe neurotherapeutic with a specific toxicity profile, which includes intraventricular hemorrhage at the highest dose tested. Additional studies should further delineate the most effective dose.
We evaluated the potential effect of IA GTN on functional outcome. Although the lower doses (3.12 and 6.25 μg/μL) initially had significant positive effects immediately following infarct induction on PSD 1, dose–response effects were no longer observed by PSD 7 (though functional outcome was still improved compared with controls). This may be due, in part, to GTN’s very short half-life (1.5–7.5 min) such that noted beneficial effects on infarct volume and neuronal viability (and decreased reactive gliosis) were not sufficiently pervasive to result in long-term functional benefit. In further support of such a conclusion, one potential mediator of GTN neuroprotection, NOx (discussed elsewhere in greater detail17–19 and below), also has an extremely short half-life of 2–6 s. We expect that further studies with post-stroke IA GTN, or perhaps combining IA GTN with other longer lasting potentially neuroprotective agents using multiple assessment tools, may demonstrate functional benefits.
Although we saw no effects of IA GTN on animal pulse distention, we further examined potential systemic effects from IA GTN administration by measuring post-stroke blood NOx levels at several points after IA GTN or vehicle administration. NOx is a good measure of IA GTN administration because GTN is converted to NOx by endothelial nitric oxide synthetase and released into the blood stream from the vascular endothelium. Ultimately, we noted a transient, but insignificant increase in blood NOx levels with IA GTN treatment. These results are consistent with efficacious GTN delivery and suggest a potential mechanism of action, while simultaneously further demonstrating a relative lack of systemic effects with IA GTN treatment.
It has been known for decades that GTN has both neuroprotective and neurotoxic properties, depending upon whether it is present in physiologic or pathologic concentrations, respectively. Post-stroke GTN-mediated neuroprotection may result from vasodilatation of the effected vasculature, which leads to increased blood flow and return of nutrients. NOx has been shown to be neuroprotective through vessel dilatation, anti-apoptotic mechanisms as a reactive oxygen species scavenger, and through inhibition of lipid peroxidation sustaining cellular membrane integrity. In our study, we noted transient, but insignificant increases in both MCA vasodilatation and blood NOx levels with IA GTN treatment. The mechanism of action of IA GTN may be a combined effect (vasodilatation and production of nitric oxide species), but one additional possibility is that IA GTN might have increased local NOx levels in the stroke-targeted brain; this analysis should be performed in additional studies. Collectively, despite the limitations of this study, our results suggest a new therapeutic modality of GTN which, independent of its blood-pressure-lowering effects, might be of benefit after ischemic stroke.
Acknowledgments
Funding
This work was supported by the National Institute on Health grant number 3R01NS065842-08S1.
Footnotes
Contributors
Study design was based on collaborative efforts of MEM, JMR, JFF, RT, and GJB. MEM and JMR performed stroke surgeries, behavior, tissue processing and analysis. AEL processed and analyzed blood samples. AG contributed to animal care and ordering of supplies. MEM, JMR, JFF, RT, and GJB compiled the manuscript, images and figure presentation. JFF, RT and GJB provided oversight for the project. A completed copy of the manuscript was provided to all authors for their input and approval.
Competing interests
None declared.
Provenance and peer review
Not commissioned; externally peer reviewed.
Data sharing statement
We agree to share data upon request. Unpublished data concerns the physiological response of IA nitroglycerin only. It was used to determine if a chosen dose was lethal.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–54. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–9. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 4.Parsons MW, Albers GW. MR RESCUE: is the glass half-full or half-empty? Stroke. 2013;44:2055–7. doi: 10.1161/STROKEAHA.113.001443. [DOI] [PubMed] [Google Scholar]
- 5.Maniskas ME, Roberts JM, Aron I, et al. Stroke neuroprotection revisited: intra-arterial verapamil is profoundly neuroprotective in experimental acute ischemic stroke. J Cereb Blood Flow Metab. 2016;36:721–30. doi: 10.1177/0271678X15608395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maniskas M, Bix G, Fraser J. Selective intra-arterial drug administration in a model of large vessel ischemia. J Neurosci Methods. 2015;240:22–7. doi: 10.1016/j.jneumeth.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bath PM, Houlton A, Woodhouse L, et al. Statistical analysis plan for the ‘Efficacy of Nitric Oxide in Stroke’ (ENOS) trial. Int J Stroke. 2014;9:372–4. doi: 10.1111/ijs.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin RC, Loscalzo J. Vascular nitric oxide: formation and function. J Blood Med. 2010;2010:147–62. doi: 10.2147/JBM.S7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Investigators ET. Bath PM, Woodhouse L, et al. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet. 2015;385:617–28. doi: 10.1016/S0140-6736(14)61121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bath PM, Robson K, Woodhouse LJ, et al. Statistical analysis plan for the ‘Triple Antiplatelets for Reducing Dependency after Ischaemic Stroke’ (TARDIS) trial. Int J Stroke. 2015;10:449–51. doi: 10.1111/ijs.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodhouse L, Scutt P, Krishnan K, et al. Effect of hyperacute administration (within 6 hours) of transdermal glyceryl trinitrate, a nitric oxide donor, on outcome after stroke: subgroup analysis of the Efficacy of Nitric Oxide in Stroke (ENOS) trial. Stroke. 2015;46:3194–201. doi: 10.1161/STROKEAHA.115.009647. [DOI] [PubMed] [Google Scholar]
- 12.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronowski J, Cho KH, Strong R, et al. Neurofilament proteolysis after focal ischemia; when do cells die after experimental stroke? J Cereb Blood Flow Metab. 1999;19:652–60. doi: 10.1097/00004647-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Lee B, Clarke D, Al Ahmad A, et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest. 2011;121:3005–23. doi: 10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 16.Stroke Therapy Academic Industry R. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–8. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- 17.O’Mahony D, Kendall MJ. Nitric oxide in acute ischaemic stroke: a target for neuroprotection. J Neurol Neurosurg Psychiatry. 1999;67:1–3. doi: 10.1136/jnnp.67.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godínez-Rubí M, Rojas-Mayorquín AE, Ortuño-Sahagún D. Nitric oxide donors as neuroprotective agents after an ischemic stroke-related inflammatory reaction. Oxid Med Cell Longev. 2013;2013:297357. doi: 10.1155/2013/297357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calabrese V, Mancuso C, Calvani M, et al. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–75. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]