Abstract
Background
Increasingly, patients are faced with farther travel distances to undergo bariatric surgery at high-volume centers.
Objective(s)
This study sought to evaluate the impact of travel distance on access to care and outcomes following bariatric surgery.
Setting
Patients who underwent Roux-en-Y Gastric Bypass (RYGB) at an academic bariatric surgery center from 1985–2004 were examined and stratified by patient travel distance.
Methods
Univariate analyses were performed for preoperative risk factors, 30-day complications, and long-term (10-year) weight loss between “local” defined as <1 hour travel time and “regional” defined as >1 hour travel time. Survival analysis was performed with Kaplan-Meier and Cox proportional hazards models.
Results
650 patients underwent RYGB, 316 (48.6%) of whom traveled <1 hour to undergo surgery and 334 (51.4%) traveled >1 hour. Median BMI (Body Mass Index) between the groups was equivalent (52.9 kg/m2 local, 53.2 regional kg/m2, p=0.76). Patients who traveled longer distances had higher rates of preoperative comorbidities, including Chronic Obstructive Pulmonary Disease (COPD), Congestive Heart Failure (CHF), Diabetes Mellitus (DM), and sleep apnea (all p<0.05). Complications within 30 days of surgery and long-term reduction of excess BMI were equivalent between groups. Travel time was an independent predictor of risk-adjusted reduced long-term survival (HR 1.23, p=0.0002).
Conclusions
A majority of patients who underwent bariatric surgery at our center traveled more than one hour. Despite longer travel time for care, 30-day complications and long-term weight loss were equivalent compared to local patients. As expected, patients who live in close proximity were more likely to adhere to yearly follow up in surgery clinic. Travel time was an independent predictor of risk-adjusted reduced long-term survival.
Keywords: Bariatric, Roux-en-Y Gastric Bypass (RYGB), Travel Distance, Access to Care, Center of Excellence
Introduction and Objective
In 2006, the Centers for Medicare & Medicaid Services (CMS) restricted the coverage of weight-loss surgery to hospitals which perform at least 125 bariatric procedures annually 1,2. This decision was made at a time when roux-en-Y gastric bypass (RYGB) comprised over four fifths of procedures and only one year after laparoscopic RYGB became more common than its open counterpart 3. Laparoscopic bariatric surgery is associated with lower rates of cardiopulmonary complications, sepsis, and anastomotic leak 4,5. Given the changing trend of procedures performed and the general improvements in technique over the past decade, it’s not surprising that the evidence that led to CMS’ 2006 decision is no longer valid. Accordingly, a 2013 study showed that there was no difference in complication rates following the implementation of CMS’ policy, and as such, CMS no longer requires that covered facilities be certified as Centers of Excellence6.
As surgeons have become more familiar and comfortable with bariatric surgery, there has been a number of studies examining safety and efficacy in broader populations including the elderly, cirrhotics, and even those with obesity and advanced heart failure who need to lose weight to meet criteria for cardiac transplantation 7–11. Although institutions with lower bariatric surgery volume have equivalent rates of morbidity and mortality for the general population, postoperative care for such complex patients requires comprehensive services such as those available at a tertiary referral center. Further, many referral centers act as safety-net hospitals which provide care regardless of coverage status. Given the fact these hospitals’ encatchment areas can span a wide region, patients often have to travel long distances to receive their care. Longer travel distance has been shown to lead to later diagnosis, increased length of stay, and increased mortality in various surgical populations12–14. In the bariatric population, travel distance has been shown to affect compliance with regular follow-up visits but its effects on long-term outcomes have not been described.
The aim of the present study was to evaluate the impact of travel distance on access to care and outcomes following bariatric surgery, including long-term weight loss, comorbidity resolution, and mortality.
Patients and Methods
Patients
All patients undergoing RYGB for morbid obesity at an academic bariatric surgery center between January 1, 1985 and January 1, 2004 were identified from a prospectively collected database approved by the Institutional Review Board (IRB# 17132). Demographic data, pre-operative weight and comorbidities, post-operative complications and comorbidity resolution, and weights from annual follow-up appointments are captured by the database. Using Electronic Medical Record (EMR) review for all encounters at our academic medical center and telephone survey, we obtained objective data for patients lost to follow-up in the database. A standardized phone script approved on the IRB protocol was followed to call the patients lost to follow-up. Phone call were conducted on different days of the week at different times of day and multiple attempts were made to contact patients using phone numbers listed in their EMR. For all disconnected numbers, an online people search tool (Intelius People Search, Bellevue, WA) was used to locate patients using publically available records such as census data, property records, and telephone records. Social security records were used to determine long-term survival.
Distance Analysis
Travel times between each patient’s home address and the medical center address were calculated using Google Maps (Google Inc. Mountain View, CA). These calculations do not factor in traffic but provide a standard travel time given distance and posted speed limit for each patient. A travel time of one hour was used to divide patients into a local (<60 minutes) and a regional (>60 minutes) group using the median travel time.
Definitions
All pre-operative comorbidity data was collected using a prospectively collected database populated during a preoperative visit. Ten-year comorbidity data was collected through a prospective database, retrospective chart review, and telephone surveys. Patients were recorded as having a comorbidity if undergoing medical therapy for a disease, it’s documented during a clinical encounter 10 years post-operatively, or through self-reporting during phone survey. We used 25 kg/m2 as ideal Body Mass Index (BMI) to calculate percent reduction in excess BMI (%REBMI).
Statistical Analysis
The primary outcome was percent reduction in excess BMI between local and regional groups. Secondary outcomes included post-operative complications and long-term comorbidity resolution between groups. Statistical analyses were performed using appropriate parametric and non-parametric tests to determine statistical differences. Additionally, survival analysis was performed with Kaplan Meier and Cox proportional hazards models. SAS version 9.4 (SAS Company, Cary NC) was used for analyses.
Results
Pre-operative Comorbidity Incidence
A total of 650 (60%) patients who underwent RYGB at our academic medical center between January 1, 1985 and January 1, 2004 had complete 10-year follow-up with a median travel time of 61 minutes [IQR 36, 92 minutes]. Of these, 316 (48.6%) traveled <1 hour to undergo surgery (local), and 334 (51.4%) traveled >1 hour (regional). Table 1 demonstrates baseline demographics with no difference between groups except a higher incidence of white race in the regional group.
Table 1.
Demographic Data
| Variables | Local | Regional | P-Value |
|---|---|---|---|
| Patients | 316 | 334 | |
| Age (Years) | 41 ± 9 | 42 ± 10 | 0.11 |
| Preoperative BMI* | 53.0 ± 10.1 | 53.2 ± 10.4 | 0.76 |
| Sex (Female) | 257 (81.6%) | 274 (82.0%) | 0.88 |
| Race (White) | 256 (81.0%) | 300 (90.1%) | 0.001 |
| Private Insurance | 186 (58.9%) | 211 (63.2%) | 0.26 |
Body Mass Index
Outcomes By Travel Distance
Pre-operative and 10-year comorbidity rates can be seen in Table 2 for both local and regional groups. Patients who traveled farther had higher rates of cardiac and pulmonary disease as well as diabetes mellitus preoperatively. At 10-years, hypertension was the only comorbidity higher in the regional group. The resolution column reports the difference in comorbidity resolution between groups; hypertension was the only variable to reach significance indicating local patients had greater amelioration of this disease. Table 3 reports post-operative complications and long-term outcomes between groups. Dumping syndrome is the only complication variable to reach statistical significance between groups. Local patients were more likely to have annual follow-ups compared to patients traveling more than an hour.
Table 2.
Preoperative and 10-Year Comorbidity Rates
| Comorbidities | Preoperative | 10-Year | Resolution P-value |
||||
|---|---|---|---|---|---|---|---|
| Local | Regional | P-value | Local | Regional | P-value | ||
| Gastroesophageal Reflux Disease | 124 (39.2%) | 126 (37.7%) | 0.69 | 61 (19.9%) | 80 (25.5%) | 0.10 | 0.08 |
| Cardiac Disease | 77 (24.4%) | 113 (33.8%) | 0.01 | 31 (10.1%) | 46 (14.7%) | 0.09 | 0.24 |
| Degenerative Joint Disease | 188 (59.5%) | 221 (66.1%) | 0.08 | 74 (24.2%) | 84 (26.8%) | 0.46 | 0.23 |
| Diabetes Mellitus | 140 (44.3%) | 174 (52.1%) | 0.046 | 40 (13.1%) | 47 (15.0%) | 0.50 | 0.34 |
| Obstructive Sleep Apnea | 117 (37.0%) | 116 (34.7%) | 0.54 | 45 (14.7%) | 35 (11.2%) | 0.19 | 0.65 |
| Hypertension | 183 (57.9%) | 202 (60.5%) | 0.51 | 98 (32.0%) | 133 (42.4%) | 0.01 | 0.004 |
| Chronic Obstructive Pulmonary Disease | 87 (27.5%) | 119 (35.6%) | 0.03 | 34 (11.1%) | 30 (9.6%) | 0.52 | 0.98 |
| Psychiatric Disease | 117 (37.0%) | 136 (40.7%) | 0.33 | 74 (24.2%) | 67 (21.3%) | 0.40 | 0.58 |
Table 3.
Postoperative Complications and Long-term Outcomes
| Variables | Local | Regional | P-Value |
|---|---|---|---|
| Wound Infection | 9 (2.8%) | 5 (1.5%) | 0.13 |
| Dumping Syndrome | 23 (7.3%) | 12 (3.6%) | 0.04 |
| Leak | 2 (0.6%) | 3 (0.9%) | 0.70 |
| Obstruction | 16 (5.1%) | 16 (4.8%) | 0.87 |
| Hernia | 21 (6.9%) | 25 (7.9%) | 0.61 |
| Major Vitamin Deficiency | 25 (8.1%) | 34 (10.8%) | 0.26 |
| Surgical Revision | 9 (2.8%) | 13 (3.9%) | 0.45 |
| Late Adverse Event | 37 (12.1%) | 53 (16.8%) | 0.09 |
| Yearly Follow-up | 88 (27.9%) | 63 (18.9%) | 0.01 |
| 10-Year Percent Reduction in Excess BMI* | 60.0 ± 44.7 | 59.9 ± 52.7 | 0.99 |
| Satisfaction Score (0–10) | 8.5 ± 2.1 | 8.7 ± 1.8 | 0.11 |
Body Mass Index
Long-term Survival By Travel Distance
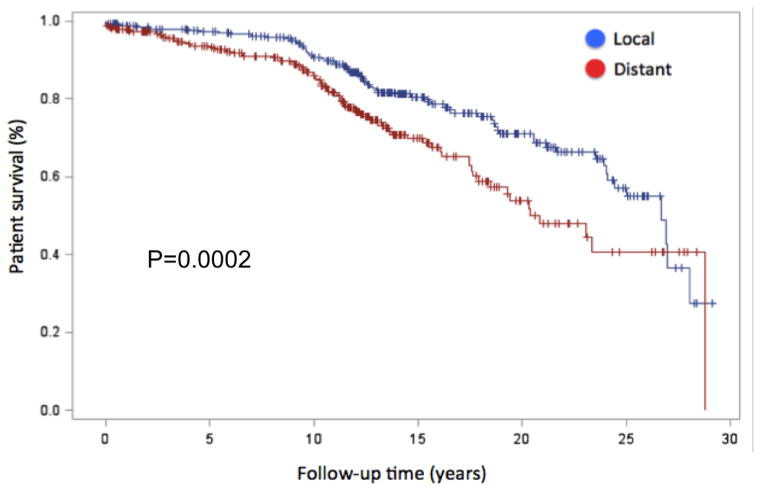
Figure 1 is the Kaplan-Meier survival curve for the local and regional groups demonstrating a statistical benefit for the local group (p<0.001). Cox proportional hazards modeling was used to evaluate predictors for risk-adjusted long-term survival. All pre-operative variables that were statistically different on univariate analysis between the groups were fit in the Cox model to determine risk-adjusted impact on survival. Hazards ratios can be seen in Table 4 with travel time (HR 1.23 per hour, p<0.001) and diabetes (HR 1.98, p=0.001) being the only independent predictors for worse long-term survival.
Figure 1.
Patient survival after RYGB
Table 4.
Cox Proportional Hazards Model
| Parameters | Hazard Ratio | P-Value |
|---|---|---|
| Travel Time (Per Hour) | 1.23 | 0.0002 |
| Race | 0.76 | 0.25 |
| Cardiac Disease | 0.68 | 0.90 |
| Degenerative Joint Disease | 1.02 | 0.90 |
| Diabetes Mellitus | 1.98 | 0.001 |
| Chronic Obstructive Pulmonary Disease | 1.06 | 0.79 |
Discussion
The present study aims to determine the impact of travel time on short- and long-term outcomes following RYGB. These results demonstrate that patients who travel farther are at higher risk pre-operatively but had no difference in %REBMI at 10 years with significant reduction in all medical comorbidities. Additionally, the local group had greater improvement of hypertension with no difference in the rate of other comorbidity resolution. Finally, we demonstrate that longer travel time predicts reduced risk-adjusted long-term survival. To our knowledge these travel time data are the first reported in the bariatric literature and have major implications on access to care in bariatric surgery.
Patients typically travel further if a service is not offered locally; they are too high risk and need to seek care in a tertiary center, or due to insurance restrictions for elective operations.2,15–17 While we do not have data regarding the availability of bariatric care close to patient’s homes, we demonstrate there is no difference in insurance status for the local and regional groups. Also, despite having higher baseline comorbidities the regional group did well postoperatively with no statistical increase in complications7. Interestingly, the rate of dumping syndrome was higher in the local group, likely due to reporting bias with the local patients more likely to seek care at our center for the symptoms. This is also seen in the disproportionate annual follow-up up rate, which is much higher in the local group5,18.
Several studies have demonstrated substantial improvement in most medical comorbidities with foregut bypass and weight loss19–21. Both groups in the present study demonstrated significant reduction in all medical comorbidities over the 10-year study period. However, when we examine the percentage of patients with improvements, we note that with the exception of hypertension both groups demonstrate similar improvement. These differences could be attributed to better diet compliance with routine follow-up but we would expect to see these effects in other comorbid diseases or %REBMI. The overall significance is likely small given hypertension is not a predictor of long-term survival in the risk adjusted multivariate cox model.
These data demonstrate a reduction in survival for patients in the regional group, which is confirmed by the risk-adjusted cox model. Several studies have demonstrated higher mortality in government-pay patients, however we did not find an insurance gap by travel distance22,23. The superior reduction in hypertension alone does not explain this finding, but in our population this difference is pronounced and increases over time. Given the proximity of the local patients to a major academic medical center, they may have better access to routine preventive care and advanced comprehensive care resulting in improved long-term survival. Further studies are required to better understand this complex result.
The limitations of this paper include the retrospective nature of this single center database study. Travel distance is a major obstacle resulting in poor follow-up after bariatric surgery. Given our large referral area and the growing dependence on primary care physicians for follow-up, many of our patients were lost to follow-up. Telephone follow-up improved the percentage of patients with 10-year data, but is limited by lack of objectivity. However, telephone follow-up has been validated in the bariatric population as demonstrated by Harper et al24.
The major finding in the present study reports improved long-term risk-adjusted survival with shorter travel time to a bariatric surgery center. Despite these benefits, there is no difference in weight loss or comorbidity amelioration between the groups with the exception of hypertension. Additionally, we demonstrate no difference in postoperative complication rates, however, the local patients do report increased dumping syndrome. Similar to findings in prior studies, local patients did have better compliance with annual follow-ups. These data have major implications for future accountable care organizations and access to care discussions as medical centers will be responsible for the long-term care of patients.
Conclusion
The majority of patients who underwent bariatric surgery at our center traveled more than one hour. When compared with local patients, regional patients had more pre-operative medical comorbidities. Despite longer travel time for care, 30-day complications and long-term weight loss were equivalent to those of local patients As expected, patients who live in close proximity were more likely to adhere to annual follow up in surgery clinic. Travel time was an independent predictor of risk-adjusted long-term survival suggesting a patient’s proximity to routine preventive and advanced comprehensive care is critical after bariatric surgery.
Acknowledgments
Source of Funding:
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Numbers T32HL007849 and NIH T32AI0074.
Footnotes
Conflicts of Interest
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schirmer B. The effect of the CMS national coverage decision on the performance and outcomes of bariatric surgery for medicare recipients in the U.S. Ann Surg. 2011;254(6):866–867. doi: 10.1097/SLA.0b013e31823b0c6e. [DOI] [PubMed] [Google Scholar]
- 2.Tunis SR, Messner DA. Medicare policy on bariatric surgery: decision making in the face of uncertainty. JAMA. 2013;310(13):1339–1340. doi: 10.1001/jama.2013.278849. [DOI] [PubMed] [Google Scholar]
- 3.Abraham A, Ikramuddin S, Jahansouz C, Arafat F, Hevelone N, Leslie D. Trends in Bariatric Surgery: Procedure Selection, Revisional Surgeries, and Readmissions. Obes Surg. 2016;26(7):1371–1377. doi: 10.1007/s11695-015-1974-2. [DOI] [PubMed] [Google Scholar]
- 4.Encinosa WE, Bernard DM, Du D, Steiner CA. Recent improvements in bariatric surgery outcomes. Med Care. 2009;47(5):531–535. doi: 10.1097/MLR.0b013e31819434c6. [DOI] [PubMed] [Google Scholar]
- 5.Mehaffey JH, LaPar DJ, Clement KC, et al. 10-Year Outcomes After Roux-en-Y Gastric Bypass. Ann Surg. 2015 doi: 10.1097/SLA.0000000000001544. [DOI] [PubMed] [Google Scholar]
- 6.Bae J, Shade J, Abraham A, et al. Effect of Mandatory Centers of Excellence Designation on Demographic Characteristics of Patients Who Undergo Bariatric Surgery. JAMA Surg. 2015;150(7):644–648. doi: 10.1001/jamasurg.2015.74. [DOI] [PubMed] [Google Scholar]
- 7.Broderick RC, Fuchs HF, Harnsberger CR, et al. Increasing the Value of Healthcare: Improving Mortality While Reducing Cost in Bariatric Surgery. Obes Surg. 2015;25(12):2231–2238. doi: 10.1007/s11695-015-1710-y. [DOI] [PubMed] [Google Scholar]
- 8.Mehaffey JH, LaPar DJ, Turrentine FE, Miller MS, Hallowell PT, Schirmer BD. Outcomes of laparoscopic Roux-en-Y gastric bypass in super-super-obese patients. Surg Obes Relat Dis. 2015;11(4):814–819. doi: 10.1016/j.soard.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Giordano S, Victorzon M. Bariatric surgery in elderly patients: a systematic review. Clin Interv Aging. 2015;10:1627–1635. doi: 10.2147/CIA.S70313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim CP, Fisher OM, Falkenback D, et al. Bariatric Surgery Provides a “Bridge to Transplant” for Morbidly Obese Patients with Advanced Heart Failure and May Obviate the Need for Transplantation. Obes Surg. 2016;26(3):486–493. doi: 10.1007/s11695-015-1789-1. [DOI] [PubMed] [Google Scholar]
- 11.Pestana L, Swain J, Dierkhising R, Kendrick ML, Kamath PS, Watt KD. Bariatric surgery in patients with cirrhosis with and without portal hypertension: a single-center experience. Mayo Clin Proc. 2015;90(2):209–215. doi: 10.1016/j.mayocp.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Chou S, Deily ME, Li S. Travel distance and health outcomes for scheduled surgery. Med Care. 2014;52(3):250–257. doi: 10.1097/MLR.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 13.Jackson KL, Glasgow RE, Hill BR, et al. Does travel distance influence length of stay in elective colorectal surgery? Dis Colon Rectum. 2013;56(3):367–373. doi: 10.1097/DCR.0b013e31827e939e. [DOI] [PubMed] [Google Scholar]
- 14.Massarweh NN, Chiang YJ, Xing Y, et al. Association between travel distance and metastatic disease at diagnosis among patients with colon cancer. J Clin Oncol. 2014;32(9):942–948. doi: 10.1200/JCO.2013.52.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon S, Wang B, Wong E, Alfonso-Cristancho R, Sullivan SD, Flum DR. The impact of accreditation on safety and cost of bariatric surgery. Surg Obes Relat Dis. 2013;9(5):617–622. doi: 10.1016/j.soard.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livingston EH, Burchell I. Reduced access to care resulting from centers of excellence initiatives in bariatric surgery. Arch Surg. 2010;145(10):993–997. doi: 10.1001/archsurg.2010.218. [DOI] [PubMed] [Google Scholar]
- 17.Telem DA, Talamini M, Altieri M, Yang J, Zhang Q, Pryor AD. The effect of national hospital accreditation in bariatric surgery on perioperative outcomes and long-term mortality. Surg Obes Relat Dis. 2015;11(4):749–757. doi: 10.1016/j.soard.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Hayes S, Napolitano MA, Lent MR, et al. The effect of insurance status on pre- and postoperative bariatric surgery outcomes. Obes Surg. 2015;25(1):191–194. doi: 10.1007/s11695-014-1478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pories WJ, Mehaffey JH, Staton KM. The surgical treatment of type two diabetes mellitus. Surg Clin North Am. 2011;91(4):821–836. viii. doi: 10.1016/j.suc.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 21.Obeid NR, Malick W, Concors SJ, Fielding GA, Kurian MS, Ren-Fielding CJ. Long-term outcomes after Roux-en-Y gastric bypass: 10- to 13-year data. Surg Obes Relat Dis. 2015 doi: 10.1016/j.soard.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Yuan X, Martin Hawver LR, Ojo P, et al. Bariatric surgery in Medicare patients: greater risks but substantial benefits. Surg Obes Relat Dis. 2009;5(3):299–304. doi: 10.1016/j.soard.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Alexander JW, Goodman HR, Martin Hawver LR, James L. The impact of medicaid status on outcome after gastric bypass. Obes Surg. 2008;18(10):1241–1245. doi: 10.1007/s11695-008-9615-7. [DOI] [PubMed] [Google Scholar]
- 24.Harper J, Madan AK, Ternovits CA, Tichansky DS. What happens to patients who do not follow-up after bariatric surgery? Am Surg. 2007;73(2):181–184. [PubMed] [Google Scholar]