Abstract
Objective:
To predict long-term disability outcomes in TEMSO core (NCT00134563) and extension (NCT00803049) studies in patients with relapsing forms of MS treated with teriflunomide.
Methods:
A post hoc analysis was conducted in a subgroup of patients who received teriflunomide in the core study, had MRI and clinical relapse assessments at months 12 (n = 552) and 18, and entered the extension. Patients were allocated risk scores for disability worsening (DW) after 1 year of teriflunomide treatment: 0 = low risk; 1 = intermediate risk; and 2–3 = high risk, based on the occurrence of relapses (0 to ≥2) and/or active (new and enlarging) T2-weighted (T2w) lesions (≤3 or >3) after the 1-year MRI. Patients in the intermediate-risk group were reclassified as responders or nonresponders (low or high risk) according to relapses and T2w lesions on the 18-month MRI. Long-term risk (7 years) of DW was assessed by Kaplan-Meier survival curves.
Results:
In patients with a score of 2–3, the risk of 12-week–confirmed DW over 7 years was significantly higher vs those with a score of 0 (hazard ratio [HR] = 1.96, p = 0.0044). Patients reclassified as high risk at month 18 (18.6%) had a significantly higher risk of DW vs those in the low-risk group (81.4%; HR = 1.92; p = 0.0004).
Conclusions:
Over 80% of patients receiving teriflunomide were classified as low risk (responders) and had a significantly lower risk of DW than those at increased risk (nonresponders) over 7 years of follow-up in TEMSO. Close monitoring of relapses and active T2w lesions after short-term teriflunomide treatment predicts a differential rate of subsequent DW long term.
ClinicalTrials.gov identifier:
TEMSO, NCT00134563; TEMSO extension, NCT00803049.
Reliable predictors of treatment response would facilitate earlier identification of patients with MS that is unresponsive to their existing therapy.1–3 Various clinical and MRI parameters have been used to classify patients according to their response to treatment. The prognostic value of the Rio score, based on outcomes after 1 year of therapy, was demonstrated in patients treated with interferon beta (IFNβ)4 or glatiramer acetate.5 A simplified version, the modified Rio score, used a score (0–3) based on the number of new T2-weighted (T2w) lesions (≤5 or >5) and clinical relapses (0, 1, or 2) occurring after 1 year of therapy.6 Patients treated with IFNβ who had higher modified Rio scores after 1 year showed increased risk of disability worsening (DW) over 4–5 years.6,7 A recent analysis of patients treated with IFNβ, performed within the MAGNIMS network, re-evaluated the modified Rio score using a large, multicenter, real-world data set from more than 1,200 patients8 and redefined the cutoffs for minimal MRI activity as ≥3 new T2w lesions on the MRI performed after 1 year from treatment start. The present study evaluated whether this revised scoring system, which was developed for IFNβ and evaluated over the short term,8 was able to predict disability outcomes for up to 7 years in patients treated with teriflunomide, a once-daily oral immunomodulator approved for the treatment of relapsing-remitting MS (AUBAGIO; Sanofi Genzyme, Cambridge, MA), using data from the TEMSO extension study (NCT00803049).
METHODS
Study population and design.
TEMSO was a multinational, multicenter, randomized, placebo-controlled, double-blind, parallel-group phase 3 study in patients with relapsing forms of MS.9 In the core study (NCT00134563), 1,088 patients were randomized 1:1:1 to receive once-daily oral placebo (n = 363), or teriflunomide 7 mg (n = 366), or 14 mg (n = 359), for 108 weeks.
Key inclusion criteria allowed for enrollment of patients aged 18–55 years who met the McDonald 200110 criteria for relapsing-remitting MS with or without progression, had an Expanded Disability Status Scale (EDSS) score of ≤5.5, and had ≥2 clinical relapses in the previous 2 years or 1 relapse during the preceding year. EDSS scores were determined at screening, baseline visit, and every 12 weeks thereafter, and at unscheduled visits when patients returned to the clinic for the assessment of potential relapse. MRIs were taken at baseline and at weeks 24, 48, 72, and 108. The primary end point was the annualized relapse rate, and the key secondary end point was 12-week–confirmed DW.
Patients completing the core study were eligible to enter the long-term extension; those already receiving teriflunomide remained on their original dose, whereas those previously receiving placebo were randomized 1:1 to teriflunomide 7 or 14 mg and were treated for up to 9 years.11
Patients were included in this post hoc analysis if they received either dose of teriflunomide in the core study, had MRI assessments at 1 year, could be assessed for clinical relapses at year 1, and entered the extension. Data are presented for the combined teriflunomide 7- and 14-mg groups (n = 552).
Standard protocol approvals, registrations, and patient consents.
TEMSO was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by central and local ethics committees. Patients provided written informed consent before the commencement of TEMSO and, again, before entry into the extension. Clinical Trial (clinicaltrials.gov) identifiers: TEMSO, NCT00134563; TEMSO extension, NCT00803049.
Defining scoring categories.
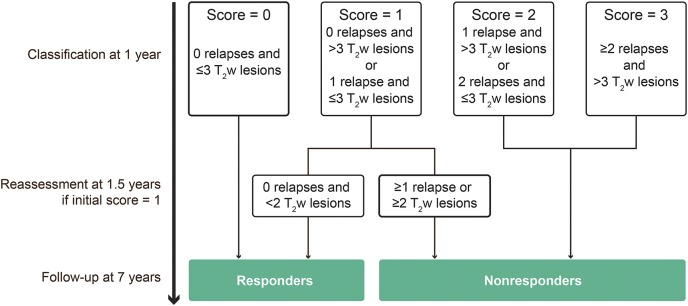
The MAGNIMS score is a recently published scoring system8 that classified patients after 1 year of treatment with IFNβ for the risk of disease progression according to the occurrence of relapse (0 to ≥2) and new and enlarging T2w lesions (<3 or ≥3) on 12-month MRIs. In the TEMSO study, MRIs were performed more frequently (every 6 months), which allowed us to increase the T2w-lesion cutoff to ≤3 or >3, according to the observation that 6-monthly scanning would detect ∼30% more lesions than 12-monthly scanning.7 The scoring assessment is reported in figure 1. Patients with a score of 0, 1, or 2–3 were categorized as having a low, intermediate, or high risk of poor treatment response, respectively. Patients with a score of 0 were classified as “treatment responders,” whereas patients with scores of 2 or 3 were classified as “nonresponders.” In addition, patients initially considered to be of intermediate risk of disease progression (score 1) were reclassified 6 months later (month 18 from treatment start) as responders or nonresponders, based on the algorithm originally used to monitor response to IFNβ.1
Figure 1. Scoring assessment.
T2w = T2-weighted.
In addition, the long-term prognostic value of the use of the composite end point of patients with no relapse and no MRI activity (based on active T2w lesions), a concept similar to NEDA (No Evidence of Disease Activity), during the first year of treatment with teriflunomide was compared with that of patients with no relapses but minimal MRI activity (i.e., 1, 2, or 3 active T2w lesions).
Statistical analysis.
Based on Kaplan-Meier survival curves, the risk of DW confirmed for 12 weeks was assessed in patients with valid scores that were classified by a score category or as responders/nonresponders. Between-group comparisons were made using a log-rank test with EDSS strata at baseline and region as stratification variables, and risk reductions were estimated from a proportional hazards model. Figures with Kaplan-Meier curves are presented until the end of year 7, after which a few patients (<10%) remained at risk of progression within each group presented.
RESULTS
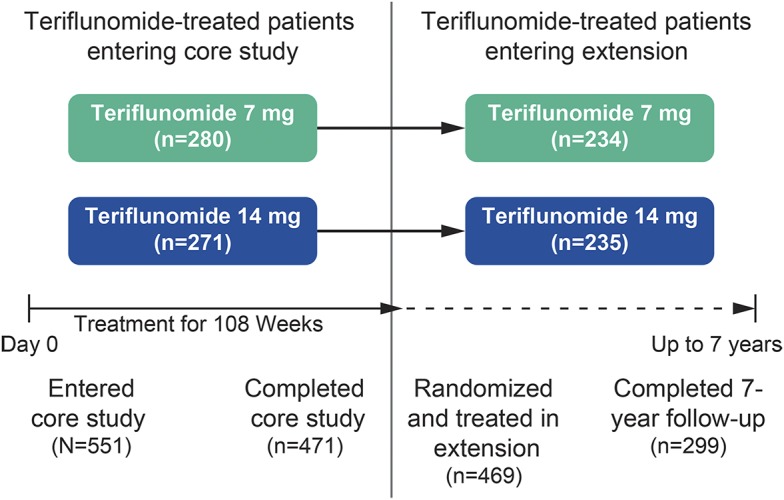
Of the 723 patients treated with teriflunomide 7 or 14 mg in the core TEMSO study, 551 (76.2%) had a computable score at year 1. Of these, 469 patients were treated in the extension and 299 completed 7 years of follow-up (figure 2).
Figure 2. Patient disposition.
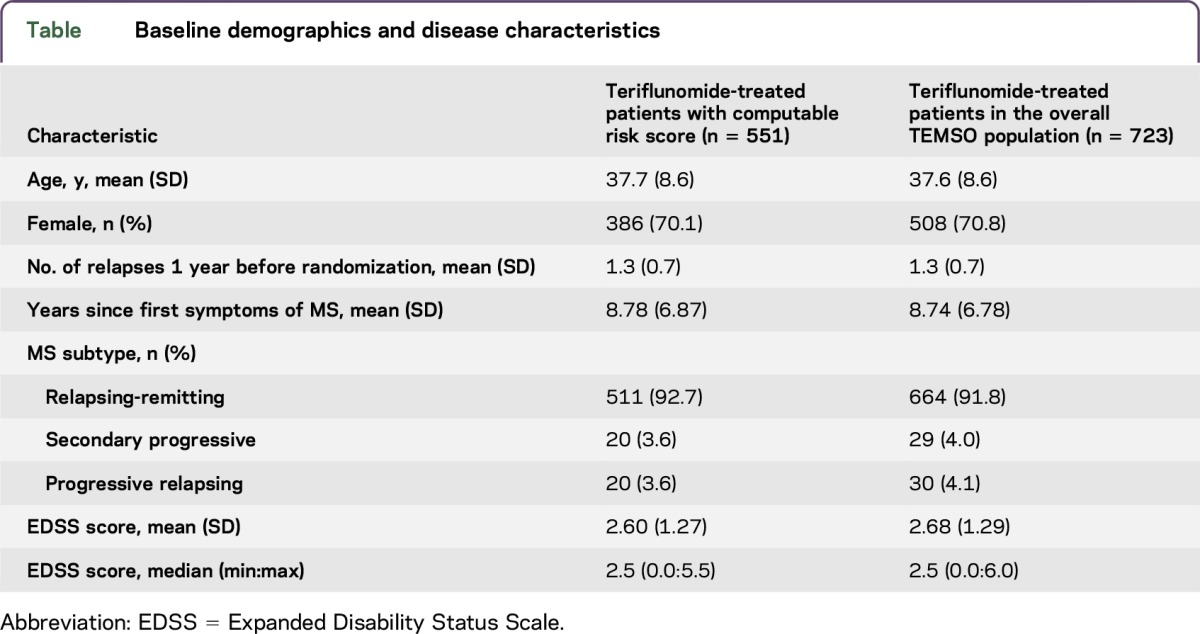
Baseline demographics and clinical disease characteristics are shown in table. Patient characteristics were consistent with those of the teriflunomide-treated patients in the overall TEMSO population.
Table.
Baseline demographics and disease characteristics
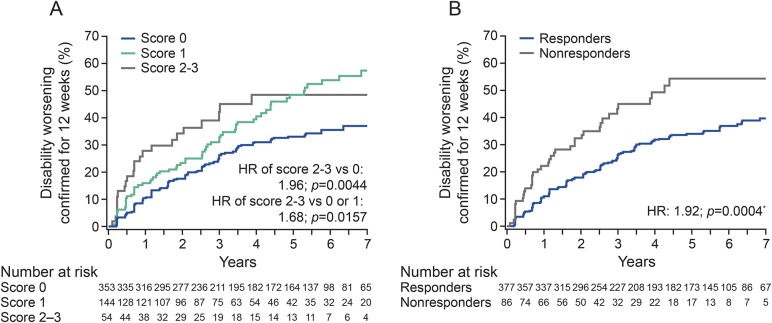
Kaplan-Meier estimates of the proportion of patients with DW at the end of year 7, categorized by scoring classification, are shown in figure 3A. The probability of disability progression was similar in the score 0 and score 1 categories over the initial years of the study, followed by a separation of the curves around 3 years. Over a period of 7 years of follow-up in the TEMSO extension, the probability of 12-week–confirmed DW was significantly increased in the high-risk categories (scores 2 or 3) compared with those in the low-risk categories (score 0) (hazard ratio [HR], 1.96; p = 0.0044), as well as in the high-risk categories compared with the combined low-risk/intermediate-risk score category (HR, 1.68; p = 0.0157).
Figure 3. Probability of disability worsening by scoring classification.
(A) At the end of year 1 and (B) on reclassification of patients with intermediate score 1, 6 months later. *Nonresponders vs responders. HR = hazard ratio.
In addition, when patients with an intermediate score of 1 were recategorized after an additional 6 months of follow-up, those classified as nonresponders had a significantly higher rate of DW over 7 years compared with those classified as responders (HR, 1.92; p = 0.0004; figure 3B).
Outcomes remained consistent for the group of patients treated with teriflunomide 14 mg only, although because of the small sample size, the differences between groups were not significant (data not shown).
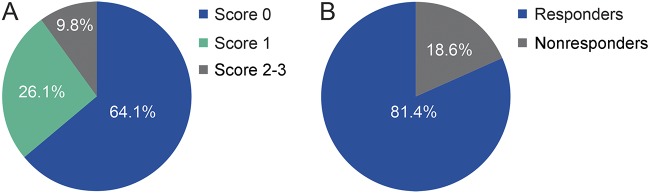
The distribution of patients by scoring category at the end of year 1 is shown in figure 4A. Most patients (∼90%) were categorized as having low or intermediate risk of disease progression. Patients with an initial score of 1 (intermediate risk) after 1 year of treatment included those with no relapses and >3 active T2w lesions or those with 1 relapse and ≤3 active T2w lesions. These patients were reclassified 6 months later as responders (no relapses and <2 new T2w lesions) or nonresponders (≥1 relapse or ≥2 new T2w lesions). The majority of these patients (∼80%) were reclassified as responders (figure 4B). The responder vs nonresponder classification had a positive predictive value of 67% and a negative predictive value of 45%, a sensitivity of 84%, a specificity of 24%, and a global accuracy of 63%.
Figure 4. Distribution of patients by scoring classification.
(A) At the end of year 1 and (B) on reclassification of patients with score 1, 6 months later.
Finally, because the absence of any disease activity, the so-called No Evidence of Disease Activity status, is the emerging target of newer therapies,12,13 we examined whether the proposed cutoff of 3 active T2w lesions might be too conservative for defining responders among teriflunomide-treated patients. This analysis showed no significant difference between patients without any relapse or MRI activity, and those with no relapses and minimal MRI activity (i.e., 1, 2, or 3 active T2w lesions), with respect to the risk of experiencing DW over the subsequent 7 years (figure 5).
Figure 5. Effect of MRI lesions on the probability of disability worsening in patients with no relapses.
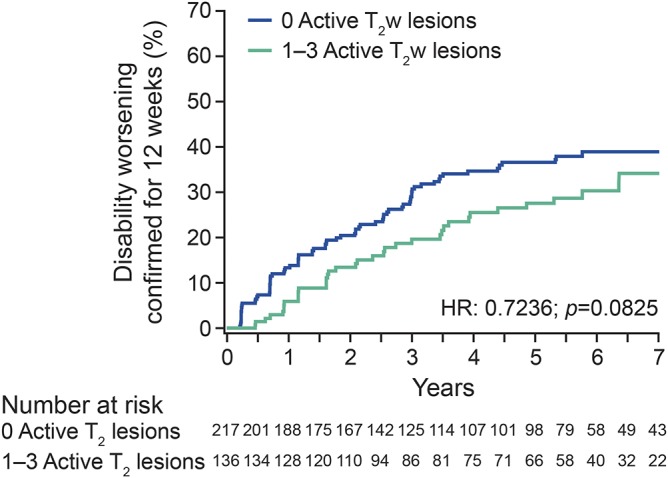
HR = hazard ratio; T2w = T2-weighted.
DISCUSSION
Reliable predictors of treatment outcomes in MS would help to identify patients who may continue to have disease activity while receiving treatment and who may therefore benefit from a change in therapy. Earlier stage treatment decisions, when alternative agents are expected to be more effective, are likely to improve longer-term outcomes in these patients.
This is the first report of predictive scoring analysis applied to patients with relapsing forms of MS treated with teriflunomide. Consistent with earlier reports of patients treated with IFNβ,4,14–16 glatiramer acetate,5,14 and fingolimod,17 clinical relapses and disease activity on MRI during the early stages of treatment were predictive of a subsequent differential rate of DW. A small number of active T2w lesions (≤3) in the absence of relapses had less predictive value, suggesting that a small amount of MRI activity can be tolerated, and a minimal amount of MRI activity alone should not be a criterion for switching treatments.
Teriflunomide-treated patients from the TEMSO study who were characterized as having low risk of disease progression after 1 year of treatment demonstrated significantly lower probability of DW over 7 years of follow-up (median follow-up time was 292 weeks), when compared with patients who were classified as having high risk. Approximately, 26% of patients were classified as having intermediate risk after 1 year; these patients may be more difficult to assess in terms of response to therapy and subsequent treatment choices. An additional evaluation of relapses and disease activity on MRI, as applied in previous analyses at 1.5 years,7,18 allowed these patients to be reclassified as responders or nonresponders, with a greater proportion of TEMSO patients (>81%) being classified as responders.
In our analysis, the predictive value of the scoring classification, particularly in the intermediate group, extended to over 7 years in the TEMSO long-term extension, which extends the finding of studies based on a shorter follow-up. A limitation of this analysis is the loss of patients to follow-up in the extension, although our results support the use of teriflunomide for the long-term treatment of most patients with relapsing forms of MS. It could also be suggested that MRI outcomes of patients at 1 year might be better compared with outcomes after steady-state levels of teriflunomide were achieved (for example, after 6 months), rather than at baseline. However, to allow for comparisons with other analyses, we followed an approach consistent with validated methodology.4,7,18 This may also better represent the real-world setting, where MRI assessments may be performed less frequently than in clinical trials. Approximately 90% of patients receiving teriflunomide in the TEMSO study were characterized as having low or intermediate risk of subsequent disease progression. Long-term observation is important in studies of response to treatment and is of particular significance in patients with MS who demonstrate heterogeneous responses to therapy over time.
ACKNOWLEDGMENT
This manuscript was reviewed by Larisa Miller, PharmD, of Sanofi Genzyme.
GLOSSARY
- DW
disability worsening
- EDSS
Expanded Disability Status Scale
- HR
hazard ratio
- IFNβ
interferon beta
- T2w
T2 weighted
AUTHOR CONTRIBUTIONS
Design or conceptualization of the study and/or analysis or interpretation of the data: M.P. Sormani, P. Truffinet, K. Thangavelu, P. Rufi, and N. De Stefano. Analysis or interpretation of the data: M.P. Sormani, K. Thangavelu, and N. De Stefano. Drafting or revising of the manuscript for intellectual content: M.P. Sormani, P. Truffinet, K. Thangavelu, P. Rufi, and N. De Stefano. Editorial and writing support: C. Simonson.
STUDY FUNDING
Editorial support for this manuscript was provided by Catherine Simonson, of Fishawack Communications Ltd., and was funded by Sanofi Genzyme.
DISCLOSURE
M.P. Sormani received travel funding and/or speaker honoraria from Merck Serono, Teva, Genzyme, Novartis, Biogen, and Roche; consulted for Merck Serono, Biogen, Teva, Genzyme, Roche, Novartis, GeNeuro, and MedDay; and received research support from Biogen, TEVA, and Merck Serono. P. Truffinet is employed; receives a salary from Genzyme/Sanofi; and holds stocks in Genzyme/Sanofi. K. Thangavelu is employed by Genzyme Corp and holds stocks in Sanofi. P. Rufi is employed by Sanofi. C. Simonson is employed by Fishawack Communications Ltd. N. De Stefano serves on the scientific advisory board for Merck Serono, Teva, Sanofi, Genzyme, Roche, Biogen, and Novartis Pharma AG; received travel funding from Biogen Idec, Merck Serono S.A., Bayer-Schering AG, Teva, Sanofi, Genzyme, Roche, Biogen, and Novartis Pharma AG; is an editorial advisory board member for Neurological Sciences; and received honoraria from Biogen Idec, Merck Serono S.A., Bayer-Schering AG, Teva, Sanofi, Genzyme, Roche, Biogen, and Novartis Pharma AG. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Sormani MP, De Stefano N. Defining and scoring response to IFN-beta in multiple sclerosis. Nat Rev Neurol 2013;9:504–512. [DOI] [PubMed] [Google Scholar]
- 2.Ziemssen T, De Stefano N, Pia Sormani M, Van Wijmeersch B, Wiendl H, Kieseier BC. Optimizing therapy early in multiple sclerosis: an evidence-based view. Mult Scler Relat Disord 2015;4:460–469. [DOI] [PubMed] [Google Scholar]
- 3.Rio J, Comabella M, Montalban X. Predicting responders to therapies for multiple sclerosis. Nat Rev Neurol 2009;5:553–560. [DOI] [PubMed] [Google Scholar]
- 4.Rio J, Castillo J, Rovira A, et al. . Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler 2009;15:848–853. [DOI] [PubMed] [Google Scholar]
- 5.Rio J, Rovira A, Tintore M, et al. . Evaluating the response to glatiramer acetate in relapsing-remitting multiple sclerosis (RRMS) patients. Mult Scler 2014;20:1602–1608. [DOI] [PubMed] [Google Scholar]
- 6.Romeo M, Martinelli V, Rodegher M, et al. . Validation of 1-year predictive score of long-term response to interferon-beta in everyday clinical practice multiple sclerosis patients. Eur J Neurol 2015;22:973–980. [DOI] [PubMed] [Google Scholar]
- 7.Sormani MP, Rio J, Tintore M, et al. . Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler 2013;19:605–612. [DOI] [PubMed] [Google Scholar]
- 8.Sormani MP, Gasperini C, Romeo M, et al. . Assessing response to interferon-beta in a multicenter dataset of patients with MS. Neurology 2016;87:134–140. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor P, Wolinsky JS, Confavreux C, et al. . Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011;365:1293–1303. [DOI] [PubMed] [Google Scholar]
- 10.McDonald WI, Compston A, Edan G, et al. . Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127. [DOI] [PubMed] [Google Scholar]
- 11.O'Connor P, Comi G, Freedman MS, et al. . Long-term safety and efficacy of teriflunomide: nine-year follow-up of the randomized TEMSO study. Neurology 2016;86:920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 2015;4:329–333. [DOI] [PubMed] [Google Scholar]
- 13.Havrdova E, Galetta S, Stefoski D, Comi G. Freedom from disease activity in multiple sclerosis. Neurology 2010;74(suppl 3):S3–S7. [DOI] [PubMed] [Google Scholar]
- 14.Romeo M, Martinelli-Boneschi F, Rodegher M, et al. . Clinical and MRI predictors of response to interferon-beta and glatiramer acetate in relapsing-remitting multiple sclerosis patients. Eur J Neurol 2013;20:1060–1067. [DOI] [PubMed] [Google Scholar]
- 15.Prosperini L, Gallo V, Petsas N, Borriello G, Pozzilli C. One-year MRI scan predicts clinical response to interferon beta in multiple sclerosis. Eur J Neurol 2009;16:1202–1209. [DOI] [PubMed] [Google Scholar]
- 16.Sormani MP, Li DK, Bruzzi P, et al. . Combined MRI lesions and relapses as a surrogate for disability in multiple sclerosis. Neurology 2011;77:1684–1690. [DOI] [PubMed] [Google Scholar]
- 17.Lattanzi S, Danni M, Cerqua R, Taffi R, Provinciali L, Silvestrini M. Prediction of disability progression in fingolimod-treated patients. J Neurol Sci 2015;358:432–434. [DOI] [PubMed] [Google Scholar]
- 18.Sormani M, Signori A, Stromillo M, De Stefano N. Refining response to treatment as defined by the modified Rio score. Mult Scler 2013;19:1246–1247. [DOI] [PubMed] [Google Scholar]