Abstract
A CD1d-binding, invariant (i) natural killer T (NKT)-cell stimulatory glycolipid, α-Galactosylceramide (αGalCer), has been shown to act as an adjuvant. We previously identified a fluorinated phenyl ring-modified αGalCer analog, 7DW8-5, displaying a higher binding activity to CD1d molecule and more potent adjuvant activity than αGalCer. In the present study, 7DW8-5 co-administered intramuscularly (i.m.) with a recombinant adenovirus expressing a Plasmodium yoelii circumsporozoite protein (PyCSP), AdPyCS, has led to a co-localization of 7DW8-5 and a PyCSP in draining lymph nodes (dLNs), particularly in dendritic cells (DCs). This occurrence initiates a cascade of events, such as the recruitment of DCs to dLNs and their activation and maturation, and the enhancement of the ability of DCs to prime CD8+ T cells induced by AdPyCS and ultimately leading to a potent adjuvant effect and protection against malaria.
Keywords: Glycolipid adjuvant, CD1d, dendritic cells, lymph node, adenovirus, malaria vaccine, co-localization, protection, CD8+ T cells, intramuscular injection
Introduction
An effective vaccine would provide an attractive strategy for preventing and controlling malaria. The adenovirus system, for a number of reasons, is attractive for the development of recombinant vaccines. The virion is relatively stable, and the foreign gene inserts remain unaltered after successive rounds of viral replication. Furthermore, the genome of adenoviruses has been extensively studied for many years and the complete DNA sequence of several serotypes is known, thus facilitating the manipulation of the adenovirus genome by recombinant DNA techniques (1). These vectors can efficiently transfer genes to non-replicating as well as to replicating cells, and the transferred genetic information remains epi-chromosomal, avoiding insertional mutagenesis and alteration of the cellular genotype (2). Another important advantage of adenoviruses as recombinant viral vectors lies in the fact that adenoviruses of serotypes 4 and 7 have been successfully and safely used for the immunization of a large number of US military recruits as prevention against acute respiratory disease outbreaks (3, 4, 5). An estimated 80% of young adults in the aggregate human population have circulating neutralizing antibodies to adenovirus serotypes 1, 2, 5 and 6 (6). In fact, such pre-existing immunity to adenovirus serotype 5 has previously been reported to inhibit the efficacy of a recombinant adenoviral vaccine (7). Due to the inhibitory effect of pre-existing immunity to adenoviruses, technologies are needed that can overcome this limitation, so that deployment of this promising vaccine vector can proceed. One solution to making adenoviral vector technology a viable vaccine platform for human use, lies in the identification of adjuvants that can enhance the immunogenicity of adenoviral vaccines.
We and others have shown that α-Galactosylceramide (αGalCer), which binds to CD1d molecules leading to stimulation of invariant natural killer T (iNKT) cells, displays significant biological activity, including an observable adjuvant effect (8, 9). αGalCer has shown its potential to act not only as a therapy for cancer, autoimmune and infectious diseases, but also as an adjuvant to enhance the efficacy of various vaccines, such as adenovirus, DNA, live-attenuated pathogen, recombinant protein and irradiated parasite vaccines (10–17).
Recently, we identified a synthetic analog of αGalCer, named 7DW8-5, having a shorter fatty acyl chain containing an aromatic group and terminal fluorine (18). 7DW8-5 was shown to have a stronger bioactivity toward iNKT cells and CD1d bearing DCs than αGalCer (18). Importantly, when co-administered with a recombinant adenovirus (Ad) expressing a major sporozoite antigen, the Plasmodium yoelii circumsporozoite protein (PyCS) protein, AdPyCS, 7DW8-5 exhibited a significantly stronger adjuvant effect than αGalCer [18]. More recently, we determined that 7DW8-5 can provide a very potent adjuvant effect on the cellular immunogenicity of an adenovirus-based malaria vaccine in non-human primates [19], guiding us to choose 7DW8-5 as a lead candidate for clinical evaluation with a malaria vaccine [20]. In view of the fact that there are currently a few adenovirus-based malaria vaccines against pre-erythrocytic stages [21–24] in Phase I/II trials, we believe it is of utmost importance to identify an adjuvant that can enhance the efficacy of current candidate adenovirus-based malaria vaccines.
Here, we investigated why 7DW8-5 displays a more potent adjuvant effect than αGalCer for an adenovirus-based malaria vaccine. Co-localization of 7DW8-5, but not αGalCer, with the vaccine upon their intramuscular conjoint administration appears to initiate a cascade of innate immune responses that lead to an induction of a more robust adaptive immune response, particularly CD8+ T cell response, against malaria.
Methods
Ethics statement
All animal experiments were carried out in strict accordance with the Policy on Humane Care and Use of Laboratory Animals of the United States Public Health Service. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at The Rockefeller University (Assurance # A3081-01). CO2 was used for euthanasia, and all efforts were made to minimize suffering.
Vaccines and parasites
Three recombinant serotype 5 adenoviruses, AdPyCS, Ad(PyCS-Luc) and Ad(PyCS-GFP) were constructed, which express P. yoelii circumsporozoite protein (PyCS), PyCS-luciferase fusion protein and PyCS-GFP fusion protein, respectively. Wild type P. yoelii parasites 17 XNL strain were maintained in the insectary facility of the Division of Parasitology, Department of Microbiology at New York University School of Medicine. Sporozoites were obtained from dissected salivary glands of infected Anopheles Stephensi mosquitoes 2 wk after infective blood meal.
Mice
Six to 8-week old female BALB/c mice were purchased from Taconic (Germantown, NY). Three tissue-specific H-2Kd transgenic mouse models, CD11c-Kd, huCD68-Kd and major histocompatibility complex-I-Kd (MHC I-Kd) transgenic mouse models, were established in our laboratory, in which H-2Kd were expressed under the control of CD11c, human CD68 and MHC I promoters, respectively, in C57BL/6 mice [25]. All of mice were maintained under standard conditions in The Laboratory Animal Research Center of The Rockefeller University.
IVIS imaging
Mice (n=5/group) were injected into anterior tibialis muscles of both legs with 5 μg of AF680-αGalCer, or AF680-7DW8-5. Mice also received i.m. administration of Ad(PyCS-Luc) by i.m. Twelve hours later, mice were anesthetized with isoflurane (Baxter, Deerfield, IL) and imaged with IVIS Lumina imaging system (Caliper, Hopkinton, MA) using cy5.5 filter. Tissue-specific autofluorescence and background were subtracted with Image Math software (Caliper). Fluorescence intensities in the region of interest (ROI) were quantified with ROI measurement tools (Caliper).
Assessment of antigen-specific CD8+ T-cell responses
The numbers of PyCS-specific, IFN-γ-secreting CD8+ T cells in the spleens of immunized mice were determined by an ELISpot assay, using a synthetic 9-mer peptide, SYVPSAEQI, corresponding to the CD8+ T cell epitope within the respective antigen, as previously described [17, 18]. The peptide was synthesized by Biosynthesis, Inc. (Lewisville, TX, USA). The level of PyCS specific CD8+ T-cell proliferation was determined by CFSE assay. Briefly, cells of PyCS-specific CD8+ T-cell line were incubated with 2 mM CFSE (Invitrogen, Eugene, OR.) for 10 min at 37°C. Cells were washed three times with 10ml complete culture medium. Five × 105 cells of CFSE-labeled PyCS-specific CD8+ T-cell line were co-cultured with 2 × 104 GFP+ DC sorted from PLN lymphocytes isolated from AdPyCS-GFP immunized mice in a 48 well plate for 4 days. Cells were harvested, washed and stained with APC-anti-CD3 antibody for evaluation of T cell proliferation.
Sporozoite challenge and assessment of protection
P. yoelii (17XNL strain) sporozoites were obtained from dissected salivary glands of infected Anopheles stephansi mosquitoes 2 weeks after infective blood meal. Sporozoite challenge experiments were performed as described previously [17, 18]. Briefly, immunized mice, as well as non-immunized mice as a control, were administered i.v. with 2 × 104 live P. yoelii sporozoites via tail vein, and 42 hr later, the parasite burden in the liver was determined by measuring parasite-specific ribosomal RNA using 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Parasite burden was described as a ratio of the absolute copy number of parasite ribosomal RNA to that of mouse GAPDH mRNA [17, 18].
Data Analysis
Statistical analyses of experimental and control data were evaluated by one-way ANOVA and Student t-test. p ≤ 0.05 was considered significant.
Results
Advancing 7DW8-5 into clinical development was based on our previous studies showing its high affinity for CD1d and stimulatory activity for iNKT cells ultimately yielding potent adjuvant activity against vaccines [18, 20]. A recombinant adenovirus vaccine expressing P. falciparum circumsporozoite protein has been shown to induce protective anti-malarial immunity in several clinical trials [21–24] leading us to determine the adjuvant effects of 7DW8-5 and its parental glycolipid, αGalCer, with a recombinant adenovirus expressing P. yoelli circumsporozoite protein (AdPyCS). Intramuscular (i.m.) administration was used, as it is one of the more practical routes for vaccine administration, compared with the intravenous (i.v.) administration. Either glycolipid co-jointly administered i.m. with AdPyCS significantly enhanced PyCS-specific CD8+ T-cell responses, with 7DW8-5 exerting nearly two-fold stronger adjuvant effect than αGalCer (Fig. 1A). However, it is noteworthy that when malaria vaccines were administered by i.m. route and glycolipids were administered by i.v. route, both glycolipids only modestly enhanced the PyCS-specific CD8+ T-cell response (Fig. 1A). Interestingly, when malaria vaccines and glycolipids were administered i.m. on opposite limbs, the adjuvant effect of 7DW8-5 was completely abolished, while αGalCer moderately enhanced the malaria-specific CD8+ T-cell response (Fig. 1A). Collectively, these results highlight the importance of co-joint administration of a vaccine and 7DW8-5 for maximizing vaccine efficacy.
Figure 1.
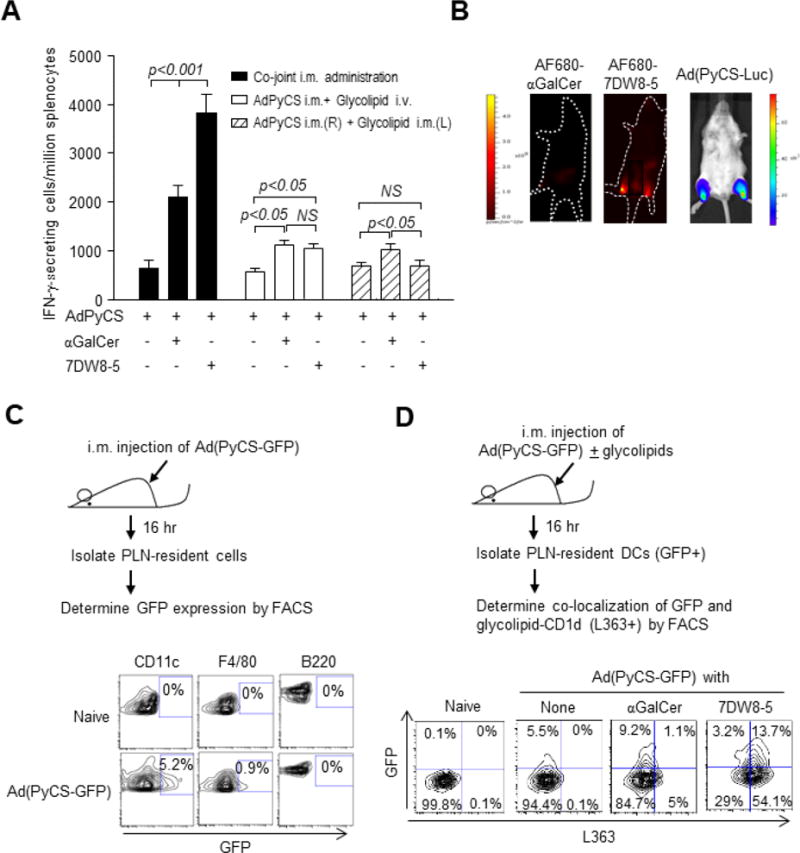
The potent 7DW8-5 adjuvant effect is dependent on the route of its administration. (A) Groups of BALB/c mice were administered i.m. (anterior tibialis muscles) with 5×108 pfu AdPyCS alone or co-jointly with 1 μg αGalCer or 7DW8-5. The solid column represents co-joint i.m. administration of the vaccine and respective glycolipid; the unfilled column represents i.m. administration of the vaccine, immediately followed i.v. administration of each glycolipid; the striped column represents i.m. administration of the vaccine and glycolipid in opposite legs. Twelve days later, splenocytes were harvested, and the relative number of PyCS-specific CD8+ T cells was determined with IFN-γ ELISpot assay. The results are expressed as mean ± S.D of four mice in each group. (B) BALB/c mice (n=4/group) were injected i.m. (anterior tibialis muscles) with 5 μg AF680-αGalCer or AF680-7DW8-5, and 12 hours later, images were collected using Lumina IVIS. Images from one representative mouse are shown. Another group of four BALB/c mice were injected i.m. with 5 × 109 v.p. of Ad(PyCS-Luc). Twelve hr later, mice were i.p. injected with 200 μL of 15 mg/mL D-luciferin, and fluorescent signal of luciferin was collected with Lumina IVIS. One representative image is shown. (C) BALB/c mice (n=4/group) were injected i.m. with 5×109 v.p. of Ad(PyCS-GFP). Sixteen hr after immunization, lymphocytes were isolated from PLNs, and GFP expression in DCs, macrophages and B cells were determined by flow cytometry. Naïve mice were used as a negative control. Histograms from one representative mouse in each group are shown. (D) Sixteen hr after Ad(PyCS-GFP) and glycolipid immunization later, PLNs were isolated and GFP-expressing DCs and CD1d bound glycolipids were determined by anti-L363 antibody. DCs collected from naïve mice were a negative control. Histograms from one representative mouse in each group are shown.
Co-localization of 7DW8-5 and PyCS by PLN-resident DCs
One of the key questions is to determine if the glycolipid and vaccine antigen need to co-localize for the glycolipid to act efficiently as an adjuvant. We first chose an adenovirus-based vaccine, because the recombinant adenovirus usually expresses only a single foreign antigen making the experimental system very simple. To address this question, infrared fluorochrome Alexa Fluor 680 (AF680)-labeled glycolipids were first synthesized, as described previously [26], and injected i.m. into the anterior tibialis muscles of both legs of BALB/c mice and biodistribution was determined using a whole body in vivo imaging system (IVIS) 6 hours later. Following AF680-7DW8-5 injection, punctate fluorescence regions were observed, and the anatomical site of the fluorescence appears to correlate with the popliteal lymph nodes (PLNs) (Fig. 1B). Punctate fluorescent regions were not observed in mice injected with either AF680-αGalCer (Fig. 1B). Using Ad(PyCS-Luc), a recombinant adenovirus expressing PyCS antigen fused to luciferase (PyCS-Luc), we determined the in vivo localization of the antigen after vaccination into anterior tibialis muscle. PyCS-Luc was expressed at dLNs, namely PLNs, which look coincide with the location of AF680-labeled 7DW8-5 (Fig. 1B). To determine the cell types that express GFP, a recombinant adenovirus expressing PyCS antigen fused to GFP, Ad(PyCS-GFP) was injected into mice i.m. (Fig. 1C). GFP was detected primarily among PLN-resident DCs, whereas only a small fraction of macrophages and no B cells expressed GFP (Fig. 1C). Following co-joint administration of each glycolipid with Ad(PyCS-GFP), we found that 13.7% of PLN-resident DCs were positive for 7DW8-5-CD1d complex as determined by L363 antibody that recognizes glycolipid:mCD1d complex [27] and express GFP, while only 1.1% co-localize the αGalCer-CD1d complex and express GFP (Fig. 1D). These results indicate that upon i.m. vaccination with AdPyCS and 7DW8-5 but not αGalCer, both antigen and adjuvant travel to PLNs and are co-expressed by resident DCs.
7DW8-5 exerts a potent adjuvant effect for AdPyCS vaccine by facilitating maturation/activation of DCs
Next, we sought to determine whether i.m. co-administration of 7DW8-5 with AdPyCS enhances the activation/maturation of antigen-presenting DCs in PLNs, ultimately leading to its potent adjuvant effect for AdPyCS vaccine. BALB/c mice were immunized i.m. with Ad(PyCS-GFP) alone or together with each glycolipid and the number and activation/maturation status of GFP-expressing PLN-resident DCs were determined. Co-administration with αGalCer moderately enhanced the number of GFP+ DCs and MHC-II and CD86 expression compared with administration of Ad(PyCS-GFP) alone (Fig. 2A–C). However, co-administration with 7DW8-5 resulted in ~2-fold more GFP+ DC recruitment to PLNs and MHC-II and CD86 expression compared to αGalCer (Fig. 2B, C). 7DW8-5 not only increased the number of antigen-presenting DCs in dLNs, but also enhanced their maturation/activation status.
Figure 2.
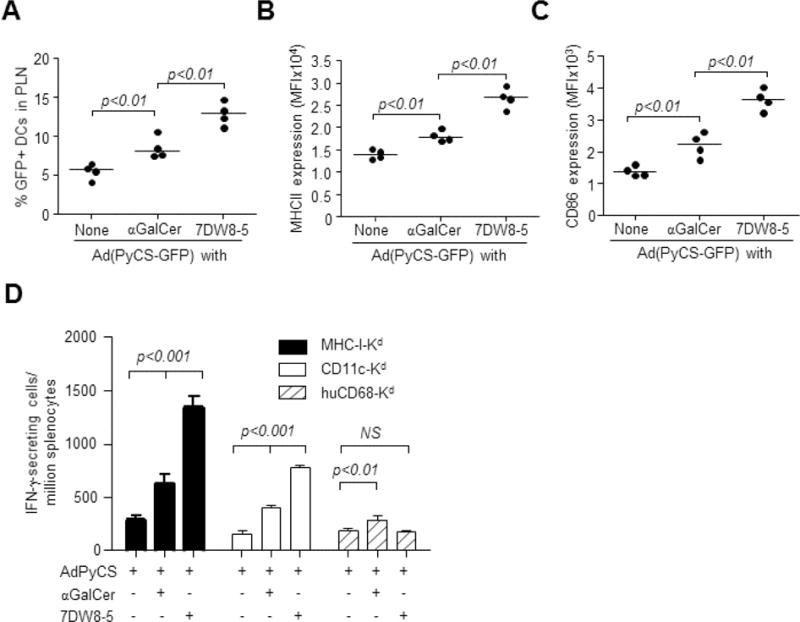
Co-localization of 7DW8-5 and AdPyCS in dLN-resident DCs facilitates activation and recruitment of the DCs and enhances T-cell immunogenicity and efficacy of AdPyCS vaccine. (A–C) BALB/c mice (n=4/group) were immunized i.m. with 5 × 109 v.p. of Ad(PyCS-GFP) alone or together with 1 μg of each glycolipid. Sixteen hr later, lymphocytes were isolated from PLNs, and the (A) the percentage of GFP+ DC, (B) MHC II expression and (C) CD86 expression were determined by FACS. Data for individual mice are shown; line represents mean. (D) MHC I-Kd, CD11c-Kd and huCD68-Kd transgenic mice (n=4/group) were immunized i.m. with 5 × 109 v.p. of AdPyCS alone or together with 1 μg each glycolipid. Twelve days later, splenocytes were harvested, and the PyCS-specific CD8+ T-cell response was determined by an IFN-γ ELIspot assay. Mean ± S.D is shown.
The potent PyCS-specific CD8+ T-cell response identified in Fig. 1A can only be elicited when SYVPSAEQI, an immunodominant epitope, is presented by MHC-class I Kd molecule to CD8+ T cells in mice. Therefore, we sought to determine the role of Kd molecules expressed by PLN-resident DCs in mediating the adjuvant effect of 7DW8-5. We used C57BL/6 transgenic (Tg) mice with tissue-specific Kd expression, including MHC-I-Kd Tg expressing Kd molecule on all nucleated cells and CD11c-Kd or huCD68-Kd Tg, expressing Kd molecule only on DCs or macrophages, respectively [25]. Tg mice were immunized i.m. with AdPyCS alone or together with a glycolipid, and the SYVPSAEQI-specific, Kd-restricted CD8+ T-cell response elicited by AdPyCS immunization was enhanced nearly 4-fold by 7DW8-5 co-administration in both MHC-I-Kd and CD11c-Kd Tg mice, but not in huCD68-Kd Tg mice (Fig. 2D). These data confirm that 7DW8-5 displays its adjuvant effect only through MHC-I, Kd molecules expressed on DCs. Interestingly, αGalCer not only enhanced the response by 2-fold in MHC-I-Kd and CD11c-Kd Tg mice, but also showed a marginal adjuvant effect in huCD68-Kd Tg mice (Fig. 2D) possibly due to the systemic biodistribution of αGalCer following i.m. injection.
To determine if antigen-presenting DCs activated/matured by 7DW8-5 have a better ability to prime and activate antigen-specific T cells, we immunized BALB/c mice i.m. with Ad(PyCS-GFP) alone or together with αGalCer or 7DW8-5. GFP+ PLN-resident DCs were isolated and co-cultured with a CFSE-labeled PyCS-specific CD8+ T-cell line, generated as previously described [28] (Fig. S1). After 4-days, the proliferation of the PyCS-specific CD8+ T-cell line was determined (Fig. 3A), and DCs isolated from PLNs of mice receiving Ad(PyCS-GFP) with 7DW8-5 stimulated the most antigen-specific CD8+ T-cell proliferation (Fig. 3B, C).
Figure 3.
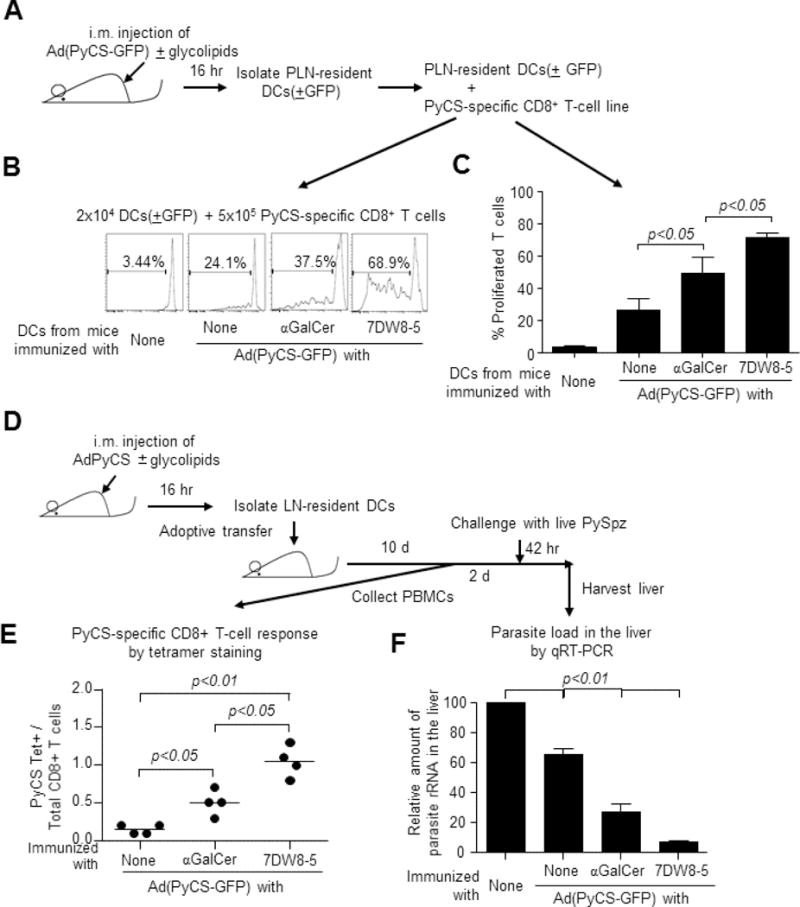
(A) BALB/c mice (n=20/group) were immunized i.m. with 5×109 v.p. of Ad(PyCS-GFP) alone or together with 1 μg of each glycolipid. Sixteen hr later, GFP+ DCs were sorted, and 2 × 104 GFP+ DCs were co-cultured with 5×105 CFSE-labeled, PyCS-specific CD8+ T cells in triplicate. Four days later, CFSE fluorescence was determined by flow cytometry. (B) Histograms from one representative experiment and (C) the mean ± S.D. of the triplicate experiments are shown. (D) BALB/c mice (n=20/group) were immunized i.m. with 5 × 109 v.p. of AdPyCS alone or together with 1 μg of each glycolipid. Sixteen hr later, PLNs-resident DCs were isolated and adoptively transferred i.v. to naïve BALB/c mice at 7.5 × 105 cells/mouse. (E) Ten days later, blood was collected and the percentage of PyCS-specific CD8+ T cells present among CD8+ T cells was determined by a flow cytometric analysis using SYVPSAEQI-loaded H-2Kd tetramer. (F) Two days later the same transferred mice, as well as naïve BALB/c mice were challenged with 2 × 104 live PySpz by i.v. and 42 hr later and the parasite burden in the liver was determined by quantifying the amount of parasite-specific rRNA by a real-time qRT-PCR.
Our ultimate question was whether quantitatively/qualitatively improved PLN-residing DCs following 7DW8-5 i.m. injection enhanced the protective efficacy of a vaccine, AdPyCS. To answer this question, PLN-resident CD11c+ DCs were harvested from BALB/c mice immunized i.m. with Ad(PyCS-GFP) alone or together with each glycolipid and adoptively transferred to naïve BALB/c mice. Antigen-specific CD8+ T cell responses in blood were assessed using SYVPSAEQI-loaded H-2Kd tetramer (Fig. 3D). Both glycolipids induced more PyCS-specific CD8+ T cells compared with Ad(PyCS-GFP) alone, and 7DW8-5 induced significantly more than αGalCer (Fig. 3E). The highest level of protective anti-malaria immunity as determined by the amount of parasite-specific rRNA following challenge with 2 × 104 viable P. yoelii sporozoites (Fig. 3D) was observed in mice adoptively transferred with DCs from mice immunized with AdPyCS and 7DW8-5 (Fig. 3F). Collectively, these results indicate that i.m. co-administration of 7DW8-5 with AdPyCS increased number and activation/maturation level of PyCS antigen-presenting DCs in PLNs, ultimately leading to a more potent PyCS-specific CD8+ T-cell response, and more importantly, protective immunity against malaria.
Discussion
We previously identified a new CD1d-binding, iNKT cell-stimulating glycolipid, named 7DW8-5, which have similar chemical structures to its parental glycolipid, αGalCer, with difference only in the fatty acyl chain [18]. 7DW8-5 binds CD1d molecules tighter, stimulates iNKT cells stronger and induces more DC activation/maturation than αGalCer in vitro, leading to display a more potent adjuvant activity than αGalCer when co-administered i.m. with an adenovirus-based malaria vaccine [18]. A number of studies have shown that αGalCer acts as an adjuvant for various vaccines in mice [12–17]. Furthermore, our group has shown 7DW8-5 could provide a significant adjuvant effect on the cellular immunogenicity of an adenovirus-based malaria vaccine in non-human primates [19]. Therefore, It is imperative for us to determine why 7DW8-5 can enhance the immunogenicity of adenovirus-based vaccine more than αGalCer as an adjuvant.
To address this question, we generated a recombinant adenovirus co-expressing PyCS and luciferase or GFP. We also used AF680-labeled glycolipids for the purpose of identifying their localization in vivo. As we previously found, 7DW8-5, but not αGalCer, retains in the dLN (Fig. 1B), likely due to a higher binding affinity to CD1d. When we performed IVIS studies after administrating Ad(PyCS-Luc), PyCS fused to luciferase was also identified in the dLN (Fig. 1B), suggesting that both 7DW8-5 and AdPyCS localize in the same LN. In order to further determine which cell type PyCS-GFP would end up with, PLN-resident cells were isolated from mice immunized with Ad(PyCS-GFP) and stained with various antibodies against cell markers. CD11c+DCs were found to be primary cells that express PyCS-GFP (Fig. 1C). Furthermore, when Ad(PyCS-GFP) was conjointly immunized with glycolipids, 7DW8-5 but not αGalCer was found to co-localized with GFP-expressing DCs (Fig. 1D).
In the present study, co-localization of 7DW8-5 with the malaria vaccine was found to induce not only maturation/activation of dLN-resident DCs, but also their recruitment (Fig. 2A–C). Using three lines of Kd Tg mice that differed in their expression of MHC-class I, Kd, molecules, which are known to present PyCS protein-derived, immunodominant epitope to CD8+ T cells [25], we confirmed that the co-localization of 7DW8-5 and malaria antigen by DCs is crucial for 7DW8-5 to display a potent adjuvant activity (Fig. 2D). 7DW8-5 was able to exert a significant adjuvant effect to enhance the CD8+ T-cell response in CD11c-Kd Tg mice, in which only DCs express Kd molecules, albeit at a lesser degree compared to the adjuvant effect seen in MHC-I-Kd Tg mice, in which all nucleated cells express Kd molecules.
The role of DCs was further supported by the studies, in which GFP+DCs isolated from mice co-administered Ad(PyCS-GFP) and 7DW8-5 were able to prime PyCS-specific CD8+ T cells in vitro stronger than GFP+DCs from mice co-administered Ad(PyCS-GFP) and αGalCer (Fig. 3B, C). Finally, the quantitatively and qualitatively improved PyCS-presenting DCs initiated a more robust malaria-specific CD8+ T-cell response in vivo which translated to the induction of more potent protective anti-malaria immunity (Fig. 3E, F). These results provide direct evidence that 7DW8-5 delivered by i.m. injection transformed DCs to better APCs in the local dLNs, thus priming and stimulating vaccine-induced CD8+ T cells more efficiently and inducing a more potent anti-malaria immunity. We anticipate that the mechanism of action elucidated here resulted in the potent adjuvant effect of 7DW8-5 previously observed with adenovirus malaria vaccines [18–20].
In conclusion, we found that in contrast with αGalCer, our novel CD1d-binding NKT cell-stimulating glycolipid, 7DW8-5, displays a localized biodistribution upon i.m. administration, by binding more tightly to CD1d molecules expressed by DCs that reside in the dLNs. This localized biodistribution of 7DW8-5 facilitates the activation of iNKT cells in the LNs, which in turn, induces activation/maturation of DCs and their recruitment to dLN. When 7DW8-5 is co-administered i.m. with an adenovirus-based malaria vaccine, AdPyCS, 7DW8-5 and the vaccine were found to be not only co-localized in PLNs, but also co-present in the PLN-resident DCs. The quantitative and qualitative improvement of PLN-resident DCs presenting malaria antigens, results in enhancing the levels of malaria-specific CD8+ T-cell response and ultimately, the level of protective anti-malaria immunity induced by the malaria vaccine. Thus, the current findings should be imperative for the future clinical applications of a CD1d-binding iNKT-cell ligand, 7DW8-5, as a potent adjuvant for various vaccines.
Supplementary Material
Acknowledgments
We thank Dr. Vincent Sahi for assisting with FACS analysis. We also thank the NIH Tetramer Core Facility at Emory University for generating and providing an Allophycocyanin-labeled H-2Kd/SYVPSAEQI-tetramer.
Funding
This work was supported by grants from NIH AI070258 and AI102891 (both to M.T.).
Abbreviations
- αGalCer
α-Galactosylceramide
- Ad
Adenovirus
- DCs
dendritic cells
- dLNs
draining lymph nodes
- i.m.
intramuscularly
- iNIKT
invariant natural killer T
- PyCSP
Plasmodium yoelii circumsporozoite protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
X.L. and M.T. designed the study. X.L. and R.F. carried out the experiments. A.K. labeled glycolipids with fluorochromes. J.H. provided several Kd-transgenic mouse lines. S.A.P. provided L363 antibody. X.L. and M.T. analyzed the data and prepared the manuscript.
Competing Financial Interests
M.T. is inventor for United States patent Nos. 7534434, 7771726, 7923013, 8163290, and 8586051. The remaining authors have no financial conflicts of interest.
References
- 1.Graham FL, Prevec L. Adenovirus based expression vectors and recombinant vaccines. In: Ellis RW, editor. Vaccines New approachs to immunological problems. Butterworth Heinemann; USA: 1992. pp. 363–389. [DOI] [PubMed] [Google Scholar]
- 2.Crystal RG. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 3.Top FH, Jr, Buescher EL, Bancroft WH, Russell PK. Immunization with live type 7 and 4 vaccines. I. Safety, infectivity, antigenicity, and potency of adenovirus type 7 vaccine in humans. J Infect Dis. 1971;124:155–160. doi: 10.1093/infdis/124.2.148. 1971. [DOI] [PubMed] [Google Scholar]
- 4.Top FH., Jr Control of adenovirus acute respiratory disease in U.S. Army trainees. Yale J Biol Med. 1975;48:185–195. [PMC free article] [PubMed] [Google Scholar]
- 5.Chaloner-Larsson G, Contrearas G, Furesz J. Immunization of Canadian Armed Forces personnel with live types 4 and 7 adenovirus vaccines. Can J Public Health. 1986;77:367–370. [PubMed] [Google Scholar]
- 6.Schaechter M, Medoff G, Eisenstein BI. Mechanisms of Microbial Disease. Second. Williams and Wilkins; USA: 1993. Adenoviruses; p. 493. [Google Scholar]
- 7.Zak DE, Andersen-Nissen E, Peterson ER, Sato A, Hamilton MK, Borgerding J, Krishnamurty AT, Chang JT, Adams DJ, Hensley TR, Salter AI, Morgan CA, Duerr AC, De Rosa SC, Aderem A, McElrath MJ. Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T-cell responses but is attenuated by preexisting Ad5 immunity. Proc Natl Acad Sci USA. 2012;109:E3503–12. doi: 10.1073/pnas.1208972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annual review of immunology. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 9.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Ann Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 10.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 11.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Ann Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 12.Venkataswamy MM, Baena A, Goldberg MF, Bricard G, Im JS, Chan J, Reddington F, Besra GS, Jacobs WR, Jr, Porcelli SA. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 2009;183:1644–1656. doi: 10.4049/jimmunol.0900858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Chen A, Li X, Chen Z, Zhang W, Song Y, Gurner D, Gardiner D, Basu S, Ho DD, Tsuji M. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosylceramide. Vaccine. 2008;26:1807–1816. doi: 10.1016/j.vaccine.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175:3309–3317. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 15.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 16.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, Tsuji M. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Fujio M, Imamura M, Wu D, Vasan S, Wong CH, Ho DD, Tsuji M. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci USA. 2010;107:13010–13015. doi: 10.1073/pnas.1006662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padte NN, Boente-Carrera M, Andrews CD, McManus J, Grasperge BF, Gettie A, Coelho-dos-Reis JG, Li X, Wu D, Bruder JT, Sedegah M, Patterson N, Richie TL, Wong CH, Ho DD, Vasan S, Tsuji M. A glycolipid adjuvant, 7DW8-5, enhances CD8+ T cell responses induced by an adenovirus-vectored malaria vaccine in non-human primates. PLoS One. 2013;8:e78407. doi: 10.1371/journal.pone.0078407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padte NN, Li X, Tsuji M, Vasan S. Clinical development of a novel CD1d-binding NKT cell ligand as a vaccine adjuvant. Clin Immunol. 2011;140:142–151. doi: 10.1016/j.clim.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimani D, Jagne YJ, Cox M, Kimani E, Bliss CM, Gitau E, Ogwang C, Afolabi MO, Bowyer G, Collins KA, Edwards N, Hodgson SH, Duncan CJ, Spencer AJ, Knight MG, Drammeh A, Anagnostou NA, Berrie E, Moyle S, Gilbert SC, Soipei P, Okebe J, Colloca S, Cortese R, Viebig NK, Roberts R, Lawrie AM, Nicosia A, Imoukhuede EB, Bejon P, Chilengi R, Bojang K, Flanagan KL, Hill AV, Urban BC, Ewer KJ. Translating the Immunogenicity of Prime-boost Immunization With ChAd63 and MVA ME-TRAP From Malaria Naive to Malaria-endemic Populations. Mol Ther. 2014;22:1992–2003. doi: 10.1038/mt.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedegah M, Tamminga C, McGrath S, House B, Ganeshan H, Lejano J, Abot E, Banania GJ, Sayo R, Farooq F, Belmonte M, Manohar N, Richie NO, Wood C, Long CA, Regis D, Williams FT, Shi M, Chuang I, Spring M, Epstein JE, Mendoza-Silveiras J, Limbach K, Patterson NB, Bruder JT, Doolan DL, King CR, Soisson L, Diggs C, Carucci D, Dutta S, Hollingdale MR, Ockenhouse CF, Richie TL. Adenovirus 5-vectored P. falciparum vaccine expressing CSP and AMA1. Part A: safety and immunogenicity in seronegative adults. PLoS One. 2011;6:e24586. doi: 10.1371/journal.pone.0024586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamminga C, Sedegah M, Regis D, Chuang I, Epstein JE, Spring M, Mendoza-Silveiras J, McGrath S, Maiolatesi S, Reyes S, Steinbeiss V, Fedders C, Smith K, House B, Ganeshan H, Lejano J, Abot E, Banania GJ, Sayo R, Farooq F, Belmonte M, Murphy J, Komisar J, Williams J, Shi M, Brambilla D, Manohar N, Richie NO, Wood C, Limbach K, Patterson NB, Bruder JT, Doolan DL, King CR, Diggs C, Soisson L, Carucci D, Levine G, Dutta S, Hollingdale MR, Ockenhouse CF, Richie TL. Adenovirus-5-vectored P. falciparum vaccine expressing CSP and AMA1. Part B: safety, immunogenicity and protective efficacy of the CSP component. PLoS One. 2011;6:e25868. doi: 10.1371/journal.pone.0025868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang I, Sedegah M, Cicatelli S, Spring M, Polhemus M, Tamminga C, Patterson N, Guerrero M, Bennett JW, McGrath S, Ganeshan H, Belmonte M, Farooq F, Abot E, Banania JG, Huang J, Newcomer R, Rein L, Litilit D, Richie NO, Wood C, Murphy J, Sauerwein R, Hermsen CC, McCoy AJ, Kamau E, Cummings J, Komisar J, Sutamihardja A, Shi M, Epstein JE, Maiolatesi S, Tosh D, Limbach K, Angov E, Bergmann-Leitner E, Bruder JT, Doolan DL, King CR, Carucci D, Dutta S, Soisson L, Diggs C, Hollingdale MR, Ockenhouse CF, Richie TL. DNA prime/Adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS One. 2013;8:e55571. doi: 10.1371/journal.pone.0055571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, Li X, Kohno K, Hatano M, Tokuhisa T, Murray PJ, Brocker T, Tsuji M. Generation of tissue-specific H-2Kd transgenic mice for the study of K(d)-restricted malaria epitope-specific CD8+ T-cell responses in vivo. J Immunol Methods. 2013;387:254–261. doi: 10.1016/j.jim.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Vo-Hoang Y, Micouin L, Ronet C, Gachelin G, Bonin M. Total enantioselective synthesis and in vivo biological evaluation of a novel fluorescent BODIPY alpha-galactosylceramide. Chembiochem. 2003;4:27–33. doi: 10.1002/cbic.200390009. [DOI] [PubMed] [Google Scholar]
- 27.Yu KO, Im JS, Illarionov PA, Ndonye RM, Howell AR, Besra GS, Porcelli SA. Production and characterization of monoclonal antibodies against complexes of the NKT cell ligand alpha-galactosylceramide bound to mouse CD1d. J Immunol Methods. 2007;323:11–23. doi: 10.1016/j.jim.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodrigues MM, Cordey AS, Arreaza G, Corradin G, Romero P, Maryanski JL, Nussenzweig RS, Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991;3:579–85. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


