Abstract
Traditional breeding for high-yielding rice has been dependent on the widespread cultivation of gibberellin (GA)-deficient semi-dwarf varieties. Dwarfism lowers the “center of gravity” of the plant body, which increases resistance against lodging and enables plants to support high grain yield. Although this approach was successful in latter half of the 20th century in rice and wheat breeding, this may no longer be enough to sustain rice with even higher yields. This is because relying solely on the semi-dwarf trait is subject to certain limitations, making it necessary to use other important traits to reinforce it. In this review, we present an alternative approach to increase lodging resistance by improving the quality of the culm by identifying genes related to culm quality and introducing these genes into high-yielding rice cultivars through molecular breeding technique.
Keywords: rice, lodging resistance, culm, quantitative trait loci, pyramiding, gibberellin
1. Introduction
It is projected that the global agricultural production must further increase by up to 60–110 percent to attain food security by 2050.1) It therefore becomes an urgent concern to improve food production if we are to avert a possible global food scarcity decades from now. Looking back into the past, remarkable success was attained in increasing rice and wheat grain production in the 1960’s through the development and widespread adoption of high-yielding varieties.2) This led to a sudden major increase in grain production, thereby avoiding an impending large-scale famine at that time.3) This milestone has since been known as the Green Revolution. Two salient features of the Green Revolution was the widespread use of semi-dwarf varieties along with the generous use of fertilizers. Normally, under heavy fertilization, traditional varieties of wheat and rice inevitably grow very tall and therefore, become prone to lodging, which eventually became much less of a problem with the advent of semi-dwarf varieties. By preventing yield losses tied to lodging, the Green Revolution was thus able to double the yield in both rice and wheat with just the same amount of agricultural land used. However, many years after the initial success of the Green Revolution, recent advances in terms of increasing yield have been apparently small, and so crop breeders are once again faced with the challenge to further develop new varieties to increase grain yield.
In addition to the population pressure, the adverse effect of climate change on global crop productivity also makes it even more necessary to increase grain yield. As estimated by Yonemura et al. (1998),4) an increase in temperature in Japan by 2 ℃ to 4 ℃, would correspond to about 5 to 12% decrease in rice yield. Also, acid rain can lead to oxidation of the soil, which inhibits root and leaf growth, hence negatively influencing crop yield (Kang and Ishii, 2003).5) High yielding rice is also advantageous from the viewpoint of nature protection and maintenance of the species. This is because high yielding cultivars can potentially reduce the necessary land area to be cultivated, which prevents further encroachment of farm lands into natural vegetation.
For these reasons, developing higher-yielding varieties promises to provide long-term solution for food security and environment protection but there remains a lot of challenges to realize it. One reason for the difficulties in developing higher-yielding cultivars is still due to lodging itself, even after the Green Revolution. Indeed, rice breeders have introduced some grain-increasing genes into rice cultivars, but such rice varieties tend to develop larger panicles with more grains than the culm could support, hence, resulting again in lodging. Thus, for continuous increase in crop yield, it is inevitable to increase lodging resistance by using novel approaches that will not cause further reduction in plant height, which has negative effects on crop productivity. In this review, we first introduce the three types of lodging, explain the mechanism of lodging resistance by dwarfism, and then present alternative approaches to improve lodging resistance.
2. Mechanisms of lodging
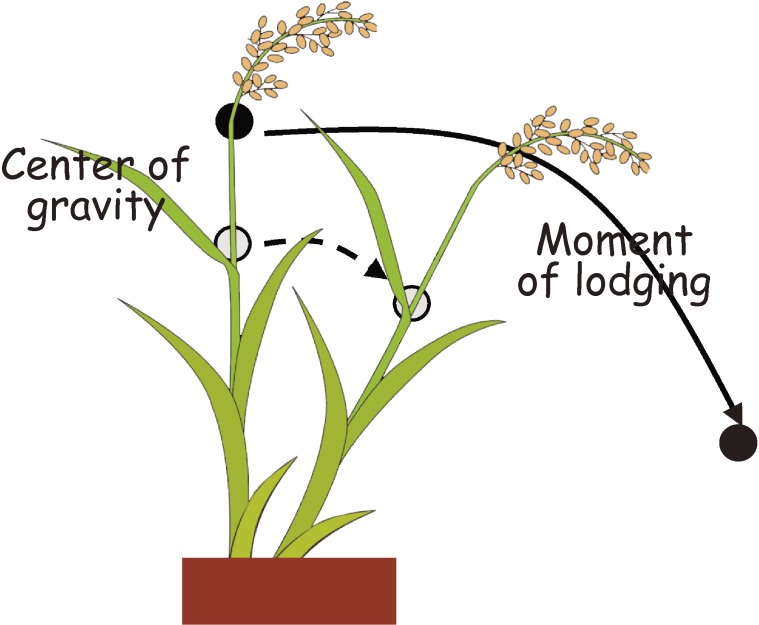
Cereal crops generally exhibit three types of lodging6) (Fig. 1). First is culm bending-type lodging which occurs when plants fail to resist bending pressure, as is often seen in the upper internodes of rice affected by strong winds and rain. Dwarfism in cereals acts to lower the “center of gravity” of the plant body, which decreases the moment of lodging (Fig. 2).7) Second is culm breaking-type lodging that usually affects the lower internodes when there is excessive bending pressure at the upper internodes. This type of lodging is mainly influenced by the morphology and quality of the culm.8,9) The last type is root lodging, which happens when the roots give in to the weight of the above-ground parts.10) Root lodging is not a major concern in transplanted rice as transplanting favors the development of a well-established root system. This may, however, seriously affect those planted through the direct-seeding system since plants have the tendency to develop shallower root systems.
Figure 1.
Three types of lodging in rice: culm bending, culm breaking, and root lodging.
Figure 2.
Mechanism for the increased lodging resistance of dwarf rice. Reduced plant height lowers the center of gravity and improves bending-type lodging resistance.
As stated above, dwarfism improves bending-type lodging resistance which is the reason why dwarf varieties show higher lodging resistance. However, recent studies revealed that dwarfism itself also has some inadequate characteristics when considering crop productivity and culm strength as presented below.
Mechanisms that lead to semi-dwarf rice in sd-1 and Rht-1.
The genes responsible for imparting short stature to rice and wheat used in the Green Revolution were identified about 15 years ago.11,12) Interestingly, both genes are related to a plant growth hormone, gibberellin (GA). Rice sd-1 mutants have loss-of-function mutations in one of the GA-synthesizing genes, namely GA 20 oxidase2 (GA20ox2) or Semi Dwarf-1 (SD-1).11) On the other hand, the wheat Green Revolution gene encodes a DELLA protein, Reduced height-1 (Rht-1), which functions as a suppressor of GA signaling.12) It is noteworthy that only GA-related dwarfing genes contributed to the Green Revolution both for rice and wheat, despite the fact that there are a lot of genes involved in stem elongation and that their mutation also cause dwarfism. This is mainly due to the unique function of GA as discussed in the literature; that is, GA deficiency specifically decreases plant height without any undesirable side effects on other essential plant morphology or physiology, which often happens when other dwarfing mechanisms are used.
3. sd-1 mutations have been repeatedly used for rice lodging resistance breeding
IR8, an indica rice known as the ‘miracle rice’, was developed by the International Rice Research Institute (IRRI) and has contributed to the rice Green Revolution in Asia.2,3) IR8 contains a 383-bp deletion from exon 1 to exon 2 in SD-1, which produces a null allele carrying a premature stop codon.11) On the other hand, some japonica varieties, such as Jikkoku, Reimei and Calrose76, which also contributed to the increased crop productivity in Japan and US, carry single-nucleotide substitutions leading to amino acid changes that only partially disrupt or weaken GA synthesis.11,13–15) The plant height of isogenic lines carrying the null allele from IR8 was lower than that of plants carrying the allele from Reimei, suggesting that the null allele from IR8 has a stronger effect on dwarfism than that of Reimei. Many indica varieties are taller than japonica varieties; and for this, the strong effect of the IR8 allele has proved to be very useful in reducing indica plant height to produce semi-dwarves. On the other hand, the milder alleles from japonica varieties such as in Reimei have been effective enough to induce semi-dwarfism in other japonica varieties. Ashikari et al. (2002)13) discussed that rice breeders were apparently able to recognize the different dwarf variants controlled by the various sd-1 alleles and consequently picked the most suitable allele for producing varieties with the desired height (Fig. 3).
Figure 3.
sd-1 mutant shows reduced plant height in rice. Original cultivars (WT) and sd-1 mutants (sd-1) are shown on the left and right side of each panel, respectively. This figure was constructed according to our original papers referred to as Ref. 13.
Asano et al. (2011)16) examined how many sd-1 alleles have been used for the generation of semi-dwarf varieties in rice breeding programs so far. They collected 53 semi-dwarf high yielding varieties from China, USA and Japan, and found 38 of them (72%) to possess an sd-1 allele (Table 1). Such sd-1 alleles were also found to be grouped into a total of seven different types, which account for all of the important semi-dwarf varieties produced in China such as Aijiao-Nante (1956), the first semi-dwarf variety ever bred in China,17) and 93-11, a paternal variety of both the hybrid rice and the major rice variety grown in China and Asia-Pacific regions.18) Such overlapping/repeated selections of different sd-1 alleles in various countries and the high frequency of sd-1 utilization in rice semi-dwarf varieties clearly demonstrate that the sd-1 mutation proved to be useful for breeders in producing the ideal rice stature in terms of increasing lodging resistance, at least during that time.16)
Table 1.
Distribution of sd-1 mutations in high-yielding semi-dwarf varieties. Of the 53 high-yielding semi-dwarf, 38 (72%) carried an sd-1 allele
| Variety | Allele-type | Variety | Allele-type | Variety | Allele-type | Variety | Allele-type |
|---|---|---|---|---|---|---|---|
| Minghui63 | IR8 | 9311 | 9311 | 98-110 | Reimei | Jia02-43 | wild |
| Teqing | IR8 | Zhenshan97B | 9311 | Bing02-105 | Reimei | ZH222 | wild |
| 9308 | IR8 | BobaiB | 9311 | R0308 | Reimei | Bing02-133 | wild |
| Peiai64 | IR8 | XieqingzaoB | 9311 | Bing02-09 | Reimei | Taihunuo | wild |
| Zhefu802 | IR8 | LongtepuB | 9311 | DiguB | Aijao-Nante | HanfenB | wild |
| Guangsi | IR8 | D297B | 9311 | V20B | Aijao-Nante | Kinmaze | wild |
| K17B | IR8 | II-32B | 9311 | Aijao-Nante | Aijao-Nante | Shirasenbon | wild |
| QingreB | IR8 | Gang46B | 9311 | Zhaiyeqing8 | Zhaiyeqing8 | Fukuhibiki | wild |
| 486B | IR8 | DshanB | 9311 | Xiushui04 | wild | ||
| 5N-76B | IR8 | H125B | 9311 | Xiushui63 | wild | ||
| GuangB | IR8 | Zhongjian100 | 9311 | Liantangzhao | wild | ||
| Guichao | IR8 | Xiushui11 | Jikkoku | 76-27B | wild | ||
| lemont | IR8 | Chunjiang026 | Jikkoku | Chunjiang025 | wild | ||
| M202 | IR8 | Bing01-223 | Jikkoku | HZ0302 | wild | ||
| M201 | IR8 | ZH233 | Jikkoku | Jia02-5 | wild |
4. Importance of lodging resistance for higher-yielding rice
As mentioned above, the widespread use of GA-related semi-dwarf plants during the Green Revolution was due to the improved resistance against bending pressure of the plant body of dwarf plants7) (Fig. 2). However, it has already been revealed that the use of GA-related semi-dwarf trait as a strategy to attain lodging resistance has certain limitations. In fact, a trend to avoid usage of semi-dwarf mutations for lodging resistance has apparently occurred in China. Zhou et al. (2016)19) investigated the changes in lodging-related traits along with the trend in rice genetics improvement in China by using 14 mega-varieties that were released and disseminated from the 1930s to 2005. According to the results, dramatic reduction in lodging index by the introduction of semi-dwarf varieties occurred in China in the mid-20th century just like the case of the Green Revolution. After 1980s, however, further shortening the plant height has ceased, but in an attempt to increase grain yield, improvement of culm strength by increasing internode diameter and culm fresh weight has been done.
Theoretically, if rice plants grew without lodging, improved semi-dwarf varieties would have lower grain yield than their taller original cultivars mainly because of their smaller biomass.20) As previously mentioned, many improved cultivars showing high crop yield already have a dwarf characteristic, and thus further dwarfism causes discernable negative effect on crop yield. In addition, recent studies have revealed that it also reduces the strength of rice culm (see below for details).20) For these reasons, as mentioned, we have to develop a novel strategy to improve the lodging resistance of rice; that is, to increase the physical strength of the culm, which should satisfy an important requirement for supporting higher grain yield.
5. Increased GA improves culm strength and biomass yield
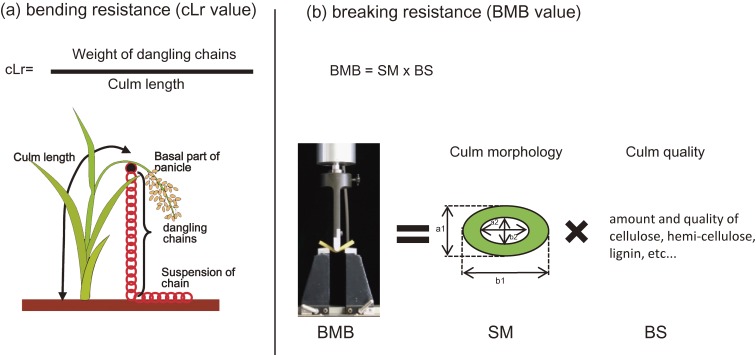
Curiously, although semi-dwarfism caused by sd-1 mutations have been repeatedly used for increasing the lodging resistance of rice, no detailed study has been conducted to explain the effect of GA-related dwarfism on rice lodging resistance and biomass yield. Okuno et al. (2014)20) performed such studies using various GA-related mutants/lines with semi-dwarf sd-1 mutants, intermediate (Tanginbozu), and severe dwarf (gid1-8 and slr1d-1) characteristics, or varieties with tall stature caused by increased level of GA (SD-1K and eui1). The bending-type lodging resistance of these rice plants were presented using the cLr parameter (Fig. 4a). The semi-dwarf sd-1 mutants showed ∼2-fold increase in bending resistance compared to their original cultivars, whereas severe dwarf lines showed much higher levels (Table 2). On the other hand, tall mutants showed reduced stem bending resistance. These results are consistent with the observation that dwarfism promotes bending-type lodging resistance.
Figure 4.
Bending and breaking-type lodging resistance and how they are analyzed. (a) To evaluate bending-type lodging resistance, a parameter called lodging resistance factor (cLr), is computed following the procedure of Grafius and Brown.47) (b) Using a load-testing machine, Tensilon RTM-25 Orientic, bending moment of the internode at breaking (BMB; g · cm) was assessed as described by Ookawa and Ishihara.20) Such stem physical property is computed as the product of the stem’s section modulus (SM) and the applied maximum bending stress (BS).41) SM = π/32 × (a13b1 − a23b2)/a1, where, a1 is the outer diameter of the minor axis in an oval cross-section, b1 is the outer diameter of the major axis in an oval cross-section, a2 is the inner diameter of the minor axis in an oval cross-section, and b2 is the inner diameter of the major axis in an oval cross-section.
Table 2.
Summary of morphological traits, lodging resistance and biomass production of GA mutants studied by Okuno et al. Values of each original cultivar was set as 1. Values improved in SD1K and eui1 compared to their original cultivar Nipponbare are shown in red. This table was constructed according to our original papers referred to as Ref. 18
| lodging resistance | ||||||||
|---|---|---|---|---|---|---|---|---|
| culm length | bending typea | breaking typebc | culm diameter (cm)c | lignin contentd | biomass (g) | tiller number | grain yield (g) | |
| sd1 mutants | 0.75 | 1.87 | 0.92 | 0.95 | 0.81 | 0.87 | 0.99 | 0.91 |
| Tanginbozu | 0.61 | 2.30 | 0.87 | 0.90 | 0.91 | 0.79 | 1.27 | 0.94 |
| gid1-8, Slr1-d1 | 0.46 | 4.01 | 0.74 | 0.77 | 0.74 | 0.76 | 1.58 | 0.60 |
| SD1K, eui1 | 1.37 | 0.67 | 1.38 | 1.05 | 1.24 | 1.41 | 0.95 | 0.97 |
acLr values (g cm−1) were measured to evaluate bending type resistance.
bBMB values (g cm−1) were measured to evaluate breaking type resistance.
c4th internodes were used to measure the breaking type resistance and culm diameters of rice.
dlignin content was calculated by total dry weight (%).
Another crucial factor for lodging resistance is the physical strength of culm, which is determined by the morphology and the quality of its components.21) Culm physical strength is often assessed in terms of bending moment at breaking (BMB) (Fig. 4b).22) Using this parameter, Okuno et al. identified that unlike taller plants, all of the dwarf lines showed decreased BMB (breaking-type lodging resistance) relative to their original cultivars (Table 2). To know the reason for this difference in BMB values, several culm traits of these mutants were further evaluated. As a result, the culm diameter of semi-dwarf mutants was slightly but significantly reduced in the lower internodes, whereas semi- and severe dwarf mutants showed a strong reduction in diameter in all internodes. In contrast, taller plants were found to have increased diameter all throughout the culm, demonstrating a positive correlation between GA and culm diameter in rice as set in red fonts in Table 2. Because lignin content positively correlates with the physical strength of the culm,21) Okuno et al. also measured the lignin content of the uppermost internode of each plant. As a result, all dwarf mutants showed reduced amount of lignin, whereas the tall ones, eui1 and SD-1K, had an increased and significantly increased lignin accumulation, respectively (Table 2). Taken together, higher GA content promotes increased culm diameter (morphology) and lignin content (quality), both of which lead to enhanced BMB. They also examined the effect of GA on other agronomic traits (Table 2). One of these is total dry weight per plant (biomass). As could be expected, dwarf and tall GA mutants/lines exhibited decreased and increased total biomass, respectively. As for tillering, the plants with intermediate to severe dwarfism showed increased tiller number per plant relative to their original cultivars, whereas the sd-1 mutants with mild dwarfism as well as the taller mutants did not show apparent differences relative to their original lines. Lastly, while the grain yield per plant was found to have decreased slightly in the mild and intermediate dwarf mutants, and severe in severe dwarf mutants, yield in taller plants remained stable and did not differ from their original cultivar.
In summary, they discussed that GA can positively or negatively affect the plant’s lodging resistance. GA deficiency and insensitivity both lead to dwarfism, resulting in improved bending-type lodging resistance. At the same time, these conditions also rob the plant of culm strength by reducing culm diameter and lignin content, thereby decreasing breaking-type lodging resistance. Apart from lodging resistance, there is also an increasing demand for biofuels which could be derived from lignocellulosic biomass. However, as plants gain lodging resistance due to GA-related dwarfism, their biomass-producing ability become compromised. These simply demonstrate the intrinsic paradox that needs to be faced by breeders when using GA-deficient dwarf plants to increase lodging resistance and biomass productivity. Since increased GA level promotes plants with thicker culm and tall plant height, which both contribute to improved lodging resistance and increased biomass, they must explore the use of tall plants with higher GA levels as part of an alternative strategy to develop new cultivars.
Similar results were also observed by Ookawa et al. (2016).23) They produced rice near-isogenic lines (NILs) carrying either loss or gain of SD-1 function. Plants carrying SD-1 (gain-of-function) showed larger outer diameter and SM than the plants carrying sd-1 (loss-of-function). These observations confirm that a defect in GA synthesis has a negative effect on the physical strength of the rice culm.
6. A breeding trial using strong culm mutant for lodging resistance
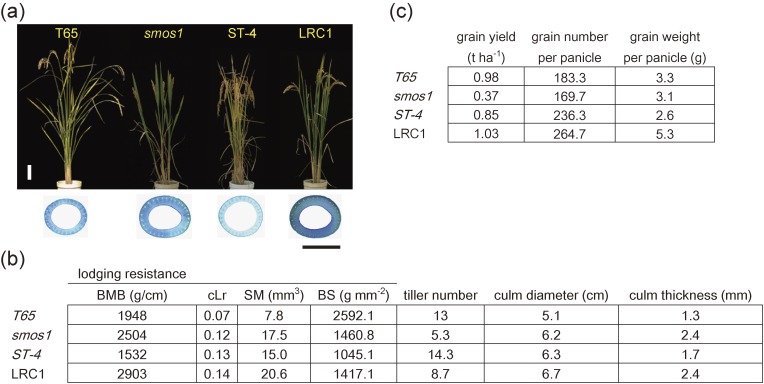
So far, there are only few trials aimed at improving the physical strength of the culm in reported rice breeding programs. This is probably due to the various unfavorable characteristics inevitably associated with higher physical strength of the culm, such as low tiller number, low grain yield, and some morphological defects. However, many varieties with various culm morphologies exist among rice cultivars, and if we can efficiently utilize these traits to increase culm strength, it should be possible to improve lodging resistance. Another reason for the lack of such experiments might depend on the lack of an efficient system to measure and quantify culm strength. Using the measurement system developed by Ookawa et al.,23) Hirano et al. (2014)24) examined whether the good effect of strong culm trait can overcome its negative side effect by doing a breeding trial. They first searched for strong culm lines from more than 3,000 rice mutants, and identified seven showing both high bending and breaking-type lodging resistance. Among them, a new rice loss-of-function mutant derived from Taichung 65 (T65), small organ size1 (smos1), was selected as a representative for the breeding trial, since it showed the highest BMB value with higher cLr value compared to its original cultivar, but also showing lower grain yield and tiller number (Fig. 5), which are often observed in rice strong culm mutants. This mutant also showed a decrease in the final size of various organs due to decreased cell size and abnormal microtubule orientation.
Figure 5.
Yield and lodging resistance traits of LRC1, its parentals (smos1 and ST-4) and T65 (original strain of smos1) (a) Gross morphology of T65, smos1, ST-4, and LRC1 at 30 days after heading. Bar = 10 cm. Culm cross-sections taken from the fourth (4th) internode of each plant is also shown. Bar = 5 mm. (b) Summary of grain yield-related traits of T65, smos1, ST-4, and LRC1. (c) Summary of morphological traits and lodging resistance of T65, smos1, ST-4, and LRC1. These figures were constructed according to our original papers referred to as Ref. 24.
Molecular analysis by Aya et al. (2014)25) revealed that SMOS1, which responds to exogenous auxin treatment, encodes an unusual APETALA2 (AP2)-type transcription factor with an imperfect AP2 domain. The gene has an auxin response element located in its promoter (cis-motif) that interacts with an auxin response factor (ARF). Based on these observations, the molecular function of SMOS1 has been considered as an auxin-dependent regulator for cell expansion during organ size control, and its loss of function leads to compact organs including the culm, which results in stiff culm structure, a favorable trait that needs to be seriously considered for lodging resistance breeding. To complement smos1’s unfavorable traits, Hirano et al. (2014)24) chose a specific line named ST-4 as a crossing partner, due to its high tiller number and high grain yield (Fig. 5b and c). After crossing smos1 with ST-4, F1 plants were self-pollinated until the F5 generation, and selected one line, named Lodging Resistant Candidate-1 (LRC1), that showed similar BMB values to that of smos1, and similar or higher grain yield relative to ST-4 (Fig. 5). LRC1 and smos1 both had increased culm diameter and culm thickness, which result in a high SM value (Fig. 5b), and this explains the mechanism for their improved lodging resistance. However, although the tiller number of LRC1 was increased compared to that of smos1, it did not come very close to that of ST-4 (Fig. 5b), demonstrating that the thick culm trait derived from smos1 is tied to the inferior trait of low tiller number. For the yield parameters, total grain yield of LRC1 was higher than that of smos1 and ST-4 (Fig. 5c). Total grain yield of rice can be calculated as grain number × average grain weight, where grain number is the total number of grains per panicle. Although the low ability of smos1 to produce tillers was not fully reversed in LRC1, this was however compensated by an increased grain weight per panicle, thereby achieving high grain yield per plant in the latter (Fig. 5c). Since smos1 possesses high BMB which is different from the traditional semi-dwarf plants utilized in the Green Revolution, an alternative approach of using thick culm lines to create rice with increased lodging resistance was proposed. However, since LRC1 fell short of the tiller number observed in its parent ST-4, this suggests that it is hard to separate traits related to culm thickness (or size) and tiller number from each other. To illustrate, rice fine culm 1 (fc1) mutant defective in strigolactone (SL) signaling possesses thin culms but they come in high number.26) The same is true for high-tillering dwarf 3 although the molecular mechanism behind it is still unknown.27) These observations therefore show that there is a trade-off between tiller number and culm size. One possible way to overcome such trade-off relationship is to increase grain productivity per tiller as that achieved in LRC1, but this does not resolve the fundamental cause of the low tiller number. Alternative approach to overcome this trade-off is presented in the succeeding sections of this review.
7. Culm strength-related gene isolation through QTL analysis
One way to avoid the negative side effects that accompany severe mutant traits is to identify and utilize mild alleles from appropriate cultivars or lines. Fortunately, identification of genes related to a certain trait from normal rice cultivars/lines has become relatively easy due to recent advances in molecular genetics. As for grain yield, for example, GN1A, GS3, DEP1 and WFP genes have been identified as related to grain yield through a technique called quantitative trait loci (QTL) analysis.28–32) Gene identification can help to accelerate breeding programs since it makes breeding more precise, in contrast to traditional breeding, which usually deal with the introgression of an unidentified genomic region into candidate varieties. In this section, we introduce an approach to identify genes related to lodging resistance by QTL analysis.
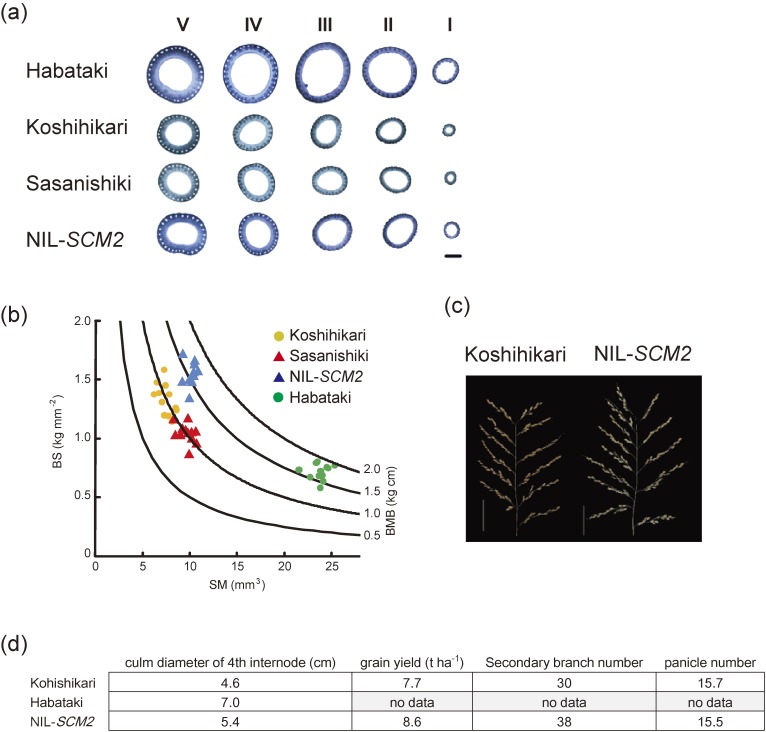
To identify genes related to lodging resistance, Ookawa et al.33) chose the indica rice variety, Habataki, as the donor parent, as it displays higher SM values compared to typical japonica varieties due to thicker culm diameter and culm wall thickness (Fig. 6a). On the other hand, the culm quality of Habataki measured in terms of BS was inferior to that of the two japonica cultivars, Sasanishiki and Koshihikari, the two leading varieties in Japan (Fig. 6b). Thus they expected that lodging resistance of japonica cultivars can be improved by the introduction of the gene from Habataki involved in conferring higher SM value. For such purpose, they first developed a rice population of Sasanishiki background with each individual having different chromosome segments replaced with that of Habataki (chromosomal segment substitution lines (CSSL)), and selected one line from them, SL420, which possesses high SM values relative to Sasanishiki. SL420 carries a chromosome 6 genome segment derived from Habataki, which harbors a QTL named STRONG CULM2 (SCM2) involved in increasing culm diameter. Finally they identified that SCM2 is identical to ABERRANT PANICLE ORGANIZATION1 (APO1) gene, which was previously identified to encode an F-box-containing protein involved in controlling the rachis branch number of the panicle.34)
Figure 6.
Culm morphology, lodging resistance and grain yield of NIL-SCM2 and control plants. (a) Culm cross-sections taken from the 5th (V; basal) to the 1st (I; panicle neck) internode of Habataki, Koshihikari, Sasanishiki, and NIL-SCM2. Scale bar = 2 mm. (b) Introgression of SCM2 increased the SM value without changing the BS value, resulting in improved BMB values similar to that of Habataki. Curved lines indicate the BMB of the 4th internode. Orange circle, Koshihikari; red triangle, Sasanishiki; blue triangle, NIL-SCM2; green circle, Habataki. (c) Panicle structure of Koshihikari and NIL-SCM2. Scale bars = 5 cm. (d) Culm and yield-related parameters and grain yield of Koshihikari, Habataki, and NIL-SCM2. These figures were constructed according to our original papers referred to as Ref. 33.
Next, to develop improved lodging-resistant japonica rice, they introduced the 454 kb Habataki genome segment containing SCM2 into Koshihikari (NIL-SCM2). Although not as big as that of Habataki, the culm diameter of NIL-SCM2 was bigger than that of Koshihikari, hence, leading to a higher SM value (Fig. 6b). The high BS value of Koshihikari was maintained in NIL-SCM2, and as a consequence, the BMB of NIL-SCM2 became similar to that of Habataki (Fig. 6b). Furthermore, the grain yield of NIL-SCM2 was surprisingly significantly higher than that of Koshihikari (Fig. 6c and d), due to increased secondary branch numbers of NIL-SCM2, which seems to be a positive pleiotropic effect of SCM2 (APO1) (Fig. 6c and d).
According to the above QTL study, it is tempting to speculate that further enhancing the function of SCM2/APO1 in rice would further improve lodging resistance and grain yield. However, this actually doesn’t seem to be true. Previously, Murai and Iizawa attempted to use an APO1-overexpressing mutant, Ur1, which produces 150% increased grain number compared to its original cultivar.35) Its tiller number per plant, however, was severely reduced by about 25%. Moreover, Ur1 caused undesirable decrease in kernel weight and spikelet fertility and abnormal panicle morphology,36) which prompted them to drop their breeding trial. Similarly, using transgenic approach, overproducing the gene SCM2/APO1 under the control of a strong promoter also led to undesirable plants with more severe abnormalities. This indicates that strong gain-of-function alleles of SCM2/APO1 can cause detrimental side effects, and so, an allele with milder effect is deemed preferable as it can increase spikelet number without reducing tiller number.
8. Improving lodging resistance of elite japonica rice by QTL pyramiding
In the above section, we presented that incorporation of an improved lodging resistance QTL, SCM2, into a Japanese elite variety, Koshihikari, improves lodging resistance and grain yield. To further enhance the lodging resistance of NIL-SCM2, Yano et al.37) attempted to pyramid another lodging resistance QTL into it. QTL pyramiding is an approach to introgress highly advantageous combinations of QTLs into one variety, and theoretically, superior alleles from a number of parental varieties can be introgressed into an elite variety, and this is exemplified by introducing favorable traits such as grain yield and resistance against pathogens as attempted by some researchers.38) For example, Ashikari et al. using QTL pyramiding, successfully introduced the rice Green Revolution gene, sd-1, which reduces plant height into the Koshikari genetic background, thereby producing a variety with two desirable traits: high yield and lodging resistance.39) The introduction of a combination of such major QTLs into rice in this manner thus provides an efficient and promising strategy for crop improvement.
Yano et al. used an indica variety, Chugoku 117, as a donor parent with culms stronger than that of Habataki but with BS inferior to that of Koshihikari (Fig. 7a and c). Using a similar procedure to that conducted by Ookawa et al.33) they performed QTL analysis and identified a QTL gene, SCM3, responsible for high SM in Chugoku 117, as the rice TEOSINTE BRANCHED 1 (OsTB1), which encodes a TCP domain-bearing transcription factor previously reported to control tiller number.26) They next produced NIL-SCM3, which contains the 163-kb Chugoku117 SCM3 region in the Koshihikari background. They then crossed this with NIL-SCM2 to obtain a pyramiding line (NIL-SCM2+SCM3) (Fig. 7b), which exceeded both of its parents and Koshihikari in terms of culm diameter and thickness, indicating the additive effect of the two QTLs (Fig. 7c and e). This consequently led to NIL-SCM2+SCM3 being able to attain a superior SM compared to NIL-SCM2 and NIL-SCM3, a similar BS to Koshihikari, and the highest BMB among the rest (Fig. 7c), demonstrating the effectiveness of pyramiding the two QTLs to improve culm strength.
Figure 7.
Culm morphology, lodging resistance and yield of rice possessing single or pyramided strong culm QTLs. (a) Gross morphology and cross-sections of the 4th internode of Koshihikari, Habataki, and Chugoku117. Scale bars = 15 cm for the upper panels, 2 mm for the lower left panels, and 100 µm for the lower right panels. (b) NIL-SCM3, NIL-SCM2 and NIL-SCM2+SCM3 were each introgressed with the Chugoku117 genomic segment carrying SCM3 (shown in red), Habataki genomic segment carrying SCM2 (blue), and both, respectively. (c) Relationship between SM, bending stress, and BMB. Curved lines indicate the BMB of the 4th internode. (d) Panicle morphology of Koshihikari, NIL-SCM3, NIL-SCM2, NIL-SCM2+SCM3, and Chugoku117. Scale bar = 5 cm. (e) Culm and yield-related morphological traits and grain yield of Koshihikari, NILs, and Chugoku117. These figures were constructed according to our original papers referred to as Ref. 37.
In terms of grain yield parameters, NIL-SCM3, although having less number of tillers per plant compared to Koshihikari (10.4 vs. 12.4), was able to produce more grains per panicle than the latter due to increased primary and secondary branch numbers (Fig. 7d and e). Such grain number was further improved when SCM3 was put in tandem with SCM2, making NIL-SCM2+SCM3 able to exceed that of the rest. Moreover, tiller number per plant in NIL-SCM2+SCM3 was improved to almost close to that of Koshihikari, possibly due to the interaction between SCM3 and SCM2 (Fig. 7e). This resulted in NIL-SCM3+SCM2 having a 20% higher total grain yield over Koshihikari. This shows that SCM3 has both positive and negative effects on grain yield, namely, increasing grain number per panicle and decreasing the tiller number per plant, respectively. On the other hand, SCM2 only has a positive effect on grain yield and has no negative effect on tiller number. Overall, the net result of this can be clearly seen in NIL-SCM2+SCM3, namely, the much desired increase in total grain yield.
9. Negative trade-off phenotype can be alleviated by using mild alleles
SCM3/OsTB1 has been considered to be negatively regulated by SL, a phytohormone which promotes tillering.40) To understand the mechanisms of how SCM3 controls culm strength and panicle development, Yano et al. overexpressed SCM3 under the control of a strong promoter, maize UBIQUITIN promoter.41) In terms of lodging resistance, the overexpressor showed favorable phenotypes of increased culm diameter and thickness, but with negative phenotypes of semi-dwarfism, reduced tiller number, and delayed heading date. In contrast, a loss-of-function mutant of SCM3, fc1, and another SL synthesis null mutant, d10,42) exhibited not only desirable SL-related phenotypes (increased tiller number) but also unfavorable reduction in culm diameter and thickness. Such a tight relationship between tiller number and culm thickness indicates that SL regulates tiller number and culm morphology in a pleiotropic manner. As far as breeders are concerned, the two traits are both crucial since tiller number directly relates to grain yield, whereas culm morphology determines lodging resistance. However, the case of fc1 and d10 shows that SL is an ambivalent target for breeding and that a trade-off between tiller number and culm morphology maybe inevitable as observed with SCM3.
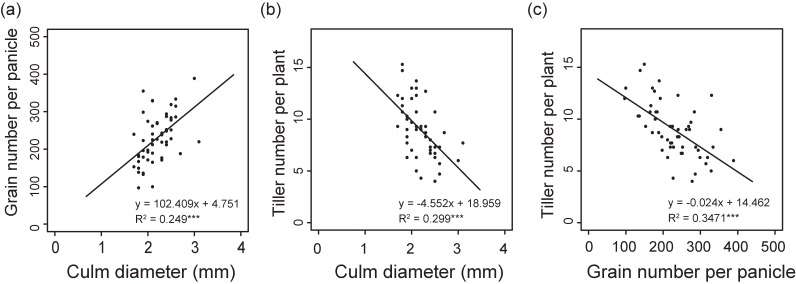
As mentioned above, although SCM3 may negatively affect crop yield by decreasing tiller number, it also has a positive side, namely, its ability to increase grain number per panicle (Fig. 7e). Meanwhile, it was found that there is a positive correlation between grain number per panicle and culm diameter as supported by previous observations using a rice core collection involving 57 accessions43) (Fig. 8a). At the same time, in such collection, negative correlation between culm diameter and tiller number per plant, which are pleiotropically controlled by SL as discussed above, was also observed (Fig. 8b). Furthermore, negative correlation between tiller number and grain number per panicle was also observed (Fig. 8c), which is logical since SL positively affects grain number per panicle and at the same time, negatively affects tiller number per plant. Such observation implies that the trade-off relationship between grain number per panicle and tiller number per plant has long been a hindrance in increasing rice crop yield. Actually, such trade-off between culm diameter/grain number and tiller number can be observed both in OsTB1 and APO1 overproducers, indicating that the overlapping mechanisms of OsTB1 and APO1 pleiotropically regulate these traits. Then, if this tradeoff is not specific to any of OsTB1 and APO1 but applies to general genetic mechanisms, which is very likely, then, we need to select suitable alleles for enhancing culm strength without inducing undesirable effects. The case of the overproducers also demonstrate that stronger alleles are not always better than milder ones, even though the individual impact of the latter may not be sufficient to readily improve agronomically important traits. Similarly, considering QTL pyramiding, one must be careful not to combine QTLs involved in similar mechanisms to avoid problems synonymous with the negative effect of stronger alleles. In the case of SCM3, for example, it had the same mild gain-of-function allelic properties of SCM2 (Fig. 6e), while being governed by a partially different mechanism from that of SCM2. Thus when developing a novel QTL pyramiding line for improved lodging resistance, it becomes important to carefully select the combination of alleles which will most effectively enhance lodging resistance without inducing detrimental side effects.
Figure 8.
Correlation between culm diameter, grain number per panicle, and tiller number per plant in 53 world rice accessions. (a) Correlation between culm diameter and grain number per panicle. (b) Correlation between culm diameter and tiller number per plant. (c) Correlation between grain number per panicle and tiller number per plant. These figures were constructed according to our original papers referred to as Ref. 37.
10. Culm quality is also an important factor for improving lodging resistance
As stated earlier, breaking-type lodging resistance is determined by SM (morphology of the culm) and BS (quality of the culm) (Fig. 4b). Although we presented studies to enhance SM, attempts to improve BS of the rice culm are also being done. BS is largely affected by the components of the secondary cell wall such as cellulose, hemicellulose, and lignin.44) Since secondary cell walls accumulate mostly in cortical fiber tissues in the culm, the quality of such secondary cell walls becomes a key factor that needs to be considered for improving culm quality. Indeed, Ookawa et al.41,45) demonstrated that both thick cortical fiber tissue and high densities of cell wall components such as cellulose and hemicellulose are responsible for the high BS in Koshihikari.41,45) To search for traits that enhance BS, Ookawa et al.45) used a unique cultivar, Leaf Star, with superior lodging resistance due to its thick layer of cortical fiber tissues. Interestingly, although Leaf Star possesses decreased lignin content due to decreased activity of the lignin biosynthetic enzyme, cinnamyl alcohol dehydrogenase2 (CAD2), it still showed high culm strength. After further analyses, they found that Leaf Star has high densities of cellulose and hemicellulose which compensated for the decreased lignin content. This suggests that rice CAD2 mutants can be studied and targeted for BS improvement. On the other hand, using a different approach, Li et al.46) performed a system biology analysis using a total of 36 distinct rice cell wall mutants to search for key factors for cell wall modification, and identified cellulose crystallinity (Crl) as the major factor that negatively determines breaking-type lodging resistance. They also found a group of glycosidase hydrolase GH9B genes that potentially reduce Crl and that arabinose within the hemicellulose negatively affect Crl. From these observations, they suggested that simultaneous overproduction of GH9B and xylan arabinosyltransferase could result in reduced amount of Crl and improved BS.
Although genes that affect BS are not yet determined, the above-mentioned factors may help provide a useful insight on how to carry out BS improvement in the future.
11. Concluding remarks
Compared to the era of the Green Revolution, it is now possible to develop rice with even more grain yield, and thus, it becomes necessary to develop rice that could sustain such higher yields through a different breeding strategy from that utilized in the past. Our group aimed to improve rice lodging resistance using genomic techniques and from our studies, we proposed one approach; that is, to identify genes that improve culm strength and to pyramid these genes into elite cultivars.
We also demonstrated that introduction of strong alleles often accompany undesirable side effects on grain yield, and thus, breeders need to be very careful when selecting the gene to be introduced. This becomes more important when pyramiding is considered. This is because even if introducing a single mild allele does not show detrimental effects, combining such alleles of the same genetic mechanism could potentially result in detrimental phenotypes similar to that observed for strong alleles. Such case may be avoided to a certain degree if we know the precise molecular function of the gene to be introduced. This therefore shows that both basic and applied researches are important for developing plants with ideal traits.
Acknowledgments
We thank Dr. Taiichiro Ookawa of Tokyo University of Agriculture and Technology for the helpful discussion. This work was supported by grants from the Japan Society for the Promotion of Science through a Grant-in-Aid for Scientific Research (A) (23248002 to M.M) and KAKENHI (grant no. 26660278 to K.H).
Profile
Makoto Matsuoka, born in Aichi in 1955, graduated from the Nagoya University School of Agricultural Science in 1978, and further went on to finish doctoral course from the same school in 1983. After receiving his Ph.D. degree, he worked as a researcher at the National Institute of Agrobiological Resources, Tsukuba from 1983 to 1994. During this period, he also became a visiting fellow at the CSIRO Division of Plant Industry in Canberra, Australia (1991–1992) as well as an adjunct associate professor in the School of Science, Tsukuba University (1993–1994).In 1994, he moved to the Nagoya University Bioscience Center as a professor, and from then on, conducted studies on the synthesis and signaling of the plant growth hormone gibberellin (GA). Using rice mutants, he successfully found the major components for GA synthesis such as the ‘green revolution’ gene, and also some important components of the GA perception machinery. With his knowledge on GA-related mechanisms, he was able to contribute to improve some agronomically important traits of rice by molecular plant breeding. For such an accomplishment, he was conferred the Tokyo Techno Forum 21 Gold Medal (1998), Kihara Foundation for the Advancement of Life Science Prize (2006), Japanese Society of Breeding Award (2010), International Plant Growth Substance Association’s Silver Medal (2012), Chunichi Cultural Award (2014), Medal with Purple Ribbon (2016), and the Duke of Edinburgh Prize from the Japan Academy (2016).
References
- 1).United Nations Report, World Population Prospects: The 2012 Revision. www.unpopulation.org.
- 2).Dalrymple D.G. (1985) The development and adoption of high-yielding varieties of wheat and rice in developing countries. Am. J. Agric. Econ. 67, 1067–1073. [Google Scholar]
- 3).Khush G.S. (1999) Green revolution: preparing for the 21st century. Genome 42, 646–655. [PubMed] [Google Scholar]
- 4).Yonemura S., Yajima M., Sakai H., Morokuma M. (1998) Estimate of rice yield of Japan under the conditions with elevated CO2 and increased temperature by using third mesh climate data. J. Agric. Meteorol. 54, 235–245. [Google Scholar]
- 5).Kang D.J., Ishii R. (2003) Studies on acid soil tolerance of rice plants (Oryza sativa) I. Morphological and physiological characteristics of acid soil tolerant varieties of rice plants. Jpn. J. Crop Sci. 72, 171–176. [Google Scholar]
- 6).Kono, M. (1995) Physiological aspects of lodging. In Science of the rice plant, Physiology 2 (eds. Matsuo, T., Kumazawa, K., Ishii, R., Ishihara, K., and Hirata, H.). Food and Agriculture Policy Research Center, Tokyo, pp. 971–982. [Google Scholar]
- 7).Kashiwagi T., Hirotsu N., Madoka Y., Ookawa T., Ishimaru K. (2007) Improvement of resistance to bending-type lodging in rice. Jpn. J. Crop Sci. 76, 1–9. [Google Scholar]
- 8).Hoshikawa K., Wang S.B. (1990) Studies lodging in rice plants. I. A general observation on lodged rice culms. Jpn. J. Crop Sci. 59, 809–814. [Google Scholar]
- 9).Islam M.S., Peng S., Visperas R.M., Ereful N., Bhuiya M.S.U. (2007) Lodging related morphological traits of hybrid rice in a tropical irrigated ecosystem. Field Crops Res. 101, 240–248. [Google Scholar]
- 10).Watanabe, T. (1997) Lodging resistance. In Science of the rice plant, Genetics 3 (eds. Matsuo, T., Futsuhara, Y., Kikuchi, F., and Yamaguchi, H.). Food and Agriculture Policy Research Center, Tokyo, pp. 567–577. [Google Scholar]
- 11).Sasaki A., Ashikari M., Ueguchi-Tanaka M., Itoh H., Nishimura A., Swapan D., Ishiyama K., Saito T., Kobayashi M., Khush G.S., Kitano H., Matsuoka M. (2002) Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416, 701–702. [DOI] [PubMed] [Google Scholar]
- 12).Peng J., Richards D.E., Hartley N.M., Murphy G.P., Devos K.M., Flintham J.E., Beales J., Fish L.J., Worland A.J., Pelica F., Sudhakar D., Christou P., Snape J.W., Gale M.D., Harberd N.P. (1999) ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400, 256–261. [DOI] [PubMed] [Google Scholar]
- 13).Ashikari M., Sasaki A., Ueguchi-Tanaka M., Itoh H., Nishimura A., Datta S., Ishiyama K., Saito T., Kobayashi M., Khush G.S., Kitano H., Matsuoka M. (2002) Loss-of-function of a rice gibberellin biosynthetic gene, GA20 oxidase (GA20ox-2), led to the rice “green revolution”. Breed. Sci. 52, 143–150. [Google Scholar]
- 14).Spielmeyer W., Ellis M.H., Chandler P.M. (2002) Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. U.S.A. 99, 9043–9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Koshio K., Inaishi Y., Hayamichi Y., Fujimaki H., Kikuchi F., Toyohara H. (2000) Character expression of isogenic lines with semidwarf genes of different origins in rice (Oryza sativa L.). J. Agr. Sci., Tokyo Nogyo Daigaku 45, 201–209. [Google Scholar]
- 16).Asano K., Takashi T., Miura K., Qian Q., Kitano H., Matsuoka M., Ashikari M. (2007) Genetic and molecular analysis of utility of sd1 alleles in rice breeding. Breed. Sci. 57, 53–58. [Google Scholar]
- 17).Yan W., Dilday R.H., Tai T.H., Gibbons J.W., McNew R.W., Rutger J.N. (2005) Differential response of rice germplasrn to straighthead induced by arsenic. Crop Sci. 45, 1223–1228. [Google Scholar]
- 18).Bao J., Lce S., Chen C., Zhang X., Zhang Y., Liu S., Clark T., Wang J., Cao M., Yang H., Wang S.M., Yu J. (2005) Serial analysis of gene expression study of a hybrid rice strain (LYP9) and its parental cultivars. Plant Physiol. 138, 1216–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Zhu G., Li G., Wang D., Yuan S., Wang F. (2016) Changes in the lodging-related traits along with rice genetic improvement in China. PLoS One 11, e0160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Okuno A., Hirano K., Asano K., Takase W., Masuda R., Morinaka Y., Ueguchi-Tanaka M., Kitano H., Matsuoka M. (2014) New approach to increasing rice lodging resistance and biomass yield through the use of high gibberellin producing varieties. PLoS One 9, e86870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Ookawa T., Ishihara K. (1993) Varietal difference of the cell wall components affecting the bending stress of the culm in relation to the lodging resistance in paddy rice. Jpn. J. Crop Sci. 62, 378–384. [Google Scholar]
- 22).Ookawa T., Ishihara K. (1992) Varietal difference of physical characteristics of the culm related to lodging resistance in paddy rice. Jpn. J. Crop Sci. 61, 419–425. [Google Scholar]
- 23).Ookawa T., Aoba R., Yamamoto T., Ueda T., Takai T., Fukuoka S., Ando T., Adachi S., Matsuoka M., Ebitani T., Kato Y., Mulsanti I.W., Kishii M., Reynolds M., Piñera F., Kotake T., Kawasaki S., Motobayashi T., Hirasawa T. (2016) Precise estimation of genomic regions controlling lodging resistance using a set of reciprocal chromosome segment substitution lines in rice. Sci. Rep. 28, 30572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Hirano K., Okuno A., Hobo T., Ordonio R., Shinozaki Y., Asano K., Kitano H., Matsuoka M. (2014) Utilization of stiff culm trait of rice smos1 mutant for increased lodging resistance. PLoS One 9, e96009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Aya K., Hobo T., Sato-Izawa K., Ueguchi-Tanaka M., Kitano H., Matsuoka M. (2014) A novel AP2-type transcription factor, SMALL ORGAN SIZE1, controls organ size downstream of an auxin signaling pathway. Plant Cell Physiol. 55, 897–912. [DOI] [PubMed] [Google Scholar]
- 26).Takeda T., Suwa Y., Suzuki M., Kitano H., Ueguchi-Tanaka M., Ashikari M., Matsuoka M., Ueguchi C. (2003) The OsTB1 gene negatively regulates lateral branching in rice. Plant J. 33, 513–520. [DOI] [PubMed] [Google Scholar]
- 27).Zhang B., Tian F., Tan L., Xie D., Sun C. (2011) Characterization of a novel high tillering dwarf 3 mutant in rice. J. Genet. Genomics 38, 411–418. [DOI] [PubMed] [Google Scholar]
- 28).Ashikari M., Sakakibara H., Lin S., Yamamoto T., Takashi T., Nishimura A., Angeles E.R., Qian Q., Kitano H., Matsuoka M. (2005) Cytokinin oxidase regulates rice grain production. Science 309, 741–745. [DOI] [PubMed] [Google Scholar]
- 29).Fan C., Xing Y., Mao H., Lu T., Han B., Xu C., Li X., Zhang Q. (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 112, 1164–1171. [DOI] [PubMed] [Google Scholar]
- 30).Huang X., Qian Q., Liu Z., Sun H., He S., Luo D., Xia G., Chu C., Li J., Fu X. (2009) Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 41, 494–497. [DOI] [PubMed] [Google Scholar]
- 31).Jiao Y., Wang Y., Xue D., Wang J., Yan M., Liu G., Dong G., Zeng D., Lu Z., Zhu X., Qian Q., Li J. (2010) Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 42, 541–544. [DOI] [PubMed] [Google Scholar]
- 32).Miura K., Ikeda M., Matsubara A., Song X.J., Ito M., Asano K., Matsuoka M., Kitano H., Ashikari M. (2010) OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 42, 545–549. [DOI] [PubMed] [Google Scholar]
- 33).Ookawa T., Hobo T., Yano M., Murata K., Ando T., Miura H., Asano K., Ochiai Y., Ikeda M., Nishitani R., Ebitani T., Ozaki H., Angeles E.R., Hirasawa T., Matsuoka M. (2010) New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield. Nat. Commun. 1, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Ikeda K., Ito M., Nagasawa N., Kyozuka J., Nagato Y. (2007) Rice ABERRANT PANICLE ORGANIZATION1, encoding an F-box protein, regulates meristem fate. Plant J. 51, 1030–1040. [DOI] [PubMed] [Google Scholar]
- 35).Murai M., Iizawa M. (1994) Effects of major genes controlling morphology of panicle in rice. Breed. Sci. 44, 247–255. [Google Scholar]
- 36).Kinoshita T., Takahashi M. (1991) The hundredth report of genetical studies on rice plants. Linkage studies and future prospects. J. Fac. Agric. Hokkaido Univ. 65, 1–61. [Google Scholar]
- 37).Yano K., Ookawa T., Aya K., Ochiai Y., Hirasawa T., Ebitani T., Takarada T., Yano M., Yamamoto T., Fukuoka S., Wu J., Ando T., Ordonio R.L., Hirano K., Matsuoka M. (2015) Isolation of a novel lodging resistance QTL gene involved in strigolactone signaling and its pyramiding with a QTL gene involved in another mechanism. Mol. Plant 8, 303–314. [DOI] [PubMed] [Google Scholar]
- 38).Takeda S., Matsuoka M. (2008) Genetic approaches to crop improvement: responding to environmental and population changes. Nat. Rev. Genet. 9, 444–457. [DOI] [PubMed] [Google Scholar]
- 39).Ashikari M., Sakakibara H., Lin S., Yamamoto T., Takashi T., Nishimura A., Angeles E.R., Qian Q., Kitano H., Matsuoka M. (2005) Cytokinin oxidase regulates rice grain production. Science 309, 741–745. [DOI] [PubMed] [Google Scholar]
- 40).Guo S., Xu Y., Liu H., Mao Z., Zhang C., Ma Y., Zhang Q., Meng Z., Chong K. (2013) The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nat. Commun. 4, 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Ookawa T., Yasuda K., Kato H., Sakai M., Seto M., Sunaga K., Motobayashi T., Tojo S., Hirasawa T. (2010) Biomass production and lodging resistance in ‘Leaf Star’, a new long-culm rice forage cultivar. Plant Prod. Sci. 13, 58–66. [Google Scholar]
- 42).Arite T., Iwata H., Ohshima K., Maekawa M., Nakajima M., Kojima M., Sakakibara H., Kyozuka J. (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 51, 1019–1029. [DOI] [PubMed] [Google Scholar]
- 43).Kojima Y., Ebana K., Fukuoka S., Nagamine T., Kawase M. (2005) Development of an RFLP-based rice diversity research set of germplasm. Breed. Sci. 55, 431–440. [Google Scholar]
- 44).Vogel J. (2008) Unique aspects of the grass cell wall. Curr. Opin. Plant Biol. 11, 301–307. [DOI] [PubMed] [Google Scholar]
- 45).Ookawa T., Inoue K., Matsuoka M., Ebitani T., Takarada T., Yamamoto T., Ueda T., Yokoyama T., Sugiyama C., Nakaba S., Funada R., Kato H., Kanekatsu M., Toyota K., Motobayashi T., Vazirzanjani M., Tojo S., Hirasawa T. (2014) Increased lodging resistance in long-culm, low-lignin gh2 rice for improved feed and bioenergy production. Sci. Rep. 4, 6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Li F., Zhang M., Guo K., Hu Z., Zhang R., Feng Y., Yi X., Zou W., Wang L., Wu C., Tian J., Lu T., Xie G., Peng L. (2015) High-level hemicellulosic arabinose predominately affects lignocellulose crystallinity for genetically enhancing both plant lodging resistance and biomass enzymatic digestibility in rice mutants. Plant Biotechnol. J. 13, 514–525. [DOI] [PubMed] [Google Scholar]
- 47).Grafius J.E., Brown H.M. (1954) Lodging resistance in oats. Agron. J. 46, 414–418. [Google Scholar]