Abstract
The brains of higher mammals such as primates and carnivores contain well-developed unique brain structures. Uncovering the physiological functions, developmental mechanisms and evolution of these brain structures would greatly facilitate our understanding of the human brain and its diseases. Although the anatomical and electrophysiological features of these brain structures have been intensively investigated, our knowledge about their molecular bases is still limited. To overcome this limitation, genetic techniques for the brains of carnivores and primates have been established, and molecules whose expression patterns correspond to these brain structures were identified recently. To investigate the functional roles of these molecules, rapid and efficient genetic manipulation methods for higher mammals have been explored. In this review, recent advances in molecular investigations of the brains of higher mammals are discussed, mainly focusing on ferrets (Mustela putorius furo).
Keywords: cerebral cortex, gyrus, outer subventricular zone, in utero electroporation, ferret
Introduction
Rodents such as mice and rats have been widely used for deciphering the molecular mechanisms underlying the development and functions of the brain, and for uncovering the pathophysiology of and treatments for various brain diseases. For example, accumulating knowledge about the processes of brain development using knockout mice in vivo has provided opportunities to recapitulate these processes in vitro. As a result, we and others successfully established techniques to make various useful types of cells, such as midbrain dopaminergic neurons and retinal pigmented epithelial cells from embryonic stem cells (ES cells) of rodents and primates in vitro.1–8) These techniques have been applied to iPS cells to make human dopaminergic neurons and retinal pigmented epithelial cells,9) which are now used for research into regenerative medicine for Parkinson’s disease and age-related macular degeneration in Japan. In addition, using rodents, we also reported that MAP kinase is essential for long-term depression in the cerebellum10) and that birth of mouse pups plays a crucial role as a trigger to start neuronal circuit formation and behavioral maturation during development.11,12) Mice have been a powerful option for investigating the mechanisms of development and physiological functions of the brain, and the pathophysiology of brain diseases.
Recently, higher mammals including primates and carnivores have attracted more attention from researchers than before. This is mainly because primates and carnivores have important unique brain structures that humans have, but mice do not. These brain structures include the folds and the outer subventricular zone (OSVZ) of the cerebral cortex, ocular dominance columns (ODCs) in the visual cortex, and the magnocellular (M) and parvocellular (P) pathways in the visual system.13–15) Because it has been proposed that they are structural bases of higher brain functions, investigation of the physiological importance, developmental mechanisms and diseases related to these structures using primates and carnivores would greatly facilitate our understanding of the human brain and its diseases. Although the anatomical and physiological characteristics of these brain structures have been intensively investigated using primates and carnivores,13,14,16) our knowledge about the molecular mechanisms underlying the formation, function, pathophysiology and evolution of these structures is still limited. This is mainly because genetic manipulation techniques for higher mammals were poorly available until recently.
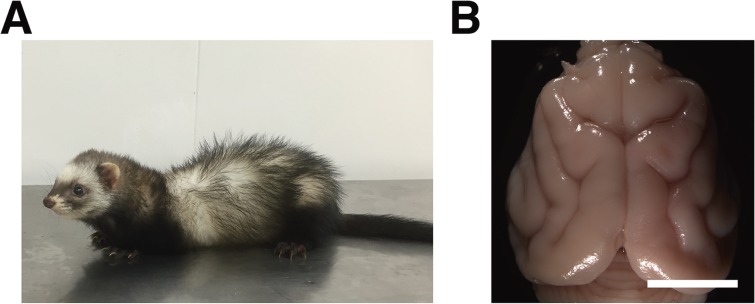
To overcome this limitation, genetic manipulation techniques for carnivores and primates were reported recently. Previous pioneering studies used virus vectors to make transgenic primates such as monkeys and marmosets.17–19) For example, the injection of a lentiviral vector into eggs resulted in marmosets expressing transgenes in several organs.19) Although the establishment of transgenic marmosets would provide new opportunities to make animal models for human diseases,20) making new transgenic marmosets requires time, effort and special animal facilities. Therefore, a rapid and simple genetic manipulation technique that can be used for carnivores and primates was desirable. We recently applied in utero electroporation to ferrets (Mustela putorius furo) (Fig. 1A) and succeeded in transgene expression in the cerebral cortex of ferrets as described below.21,22) Pioneering studies from another group also reported transgene expression in the cerebral cortex of ferrets using postnatal electroporation.23,24) The ferret belongs to Mustelidae, a family of carnivorous mammals, and adult ferrets have an average length of about 50 cm and a weight of about 1–2 kg. Ferrets have a long history in research because they have well-developed brain structures that mice do not have (Fig. 1B). Because marmosets and ferrets have their own unique features, combining transgenic marmosets and electroporated ferrets would facilitate our understanding of the brain of higher mammals. In this review, recent advances in the molecular understanding of the brains of higher mammals are summarized, primarily focusing on ferrets.
Figure 1.
The ferret and its brain. (A) An adult ferret. The ferret has an average length of about 50 cm and weight of about 1–2 kg. (B) A dorsal view of the ferret brain. The cortical gyri and sulci are clearly present. Scale bar, 1 cm. (Adapted from Kawasaki et al., 2012)21)
Investigation of the cerebral cortex using ferrets
One of the most prominent characteristics of the brains of higher mammals is the folds of the cerebral cortex (i.e., cortical gyri and sulci) (Fig. 1B). The brains of humans, monkeys and ferrets are gyrencephalic (i.e., brains with cortical folds), whereas those of rodents are often lissencephalic (i.e., brains without cortical folds). It has been believed that the acquisition of cortical folds during evolution played important roles in obtaining higher brain functions.25) Furthermore, human patients with malformations of cortical folds often exhibit intellectual disability and epilepsy.26,27) Therefore, the molecular mechanisms of the formation and malformation of cortical folds during development have been of great interest.
During development, neurons in the cerebral cortex are produced from radial glial cells (RG cells, also known as apical progenitors/apical RG cells/ventricular RG cells). RG cells are neural stem cells in the ventricular zone (VZ), which is located along the cerebral ventricles. RG cells undergo multiple rounds of asymmetric cell divisions, and, as a result, intermediate progenitor cells (IP cells/basal progenitors) are produced. IP cells then migrate into the subventricular zone (SVZ) and further proliferate to produce post-mitotic neurons. One of the characteristic features of the developing cerebral cortex of higher mammals is the presence of an enlarged SVZ containing an inner region (ISVZ) and an outer region (OSVZ).28,29) Several pioneering groups have identified new progenitor cells in the OSVZ: OSVZ radial glial cells (oRG cells/outer RG cells/basal RG cells/intermediate RG cells/translocating RG cells).30–32) While RG cells and IP cells are abundantly found in both mice and humans, oRG cells are preferentially observed in humans rather than in mice. It would be intriguing to compare neuronal cell types produced from IP cells and oRG cells in mice and humans during development. Because it has been proposed that neural progenitors in the SVZ play crucial roles in cortical folding,15,33–35) it would be important to investigate the mechanisms underlying their proliferation, migration and differentiation.
The ferret is an attractive option for investigating such mechanisms because genetic manipulation techniques for ferrets have become feasible, as described below.21,22,36) Recently, several groups reported genes expressed in the VZ and the SVZ of mice and in various regions of the cerebral cortex in monkeys.37,38) Examining the roles of these genes in the cerebral cortex using ferrets should lead to uncovering the molecular mechanisms of the formation and malformation of both cortical folding and the OSVZ in the cerebral cortex of higher mammals.
Genetic manipulation for the ferret brain using in utero electroporation
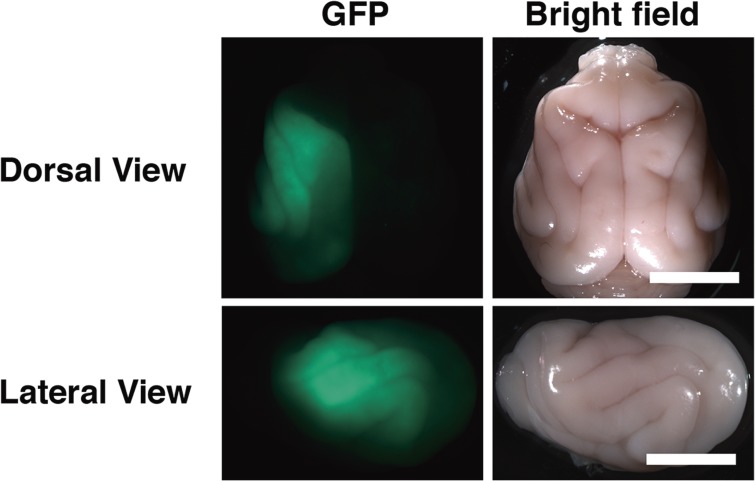
Although transgenic marmosets had offered a novel avenue to investigate the pathophysiological mechanisms of human diseases,20) much more rapid and simple genetic manipulation techniques that can be used for carnivores and primates were desirable. Because in utero electroporation was widely used for expressing transgenes in the brain of rodents,39–41) we tried to apply in utero electroporation to ferrets. Fortunately, we succeeded in establishing a rapid and efficient procedure of in utero electroporation for the ferret brain (Fig. 2).21,22) It takes only a few hours to perform electroporation using ferret embryos, and after a couple of days, ferret babies expressing transgenes can be obtained. Expression of transgenes is detectable even in the embryo and persists at least several months after ferret babies are born. Transgenes can be introduced in both superficial and deep neurons in the cerebral cortex, depending on at which age of the ferret embryo in utero electroporation is performed during development. In utero electroporation performed at embryonic day 31 (E31) and at E37 results in transgene expression in superficial and deep cortical neurons, respectively. Using our electroporation procedure, transgenes can be expressed not only in post-mitotic neurons but also neural progenitors including RG cells, oRG cells and IP cells.21,22)
Figure 2.
GFP in the ferret brain expressed using in utero electroporation. Scale bars, 1 cm. (Adapted from Kawasaki et al., 2012)21)
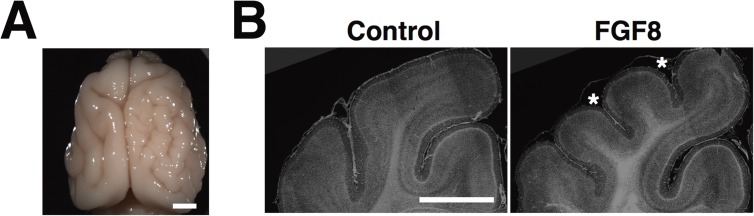
We thought that it would be intriguing to investigate the molecular mechanisms of the formation and malformation of cortical folds using our in utero electroporation technique for ferrets. Thanatophoric dysplasia (TD) is a relatively common skeletal dysplasia in which the cerebral cortex displays polymicrogyria, megalencephaly and neuronal heterotopia.42,43) TD is caused by activating mutations of the fibroblast growth factor receptor 3 (FGFR3) gene.44–49) Because knockin mice that have the same activating mutation in the FGFR3 gene did not exhibit polymicrogyria,50,51) appropriate animal models would be extremely important for examining the pathophysiology of TD. We utilized fibroblast growth factor 8 (FGF8), which has a high affinity to FGFR3 and activates it. We successfully recapitulated the cortical phenotypes of thanatophoric dysplasia (TD) by expressing FGF8 in the ferret cerebral cortex.52) Strikingly, our TD ferret model showed not only megalencephaly but also polymicrogyria (Fig. 3A, B).52) We further uncovered that SVZ progenitors (i.e., oRGs and IP cells) were markedly increased, and it seemed likely that these increases underlie the pathogenesis of polymicrogyria. Our findings indicate that ferrets are useful for investigating the molecular mechanisms underlying the malformation of cortical gyri.
Figure 3.
The brain of TD ferrets. (A) A dorsal view of the TD ferret brain. FGF8 was electroporated into the right cortex. (B) A coronal section of the cerebral cortex of TD ferrets stained with Hoechst 33342. Note that additional sulci (asterisks) and gyri with normal layer structures were formed by expressing FGF8. Scale bars, 4 mm (A), 6 mm (B). (Adapted from Masuda et al., 2015)52)
The mechanisms underlying cortical folding during normal development are still largely unknown. Because it has been proposed that an increase in the numbers of SVZ progenitors is responsible for the acquisition of cortical folds during evolution,15,33–35) we investigated the role of SVZ progenitors in cortical folding during development.53) We found that the numbers of SVZ progenitors were markedly reduced by inhibiting Tbr2 transcription factor. Interestingly, when the numbers of SVZ progenitors were reduced by inhibiting Tbr2, cortical folding was significantly impaired, suggesting an essential role of SVZ progenitors in cortical folding. We also found that there were regional differences in the abundance of SVZ progenitors in the developing ferret cortex even before cortical folds were formed.53) Because Tbr2-positive cells were increased by FGF8, Tbr2 seems to be a downstream target of FGF signaling.52) Our findings indicate that our electroporation procedure for ferrets will aid in uncovering the entire picture of mechanisms underlying cortical folding.
In utero electroporation is applicable not only for investigating molecular mechanisms but also for examining neuronal circuitry.54,55) Development of the cerebral cortex in higher mammals is characterized by the appearance of the large OSVZ. The OSVZ is often split into the ISVZ and the OSVZ by the inner fiber layer (IFL), a fiber layer located between the ISVZ and the OSVZ.28,29) However, a fiber layer corresponding to the IFL of the primate cerebral cortex had not been identified in the ferret cerebral cortex. In addition, it was unknown from which neurons fibers in the IFL are derived.34) We therefore expressed GFP in layer 2/3 neurons of the ferret cerebral cortex using in utero electroporation and found GFP-positive fibers just above the ISVZ (Fig. 4). Because both the GFP-positive fibers in ferrets and the IFL in primates are located just above the ISVZ, our result raised the possibility that a fiber layer corresponding to the IFL in primates also exists in the cerebral cortex of ferrets.22) Because the properties of the IFL are currently largely unknown,34) future investigations would be necessary to determine if the GFP-positive fibers in ferret and the IFL in primate share similar properties. This result also raised the possibility that fibers in the IFL are, at least partially, derived from layer 2/3 neurons of the cerebral cortex.22) Because the thickness of superficial layers of the cerebral cortex preferentially increased in higher mammals during evolution,56) a possible hypothesis about the creation of the IFL during evolution is that an increase in the number of layer 2/3 neurons resulted in the formation of a thick fiber bundle comprising the IFL in the developing cerebral cortex of higher mammals.
Figure 4.
IFL-like fibers in the developing ferret cerebral cortex. When GFP was expressed in layer 2/3 neurons using in utero electroporation, GFP-positive fibers were observed in the inner OSVZ (arrows). Low magnification images (upper panels) and high magnification images (lower panels) are shown. CP, cortical plate. Scale bars, 500 µm (upper) and 200 µm (lower). (Adapted from Kawasaki et al., 2013)22)
In utero electroporation has several important advantages compared with transgenic animals. First, the procedure of in utero electroporation is simple and rapid. It requires relatively little time and effort to express genes of interest. It takes only a few hours for the surgery of electroporation, and transfected embryos can be collected within a few days. Second, co-transfection is easy to perform, and as a result, multiple genes can be expressed simultaneously. Electroporation using a mixture of GFP and mCherry expression plasmids resulted in most GFP-positive neurons also being mCherry-positive in the ferret cerebral cortex, indicating efficient co-transfection. Third, by adjusting the direction of the electrodes and by changing the age of the embryo at which in utero electroporation is performed, the position of the transfected area can be controlled. While we mainly transfected the cerebral cortex of ferrets, based on the results obtained using rodents,57–62) it seems plausible that in utero electroporation is applicable to other brain regions, such as the hippocampus, the thalamus, the retina and the amygdala in ferrets. Finally, large plasmids can be introduced using in utero electroporation. Cell type-specific promoters, which are often quite large, seem to be useful for controlling the expression patterns of transgenes. For example, we recently transfected only a small number of neurons in mice by combined in utero electroporation and the Thy1S promoter.63) In addition, because in utero electroporation works well not only in rodents but also in carnivores, it could also be applicable to other higher mammals such as primates.
Besides in utero electroporation, postnatal electroporation can be used for expressing transgenes in the ferret cerebral cortex.24) It should be noted that when postnatal electroporation was performed, transfected neurons were mostly distributed in superficial layer 2/3 in the ferret cerebral cortex. This is presumably because most post-mitotic neurons had already migrated into the cortical plate from the VZ when postnatal electroporation was performed. Almost all excitatory neurons in the cerebral cortex can be transfected using in utero electroporation and postnatal electroporation in ferrets.
Ferrets and marmosets differ in several important points. The gestation period of ferrets is shorter than that of marmosets (42 days for ferrets, 150 days for marmosets). About 6–8 babies are born from one pregnant ferret mother, while 1–2 are born from a pregnant marmoset. Because of the larger number of ferret babies per pregnant mother, many experimental samples can be obtained using ferrets. Ferrets and marmosets become sexually mature at about 8 months old and 14 months old, respectively, and the average life span is 5–10 years in ferrets and 10–15 years in marmosets. The ferret is an attractive option for exploring the brain structures unique to higher mammals.
Investigation of ocular dominance columns in the visual system using ferrets
The visual system is more developed in higher mammals such as carnivores and primates than in rodents. Using the well-developed visual system of higher mammals, important concepts about the intrinsic and extrinsic factors regulating brain development and maturation have been uncovered.64) For instance, the anatomical and physiological properties of ocular dominance columns (ODCs) in the primary visual cortex (V1) have been investigated. Hubel and Wiesel demonstrated ODCs in V1 of cats in the early 1960s.65) Cortical neurons in V1 are activated differentially by one of the two eyes, and neurons with similar eye preference are clustered into cortical columns called ODCs in V1. ODCs were demonstrated with electrophysiological experiments and trans-neuronal tracers such as tritiated amino acids.66,67) Importantly, ODCs in V1 are found in both primates and carnivores including ferrets, but not in rats.68) Because it is still unclear whether ODCs are functionally important or are just a byproduct, more research is needed to examine the functional importance of ODCs.
Using ODCs in V1, the mechanisms underlying developmental plasticity have been investigated. When one of the two eyes was closed during early periods of postnatal life (i.e., the critical period), neurons activated by the closed eye decreased, and those activated by the intact open eye increased in V1.69–71) Pre-existing neuronal connections were substantially modified by an activity-dependent competitive process.72) Interestingly, when thalamic axons projecting to V1 were visualized by direct tracer injections into the lateral geniculate nucleus (LGN) of the thalamus, segregated ODC-like patterns were visible even before the critical period.73) These results were explained by the following hypothesis; the initial formation of ODC architecture is determined by intrinsic genetic mechanisms, and later modification of ODCs during the critical period is regulated by experience-dependent refinement.
Investigation of parallel pathways in the visual system using ferrets
The parallel visual pathways are also prominent in the visual system of higher mammals. Visual information from the outside world detected by the retina is transferred to the LGN of the thalamus and then to V1 in the cerebral cortex along the parallel visual pathways. The parallel visual pathways mainly consist of three kinds of pathways with distinct anatomical and physiological features. The three pathways are known as the parvocellular (P), magnocellular (M) and koniocellular (K) pathways in primates, and the X, Y and W pathways in carnivores, such as ferrets and cats. It has been proposed that each pathway contributes to visual perception in a different way. Visual functions were investigated using monkeys after damaging either the M or P pathway of the LGN selectively by injecting pharmacological drugs. When the M pathway was selectively damaged, there was little effect on visual acuity or color vision, but the ability to recognize moving stimuli was markedly impaired.74) In contrast, damage to the P pathway did not show obvious effects on motion perception but markedly inhibited visual acuity and color perception.74) These results suggest that the P pathway is mainly involved in the detailed analysis of the shape, size and color of visual objects, whereas the M pathway is preferentially related to information about the movement of visual objects.
It has been believed that the parallel visual pathways in mice are not well-developed than those in primates and carnivores. Although some evidence for the parallel visual pathways of rats was reported, it is unclear whether functionally and morphologically distinct parallel visual pathways, which correspond to the M, P and K pathways in primates and the X, Y and W pathways in carnivores, also exist in mice. Recently, a morphological study reported that LGN neurons in the mouse LGN could be classified into three distinct classes.75) However, it remains unclear whether morphologically distinct classes of LGN neurons in mice possess different functional properties. Future investigations would be necessary to uncover the relationship between the distinct classes of LGN neurons in mice and the parallel visual pathways in primate and carnivores.
Despite the physiological importance of the parallel visual pathways, molecular investigations of the parallel visual pathways are still limited, mainly because the parallel visual pathways are unclear in mice. Using the LGN of ferrets, we recently demonstrated that the Forkhead transcription factor FoxP2 was specifically expressed in X cells of the adult ferret LGN and in the P layers in the adult monkey LGN.76) FoxP2 is the first transcription factor found to be specifically expressed in one of the three pathways in the visual system. Further investigations of the roles of FoxP2 in the formation and functions of the parallel visual pathways would be important. In addition, detailed investigation of the visual functions of the human KE family patients, who have a mutated FOXP2 gene and developmental speech-language disabilities,77) could help deciphering the roles of FoxP2 in the parallel visual pathways. Another application of FoxP2 would be selective expression of genes of interest, such as GFP, Kir2.1 and NaChBac, in the P pathway using the FoxP2 promoter. Kir2.1 and NaChBac are useful for suppressing and enhancing neuronal activities, respectively. Selective expression of such genes in the P pathway would help in understanding the precise neuronal circuits and functional roles of the P pathway.
One of the long-lasting important questions about the evolution of the parallel visual pathways is the relationship between M and P cells of primates, and Y and X cells of carnivores (Fig. 5). One hypothesis is that X and Y cells of carnivores correspond to P and M cells of primates, respectively. Another hypothesis is that X and Y cells are homologous to linear and non-linear M cells of primates, respectively, and that P cells are unique to primates.78) Our results showing selective expression of FoxP2 in X cells of the ferret LGN and in the P layers of the monkey LGN provide new evidence for a homology between X cells of ferrets and P cells of monkeys76) (Fig. 5). Further molecular investigations of the parallel visual pathways of higher mammals will not only facilitate our understanding of the mechanisms of visual recognition in humans but will also contribute to uncovering the development and evolution of the visual system.
Figure 5.
Two hypotheses about the relationship between M and P cells in primates and Y and X cells in carnivores. The expression pattern of FoxP2 is consistent with hypothesis 1.
Conclusions
In this review, recent advances in the molecular understanding of the development and evolution of the brain of higher mammals have been summarized, mainly focusing on ferrets. One of the ultimate goals of neuroscience is to understand the human brain, and therefore molecular investigations of the brain of carnivores and primates are of great importance. Because a rapid and efficient genetic manipulation is now available for ferrets, the ferret should be an important option for neuroscience research involving higher mammals.
Acknowledgments
We apologize to the authors whose valuable work we were not able to cite due to space limitations. We thank Kawasaki lab members for their contributions. Some research introduced in this review was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology-Japan (MEXT), Japan Agency for Medical Research and Development (AMED), Life Science Foundation of Japan, the Uehara Memorial Foundation and Takeda Science Foundation.
Abbreviations
- ES cells
embryonic stem cells
- IFL
inner fiber layer
- IP cells
intermediate progenitor cells
- ISVZ
inner subventricular zone
- K
koniocellular
- LGN
lateral geniculate nucleus
- M
magnocellular
- ODC
ocular dominance column
- oRG cells
OSVZ radial glial cells
- OSVZ
outer subventricular zone
- P
parvocellular
- RG cells
radial glial cells
- SVZ
subventricular zone
- V1
primary visual cortex
- VZ
ventricular zone
Profile
Hiroshi Kawasaki was born in Kanazawa in 1965. He graduated from Kyoto University School of Medicine in 1990 and worked as a clinical neurologist for 4 years. In 1998, he received his Ph.D. from Kyoto University Graduate School of Medicine, where he uncovered roles of MAP kinases in the brain under the supervision of Prof. Eisuke Nishida and Prof. Jun Kimura. He then became an assistant professor, and later a lecturer, in the late Yoshiki Sasai’s laboratory at the Institute for Frontier Medical Sciences, Kyoto University, and succeeded in making dopaminergic neurons and retinal pigmented epithelial cells from embryonic stem cells in vitro. In 2002, he moved to the late Prof. Lawrence C. Katz’s laboratory as a research fellow at the Howard Hughes Medical Institute/Duke University in the U.S.A. In 2004, he became a project associate professor and started his own laboratory at the University of Tokyo Graduate School of Medicine, where he investigated genetic and environmental factors regulating brain formation and maturation. He also worked as a PRESTO researcher of the Japan Society for the Promotion of Science from 2006 to 2010. In 2013, he became a professor at Kanazawa University School of Medicine. Recently, his work has been focused on investigating brain structures unique to higher mammals using the carnivore ferret. He is now the director of Kanazawa University Brain/Liver Interface Medicine Research Center and a vice dean of Kanazawa University School of Medicine.
References
- 1).Kawasaki H., Mizuseki K., Nishikawa S., Kaneko S., Kuwana Y., Nakanishi S., Nishikawa S.I., Sasai Y. (2000) Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 28, 31–40. [DOI] [PubMed] [Google Scholar]
- 2).Kawasaki H., Suemori H., Mizuseki K., Watanabe K., Urano F., Ichinose H., Haruta M., Takahashi M., Yoshikawa K., Nishikawa S., Nakatsuji N., Sasai Y. (2002) Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc. Natl. Acad. Sci. U.S.A. 99, 1580–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Mizuseki K., Sakamoto T., Watanabe K., Muguruma K., Ikeya M., Nishiyama A., Arakawa A., Suemori H., Nakatsuji N., Kawasaki H., Murakami F., Sasai Y. (2003) Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 100, 5828–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Watanabe K., Kamiya D., Nishiyama A., Katayama T., Nozaki S., Kawasaki H., Watanabe Y., Mizuseki K., Sasai Y. (2005) Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 8, 288–296. [DOI] [PubMed] [Google Scholar]
- 5).Haruta M., Sasai Y., Kawasaki H., Amemiya K., Ooto S., Kitada M., Suemori H., Nakatsuji N., Ide C., Honda Y., Takahashi M. (2004) In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest. Ophthalmol. Vis. Sci. 45, 1020–1025. [DOI] [PubMed] [Google Scholar]
- 6).Lee S.H., Lumelsky N., Studer L., Auerbach J.M., McKay R.D. (2000) Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat. Biotechnol. 18, 675–679. [DOI] [PubMed] [Google Scholar]
- 7).Wichterle H., Lieberam I., Porter J.A., Jessell T.M. (2002) Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385–397. [DOI] [PubMed] [Google Scholar]
- 8).Tropepe V., Hitoshi S., Sirard C., Mak T.W., Rossant J., van der Kooy D. (2001) Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 30, 65–78. [DOI] [PubMed] [Google Scholar]
- 9).Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- 10).Kawasaki H., Fujii H., Gotoh Y., Morooka T., Shimohama S., Nishida E., Hirano T. (1999) Requirement for mitogen-activated protein kinase in cerebellar long term depression. J. Biol. Chem. 274, 13498–13502. [DOI] [PubMed] [Google Scholar]
- 11).Toda T., Homma D., Tokuoka H., Hayakawa I., Sugimoto Y., Ichinose H., Kawasaki H. (2013) Birth regulates the initiation of sensory map formation through serotonin signaling. Dev. Cell 27, 32–46. [DOI] [PubMed] [Google Scholar]
- 12).Toda T., Kawasaki H. (2014) The development of suckling behavior of neonatal mice is regulated by birth. Mol. Brain 7, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Katz L.C., Crowley J.C. (2002) Development of cortical circuits: lessons from ocular dominance columns. Nat. Rev. Neurosci. 3, 34–42. [DOI] [PubMed] [Google Scholar]
- 14).Nassi J.J., Callaway E.M. (2009) Parallel processing strategies of the primate visual system. Nat. Rev. Neurosci. 10, 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Namba T., Huttner W.B. (2017) Neural progenitor cells and their role in the development and evolutionary expansion of the neocortex. WIREs Dev. Biol. 6, e256. [DOI] [PubMed] [Google Scholar]
- 16).Homman-Ludiye J., Bourne J.A. (2014) Mapping arealisation of the visual cortex of non-primate species: lessons for development and evolution. Front. Neural Circuits 8, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Chan A.W., Chong K.Y., Martinovich C., Simerly C., Schatten G. (2001) Transgenic monkeys produced by retroviral gene transfer into mature oocytes. Science 291, 309–312. [DOI] [PubMed] [Google Scholar]
- 18).Lois C., Hong E.J., Pease S., Brown E.J., Baltimore D. (2002) Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295, 868–872. [DOI] [PubMed] [Google Scholar]
- 19).Sasaki E., Suemizu H., Shimada A., Hanazawa K., Oiwa R., Kamioka M., Tomioka I., Sotomaru Y., Hirakawa R., Eto T., Shiozawa S., Maeda T., Ito M., Ito R., Kito C., Yagihashi C., Kawai K., Miyoshi H., Tanioka Y., Tamaoki N., Habu S., Okano H., Nomura T. (2009) Generation of transgenic non-human primates with germline transmission. Nature 459, 523–527. [DOI] [PubMed] [Google Scholar]
- 20).Okano H., Hikishima K., Iriki A., Sasaki E. (2012) The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin. Fetal Neonatal. Med. 17, 336–340. [DOI] [PubMed] [Google Scholar]
- 21).Kawasaki H., Iwai L., Tanno K. (2012) Rapid and efficient genetic manipulation of gyrencephalic carnivores using in utero electroporation. Mol. Brain 5, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Kawasaki H., Toda T., Tanno K. (2013) In vivo genetic manipulation of cortical progenitors in gyrencephalic carnivores using in utero electroporation. Biol. Open 2, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Borrell V., Kaspar B.K., Gage F.H., Callaway E.M. (2006) In vivo evidence for radial migration of neurons by long-distance somal translocation in the developing ferret visual cortex. Cereb. Cortex 16, 1571–1583. [DOI] [PubMed] [Google Scholar]
- 24).Borrell V. (2010) In vivo gene delivery to the postnatal ferret cerebral cortex by DNA electroporation. J. Neurosci. Methods 186, 186–195. [DOI] [PubMed] [Google Scholar]
- 25).Zilles K., Armstrong E., Schleicher A., Kretschmann H.J. (1988) The human pattern of gyrification in the cerebral cortex. Anat. Embryol. (Berl.) 179, 173–179. [DOI] [PubMed] [Google Scholar]
- 26).Poduri A., Evrony G.D., Cai X., Walsh C.A. (2013) Somatic mutation, genomic variation, and neurological disease. Science 341, 1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Barkovich A.J., Guerrini R., Kuzniecky R.I., Jackson G.D., Dobyns W.B. (2012) A developmental and genetic classification for malformations of cortical development: update 2012. Brain 135, 1348–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Smart I.H., Dehay C., Giroud P., Berland M., Kennedy H. (2002) Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex 12, 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Zecevic N., Chen Y., Filipovic R. (2005) Contributions of cortical subventricular zone to the development of the human cerebral cortex. J. Comp. Neurol. 491, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Fietz S.A., Kelava I., Vogt J., Wilsch-Brauninger M., Stenzel D., Fish J.L., Corbeil D., Riehn A., Distler W., Nitsch R., Huttner W.B. (2010) OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci. 13, 690–699. [DOI] [PubMed] [Google Scholar]
- 31).Hansen D.V., Lui J.H., Parker P.R., Kriegstein A.R. (2010) Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561. [DOI] [PubMed] [Google Scholar]
- 32).Reillo I., de Juan Romero C., Garcia-Cabezas M.A., Borrell V. (2011) A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb. Cortex 21, 1674–1694. [DOI] [PubMed] [Google Scholar]
- 33).Lui J.H., Hansen D.V., Kriegstein A.R. (2011) Development and evolution of the human neocortex. Cell 146, 18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Molnar Z., Clowry G. (2012) Cerebral cortical development in rodents and primates. Prog. Brain Res. 195, 45–70. [DOI] [PubMed] [Google Scholar]
- 35).Rakic P. (2009) Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. 10, 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Nonaka-Kinoshita M., Reillo I., Artegiani B., Martinez-Martinez M.A., Nelson M., Borrell V., Calegari F. (2013) Regulation of cerebral cortex size and folding by expansion of basal progenitors. EMBO J. 32, 1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Ayoub A.E., Oh S., Xie Y., Leng J., Cotney J., Dominguez M.H., Noonan J.P., Rakic P. (2011) Transcriptional programs in transient embryonic zones of the cerebral cortex defined by high-resolution mRNA sequencing. Proc. Natl. Acad. Sci. U.S.A. 108, 14950–14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Bernard A., Lubbers L.S., Tanis K.Q., Luo R., Podtelezhnikov A.A., Finney E.M., McWhorter M.M.E., Serikawa K., Lemon T., Morgan R., Copeland C., Smith K., Cullen V., Davis-Turak J., Lee C.-K., Sunkin S.M., Loboda A.P., Levine D.M., Stone D.J., Hawrylycz M.J., Roberts C.J., Jones A.R., Geschwind D.H., Lein E.S. (2012) Transcriptional architecture of the primate neocortex. Neuron 73, 1083–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Saito T., Nakatsuji N. (2001) Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol. 240, 237–246. [DOI] [PubMed] [Google Scholar]
- 40).Tabata H., Nakajima K. (2001) Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience 103, 865–872. [DOI] [PubMed] [Google Scholar]
- 41).Fukuchi-Shimogori T., Grove E.A. (2001) Neocortex patterning by the secreted signaling molecule FGF8. Science 294, 1071–1074. [DOI] [PubMed] [Google Scholar]
- 42).Hevner R.F. (2005) The cerebral cortex malformation in thanatophoric dysplasia: neuropathology and pathogenesis. Acta Neuropathol. 110, 208–221. [DOI] [PubMed] [Google Scholar]
- 43).Vogt C., Blaas H.G. (2013) Thanatophoric dysplasia: autopsy findings over a 25-year period. Pediatr. Dev. Pathol. 16, 160–167. [DOI] [PubMed] [Google Scholar]
- 44).Shiang R., Thompson L.M., Zhu Y.Z., Church D.M., Fielder T.J., Bocian M., Winokur S.T., Wasmuth J.J. (1994) Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 78, 335–342. [DOI] [PubMed] [Google Scholar]
- 45).Tavormina P.L., Shiang R., Thompson L.M., Zhu Y.Z., Wilkin D.J., Lachman R.S., Wilcox W.R., Rimoin D.L., Cohn D.H., Wasmuth J.J. (1995) Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat. Genet. 9, 321–328. [DOI] [PubMed] [Google Scholar]
- 46).Bellus G.A., McIntosh I., Smith E.A., Aylsworth A.S., Kaitila I., Horton W.A., Greenhaw G.A., Hecht J.T., Francomano C.A. (1995) A recurrent mutation in the tyrosine kinase domain of fibroblast growth factor receptor 3 causes hypochondroplasia. Nat. Genet. 10, 357–359. [DOI] [PubMed] [Google Scholar]
- 47).Rousseau F., Bonaventure J., Legeai-Mallet L., Pelet A., Rozet J.M., Maroteaux P., Le Merrer M., Munnich A. (1994) Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature 371, 252–254. [DOI] [PubMed] [Google Scholar]
- 48).Rousseau F., Saugier P., Le Merrer M., Munnich A., Delezoide A.L., Maroteaux P., Bonaventure J., Narcy F., Sanak M. (1995) Stop codon FGFR3 mutations in thanatophoric dwarfism type 1. Nat. Genet. 10, 11–12. [DOI] [PubMed] [Google Scholar]
- 49).Naski M.C., Wang Q., Xu J., Ornitz D.M. (1996) Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nat. Genet. 13, 233–237. [DOI] [PubMed] [Google Scholar]
- 50).Inglis-Broadgate S.L., Thomson R.E., Pellicano F., Tartaglia M.A., Pontikis C.C., Cooper J.D., Iwata T. (2005) FGFR3 regulates brain size by controlling progenitor cell proliferation and apoptosis during embryonic development. Dev. Biol. 279, 73–85. [DOI] [PubMed] [Google Scholar]
- 51).Lin T., Sandusky S.B., Xue H., Fishbein K.W., Spencer R.G., Rao M.S., Francomano C.A. (2003) A central nervous system specific mouse model for thanatophoric dysplasia type II. Hum. Mol. Genet. 12, 2863–2871. [DOI] [PubMed] [Google Scholar]
- 52).Masuda K., Toda T., Shinmyo Y., Ebisu H., Hoshiba Y., Wakimoto M., Ichikawa Y., Kawasaki H. (2015) Pathophysiological analyses of cortical malformation using gyrencephalic mammals. Sci. Rep. 5, 15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Toda T., Shinmyo Y., Dinh Duong T.A., Masuda K., Kawasaki H. (2016) An essential role of SVZ progenitors in cortical folding in gyrencephalic mammals. Sci. Rep. 6, 29578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Sehara K., Toda T., Iwai L., Wakimoto M., Tanno K., Matsubayashi Y., Kawasaki H. (2010) Whisker-related axonal patterns and plasticity of layer 2/3 neurons in the mouse barrel cortex. J. Neurosci. 30, 3082–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Sehara K., Wakimoto M., Ako R., Kawasaki H. (2012) Distinct developmental principles underlie the formation of ipsilateral and contralateral whisker-related axonal patterns of layer 2/3 neurons in the barrel cortex. Neuroscience 226, 289–304. [DOI] [PubMed] [Google Scholar]
- 56).DeFelipe J., Alonso-Nanclares L., Arellano J.I. (2002) Microstructure of the neocortex: comparative aspects. J. Neurocytol. 31, 299–316. [DOI] [PubMed] [Google Scholar]
- 57).Borrell V., Yoshimura Y., Callaway E.M. (2005) Targeted gene delivery to telencephalic inhibitory neurons by directional in utero electroporation. J. Neurosci. Methods 143, 151–158. [DOI] [PubMed] [Google Scholar]
- 58).Kataoka A., Shimogori T. (2008) Fgf8 controls regional identity in the developing thalamus. Development 135, 2873–2881. [DOI] [PubMed] [Google Scholar]
- 59).Soma M., Aizawa H., Ito Y., Maekawa M., Osumi N., Nakahira E., Okamoto H., Tanaka K., Yuasa S. (2009) Development of the mouse amygdala as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J. Comp. Neurol. 513, 113–128. [DOI] [PubMed] [Google Scholar]
- 60).Nakahira E., Yuasa S. (2005) Neuronal generation, migration, and differentiation in the mouse hippocampal primoridium as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J. Comp. Neurol. 483, 329–340. [DOI] [PubMed] [Google Scholar]
- 61).Hatanaka Y., Hisanaga S., Heizmann C.W., Murakami F. (2004) Distinct migratory behavior of early- and late-born neurons derived from the cortical ventricular zone. J. Comp. Neurol. 479, 1–14. [DOI] [PubMed] [Google Scholar]
- 62).Garcia-Frigola C., Carreres M.I., Vegar C., Herrera E. (2007) Gene delivery into mouse retinal ganglion cells by in utero electroporation. BMC Dev. Biol. 7, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Ako R., Wakimoto M., Ebisu H., Tanno K., Hira R., Kasai H., Matsuzaki M., Kawasaki H. (2011) Simultaneous visualization of multiple neuronal properties with single-cell resolution in the living rodent brain. Mol. Cell. Neurosci. 48, 246–257. [DOI] [PubMed] [Google Scholar]
- 64).Chalupa, L.M. and Werner, J.S. (2003) The Visual Neuroscience. The MIT Press, Cambridge. [Google Scholar]
- 65).Hubel D.H., Wiesel T.N. (1962) Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. 160, 106–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Hubel D.H., Wiesel T.N. (1968) Receptive fields and functional architecture of monkey striate cortex. J. Physiol. 195, 215–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Hubel D.H., Wiesel T.N., LeVay S. (1977) Plasticity of ocular dominance columns in monkey striate cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 278, 377–409. [DOI] [PubMed] [Google Scholar]
- 68).Ohki K., Chung S., Ch’ng Y.H., Kara P., Reid R.C. (2005) Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603. [DOI] [PubMed] [Google Scholar]
- 69).Wiesel T.N., Hubel D.H. (1963) Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017. [DOI] [PubMed] [Google Scholar]
- 70).Wiesel T.N., Hubel D.H. (1965) Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J. Neurophysiol. 28, 1029–1040. [DOI] [PubMed] [Google Scholar]
- 71).Wiesel T.N., Hubel D.H. (1965) Extent of recovery from the effects of visual deprivation in kittens. J. Neurophysiol. 28, 1060–1072. [DOI] [PubMed] [Google Scholar]
- 72).Antonini A., Stryker M.P. (1993) Rapid remodeling of axonal arbors in the visual cortex. Science 260, 1819–1821. [DOI] [PubMed] [Google Scholar]
- 73).Crowley J.C., Katz L.C. (2000) Early development of ocular dominance columns. Science 290, 1321–1324. [DOI] [PubMed] [Google Scholar]
- 74).Schiller P.H., Logothetis N.K. (1990) The color-opponent and broad-band channels of the primate visual system. Trends Neurosci. 13, 392–398. [DOI] [PubMed] [Google Scholar]
- 75).Krahe T.E., El-Danaf R.N., Dilger E.K., Henderson S.C., Guido W. (2011) Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. J. Neurosci. 31, 17437–17448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Iwai L., Ohashi Y., van der List D., Usrey W.M., Miyashita Y., Kawasaki H. (2013) FoxP2 is a parvocellular-specific transcription factor in the visual thalamus of monkeys and ferrets. Cereb. Cortex 23, 2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Lai C.S., Fisher S.E., Hurst J.A., Vargha-Khadem F., Monaco A.P. (2001) A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413, 519–523. [DOI] [PubMed] [Google Scholar]
- 78).Kaplan, E. (2004) The M, P, and K pathways of the primate visual system. In The Visual Neurosciences Vol. 1 (eds. Chalupa, L.M. and Werner, J.S.). MIT Press, Cambridge, pp. 481–493. [Google Scholar]