Abstract
Biomass of Aspergillus awamori was investigated for mycochemicals, total phenolic compounds (TPC), condensed tannin content (CTC), free-radical scavenging potential (FRSP), and DNA damage protection activity. FRSP was determined using DPPH, ABTS, FRAP (Ferric reducing antioxidant power), metal chelating activity, and cupric reducing antioxidant capacity) assays. Water (Aq), aqueous ethanol 50% (AqE), and methanol were used as extraction phase at 44.5 °C for 23.8 min. AqE shows the presence of maximum mycochemicals (coumarins, glucose, saponins, flavonoids, and tannin). Further quantitative analysis shows maximum TPC (23.17 mg GAE/g dwb) in AqE and CTC (.89 mg CE/g dwb) in ME. Qualitative and quantitative analysis for identification of specific bioactive compound in AqE was carried out using HPLC. HPLC analysis confirmed the presence of bioactive compounds: p′-Coumaric acid (5.96 mg/g dwb), cinnamic acid (4.31 mg/g dwb), gallic acid (2.27 mg/g dwb), and ascorbic acid (.98 mg/g dwb). All the extracts show significant DNA damage protection activity; however, AqE showed the maximum activity. Pearson correlations were also calculated to find the relationships between bioactive compounds and antioxidant potential.
Keywords: GRAS fungi, Mycochemicals, HPLC, DNA protection, Phenolic compounds
Introduction
The use of microorganisms as nutritious food and secondary products developed from them has generated considerable interest from food and nutraceutical industries. Utilization of microbial capability to produce products of medicinal value is of utmost importance (Smith et al. 2015; Chang and Buswell 1996). Because of the health concerns and lesser side effects, the antioxidant-rich food and food products of natural origin are preferred over synthetic ones (Siroha et al. 2016; Salar and Purewal 2016; Bhanja et al. 2009). Bioactive compounds mainly include extractable phenolic compounds, flavonoids, condensed tannin content, and other important derivatives (Salar et al. 2015; Lahouar et al. 2014; Sharma and Gujral 2010). Oxidative stress is an indicator of imbalance between the antioxidants and free-radical molecule inside the body. Antioxidants can switch off or reduce the activity of free radicals due to electron donating nature (Dhull et al. 2016). Once free-radical molecules get an electron from antioxidants, they become stabilized and further not able to produce any kind of damage within body (Sandhu et al. 2016; Salar and Purewal 2016). Sometimes, the natural protective mechanism became insufficient; hence, body requires these antioxidants from dietary intakes. Intake of antioxidant-rich food and food products is strongly associated with reduced risk of chronic diseases and ageing problems (Salar et al. 2013; Ferreira et al. 2009).
Secondary products isolated from microorganisms especially from fungi is as important as medicine, indicating their role in understanding the mechanism of occurrence of neurodegenerative disorders and other diseases (Chandra and Arora 2014; Arora and Chandra 2010; Rodrigues et al. 2005). Fungal strains are remarkably assessed for the presence of alkaloids, tannins, phenolic content, steroids, and flavonoids (Archer 2000). Fungal strains are widely used for the production of secondary metabolites in conjunction with natural substrates and microorganisms themselves act as a source of secondary metabolites (Salar et al. 2012, 2013; Bhanja et al. 2009). Bioactive compounds from several microorganisms have been reported with their ability to scavenge reactive oxygen species and can function as a source of natural antioxidants (Abubakr et al. 2012; Afify et al. 2012; Arora and Chandra 2011; Demirel et al. 2009; Malpure et al. 2006).
In the present investigation, Aspergillus awamori was cultivated in submerged flask cultures. Scarcity of information on bioactive compounds, antioxidant potential and DNA damage protection activity from the microbial strain prompted us to design the current study. Accordingly, the objective of the present investigation was to evaluate the fungal extracts for the presence of mycochemicals, total phenolic compounds, condensed tannin content, free-radical scavenging potential, and DNA damage protection activity. HPLC analysis was also carried out to determine major bioactive compounds in A. awamori.
Materials and methods
Organism, inoculums, and media
Fungal strain A. awamori (MTCC 548) was procured from the Institute of Microbial Technology (IMTECH) Chandigarh, India. The strain was maintained on czapek broth and czapek agar media. Media were prepared using four different stock solutions separately in volumetric flasks and all of these were prepared in 1 l of distilled water [Stock A: NaNO3 (40 g), KCl (10 g), MgSO4·7H2O (10 g), FeSO4·7H2O (.2 g) Stock B: K2HPO4 (20 g), Stock C: ZnsO4·7H2O (.1 g), Stock D: CuSO4·5H2O (.05 g)]. Final media were prepared using stock A (50 ml), stock B (50 ml), stock C (1 ml), and stock D (1 ml) and final volume (1000 ml) was prepared with distilled water. Fungal strain was incubated at 25 ± 2 °C under shaking conditions (120 rpm) in orbital shaker (Scigenics Biotech, India).
Preparation of samples
Biomass recovered was dried in an oven at 40 °C for 24 h. To obtain fine powdered form, dried fungal biomass was ground in an electric grinder (Sujata, India) and stored at −20 °C for further analysis (Salar et al. 2012).
Preparation of extracts
Dried biomass (powdered form) was extracted with water (Aq), aqueous ethanol (50%) (AqE), and methanol (ME) in an Erlenmeyer flask (1:20 w/v) under optimized conditions: 44.5 °C for 23.8 min (Salar et al. 2016) and the extracts were prepared accordingly.
Determination of total phenolics content (TPC)
Total phenolic content of the extracts was determined using Folin–Ciocalteu method as described by Salar et al. (2012). The amount of total phenolic content was calculated from the equation generated from the standard calibration curve of gallic acid and expressed as mg gallic acid equivalents/g dry weight basis (mg GAE/g dwb).
Free-radical scavenging potential (FRSP)
The FRSP of the extracts was evaluated by employing different antioxidant assays as elaborated below.
DPPH (2,2′ Diphenyl–1′ picrylhydrazyl) radical scavenging assay
The radical scavenging capacity of extracts was measured by the DPPH scavenging method described by Yen and Chen (1995) with slight modifications (Salar and Purewal 2017). Absorbance (A) at 517 nm was read at 0 and 30 min. against a blank. Antioxidant activity was calculated as % discoloration:
ABTS radical cation depolarization assay
ABTS, 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (HiMedia) radical cation decolorization test is also a spectrophotometric method widely used for the assessment of antioxidant activity in various extracts. Antioxidant activity was measured using method described by Salar et al. (2012). Absorbance (A) at 732 nm was read at 0 and 10 min. Antioxidant activity was calculated in %:
FRAP (ferric reducing antioxidant power) assay
Ferric reducing antioxidant power of extracts was estimated using FRAP reagent according to the method described by Benzie and Strain (1996) with slight modifications (Salar and Purewal 2017). Reading of the colored product (ferrous-tripyridyltriazine complex) was then taken at 595 nm. The standard curve was linear between .1 mM and 1.0 mM FeSO4·7H2O. FRAP value of extracts was calculated using formula:
where A E is the absorbance of extracts and A C is the absorbance of control, respectively.
MCA [metal chelating (Fe+2) activity]
The extract (100 µl) was mixed with 50 μl of ferrous chloride (2 mM/l) followed by addition of 1.5 ml of 50% ethanol. After 5 min, the reaction was initiated by the addition of 5 mM/l ferrozine (100 μl) and the mixture was shaken on vortex mixer. The mixture was incubated at room temperature for 10 min. Absorbance of solution was measured at 560 nm on a spectrophotometer. The chelating activity of the extract for Fe+2 was calculated as follows:
CTC (condensed tannin content)
Condensed tannins content was determined using Vannilin–HCl method described by Julkunen-Titto (1985). The absorbance against blank was read at 500 nm. Catechin was used for the preparation of standard curve. The results were expressed as mg catechin equivalent/g dry weight basis (mg CE/g dwb).
CUPRAC (cupric ion reducing capacity)
Cupric ion reducing capacity was measured by the method described by Salar and Purewal (2017). Aliquot of extracts (100 µl) was added to different storage vials followed by the addition of CUPRAC reagent (3 ml). The reaction mixture was incubated at room temperature for 30 min. CUPRAC reagent was prepared using ammonium acetate buffer (1 M), cupric chloride (10 mM), and neocuproine (7.5 mM) in ratio (1:1:1). After incubation, the absorbance was recorded at 450 nm. Ascorbic acid was used as a positive control.
HPLC analysis
HPLC analysis for the detection of specific bioactive compounds in AqE was carried out on a Shimadzu 10 AVP HPLC system comprising an SCL10 AVP system controller, two LC-10 AVP pumps CTO-10 AVP column oven with Rheodyne 7120 injection value (20 µl sample loop), and SPD-M10 AVP photodiode-array detector (all from Shimadzu, Tokyo, Japan). Gemini-NX C18 analytical HPLC column (250 × 4.6 mm, 3 µm) with a guard column (40 × 3 mm, 3 µm) both from Phenomenex (Torrance, CA, USA) was used. Analysis was performed at a rate of .5 ml/min using 1.5% v/v acetic acid (solvent A) and aqueous ethanol:acetonitrile (40:50 v/v) mixture (solvent B) under the following gradient program: 0–8 min. 70% acetic acid, 8–19 min. 60% acetic acid, and 19–30 min. 30% acetic acid. Injection volume was 10 µl. The analytes were detected at 280 nm.
DDPA (DNA damage protection activity)
The ability of different extracts prepared in water (Aq), aqueous ethanol 50% (AqE), and methanol (ME) to protect DNA from damaging effects of free radicals generated by Fenton’s reagent was analyzed according to method described by Kumar et al. (2013) and further modified by Salar and Purewal (2016). The results were analyzed on 1% Agarose gel electrophoresis stained with Ethidium bromide (4 µl). Quercetin was used as a positive control.
Mycochemical screening
Mycochemical screening of various extracts/fractions was carried out according to the standard methods as described by Trease and Evans (1996).
Statistical analysis and correlations
The mean values and the standard deviations were calculated from the data obtained from three independent experiments. Correlations between bioactive compounds, antioxidant potential of extracts, and their means were compared using the SPSS 16.0 (IBM, New York) statistical software. Principal component loading plot for determining the relationship between bioactive compounds and antioxidant potential of extracts prepared in different extraction phase was drawn using the Minitab statistical software version 14 (Minitab Inc, USA).
Results and discussion
Mycochemicals and bioactive compounds
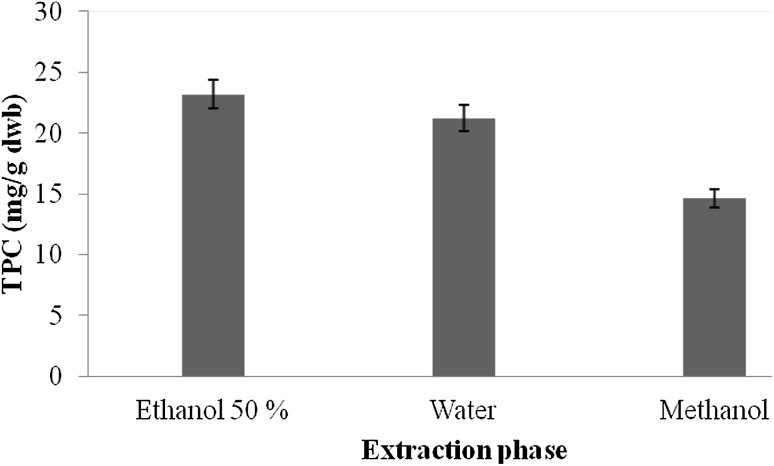
Preliminary screening for the presence of various mycochemicals in extracts of A. awamori was carried out and results of mycochemical analysis are presented in Table 1. Mycochemical analysis of A. awamori revealed the presence of coumarins, glucose, saponins, flavonoids, and tannin in AqE, whereas Aq extracts showed the presence of coumarins, reducing sugar, glucose, and saponins. ME, however, showed the presence of only two compounds, i.e., flavonoids and flavonone. The results from the mycochemical analysis thus revealed that the extracts of A. awamori contained many bioactive agents. The total phenolic content of different extracts of A. awamori is presented in Fig. 1. The total phenolics in extracts ranged from .56 to 23.17 mg GAE/g dwb (mg gallic acid equivalent/g dry weight basis). The amount of extractable phenolic compounds in AqE extracts was 23.17 mg GAE/g dwb followed by Aq extracts (21.23 mg GAE/g dwb) and ME (.56 mg GAE/g dwb). From the results, it was observed that the amount of extractable phenolic compounds was the highest in AqE and the lowest in ME. Various workers have reported significant amount of phenolic compounds in different microbial strains. They reported the phenolic compounds of 62.72 mg GAE/g in Rhodotorula glutinis (Salar et al. 2013), 34.56 mg CE/g in Aspergillus candidus (Malpure et al. 2006), 35.2 mg/ml in Aspergillus fumigates (Arora and Chandra 2011), and 18 mg/g in Aspergillus terreus (Chandra and Arora 2014).
Table 1.
Mycochemical analysis of Aspergillus awamori extracts prepared using water, ethanol (50%), and methanol
| S. No. | Mycochemical | Ethanol (50%) | Water | Methanol |
|---|---|---|---|---|
| 1 | Phlabotannin | − | − | − |
| 2 | Coumarins | + | + | − |
| 3 | Flavonoids | + | − | + |
| 4 | Steroids | − | − | − |
| 5 | Reducing sugar | − | + | − |
| 6 | Glucose | + | + | − |
| 7 | Tannin | + | − | − |
| 8 | Protein | − | − | − |
| 9 | Anthocyanin | − | − | − |
| 10 | Saponins | + | + | − |
| 11 | Flavonon | − | − | + |
| 12 | Starch | − | − | – |
Fig. 1.
Total phenolic compounds (mg GAE/g dwb) in different extracts
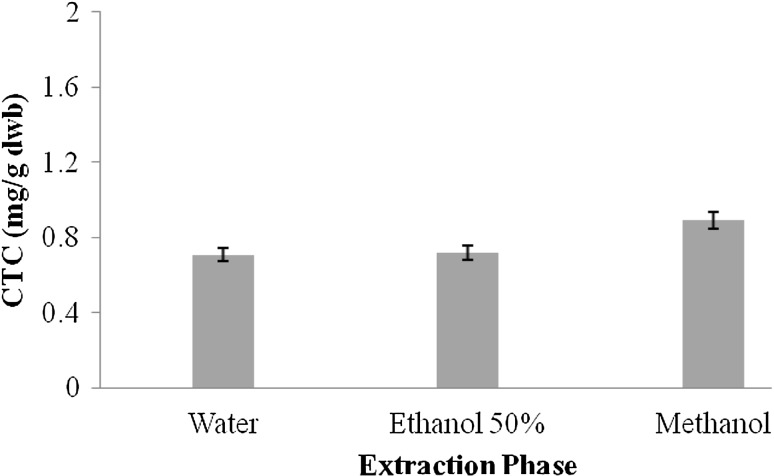
Tannins are another important class of bioactive compounds present in natural resources. They possess high molecular weight and have capability to form various complexes with protein molecules. The presence of two major classes of tannins in natural resources has been reported and these include (a) condensed tannin content (CTC) and (b) hydrolysable tannin content (HTC) (Hagerman et al. 1998). CTC in extracts of A. awamori followed the order: ME > AqE > Aq. Figure 2 shows the CTC of Aq, AqE, and ME.
Fig. 2.
Condensed tannin content (mg GAE/g dwb) in different extracts
Free-radical scavenging potential (FRSP)
Determination of FRSP is a critical process and needs many tests to be performed (Siroha et al. 2016). A number of antioxidant assays are currently being used by the researchers/scientists. The uses of antioxidant potential assays ease the product formulation that are of medicinal value. However, the results of antioxidant potential depend on the testing system being employed. Until date, there is no single method that can be use to determine the complete antioxidant capacity of extracts (Salar and Seasotiya 2012; Salar et al. 2015). Furthermore, the antioxidant potential of extracts also depends on the extraction phase used to leach out phenolic compounds from experimental samples. Sometimes, absolute solvent yields maximum bioactive compounds, whereas in some cases, the combination of aqueous phase along with solvent results in maximal recovery of the bioactive compounds from the experimental material. Results of FRSP also depend on the specific free radical being used as reactant. The antioxidant potential of phenolic compounds present in microorganisms has been associated with the health benefits attributed to the product derived from them (Salar et al. 2013).
The results of FRSP analysis of extracts are shown in Table 2. The FRSP established by DPPH test was found to be the highest in AqE (85.0%) followed by Aq (66.7%) and ME (60.6%). Similarly, the percent inhibition during ABTS assay was maximum in AqE (99.4%) followed by Aq (99.2%) and ME (93.8%). FRAP assay is widely used by researchers to determine the potential of extracts to reduce the tripyridyltriazine complex to dark blue-colored ferrous-tripyridyltriazine complex. FRAP values of extracts studied ranged from 1.0 to 1.3 mM Fe2+/g. The MCA of A. awamori extracts followed the order: ME > AqE > Aq. Ascorbic acid was used as a standard to determine the CUPRAC value of A. awamori extracts. CUPRAC value of extracts prepared in different extraction phase ranged from 1.7 to 11.6 (mg AAE/g dwb). Several researchers have reported the presence of DPPH radical scavenging activity in Aspergillus terrus (85.2%) (Chandra and Arora 2014); Aspergillus fumigatus (69%) (Arora and Chandra 2011). Malpure et al. (2006) reported the presence of ABTS scavenging activity (5.52–92.30%) in A. candidus. Depending on the type of microorganisms and the media used for cultivation and maintenance, the FRSP varies accordingly (Smith et al. 2015; Salar et al. 2013).
Table 2.
Free-radical scavenging potential in extracts of Aspergillus awamori
| Extraction phase | DPPH (% inhibition) | ABTS (% inhibition) | FRAP (mM Fe2+/g) | MCA (%) | CUPRAC (mg AAE/g DWB) |
|---|---|---|---|---|---|
| Ethanol (50%) | 85.0 ± .07af | 99.4 ± .24bf | 1.3 ± .03cf | 89.2 ± .08df | 11.6 ± .04ef |
| Water | 66.7 ± .11ag | 99.2 ± .41bf | 1.2 ± .01cf | 25.8 ± .14dg | 4.8 ± .07eg |
| Methanol | 60.6 ± .19ah | 93.8 ± .36bg | 1.0 ± .05cf | 90.1 ± .05dh | 1.7 ± .01eh |
DPPH 2,2-diphenyl-1-picrylhydrazyl, ABTS 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt, FRAP ferric reducing antioxidant power, MCA metal chelating activity, CUPRAC cupric ion reducing capacity
Values (±SD) are the means of triplicate experiments. Values in each row and column having the same superscript letter are not significantly different (p < .05)
Phenolic profile from HPLC
Identification of specific bioactive compounds in fungal extracts was carried out using HPLC. Six standards viz. ascorbic acid, benzoic acid, catechol, p-coumaric acid, cinnamic acid, and gallic acid were used for the detection of same compounds in extracts prepared in AqE. Data from bioactive compounds in A. awamori extracts are reported in Table 3 and the chromatograms are shown in Fig. 3. HPLC analysis of AqE extract of A. awamori revealed the presence of several bioactive compounds. Figure 3a, b represents two types of peaks: first-type peaks are larger in size, whereas the other is smaller as compared to first. The larger peaks in the Fig. 3a, b is of standards, whereas the smaller peaks were generated due to the amount of specific bioactive compounds present in AqE extract. Some peaks are superimposed in the figure and they denote the purity of specific compounds as that of standard. Generation of smaller peaks indicates the presence of specific compounds in small amount. However, the particular compound present in amount that is enough to generate their peak. The retention time of peak, area, and height and other important factors are explained in Table 4. The main phenolic compound in AqE extract was p′-Coumaric acid, cinnamic acid, gallic acid, and ascorbic acid, whereas more than five unknown peaks were also detected (Fig. 3c). The presence of specific bioactive compounds in AqE extract was identified by comparing the chromatographic peaks with the retention time (R t) of individual standard and further confirmed by co-injection with isolated standards. The unknown peaks might be playing an important role in providing antioxidant potential to the extract tested. All these specific bioactive compounds are reported for the first time in A. awamori. Amount of specific bioactive compounds present in the extract is expressed as mg/g. Data from the quantitative analysis of extract showed that the amount of identified bioactive compounds ranged from .98 to 5.96 mg/g (Table 3). p′-Coumaric acid was found as the major bioactive compound followed by cinnamic acid, gallic acid, and ascorbic acid. In earlier report (Salar et al. 2013), the presence of specific compounds viz., gallic acid, benzoic acid, catechol, caffeic acid, and ferulic acid was confirmed by HPLC analysis of extracts R. glutinis CCY 20-2-26. The difference in types of specific compounds present in the studied fungus might be due to the difference in type of fungus as well as the conditions provided for the growth.
Table 3.
Quantitative profile of bioactive compounds derived via HPLC
| Specific bioactive compounds | Amount (mg/g dwb) |
|---|---|
| p′-Coumaric acid | 5.96 |
| Cinnamic acid | 4.31 |
| Gallic acid | 2.27 |
| Ascorbic acid | .98 |
Fig. 3.
HPLC chromatogram of Aspergillus awamori extract from AqE (50%) (above) and standard phenolic compounds (below)
Table 4.
Peak table (detector A Ch1 280 nm)
| Peak | Name | Retention time | Area | Height | Area % | Height % | Theoretical plate/m | Tailing factor |
|---|---|---|---|---|---|---|---|---|
| 1 | p′-Coumaric acid | 3.332 | 48,936 | 11,482 | 16.900 | 18.615 | 71,879.276 | 1.196 |
| 2 | Cinnamic acid | 4.334 | 182,054 | 44,502 | 62.872 | 72.150 | 143,947.887 | 1.470 |
| 3 | Gallic acid | 6.476 | 49,063 | 5150 | 16.944 | 8.349 | 63,926.427 | .940 |
| 4 | Ascorbic acid | 8.243 | 9510 | 547 | 3.284 | .886 | 58,916.315 | .696 |
| Total | 289,563 | 61,681 | 100.00 | 100.00 |
DNA damage protection activity
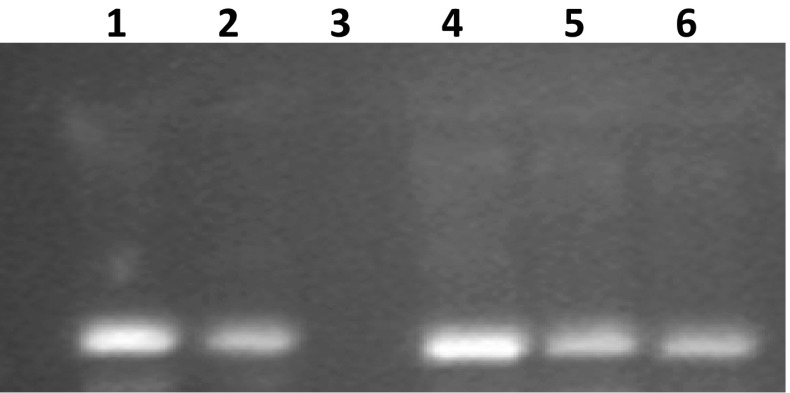
All the extracts of A. awamori prepared using different extraction phases were assessed for the presence of DNA damage protection activity. DNA damage protection activity was determined using agarose gel (1%) electrophoresis. Fenton’s reagent was used for DNA damage during the electrophoresis process. A model DNA pBR322 was used to check the activity against DNA damage by fenton’s reagent. Confirmation of damaging effect of fenton’s reagent towards DNA was shown by the Lane 3 (Fig. 4), although all the extracts possess DNA damage protection activity as confirmed by the presence of visible bands (Fig. 4). Among extracts, the maximal DNA damage protection activity was shown by the AqE (Lane 4), whereas the minimal activity was observed in ME (Lane 6). Sharpness of the band indicates the maximal activity, whereas the faint band indicates the lesser/minimum DNA damage protection activity. Quercetin was used as a positive control (Lane 2). DNA damage is solely responsible for the generation of oxidative stress-related chronic diseases (Salar et al. 2012). DNA damage protection activity of plant extracts and fermented products against fenton’s reagent has been reported in earlier publications (Salar and Purewal 2016, 2017; Kumar et al. 2013). This is the first report which confirms the DNA damage protection activity in filamentous fungal strain A. awamori (MTCC 548). DNA damage protection activity in extracts might be attributed to the presence of specific bioactive compounds.
Fig. 4.
DNA damage protecting activity in different extracts of Aspergillus awamori (MTCC 548) against hydroxyl radicals induced DNA damage of pBR322. Lane 1 Native pBR322 plasmid DNA, Lane 2 DNA + Fenton’s reagent + Quercetin (mg/ml positive control), Lane 3 DNA + Fenton’s reagent, Lane 4 DNA + Fenton’s reagent + ethanol 50% extract, and Lane 5 DNA + Fenton’s reagent + Water extract, Lane 6 DNA + Fenton’s reagent + methanol extract
Correlation between TPC and FRSP
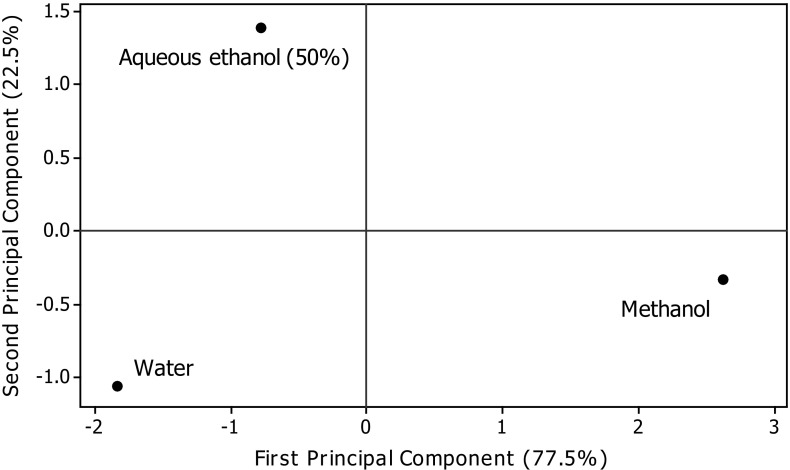
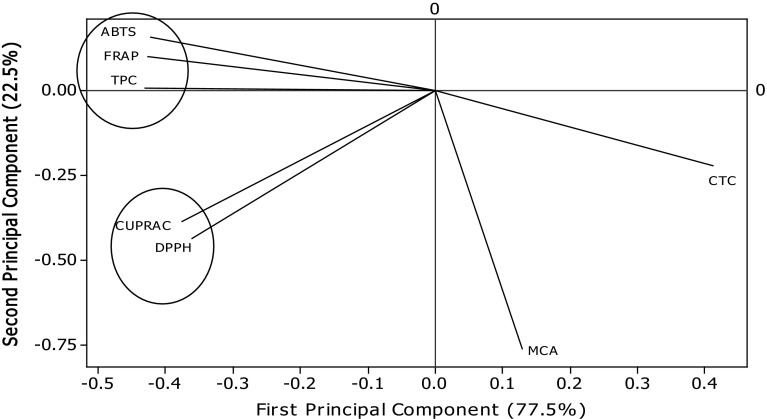
Table 5 presents the values of correlation of TPC with the FRSP determinants used in the present study. Significant correlations were found between TPC and antioxidant assays (FRAP, r = .993 p < .05; ABTS, r = .982 p < .05; CUPRAC, r = .869 p < .05; DPPH, r = .832 p < .05) (Table 5). Several workers have reported the positive relationships between bioactive compounds and antioxidant potential of natural resources. Salar and Purewal (2016) reported significant correlations between TPC and antioxidant activity of pearl millet cultivar fermented with Aspergillus oryzae (FRAP, r = .983 p < .05; ABTS, r = .956 p < .05; CUPRAC, r = .990 p < .05; DPPH, r = .985 p < .05. The results of principal component analysis are shown in Figs. 5 and 6. Significant variations among TPC, CTC, and FRSP of A. awamori extracts were observed. The distance between the locations on score plot is directly proportional to the different and similarity among the extraction phase used. The distance between the lines of curve indicates the degree of differences and similarity among various properties studied (TPC, CTC, DPPH, ABTS, FRAP, CUPRAC, and MCA). The curves which are close to each other on the plot are positively correlated, whereas those in opposite directions are negatively correlated. Figure 6 clearly explains the fact that MCA of extracts prepared using different extraction phase was not mainly due to phenolic compounds, and it may be affected by another important class of compounds present in A. awamori.
Table 5.
Correlation between total phenolic compounds, condensed tannin content, and free-radical scavenging potential of Aspergillus awamori extracts
| TPC | CTC | DPPH | ABTS | FRAP | MCA | CUPRAC | |
|---|---|---|---|---|---|---|---|
| TPC | 1 | −.964 | .832 | .982* | .993* | −.311 | .869 |
| CTC | −.964 | 1 | −.655 | −.997 | -.988 | .552 | −.707 |
| DPPH | .832 | −.655 | 1 | .714 | .764 | .268 | .998* |
| ABTS | .982* | −.997 | .714 | 1 | .997* | −.483 | .762 |
| FRAP | .993* | −.988 | .764 | .997* | 1 | −.417 | .807 |
| MCA | −.311 | .552 | .268 | −.483 | −.417 | 1 | .200 |
| CUPRAC | .869 | −.707 | .998* | .762 | .807 | .200 | 1 |
TPC total phenolic compounds, CTC condensed tannin content, DPPH 2,2-diphenyl-1-picrylhydrazyl, ABTS 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt, FRAP ferric reducing antioxidant power, MCA metal chelating activity, CUPRAC cupric ion reducing capacity
* Correlation is significant at the .05 level (1-tailed)
Fig. 5.
Principal control analysis: Score plot of first principal component and second principal component
Fig. 6.
Principal component analysis: loading plot of PC1 and PC2 describing the relationship among TPC, CTC, and antioxidant potential of Aspergillus awamori
Conclusions
Aspergillus awamori was successfully grown and good biomass yield was recovered. All the extracts (Aq, AqE, and ME) showed good amount of bioactive compounds with antioxidant potentials. AqE seems to be effective solvent for leaching out of bioactive mycochemicals from A. awamori. HPLC analysis confirmed the presence of four specific compounds viz. p-Coumaric acid, cinnamic acid, gallic acid, and ascorbic acid. All the extracts show significant DNA damage protection activity; however, AqE showed the maximum activity. Pearson correlations and principal control analysis confirmed the relationships between bioactive compounds and antioxidant potential of extracts. Further study needs to be carried out on toxicological analysis of the studied fungal strain, so that it can be utilized in different food products to enhance their bioactive constituents.
Acknowledgements
The study was supported by Grant received from University Grants Commission, New Delhi for a Major Research Project sanctioned to Prof. Raj Kumar Salar vide letter F No. 41-541/2012 (SR).
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
References
- Abubakr MAS, Hassan Z, Imdakim MMA, Sharifah NRS. Antioxidant activity of lactic acid bacteria (LAB) fermented skim milk as determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) and ferrous chelating activity (FCA) Afr J Microbiol Res. 2012;6:6358–6364. [Google Scholar]
- Afify AEMM, El-Beltagi HS, El-Salam SM, Omran AA. Biochemical changes in phenols, flavonoids, tannins, vitamin E, β-carotene and antioxidant activity during soaking of three white sorghum varieties. Asian Pac J Trop Med. 2012;2:203–209. doi: 10.1016/S2221-1691(12)60042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DB. Filamentous fungi as microbial cell factories for food use. Curr Opin Biotechnol. 2000;11:478–483. doi: 10.1016/S0958-1669(00)00129-4. [DOI] [PubMed] [Google Scholar]
- Arora DS, Chandra P. Assay of antioxidant potential of two Aspergillus isolates by different methods under various physio-chemical conditions. Braz J Microbiol. 2010;41:765–777. doi: 10.1590/S1517-83822010000300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora DS, Chandra P. In vitro antioxidant potential of some soil fungi: screening of functional compounds and their purification from Penicillium citrinum. Appl Biochem Biotechnol. 2011;165:639–651. doi: 10.1007/s12010-011-9282-3. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bhanja T, Kumari A, Banerjee R. Enrichment of phenolics and free radical scavenging property of wheat koji prepared with two filamentous fungi. Bioresour Technol. 2009;100:2861–2866. doi: 10.1016/j.biortech.2008.12.055. [DOI] [PubMed] [Google Scholar]
- Chandra P, Arora DS. Antioxidant potential of fungal isolates assayed through various procedures, screening of functional compounds and their purification from Aspergillus terreus. J Microbiol Biotech Res. 2014;4:15–24. [Google Scholar]
- Chang ST, Buswell JA. Mushroom nutriceuticals. World J Microbiol Biotechnol. 1996;12:473–476. doi: 10.1007/BF00419460. [DOI] [PubMed] [Google Scholar]
- Demirel Z, Yilmaz-Koz FF, Karabay-Yavasoglu UN, Ozdemir G, Sukatar A. Antimicrobial and antioxidant activity of brown algae from Aegean Sea. J Serb Chem Soc. 2009;74:619–628. doi: 10.2298/JSC0906619D. [DOI] [Google Scholar]
- Dhull SB, Kaur P, Purewal SS. Phytochemical analysis, phenolic compounds, condensed tannin content and antioxidant potential in Marwa (Origanum majorana) seed extracts. Resour Eff Technol. 2016;2:168–174. [Google Scholar]
- Ferreira ICFR, Barros L, Abreu RMV. Antioxidants in wild mushrooms. Curr Med Chem. 2009;16:1543–1560. doi: 10.2174/092986709787909587. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Julkunen-Titto R. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem. 1985;33:213–217. doi: 10.1021/jf00062a013. [DOI] [Google Scholar]
- Kumar V, Lemos M, Sharma M, Shriram V. Antioxidant and DNA damage protection activities of Eulophia nuda Lindl. Free Radic Antioxid. 2013;3:55–60. doi: 10.1016/j.fra.2013.07.001. [DOI] [Google Scholar]
- Lahouar L, Arem AE, Ghrairi F, Chahdoura H, Salem HB, Felah ME, Achour L. Phytochemical content and antioxidant properties of diverse varieties of whole barley (Hordeum vulgare L.) grown in Tunisia. Food Chem. 2014;145:578–583. doi: 10.1016/j.foodchem.2013.08.102. [DOI] [PubMed] [Google Scholar]
- Malpure PP, Shah AS, Juvekar AR. Antioxidant and anti-inflammatory activity of extract obtained from Aspergillus candidus MTCC 2202 broth filtrate. Indian J Exp Biol. 2006;44:468–473. [PubMed] [Google Scholar]
- Rodrigues KF, Costa GL, Carvalho MP, Epifanio RDA. Evaluation of extracts produced by some tropical fungi as potential cholinesterase inhibitors. World J Microbiol Biotechnol. 2005;21:1617–1621. doi: 10.1007/s11274-005-8344-5. [DOI] [Google Scholar]
- Salar RK, Purewal SS. Improvement of DNA damage protection and antioxidant activity of biotransformed pearl millet (Pennisetum glaucum) cultivar PUSA-415 using Aspergillus oryzae MTCC 3107. Biocatal Agric Biotechnol. 2016;8:221–227. [Google Scholar]
- Salar RK, Purewal SS. Phenolic content, antioxidant potential and DNA damage protection of pearl millet (Pennisetum glaucum) cultivars of North Indian region. Food Meas. 2017;11:126–133. doi: 10.1007/s11694-016-9379-z. [DOI] [PubMed] [Google Scholar]
- Salar RK, Seasotiya L. Free radical scavenging activity, phenolic contents and phytochemical evaluation of different extracts of stem bark of Butea monosperma (Lam.) Kuntze. Front Life Sci. 2012;5:107–116. doi: 10.1080/21553769.2011.635813. [DOI] [Google Scholar]
- Salar RK, Certik M, Brezova V. Modulation of phenolic content and antioxidant activity of maize by solid state fermentation with Thamnidium elegans CCF-1456. Biotechnol Bioprocess Eng. 2012;17:109–116. doi: 10.1007/s12257-011-0455-2. [DOI] [Google Scholar]
- Salar RK, Certik M, Brezova V, Brlejova M, Hanusova V, Breierova E. Stress influenced increase in phenolic content and radical scavenging capacity of Rhodotorula glutinis CCY 20-2-26. 3. Biotech. 2013;3:53–60. doi: 10.1007/s13205-012-0069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salar RK, Sharma P, Purewal SS. In vitro antioxidant and free radical scavenging activities of stem extract of Euphorbia trigona Miller. TANG [Humanitas Med] 2015;5:1–6. [Google Scholar]
- Salar RK, Purewal SS, Bhatti MS. Optimization of extraction condition and enhancement of phenolic content and antioxidant activity of pearl millet fermented with Aspergillus awamori MTCC-548. Resour Eff Technol. 2016;2:148–157. [Google Scholar]
- Sandhu KS, Punia S, Kaur M. Effect of duration of solid state fermentation by Aspergillus awamori Nakazawa on antioxidant properties of wheat cultivars. LWT Food Sci Technol. 2016;71:323–328. doi: 10.1016/j.lwt.2016.04.008. [DOI] [Google Scholar]
- Sharma P, Gujral HS. Antioxidant and polyphenol oxidase activity of germinated barley and its milling fractions. Food Chem. 2010;120:673–678. doi: 10.1016/j.foodchem.2009.10.059. [DOI] [Google Scholar]
- Siroha AK, Sandhu KS, Kaur M. Physicochemical, functional and antioxidant properties of flour from pearl millet varieties grown in India. Food Meas. 2016;10:1–8. doi: 10.1007/s11694-015-9270-3. [DOI] [Google Scholar]
- Smith H, Doyle S, Murphy R. Filamentous fungi as a source of natural antioxidants. Food Chem. 2015;185:389–397. doi: 10.1016/j.foodchem.2015.03.134. [DOI] [PubMed] [Google Scholar]
- Trease GE, Evans WC. Pharmacognosy. 13. London (UK): Bailliere Tindall; 1996. pp. 282–396. [Google Scholar]
- Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their mutagenicity. J Agric Food Chem. 1995;43:27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]