Abstract
Both hyperglycaemia and hyperlipidaemia are major risk factors for the development of coronary artery diseases and atherosclerosis, and therefore therapeutic drugs must be developed for treatment of them. Pumpkin polysaccharides (PPs) are biomacromolecules with varying bioactivities. In this study, PPs were extracted with commercial thermostable α-amylase, and their hypolipidaemic and hypoglycaemic activities were evaluated. Twenty four KKAy mice were divided into two groups: control was fed with high-fat diet; while the PP group was fed with high-fat diet with the addition of PPs at the same time, for 6 weeks. PP diet reduced body weight gain, the levels of plasma insulin, serum triglyceride, cholesterol, low-density lipoprotein cholesterol and blasting blood glucose in mice and improved the level of high-density lipoprotein cholesterol and liver glycogen. Results indicate that PPs had high hypolipidaemic and hypoglycaemic activities and could be used as potential drugs for treatment of hyperlipidaemia and hyperglycaemia.
Keywords: Pumpkin, Polysaccharide, Hypolipidaemic, Hypoglycaemic
Introduction
Diabetes mellitus is one of the most costly chronic diseases in modern society (Zhou et al. 2015). Most diabetics are diagnosed as non-insulin-dependent diabetes, i.e., type 2 diabetes, which are characterised by hyperglycaemia and dyslipidaemia, resulting from defects in insulin secretion and action (Huang et al. 2015). Considering the accompanying side effects and adverse reactions of chemosynthetic drugs, such as acarbose and biguanides, edible and medicinal resources have become potential candidates for new natural compounds having hypoglycaemic and hypolipidaemic activities (Pan et al. 2014).
A number of studies reported that some plant polysaccharides exhibit hypoglycaemic activities; these plant polysaccharides include those derived from Trichosanthes peel, bamboo shoots (Leleba oldhami Nakal) shells, Lycium barbarum L, loach, Grifola frondosa and Moringa oleifera Lam. leaves (Chen et al. 2016, 2017; Huang et al. 2015; Tang et al. 2015; Xiao et al. 2015; Zhou et al. 2015; Zheng et al. 2016).
Pumpkin (Cucurbita moschata Duch), an annual herbaceous plant of the family Cucurbitacea, is widely grown and consumed in the world (Maran et al. 2013). The fruit of pumpkin is rich in polysaccharides, carotene, minerals, vitamins and other components beneficial to health, resulting in development of varying processed food products (Jun et al. 2006).
Recently, pumpkin polysaccharides (PPs) attracted more attention because they have many biological effects such as detoxification, anti-oxidation, reducing blood pressure, and reducing blood lipids (Li et al. 2010; Wang et al. 2012). However, data on regarding the hypoglycaemic and hypolipidaemic activities of the PPs are limited.
In this study, the PPs were extracted using a commercial thermostable α-amylase-assisted method and their hypoglycaemic and hypolipidaemic activities were evaluated in rats.
Materials and methods
Ethics statement
This study was approved by the ethics committee of The First People’s Hospital of Lianyungang, China.
Materials
Fresh pumpkins (C. moschata), with similar maturity and weight, were purchased from a farmers market (Xinpu, China). KKAy mice were purchased from the Experimental Animal Center of Nanjing University (Jiangsu, China). Total cholesterol (TC), total triglyceride (TG), low-density lipoprotein cholesterol (LDL-c) and high-density lipoprotein cholesterol (HDL-c), glucose, insulin, and glycogen kits were purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). The commercial thermostable α-amylase, with 6000 U/g enzyme activities, was purchased from Beijing Shengshi Jiaming Technology Development Co., Ltd. (Beijing, China). All other chemicals were reagent grade.
PP preparation
Pumpkins were cleaned to remove peels and pulps, washed with tap water, sliced, dried in a hot air oven (JK-OOI-240A, China) at 60 °C for 4 h, pulverised and sifted through a 80 mesh sieve to obtain a fine powder. The pumpkin powder (100 g) was suspended 1 L of distilled water. After the pH of the suspension was adjusted to 6.5, 60,000 U of commercial thermostable α-amylase was added. The mixture was extracted in a water bath at 95 °C for 2 h. The extracts were filtered through a Whatman GF/A filter paper, concentrated to ~20% (w/v), proteins removed using the Sevag method and precipitated with three volumes of absolute ethanol. The precipitates were freeze dried to yield crude PPs, which were dissolved in distilled water and passed through a DEAE-52 cellulose anion-exchange chromatography column (30 cm × 2.6 cm, GE Healthcare, UK), eluted with 0.05 mol/L PBS, and followed with gradient solution of 0.05–1.0 mol/L NaCl at a flow rate of 1.0 mL/min. The eluted fraction was collected for subsequent experiments.
PP characterisation
Ash, moisture, lipid, protein and total sugar contents of the samples were determined according to standard methods (Hou 2004). The monosaccharide component of PP was determined according to the method of Sheng et al. (2007). The Fourier transform infrared (FTIR) spectra of the resulting PP sample were recorded in KBr pellets by a Nexus FTIR 470 spectrophotometer (Nicolet, USA) over a wavelength range of 400–4000 cm−1. The UV spectra were recorded on a UV spectrometer (Spectra test, German).
Experimental design
KKAy mice, with similar initial blood glucose levels and weights, were used as models of type 2 diabetes. Twenty four KKAy mice were divided into control and PP groups: the control group was given high-fat diet containing 10% (w/w) lard, 15% (w/w) egg yolk powder, 1% (w/w) cholesterol and 76% (w/w) basic diet with composition conforming to AIN 76 (Shanghai SLAC Laboratory Animal Co. Ltd., Shanghai, China); while the PP group received 90% (w/w) high-fat diet and 10% (w/w) PPs. The diet pellets were prepared by mixing the powdered commercial basal diet with other ingredients and test materials (Teng et al. 2013).
The mice were fasted overnight, and blood samples were collected to measure initial plasma lipid and glucose levels prior to the experiments. After 12 h of fasting, blood glucose levels were tested once a week using One-Touch basic glucose monitor (Johnson, USA). The body weights of the mice and food intake were also recorded once a week. At the end of the 6th week, the mice were fasted overnight and killed by cervical dislocation. Blood samples were used to determine plasma biomarker assay. After the blood was collected, the liver was removed, rinsed with a physiological saline solution, and immediately stored at −80 °C.
Oral glucose tolerance test (OGTT)
At the end of the 6th week, the mice were fasted for 12 h and orally administered with glucose (2.0 g/kg body weight). The blood drops were obtained by clipping the tail of the mice, and serum glucose levels were recorded at 0, 20, 40, 60, 80, 100 and 180 min, respectively, on a One-Touch glucose monitor (Lifescan, USA).
Blood biochemical measurement
At the end of the 6th week, the mice were fasted for 12 h, blood samples were collected under anaesthetized condition, placed into prechilled tubes, immediately centrifuged at 3000×g for 10 min. The serum was used for further analyses. Serum insulin level was determined by mouse insulin enzyme-linked immunosorbent assay (ELISA) kit. The levels of serum lipid including TC, TG, LDL-c and HDL-c were assayed by an automatic biochemical analyzer.
Liver glycogen measurement
At the end of the 6th week of treatment with PPs, the livers were separated from the killed mice, weighed and washed with cold physiological saline. Glycogen levels were determined according to the anthracenone method after the removal of visible fats and connective tissues in an ice bath.
Statistical analysis
Data are presented as mean ± SD (n = 3), and ANOVA was performed to compare the means of the two groups. Statistical significance at the 95% probability level was set at p < 0.05.
Results
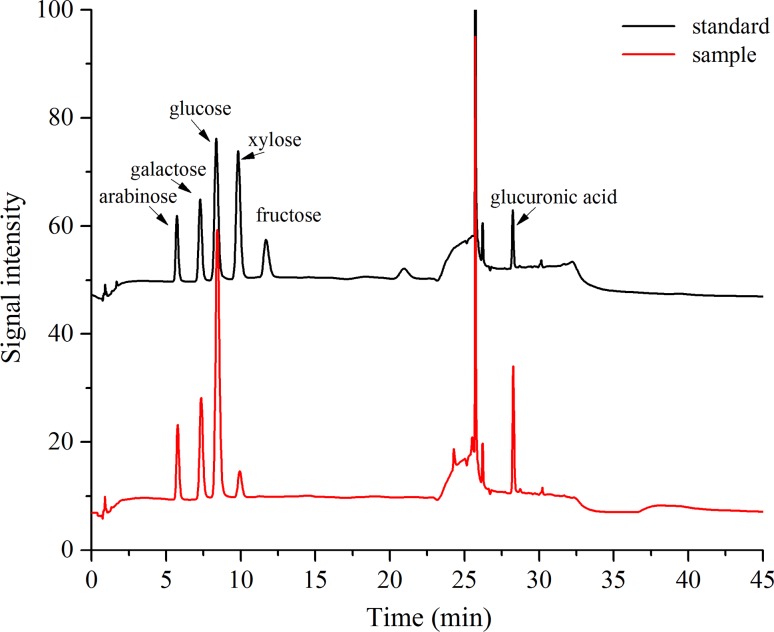
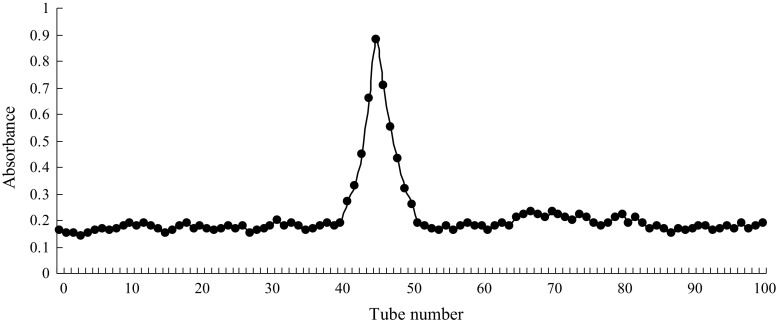
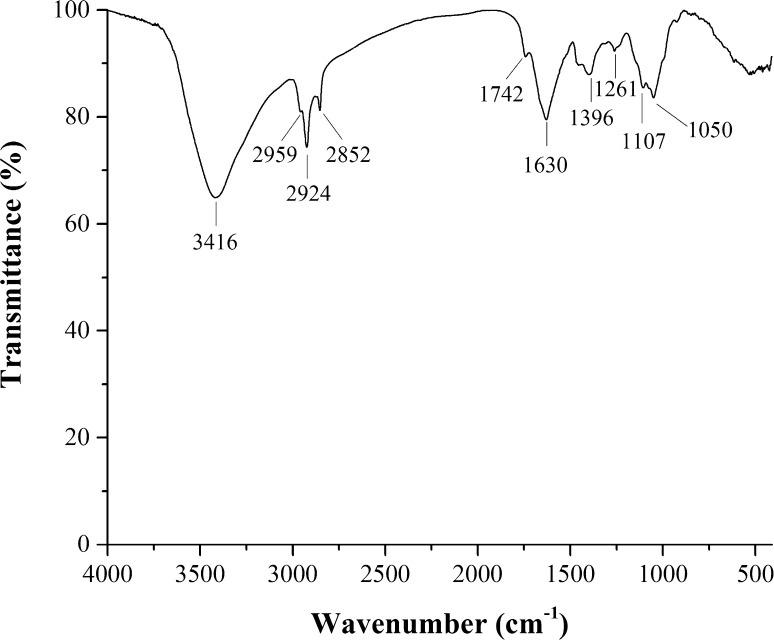
The PP products were light yellow and water soluble powders, contained 2.14% moisture, 2.26% protein and 94.02% total sugar and did not contain any lipids. Monosaccharide composition analysis with GC showed that the PPs consisted of glucose, galactose, arabinose, xylose and glucuronic acid in a molar ration of 5.8:3.2:2.1:1.0:5.9 (Fig. 1). Analysis of the elution curve of pumpkin polysaccharides on a DEAE Sepharose Fast Flow ion exchange chromatography column indicated that there was only a fraction in the PPs (Fig. 2). Figure 3 showed that the absorption bands of PPs appeared at 3416 cm−1 (hydroxyl stretching vibration), 2924 cm−1 (C−H stretching vibration), 1630 cm−1 (C=O vibration), 1396 cm−1 (carboxyl groups), and 1107–1050 cm−1 (pyranose ring).
Fig. 1.
Monosaccharide component of pumpkin polysaccharides
Fig. 2.
Elution curve of pumpkin polysaccharides on a DEAE Sepharose Fast Flow ion exchange chromatography column
Fig. 3.
Fourier transform infrared spectrum of pumpkin polysaccharides
The WG, FI and FER of the mice for the two groups at the end of the experimental period were showed in Table 1. Though there was no significant difference in FI between the two groups (p > 0.05), WG and PER of the PP group were lower than those of the control group (p < 0.05), respectively.
Table 1.
Body weight gain (WG), food intake (FI), food efficiency ratio (FER), serum total cholesterol (TC), total triglyceride (TG), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), insulin and glycogen levels of KKAy mice received high-fat (HF) and pumpkin polysaccharides (PPs)
| Parameters | Control | PPs |
|---|---|---|
| WG (g) | 45.8 ± 1.87a | 34.6 ± 1.61b |
| FI (g) | 161.3 ± 6.93a | 162.4 ± 7.03a |
| FER | 28.39 ± 1.49a | 21.34 ± 0.86b |
| TG (mmol/L) | 1.3 ± 0.06a | 0.6 ± 0.05b |
| TC (mmol/L) | 3.6 ± 1.54a | 2.1 ± 0.02b |
| LDL-c (mmol/L) | 1.5 ± 0.07a | 0.7 ± 0.04b |
| HDL-c (mmol/L) | 2.6 ± 5.37a | 3.5 ± 5.92b |
| Insulin (mU/L) | 17.9 ± 0.78a | 13.7 ± 0.71b |
| Glycogen (mg/g) | 1.8 ± 0.23a | 4.3 ± 0.71b |
Values are expressed as mean ± SD (n = 3). Means with different superscripts within a row indicate significant differences (p < 0.05)
Table 1 shows the effect of PP oral administration on serum insulin and levels and glycogen contents in the liver of KKAy mice. The levels of serum insulin TG, TC and LDL-c of the KKAy mice decreased, while level of serum LDL-c and glycogen contents in the livers of KKAy mice for PP oral administration group increased significantly compared with the control group (p < 0.05).
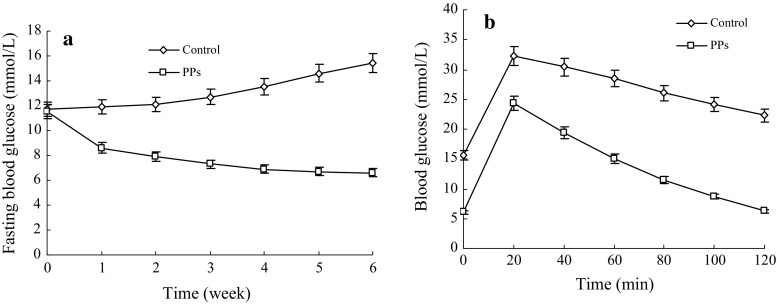
The effect of oral administration of PP on the fasting blood glucose level of mice was shown in Fig. 4. The fasting blood glucose level of the KKAy mice for control group stably increased till end the experimental period (Fig. 4a). In contrast, the fasting blood glucose level of the KKAy mice for PP oral administration group sharply decreased and then levelled off (Fig. 4a). As a result, the fasting blood glucose level of the KKAy mice for the PP group was consistently lower than that of the control group during the whole experimental period (p < 0.05).
Fig. 4.
Effect of the pumpkin polysaccharides (PPs) on fasting blood glucose levels and oral glucose tolerance of the KKAy mice. Bars represent the standard deviation. Data are shown as mean ± SD (n = 3)
We investigated the effect of PP oral administration on the glucose tolerance of the KKAy mice. The changes in blood glucose level are shown in Fig. 4. After oral administration of glucose, the blood glucose level of the KKAy mice for both the control and PP oral administration groups increased initially in 20 min and then decreased (Fig. 4b). Nevertheless, the blood glucose level of the KKAy mice for the PP group was consistently lower than that of the control group during the entire experimental period (Fig. 4b; p < 0.05).
Discussion
The results indicate that the monosaccharide composition was similar to that reported by Kong et al. (2000). FTIR spectrum of PPs indicated that PPs were acidic polysaccharides. Therefore, the PPs were acidic hetero-polysaccharides composed of five different monosaccharides, which was also similar to that reported by Kong et al. (2000).
Pumpkin polysaccharide oral administration inhibited the body weight gain of the mice received HF diets. Similarly, many polysaccharides, such as polysaccharides from porphyra yezoensis, polysaccharide from Cyclocarya paliurus, polysaccharide fractions from Fortunella margarita (Lour.) Swingle, polysaccharides from Enterobacter cloacae Z0206 and polysaccharides from Enteromorpha prolifera, were reported to have inhibited the body weight gain of the mice (Huang et al. 2015; Qian et al. 2014; Teng et al. 2013; Yang et al. 2016; Zeng et al. 2016).
Hyperglycaemia and dyslipidaemia are the main characteristics of diabetes mellitus and major risk factors for the development of coronary artery diseases and atherosclerosis. Insulin regulates glycogen deposition in liver by stimulating glycogen synthase and suppressing glycogen phosphorylase; thus, glycogen reflects insulin activity (Muthulakshmi and Saravanan 2013). In this study, oral administration of PPs decreased levels of serum insulin TG, TC and LDL-c of the KKAy mice and increased serum LDL-c level and glycogen contents in the livers of the KKAy mice compared with the control group.
High-fat diet aggravated the state of an illness of the KKAy mice. Our results showed that the fasting blood glucose level of the KKAy mice for the PP group was lower than that of the control group during the whole experimental period, indicating that PP alleviated illness of the KKAy mice aggravated by HF diet. Diabetes mellitus decreases glucose responses capacity. However, PP increased glucose responses capacity of the KKAy mice. Similarly, many plant polysaccharides have hypoglycaemic activities, e.g., the polysaccharides derived from Trichosanthes peel, bamboo shoots (Leleba oldhami Nakal) shells, Lycium barbarum L, loach, Grifola frondosa and Moringa oleifera Lam. leaves (Chen et al. 2016, 2017; Huang et al. 2015; Tang et al. 2015; Xiao et al. 2015; Zhou et al. 2015; Zheng et al. 2016).
In conclusion, the PPs were extracted with commercial thermostable α-amylase, and their hypolipidaemic and hypoglycaemic activities were investigated. The PPs showed high hypoglycaemic and hypolipidaemic activities for KKAy mice. Thus, PPs might be developed into a promising hypoglycaemic and hypolipidaemic drug for diabetes treatment. However, the mechanisms of the hypoglycaemic and hypolipidaemic activities of PPs should be further investigated.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- Chen TL, Zhang M, Li JL, Surhio MM, Li BB, Ye M. Structural characterization and hypoglycemic activity of Trichosanthes peel polysaccharide. LWT-Food Sci Technol. 2016;70:55–62. doi: 10.1016/j.lwt.2016.02.024. [DOI] [Google Scholar]
- Chen C, Zhang B, Huang Q, Fu X, Liu RH. Microwave-assisted extraction of polysaccharides from Moringa oleifera Lam. leaves: characterization and hypoglycemic activity. Ind Crop Prod. 2017;100:1–11. doi: 10.1016/j.indcrop.2017.01.042. [DOI] [Google Scholar]
- Hou ML. Food analysis. Beijing: Chemical Industry Press; 2004. [Google Scholar]
- Huang M, Wang F, Zhou X, Yang H, Wang Y. Hypoglycemic and hypolipidemic properties of polysaccharides from Enterobacter cloacae Z0206 in KKAy mice. Carbohydr Polym. 2015;117:91–98. doi: 10.1016/j.carbpol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Jun HI, Lee CH, Song GS, Kim YS. Characterization of the pectic polysaccharides from pumpkin peel. LWT-Food Sci Technol. 2006;39:554–561. doi: 10.1016/j.lwt.2005.03.004. [DOI] [Google Scholar]
- Kong QS, Wang YY, Jiang Y. Studies on extraction and hypolipidemic activity of polysaccharides from pumpkin. Chin J Biochem Pharm. 2000;21:130–132. [Google Scholar]
- Li J, Wang Y, Zhang D, Hu X, Zhang Z, Xiang C. Characterization and bioactivity of water-soluble polysaccharides from the fruit of pumpkin. J Food Agric Environ. 2010;8:20692–20703. [Google Scholar]
- Maran JP, Mekala V, Manikandan S. Modeling and optimization of ultrasound-assisted extraction of polysaccharide from Cucurbita moschata. Carbohydr Polym. 2013;92:2018–2026. doi: 10.1016/j.carbpol.2012.11.086. [DOI] [PubMed] [Google Scholar]
- Muthulakshmi S, Saravanan R. Efficacy of azelaic acid on hepatic key enzymes of carbohydrate metabolism in high fat diet induced type 2 diabetic mice. Biochimie. 2013;95:1239–1244. doi: 10.1016/j.biochi.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Pan LH, Li XF, Wang MN, Zha XQ, Yang XF, Liu ZJ, Luo YB, Luo JP. Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int J Biol Macromol. 2014;64:420–427. doi: 10.1016/j.ijbiomac.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Qian L, Zhou Y, Ma JX. Hypolipidemic effect of the polysaccharides from porphyra yezoensis. Int J Biol Macromol. 2014;68:48–49. doi: 10.1016/j.ijbiomac.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Sheng J, Yu F, Xin Z, Zhao L, Zhu X, Hu Q. Preparation, identification and their antitumor activities in vitro of polysaccharides from Chlorella pyrenoidosa. Food Chem. 2007;105:533–539. doi: 10.1016/j.foodchem.2007.04.018. [DOI] [Google Scholar]
- Tang HL, Chen C, Wang SK, Sun GJ. Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int J Biol Macromol. 2015;77:235–242. doi: 10.1016/j.ijbiomac.2015.03.026. [DOI] [PubMed] [Google Scholar]
- Teng Z, Qian L, Zhou Y. Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera. Int J Biol Macromol. 2013;62:254–256. doi: 10.1016/j.ijbiomac.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang LZ, Dong LL. Inhibitory effect of polysaccharides from pumpkin on advanced glycation end-products formation and aldose reductase activity. Food Chem. 2012;130:821–825. doi: 10.1016/j.foodchem.2011.07.064. [DOI] [Google Scholar]
- Xiao C, Wu Q, Xie Y, Zhang J, Tan J. Hypoglycemic effects of Grifola frondosa (Maitake) polysaccharides F2 and F3 through improvement of insulin resistance in diabetic rats. Food Funct. 2015;6:3567–3575. doi: 10.1039/C5FO00497G. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Ouyang KH, Zhao J, Chen H, Xiong L, Wang WJ. Structural characterization and hypolipidemic effect of Cyclocarya paliurus polysaccharide in rat. Int J Biol Macromol. 2016;91:1073–1080. doi: 10.1016/j.ijbiomac.2016.06.063. [DOI] [PubMed] [Google Scholar]
- Zeng H, Miao S, Zhang Y, Lin S, Jian Y, Tian Y, Zheng B. Isolation, preliminary structural characterization and hypolipidemic effect of polysaccharide fractions from Fortunella margarita (Lour.) Swingle. Food Hydrocoll. 2016;52:126–136. doi: 10.1016/j.foodhyd.2015.05.028. [DOI] [Google Scholar]
- Zheng YF, Zhang S, Wang Q, Lu X, Lin LM, Tian YT, Xiao JB, Zheng BD. Characterization and hypoglycemic activity of β-pyran polysaccharides from bamboo shoot (Leleba oldhami Nakal) shells. Carbohydr Polym. 2016;144:438–446. doi: 10.1016/j.carbpol.2016.02.073. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yan JY, Bai ZS, Li KC, Huang KX. Hypoglycemic activity and potential mechanism of a polysaccharide from the loach in streptozotocin-induced diabetic mice. Carbohydr Polym. 2015;121:199–206. doi: 10.1016/j.carbpol.2014.12.037. [DOI] [PubMed] [Google Scholar]