Abstract
Research into adipose tissue-derived mesenchymal stem cells (AD-MSCs) has demonstrated the feasibility of their use in clinical applications due to their ease of isolation and abundance in adipose tissue. We isolated AD-MSCs from young and old dogs, and the cells were subjected to sequential sub-passaging from passage 1 (P1) to P7. Canine AD-MSCs (cAD-MSCs) were examined for proliferation kinetics, expression of molecules associated with self-renewal, expression of cell surface markers, and differentiation potentials at P3. Cumulative population doubling level was significantly higher in cAD-MSCs of young donors than in those of old donors. In addition, expressions of CD73, CD80, Oct3/4, Nanog, cell survival genes and differentiation potentials were significantly higher in young donors than in old donors. The present study suggests that donor age should be considered when developing cell-based therapies for clinical application of cAD-MSCs.
Keywords: adipose mesenchymal stem cells, age, canine, differentiation, multipotency
Introduction
The clinical potential of mesenchymal stem cells (MSCs) has been recognized due to their capacities for multilineage differentiation, homing, and immune modulation effects. Adipose tissue-derived MSCs (AD-MSCs) have morphological, phenotypic, and functional characteristics similar to those of bone marrow-derived MSCs (BM-MSCs) [36]. The variety of advantages of AD-MSCs includes simplicity of isolation with minimal risk to the donor and the sufficient quantity of the multipotent population within adipose tissue [7]. AD-MSCs have been used in regenerative therapies for the treatment of various tissue disorders, including those affecting bone, cartilage, and fat [2,6,19,33]. AD-MSCs are capable of long-term self-renewal in culture, while maintaining multipotency, in several species including human, bovine, rat, and mouse [20,23,34]. Recently, canine AD-MSCs (cAD-MSCs) have been shown to have the capacity to differentiate into adipogenic, osteogenic, chondrogenic, myogenic, and neuronal lineages [14,20,25,29].
Donor age is closely associated with MSC functions such as proliferation, differentiation, and wound healing properties in rats [16], mice [3,12], and humans [12,30,35]. The influence of donor age has also been observed in canine mobilized dental pulp stem cells (cMDP-SCs) [9]. Interestingly, despite many studies into the effect of age on differentiation capacities of AD-MSCs in human and other species, Guercio et al. [8] observed no age effect on stemness characteristics or differentiation capacities of cAD-MSCs. Since variations in isolation and/or growing methods and animal source (age, species, etc.) are critical to the characteristics of MSCs, it is important to define the age effect on cAD-MSCs. Thus, our study was conducted to isolate cAD-MSCs from young and old donors and investigate age-dependent change in the multipotency of cAD-MSCs.
Materials and Methods
Isolation of cAD-MSCs and cell culture
Adipose tissues were individually collected from 7-month-old (n = 3) and 10- to 11-year-old male beagle dogs (n = 3). Abdominal subcutaneous adipose tissues were sampled from each dog. All animal experimental protocols were approved by the Animal Welfare Committee of the Animal and Plant Quarantine Agency (registry No. QIA-2012-493). Canine adipose tissues (n = 6) were washed with phosphate-buffered saline (PBS; Gibco, USA) to remove blood. Blood vessels were removed with sterile scissors and forceps. The tissue was then incubated in PBS containing 1× P/S (100 unit/mL penicillin, 100 µg/mL streptomycin; Gibco). The tissues were minced into 1–2 mm pieces and incubated in PBS containing 0.1% collagenase type I (Sigma, USA) at 37℃ for 30 min. The digested tissues were filtered through a cell strainer (100 µm) to remove undigested tissues and centrifuged at 450 × g for 10 min. The cell pellet was resuspended in low Dulbecco's Modified Eagle's Medium (DMEM; Invitrogen, USA) containing 1× P/S and 10% fetal bovine serum, and cultured in a cell culture flask at 37℃ in a 5% CO2 incubator. The following day, culture medium was replaced with fresh low DMEM, and the cells were passaged after five days. Cells at each passage were maintained until 80% confluence was reached and sub-passaged.
Cumulative population doubling level
During continuous sub-passaging, the number of cAD-MSCs at both seeding and harvesting time were determined in order to calculate cumulative population doubling level (CPDL) from the formula: CPDL = {log10(NH) − log10(NI)}log10, where NI = initial cell number and NH = harvested cell number. Cumulative doubling level was obtained by adding the CPDL of each passage to that of the previous passage [4].
Reverse transcriptase polymerase chain reaction (RT-PCR) gene expression analysis
The list of primers used for the pluripotent markers (Oct3/4, Sox2, and Nanog) and for GAPDH, the internal control, are shown in Table 1. Total RNA was isolated from the cAD-MSCs by applying the TRIzol method (Invitrogen). RNA concentration was measured by using a spectrophotometer (Thermo, USA). RT-PCR was conducted by using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions. For pluripotent marker analysis, target genes were amplified in 35 cycles of 94℃ (30 sec), 55℃ to 60℃ (30 sec), 72℃ (20 sec), and 72℃ for 10 min. The PCR products were separated by performing electrophoresis in 2% agarose gel, marked with Red Safe stain (iNtRon Biotechnology, Korea), and visualized under UV light. Images were digitally observed and recorded by using a CCD camera (Gel Documentation System, USA).
Table 1. List of primers used for reverse transcription-polymerase chain reactions.
F, forward; R, reverse.
Real-time quantitative RT-PCR (qRT-PCR)
Primers for pluripotent markers (Oct3/4, Sox2, and Nanog), cell surface markers (CD29, CD34, CD44, CD54, CD61, CD73, CD80, CD90, CD105, CD117, and MHC-class II), differentiation potential markers (lipoprotein lipase [LPL], leptin, osteopontin [OP], osteocalcin [OC], aggrecan, and type II-collagen [COL2A]), cell survival-related genes (telomerase reverse transcriptase [TERT], dyskerin pseudouridine synthase 1 [DKC1], histone deacetylase 1 [HDAC1], DNA (cytosine-5)-methyltransferase 1 [DNMT1], Bcl-2, lactate dehydrogenase A [LDHA], glucose transporter member 1 [SLC2A1]), and GAPDH as an internal control are listed in Table 1. Total RNA was isolated from the cells and reversed transcribed to cDNA (5 µL), which was used for PCR analysis. qRT-PCR analysis was performed in 96-well plates with a lightCycler 480 (Roche Applied Science, Germany) using SYBR Green I Master kit (Roche Diagnostics, Germany) according to the manufacturer's instructions. A thermocycling program was used for amplification: Pre-denaturation (95℃, 10 min), followed by 40 cycles of denaturation (95℃, 10 sec), annealing (55℃–64℃, 10 sec), and elongation (72℃, 10 sec). Melting curve analysis was performed from 65℃ to 97℃ to assess the distinction of the qRT-PCR products. Using the Ct value, we calculated the qRT-PCR results. Relative quantification was conducted as previously described using GAPDH as a reference gene. The 2−ΔCT method described by Livak and Schmittgen [18] was employed to normalize gene expression values.
In vitro differentiation
For adipogenic differentiation, cAD-MSCs were seeded at 2 × 104 cells/cm on tissue culture plates (4-well) and cultured for 21 days in adipogenic differentiation medium and adipogenic maintenance medium according to the manufacturers protocols (Lonza, USA). Medium changes were made every 2–3 days. Differentiated or undifferentiated cells were washed twice with PBS, fixed with 4% formalin for 10 min and washed with PBS. The fixed cells were stained with an Oil Red O stain kit (IHC World, USA) and red colored lipid vacuoles that accumulated in the differentiated cells were observed under an inverted microscope. For osteogenic differentiation, the cells of 3 × 103 cells/cm were plated on tissue culture plates (4-well) and grown in osteogenic differentiation medium (Lonza) for 21 days. Medium changes were made every 2–3 days. Differentiated or undifferentiated cells were washed twice with PBS, fixed with 4% formalin for 10 min, and washed with PBS. The fixed cells were stained with an Alizarin Red stain kit (IHC World) and the deposited calcium (orange-red colored) was visualized. For chondrogenic differentiation, 5 × 105 cells were seeded in a 15 mL polypropylene tube and centrifuged to form a pellet. Pellets were cultured in 1 mL of chondrogenic differentiation medium plus TGF-β3 (Lonza) for 21 days. Medium changes were made every 2–3 days. After differentiation, the pellet was embedded in paraffin, cut into 3 µm sections, and stained with an Alcian Blue stain kit (IHC World) to detect the presence of glycosaminoglycan, which was stained to a blue color.
Statistical analysis
Cell doubling time and relative expression of differentiation potential markers were analyzed by one-way ANOVA test or Student's t-test (JMP 6.0; SAS Institute, USA). The graphs were prepared by using SigmaPlot (ver. 8.0; Systat Software, USA). A value of p < 0.05 was considered statistically significant.
Results
Cell growth and proliferation kinetics
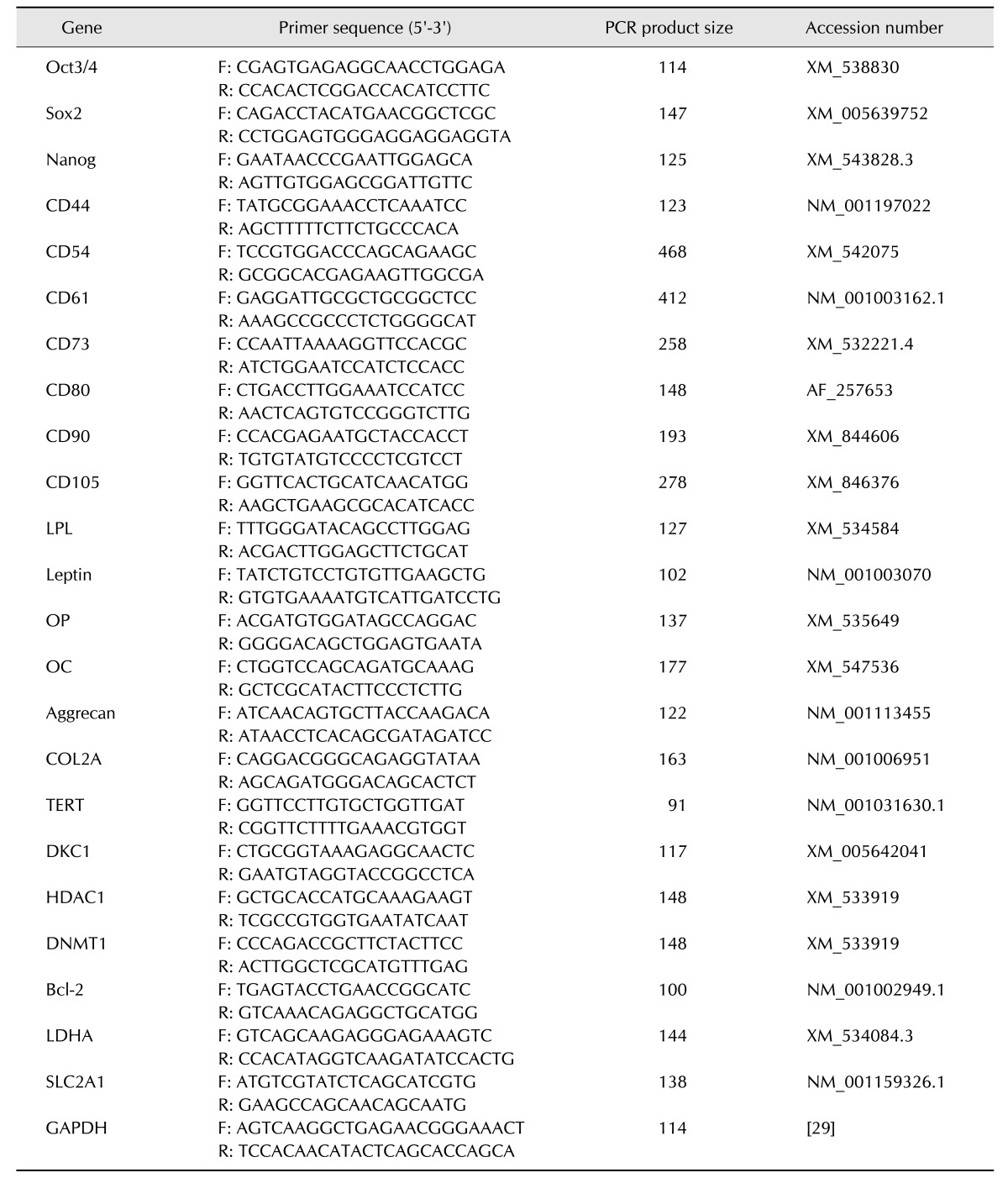
The cAD-MSCs were isolated from canine adipose tissue and grown as adherent populations in plastic tissue culture flasks. The adherent cells had a fibroblast-like morphology and spindle shape, and routinely formed homogenous monolayers (panel A in Fig. 1). The cells were sub-passaged every 5 days and the number of cells grown was determined after trypsinization. During 7 passages, CPDL of the cells linearly increased until passage 3 (P3) and that level was maintained until P5, after which the CPDL decreased in cAD-MSCs obtained from both young and old donors (panel B in Fig. 1). Cell growth was 2.4-fold higher for cAD-MSCs from young donors than that from old donors, evidence of a positive correlation with age.
Fig. 1. Morphology and proliferation of canine adipose tissue-derived mesenchymal stem cells (cAD-MSCs). (A) Morphology of cells passage 1–6 (P1–P6) derived from adipose tissues typically appeared as fibroblast-like. (B) Cumulative population doubling level (CPDL) of cAD-MSCs during continuous passages. CPDL of the cAD-MSCs increased at each passage until P3. Cells were grown in two age groups, YAD-MSCs and OAD-MSCs. GAPDH was used as a housekeeping control gene. The results are shown as the mean ± standard error of the mean (n = 5) obtained by three determinations. YAD, 7-month-old dogs; OAD, 10- to 11-year-old dogs. 100× (A).
Expression of pluripotent markers
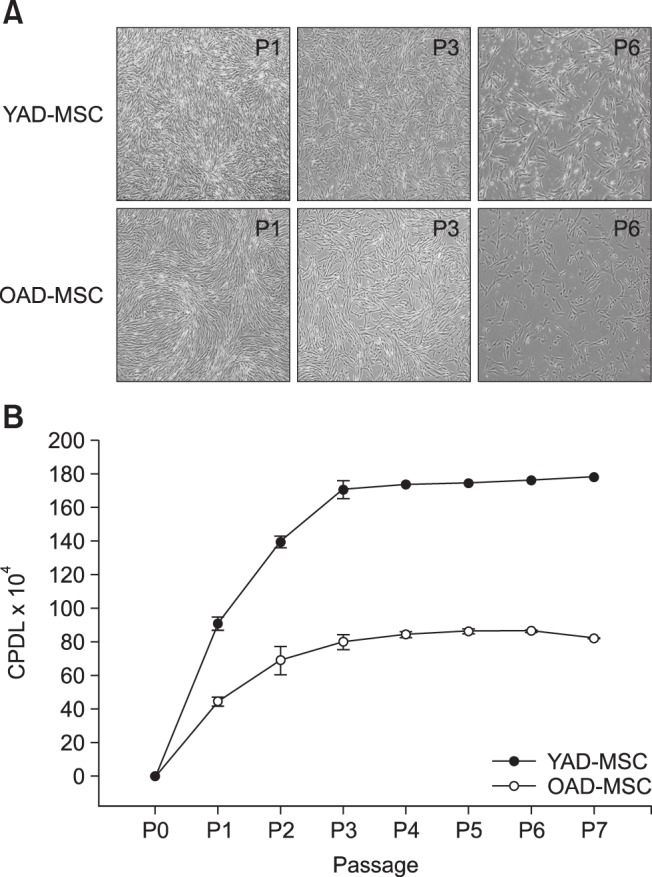
To observe the effect of age on the pluripotency of cAD-MSCs, transcriptional patterns of pluripotent markers (Oct3/4, Sox2, and Nanog) of cAD-MSCs from young and old donors at P3 were compared by performing RT-PCR (panel A in Fig. 2) and qRT-PCR (panel B in Fig. 2). Expressions of Oct3/4 and Nanog of cAD-MSCs from young donors were significantly (p < 0.05) higher than those from old donors.
Fig. 2. Expression pluripotency markers (Oct3/4, Sox2, and Nanog) of canine adipose tissue-derived mesenchymal stem cells (cAD-MSCs). Using reverse transcriptase polymerase chain reaction (RT-PCR; A) and real-time quantitative RT-PCR (qRT-PCR; B), the expressions of stemness genes of cAD-MSCs were examined in two age groups (young and old) at passage 3. GAPDH was used as a housekeeping control gene. All mRNA data were normalized to the GAPDH levels, and the relative fold change in expression level is shown as a mean ± SEM (n = 3). *p < 0.05. YAD, 7-month-old dogs; OAD, 10- to 11-year-old dogs.
Immunophenotyping
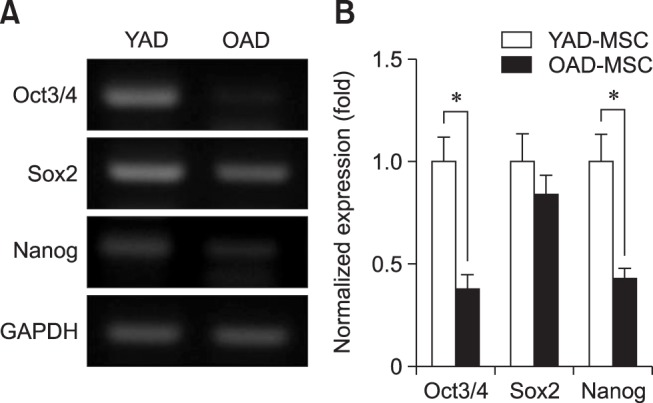
The qRT-PCR results revealed that the cAD-MSCs were strongly positive for CD44 and CD90, and weakly positive for CD54, CD61, CD73, CD80, and CD105. Expressions of CD29, CD34, CD117, and MHC-II markers were not detected in cAD-MSCs from young or old donors. The expressions of the detected surface markers CD73 and CD80 were significantly (p < 0.05) higher in young donors than in old donors, while other makers did not show significant differences associated with donor age (Fig. 3).
Fig. 3. Surface markers of canine adipose tissue-derived mesenchymal stem cells (cAD-MSCs). MSC-specific cell surface markers (positive: CD44, CD54, CD61, CD73, CD80, CD90, and CD105; not detectable: CD29, CD34, CD117, and MHC-class II) of cAD-MSCs were observed by real-time quantitative reverse transcriptase polymerase chain reaction in two age groups (young and old) at passage 3. GAPDH was used as a housekeeping control gene. All mRNA data were normalized to the levels of GAPDH, and relative fold changes in expression levels are shown as the mean ± SEM (n = 3). *p < 0.05. YAD, 7-month-old dogs; OAD, 10- to 11-year-old dogs.
Mesodermal differentiation
Adipogenic differentiation
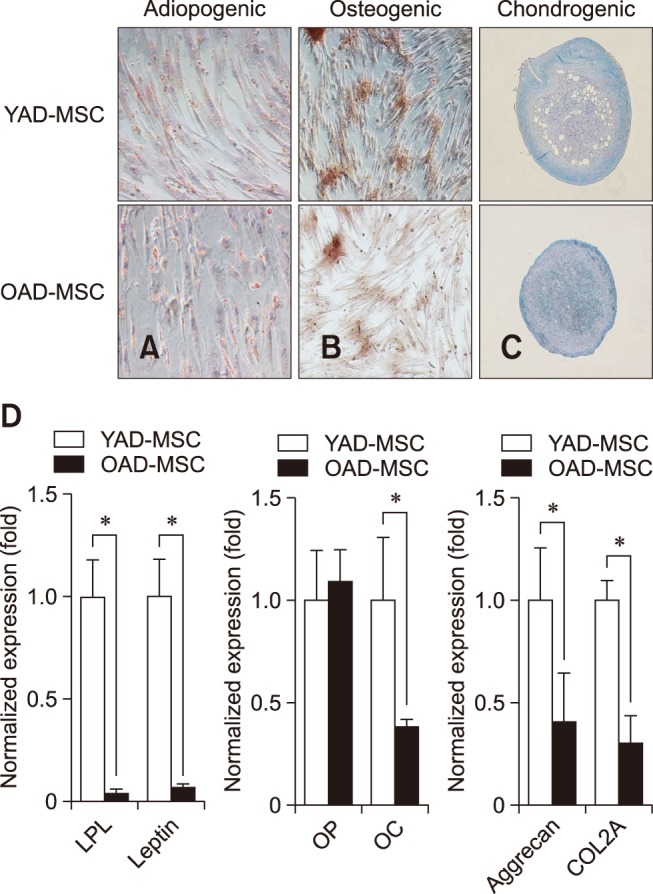
cAD-MSCs of young and old donors at P3 were cultured for 21 days in specific induction medium for assessment of adipogenic differentiation. The differentiated cells were analyzed for the presence of intracellular lipid accumulation by staining with Oil Red O and for mRNA expression of adipogenic markers by qRT-PCR to determine the adipogenic ability of cAD-MSCs according to donor age (panel A in Fig. 4). The cAD-MSCs in young donors displayed many small lipid droplets compared to those in old donors. Also, gene expression levels of specific markers associated with adipogenic differentiation such as LPL and leptin were determined in the differentiated cells (panel D in Fig. 4). Compared to old donors, significantly higher expressions of LPL (23-fold) and leptin (6.5-fold) mRNA transcripts were evident in young donors.
Fig. 4. Differentiation potentials of canine adipose tissue-derived mesenchymal stem cells (cAD-MSCs). Adipocytes (Oil Red O; A), osteocytes (Alizarin Red; B), and chondrocytes (Alcian Blue; C) were positively stained in two age groups (young and old) at passage 3. (D) The mRNA expressions of adipocyte, osteocyte, and chondrocyte-related genes were detected by real-time quantitative reverse transcriptase polymerase chain reaction and compared between undifferentiated and differentiated cells in the two groups at passage 3. All mRNA data were normalized to the levels of GAPDH, and relative fold changes in expression levels are shown as the mean ± SEM (n = 3). *p < 0.05. 100× (A–C). YAD, 7-month-old dogs; OAD, 10- to 11-year-old dogs; LPL, lipoprotein lipase; OP, osteopontin; OC, osteocalcin; COL2A, type II-collagen.
Osteogenic differentiation
cAD-MSCs of young and old donors at P3 were cultured for osteogenic differentiation for 21 days in specific induction medium. The differentiated cells were then examined for the presence of calcium deposit accumulation by staining with Alizarin Red (panel B in Fig. 4) and for mRNA expression of osteogenic markers by using qRT-PCR to determine the osteogenic ability of cAD-MSCs according to donor age. The cAD-MSCs in young donors displayed more intense staining of calcium deposits than those in old donors. In addition, gene expression levels of specific markers associated with osteogenic differentiation, such as OP and OC, were determined in the differentiated cells. Significantly higher expression of OC mRNA transcripts (2.6-fold) was evident in the young donors compared to that in old donors. But, expression of OP mRNA transcript was not significantly different in cAD-MSCs from young and old donors (panel D in Fig. 4).
Chondrogenic differentiation
All pellets of cAD-MSCs in specific medium were well maintained and exhibited a packed and rounded morphology during the 21 day culture period with no differences in pellet size between young and old donors. Glycosaminoglycans formed in the differentiated cells were stained a blue color by Alcian Blue (panel C in Fig. 4). All cAD-MSCs from P3 underwent chondrogenic differentiation, but the cells in young donors displayed more intense glycosaminoglycan staining than those in old donors. Chondrocyte-specific markers such as aggrecan and COL2A as a chondrogenic marker were analyzed by qRT-PCR in the differentiated cells. Significantly higher expressions of mRNA transcripts encoding aggrecan (2.4-fold) and COL2A (3.36-fold) were evident in cAD-MSCs from young donors compared those in old donors (panel D in Fig. 4).
Expression of cell survival genes
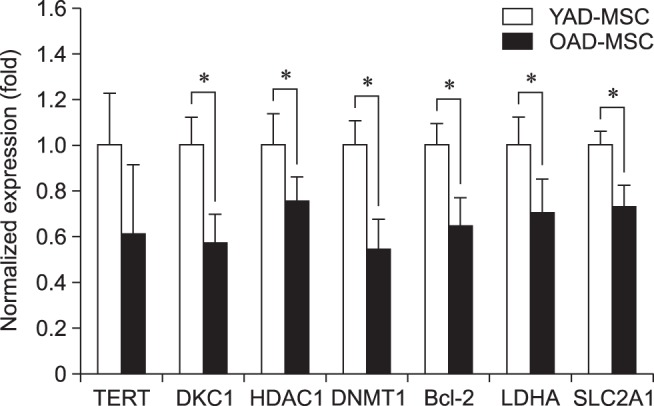
To further confirm the involvement of cell survival signals in proliferating cAD-MSCs from both young and old donors, the expressions of genes such as TERT, DKC1, HDAC1, DNMT1, Bcl-2, LDHA, and SLC2A1 were examined using qRT-PCR. We observed that cAD-MSCs from young donors had significantly higher expressions of cell survival-related genes such as TERT, DKC1, HDAC1, DNMT1, Bcl-2, LDHA, and SLC2A1 than those in cAD-MSCs from old donors (Fig. 5).
Fig. 5. Cell survival-related genes of canine adipose tissue-derived mesenchymal stem cells (cAD-MSCs). The mRNA expressions of cell survival-related genes such as TERT, DKC1, HDAC1, DNMT1, Bcl-2, LDHA, and SLC2A1 were examined by real-time quantitative reverse transcriptase polymerase chain reaction in two groups (young and old) at passage 3. GAPDH was used as a housekeeping control gene. All mRNA data were normalized to the levels of GAPDH, and relative fold changes in expression levels are shown as the mean ± SEM (n = 3). *p < 0.05. YAD, 7-month-old dogs; OAD, 10- to 11-year-old dogs; TERT, telomerase reverse transcriptase; DKC1, dyskerin pseudouridine synthase 1; HDAC1, histone deacetylase 1; DNMT1, DNA (cytosine-5)-methyltransferase 1; LDHA, lactate dehydrogenase A; SLC2A1, glucose transporter member 1.
Discussion
Many researchers have reported on the multilineage differentiation potential of AD-MSCs. However, recent studies using MSCs from mice and humans have raised questions about the plasticity of MSCs collected from aged donors. Such concerns have been mainly attributed to reduced MSC quantity and altered differentiation potential. Alt et al. [1] reported that the number of colony-forming units (CFU) of MSCs from human adipose tissue decreased in a donor age-dependent manner. Li et al. [17] also reported that proliferation of MSCs from human BM-MSCs decreased with increasing donor age. Paxson et al. [24] demonstrated relatively low CFU, growth potential, and telomerase activity in lung-derived MSCs obtained from older mice. In our study, cAD-MSCs from young and old canine donors were spindle-shaped, well adherent, able to be cultured in vitro, and increasing in cell number up to P5. The number of cAD-MSCs in in vitro culture of isolated cells from adipose tissue at P0 was 2.7-fold higher in young donors than in old donors. The CPDL results indicated that the proliferative activity in young donors was much higher than that in old donors, but proliferation markedly dropped at P4 in both donors. That decrease in proliferating activity was anticipated, as similar observations were reported in MSCs derived from canine umbilical cord matrix [15] and adipose tissue [14]. Collectively, these results are important as they indicate that cAD-MSCs harvested from older donors may need to be harvested at an increased harvest level to obtain sufficient adipose tissue or a pretreatment strategy to enhance cell proliferation and expansion may be needed. In addition, molecular markers associated with self-renewal were also affected by donor age. Similar to human embryonic stem cells (ESCs), cESCs express Oct3/4, Sox2, Nanog, stage-specific embryonic antigen 3 (SSEA-3), SSEA-4, TRA-1-60, TRA-1-81, and alkaline phosphatase, whereas they only express very low levels of SSEA-1 [13,27,28,32]. Among three pluripotent markers (Oct3/4, Sox2, and Nanog) compared in this study, the expression levels of Oct3/4 and Nanog in the young donors were significantly higher than those in the old donors, which suggests that the cAD-MSCs from the young donors have better MSC multipotency characteristics.
Expression patterns of cell surface markers for the cAD-MSCs in the present study were similar to those in a recent study [14]. No difference between young and old donors was observed in expression patterns, which has also been reported in mouse MSCs [24]. Meanwhile, the expression levels of CD73 and CD80 were significantly higher in young donors than in old donors. The roles of CD73- and/or CD80-expressing cells have not been studied in cMSCs. However, some studies have suggested that CD73-expressing MSCs, which have been isolated from some animal species, exist as an undifferentiated sub-population. Li et al. [16] demonstrated that the CD73-positive MSCs from rat adipose tissue had better proliferative activity than CD73-negative MSCs, which supports our observation that young donor cells, which contain a higher level of CD73-expressing MSCs, showed better proliferative activity than old donor cells.
Importantly, differentiation potential of cAD-MSCs was observed to be age-dependent. Our results revealed that differentiation capacities of cAD-MSCs for various mesodermal lineages significantly (p < 0.05) declined in the old donors, even though expression of OP (one of the molecular markers) used to determine osteogenic differentiation was not different between young and old donors. The change of differentiation potential with age could be expected in the similar study. Kretlow et al. [12] reported a decreased differentiation capacity of the three mesodermal lineage cells with increasing age in murine BM-MSCs. Kanawa et al. [11] confirmed that human BM-MSCs showed a decrease in chondrogenic differentiation in aged donors, but no age-dependent changes in osteogenic or adipogenic differentiation were observed. Meanwhile, as CD73-positive AD-MSCs isolated from rat adipose tissue showed a high differentiation potential into cardiomyocyte [16], cAD-MSCs of the young donors with their higher CD73 expression level were also expected to have better differentiation potential than that of the old donors in our study.
Alteration of DNA methylation is greatly associated with aging. It has been reported that SLC2A9 reduces reactive oxygen species and protects against DNA damage and cell death [10]. TERT and DKC1, two anti-senescence genes, may improve cell migration, protection, and cellular senescence, which may provide improved efficacy in stem cell plasticity [22]. Wang et al. [31] revealed that LDHA deletion inhibits the function of both hematopoietic stem cells and precursor cells during hematopoiesis. We observed that cAD-MSCs from young donors had significantly increased expression levels of TERT, DKC1, HDAC1, DNMT1, Bcl-2, LDHA, and SLC2A1 compared to those in the cAD-MSCs from old donors.
The age effect on differentiation potential for a specific cell type has been studied using MSCs of humans and other animals. For example, lung-derived MSCs of old mice donors have better regenerative activity than that of the young mice donors [24]. Stolzing et al. [26] observed an age-related decline of Notch-1 receptor levels in MSCs originated from human BM, leading to a decrease in bone formation. Feng et al. [5] revealed the negative effect of aging on neurogenic differentiation of human dental MSCs. In addition to better differentiation potential, the young canine donors in our study, with the higher level of CD73-expressing MSCs, were also expected to provide a greater benefit to clinical applications, since Ode et al. [21] reported that migration of MSCs isolated from rat BM were controlled by the expression of CD73. In contrast to our results, Guercio et al. [8] reported no age effect on stemness marker expression and differentiation potential of cAD-MSCs isolated from subcutaneous and visceral fat tissue of young (1–4 years old) and old (8–14 years old) canine donors. However, the apparent difference existed in age of young donors (1–4 years old vs 7 months old). Iohara et al. [9] demonstrated an age effect on regenerative potential of cMDP-SCs between young (8–10 months old) and old (5–6 years old) dogs. The ages of old dogs in the study by Iohara et al. [9] was similar to that by Guercio et al. [8]. Therefore, we speculate that if the latter study used donors under one year of age, the authors may have observed an age effect.
The present study is the first to report the presence of age-dependent changes in multipotency of cAD-MSCs. Since the success of utilizing stem cells in tissue-engineering applications is highly dependent on preparation of cells with a satisfactory level of differentiation potential, donor age should be considered when cAD-MSCs are being harvested and prepared.
Acknowledgments
This work was funded by the Animal and Plant Quarantine Agency (N-1541780-2013-22-02), Republic of Korea.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R. Aging alters tissue resident mesenchymal stem cells properties. Stem Cell Res. 2012;8:215–225. doi: 10.1016/j.scr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 3.Choudhery MS, Khan M, Mahmood R, Mehmood A, Khan SN, Riazuddin S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol Int. 2012;36:747–753. doi: 10.1042/CBI20110183. [DOI] [PubMed] [Google Scholar]
- 4.Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, Beck JC. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad Sci U S A. 1998;95:10614–10619. doi: 10.1073/pnas.95.18.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng X, Xing J, Feng G, Sang A, Shen B, Xu Y, Jiang J, Liu S, Tan W, Gu Z, Li L. Age dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/XMLLink_XYZ-catenin signaling. Cell Mol Neurobiol. 2013;33:1023–1031. doi: 10.1007/s10571-013-9965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotheraphy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 8.Guercio A, Di Bella S, Casella S, Di Marco P, Russo C, Piccione G. Canine mesenchymal stem cells (MSCs): characterization in relation to donor age and adipose tissue-harvesting site. Cell Biol Int. 2013;37:789–798. doi: 10.1002/cbin.10090. [DOI] [PubMed] [Google Scholar]
- 9.Iohara K, Murakami M, Nakata K, Nakashima M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp Gerontol. 2014;52:39–45. doi: 10.1016/j.exger.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Itahana Y, Han R, Barbier S, Lei Z, Rozen S, Itahana K. The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense. Oncogene. 2015;34:1799–1810. doi: 10.1038/onc.2014.119. [DOI] [PubMed] [Google Scholar]
- 11.Kanawa M, Igarashi A, Ronald VS, Higashi Y, Kurihara H, Sugiyama M, Saskianti T, Pan H, Kato Y. Age-dependent decrease in the chondrogenic potential of human bone marrow mesenchymal stromal cells expanded with fibroblast growth factor-2. Cytotherapy. 2013;15:1062–1072. doi: 10.1016/j.jcyt.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KS, Cha SH, Kang HW, Song JY, Lee KW, Ko KB, Lee HT. Effects of serial passage on the characteristics and chondrogenic differentiation of canine umbilical cord matrix derived mesenchymal stem cells. Asian-Australas J Anim Sc. 2013;26:588–595. doi: 10.5713/ajas.2012.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KS, Kang HW, Lee HT, Kim HJ, Kim CL, Song JY, Lee KW, Cha SH. Sequential sub-passage decreases the differentiation potential of canine adipose-derived mesenchymal stem cells. Res Vet Sci. 2014;96:267–275. doi: 10.1016/j.rvsc.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Lee KS, Nah JJ, Lee BC, Lee HT, Lee HS, So BJ, Cha SH. Maintenance and characterization of multipotent mesenchymal stem cells isolated from canine umbilical cord matrix by collagenase digestion. Res Vet Sci. 2013;94:144–151. doi: 10.1016/j.rvsc.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Qi LJ, Guo ZK, Li H, Zuo HB, Li NN. CD73+ adipose-derived mesenchymal stem cells possess higher potential to differentiate into cardiomyocytes in vitro. J Mol Histol. 2013;44:411–422. doi: 10.1007/s10735-013-9492-9. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Charif N, Mainard D, Bensoussan D, Stoltz JF, de Isla N. Donor's age dependent proliferation decrease of human bone marrow mesenchymal stem cells is linked to diminished clonogenicity. Biomed Mater Eng. 2014;24(Suppl 1):47–52. doi: 10.3233/BME-140973. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch. 2009;76:55–66. doi: 10.1272/jnms.76.56. [DOI] [PubMed] [Google Scholar]
- 20.Neupane M, Chang CC, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng Part A. 2008;14:1007–1015. doi: 10.1089/ten.tea.2007.0207. [DOI] [PubMed] [Google Scholar]
- 21.Ode A, Kopf J, Kurtz A, Schmidt-Bleek K, Schrade P, Kolar P, Buttgereit F, Lehmann K, Hutmacher DW, Duda GN, Kasper G. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. Eur Cell Mater. 2011;6:26–42. doi: 10.22203/ecm.v022a03. [DOI] [PubMed] [Google Scholar]
- 22.Oh YS, Jeong SG, Cho GW. Anti-senescence effects of DNA methyltransferase inhibitor RG108 in human bone marrow mesenchymal stromal cells. Biotechnol Appl Biochem. 2015;62:583–590. doi: 10.1002/bab.1393. [DOI] [PubMed] [Google Scholar]
- 23.Ohsaki H, Sawa T, Sasazaki S, Kano K, Taniguchi M, Mukai F, Mannen H. Stearoyl-CoA desaturase mRNA expression during bovine adipocyte differentiation in primary culture derived from Japanese Black and Holstein cattle. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:629–634. doi: 10.1016/j.cbpa.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Paxson JA, Gruntman AM, Davis AM, Parkin CM, Ingenito EP, Hoffman AM. Age dependence of lung mesenchymal stromal cell dynamics following pneumonectomy. Stem Cells Dev. 2013;22:3214–3225. doi: 10.1089/scd.2012.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Requicha JF, Viegas CA, Albuquerque CM, Azevedo JM, Reis RL, Gomes ME. Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev. 2012;8:1211–1222. doi: 10.1007/s12015-012-9397-0. [DOI] [PubMed] [Google Scholar]
- 26.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Takemitsu H, Zhao D, Yamamoto I, Harada Y, Michishita M, Arai T. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet Res. 2012;8:150. doi: 10.1186/1746-6148-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaags AK, Rosic-Kablar S, Gartley CJ, Zheng YZ, Chesney A, Villagómez DAF, Kruth SA, Hough MR. Derivation and characterization of canine embryonic stem cell lines with in vitro and in vivo differentiation potential. Stem Cells. 2009;27:329–340. doi: 10.1634/stemcells.2008-0433. [DOI] [PubMed] [Google Scholar]
- 29.Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010;19:279–289. doi: 10.3727/096368909X481764. [DOI] [PubMed] [Google Scholar]
- 30.Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, Eckstein V, Boukamp P, Ho AD. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One. 2009;4:e5846. doi: 10.1371/journal.pone.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YH, Israelsen WJ, Lee D, Yu VWC, Jeanson NT, Clish CB, Cantley LC, Vander Heiden MG, Scadden DT. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014;158:1309–1323. doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenceslau CV, Miglino MA, Martins DS, Ambrósio CE, Lizier NF, Pignatari GC, Kerkis I. Mesenchymal progenitor cells from canine fetal tissues: yolk sac, liver, and bone marrow. Tissue Eng Part A. 2011;17:2165–2176. doi: 10.1089/ten.TEA.2010.0678. [DOI] [PubMed] [Google Scholar]
- 33.Yang CC, Ellis SE, Xu F, Burg KJL. In vitro regulation of adipogenesis: tunable engineered tissues. J Tissue Eng Regen Med. 2007;1:146–153. doi: 10.1002/term.17. [DOI] [PubMed] [Google Scholar]
- 34.Zheng B, Cao B, Li G, Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng. 2006;12:1891–1901. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- 35.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]