Abstract
Goose parvovirus (GPV) continues to be a threat to goose farms and has significant economic effects on the production of geese. Current commercially available vaccines only rarely prevent GPV infection. In our study, Lactobacillus (L.) plantarum NC8 was selected as a vector to express the VP2 gene of GPV, and recombinant L. plantarum pSIP409-VP2/NC8 was successfully constructed. The molecular weight of the expressed recombinant protein was approximately 70 kDa. Mice were immunized with a 2 × 109 colony-forming unit/200 µL dose of the recombinant L. plantarum strain, and the ratios and numbers of CD11c+, CD3+CD4+, CD3+CD8+, and interferon gamma- and tumor necrosis factor alpha-expressing spleen lymphocytes in the pSIP409-VP2/NC8 group were higher than those in the control groups. In addition, we assessed the capacity of L. plantarum SIP409-VP2/NC8 to induce secretory IgA production. We conclude that administered pSIP409-VP2/NC8 leads to relatively extensive cellular responses. This study provides information on GPV infection and offers a clear framework of options available for GPV control strategies.
Keywords: Lactobacillus plantarum, VP2 gene, goose parvovirus, immunization
Introduction
Goose parvovirus (GPV) has been identified as a member of the genus Dependovirus in the family Parvoviridae [4]. The first sample of this virus was obtained in China in 1956. Since then, sporadic outbreaks of GPV infection have occurred in all of the major goose-farming countries in the world [21,28], which has led to tremendous economic loss. GPV is a well-known causative agent of gosling plague (GP), which is characterized by a highly contagious nature and high mortality, and both geese and Muscovy ducks are susceptible to GP. Thus, there is an urgent need to control or prevent GPV infection. Nevertheless, none of the currently available commercial vaccines are able to completely prevent GPV infection. Therefore, certain investigators are pursuing comparatively avirulent, convenient, and efficacious approaches to constructing new vaccine candidates to prevent GPV infection.
The genome of GPV is a linear, single-stranded DNA molecule that is 5106 nucleotides long and that includes two major open reading frames (ORFs) [36]. One ORF encodes regulatory proteins, and the other ORF encodes three capsid proteins designated as VP1, VP2, and VP3 [40]. VP2 and VP3 are included in the carboxyl terminal portion of VP1. The VP2 protein is the primary functional immunological area of GPV, containing an immunodominant region that can participate in neutralizing the virus [5]. Therefore, the VP2 protein may be a suitable target for research strategies aimed at developing new prophylactic vaccines against GPV.
Lactobacillus is a microaerophilic, rod-shaped, Gram-positive, facultative anaerobic bacterium [37]. Lactobacillus has been demonstrated to have a strong propensity to promote good health and alleviate many health problems [8,10,34]. Certain Lactobacillus (L.) plantarum strains have been shown to have probiotic effects on human health [26,29]. Increasing the use of genetic tools to produce efficient and convenient gene expression in Lactobacillus is important. Recombinant lactobacilli that express exogenous antigens have been administered to patients [6,17,38] at particular dosages in accordance with the expression of antigen and duration of persistence in the intestine. The L. plantarum strain NC8 was isolated from grass silage and was recognized as being naturally plasmid-free [2]. As a model strain useful in the development of genetic tools, many reports indicate that NC8 can be widely used in transformation [33], vector-based expression [1,31,32], general fermentation [27], and bacteriocin production [25] in lactobacilli. This strain has also received considerable attention as an expression vector. For example, recombinant viral and immunomodulatory genes have been expressed in NC8 in order to assess their potential as vaccines or therapeutics [30]. As genetic engineering technology progresses, recombinant NC8 has great potential in the prevention and treatment of many diseases.
To develop a relatively safe expression system for an oral/nasal vaccine against GPV infection, L. plantarum NC8 was selected as a vector to express the VP2 gene of GPV as a recombinant protein. The recombinant expression system pSIP-409-VP2/NC8 was successfully constructed, and SDS-PAGE and western blotting analyses revealed that the VP2 protein was highly expressed by L. plantarum NC8 and had favorable immunogenicity. We also assessed the immunogenicity of the recombinant pSIP409-VP2/NC8 in BALB/c mice. Our aim was to provide theoretical and experimental bases for developing genetically engineered L. plantarum mucosal vaccines against GPV.
Materials and Methods
Virus and bacterial strains, plasmid vector and main reagents
Both GPV and L. plantarum NC8 were maintained by and obtained from Jilin Agricultural University in Changchun, China. Induction of the expression vector pSIP-409 was kindly provided by Kolandaswamy Anbazhagan (Madurai Kamaraj University, India). The pGEM-T plasmid vector was obtained from Promega (USA) Rabbit anti-GPV IgG was produced in our laboratory. Horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG was purchased from Bioss (China).
Amplification and sequence identification of the VP2 gene
To amplify the GPV VP2 gene, one pair of PCR primers was designed based on sequences published in GenBank (National Center for Biotechnology Information, USA). The forward primer (primer P1: 5′-GGGTCTAGAACGGCACCTGCAAAAAA-3′) contained the XbaI restriction endonuclease site, and the reverse primer (primer P2: 5′-GCGGGTACCTTACAGA TTTTGAGTTAGAT-3′) contained the KpnI restriction endonuclease site. Based on these primers, the VP2 structural protein gene of GPV containing the XbaI and KpnI restriction endonuclease sites was amplified by using PCR and was purified by using an agarose gel DNA purification kit, as described previously [39]. The purified specific fragment was then cloned into the pGEM-T vector and transformed into Escherichia (E.) coli JM109 competent cells. Suspected positive E. coli colonies were screened, and the plasmids identified and extracted by using specific restriction enzyme digestion and sequencing.
Construction of the recombinant expression plasmid pSIP-409-VP2
Positive pGEMT-VP2 plasmids were double-digested with XbaI and KpnI, and, after recycling and purification, the target fragments were ligated with T4 DNA ligase overnight at 16℃ with the pSIP-409 plasmid. The ligated products were then transformed into E. coli DH5α competent cells [23]. After screening the transformants by restriction enzyme digestion and PCR, the positive recombinant plasmids were defined as pSIP-409-VP2.
Construction of recombinant L. plantarum NC8
Electroporation was used to introduce the recombinant plasmid pSIP409-VP2 into L. plantarum NC8. Subsequently, 100 µL of the electrotransformation product was plated onto MRS solid medium containing erythromycin at a concentration of 10 µg/mL and was cultured anaerobically at 30℃ for 24 to 36 h. Clones that were screened positive were classified as pSIP-409-VP2/NC8. In addition, samples were analyzed by SDS-PAGE and western blotting.
SDS-PAGE and western blotting analyses
To observe the expression levels of the VP2 antigen, the recombinant pSIP409/NC8 and pSIP409-VP2/NC8 plasmids were cultured and Sakacin P (SppIP) (50 ng/mL) was added to the culture medium when the OD600 reached 0.3 to induce antigen expression [13]. Next, the cells were disrupted on ice with sonication, and the proteins were separated by SDS-PAGE (12% acrylamide). After being transferred to nitrocellulose membranes, the VP2 protein was detected by using rabbit anti-GPV IgG as a primary antibody and HRP-conjugated labeled goat anti-rabbit IgG as a secondary antibody. An ECL Plus detection kit was used for enhanced chemiluminescence (ECL) visualization of the immunobinding [30].
Animals and ethics statement
All animal experiments were accredited by the Ethics Committee (No. JLAU08201007) for Animal Care of Jilin Agriculture University (China), where the animal facility is approved by the National Association of Laboratory Animal Care. Specific pathogen-free 6- to 8-week-old female BALB/c mice were obtained from Beijing HFK Bioscience, China.
Administration of recombinant L. plantarum NC8/VP2 to BALB/c mice
To assess the immunogenicity of the recombinant L. plantarum NC8, 200 µL of recombinant pSIP409-VP2/NC8 or pSIP409/NC8 suspensions at 2 × 109 colony-forming unit were orally administered to the BALB/c mice once/day for 14 days. PBS was given in the same manner to the control groups for 14 days.
Flow cytometric analysis
Mice were euthanized 7 days after then end of the 14 day vaccination period. The spleen was obtained and single-lymphocyte suspensions were prepared. Spleen lymphocytes (1 × 106) in 100 µL of fluorescence-activated cell sorting (FACS) buffer were combined with 10 µL of monoclonal fluorescently labeled anti-mouse antibodies (CD3-APC, CD4-FITC, and CD8-PE), mixed well, and incubated at 4℃ for 30 min in the dark. The cells were then incubated with 10 µL of anti-mouse antibody CD11-PE in the same way. After cell suspensions were washed with FACS buffer twice, cell sorting was performed to quantify the cells by using BD FACS Calibur (BD Biosciences, USA), and the data were analyzed by using FlowJo 7.6.1 software [22].
Measurement of cytokine and secretory IgA (sIgA) production by enzyme-linked immunosorbent assay (ELISA)
Splenic lymphocytes were used for analysis of cytokine secretion in vitro following re-stimulation with purified VP2 protein. For this purpose, splenic lymphocyte cultures were seeded into 24-well plates (5 × 106 cells) and treated with purified recombinant VP2 (final concentration, 10 µg/mL) in vitro for 72 h, as described previously [37]. The supernatants of the spleen lymphocytes were collected after stimulation, and cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α were analyzed by performing ELISA.
The sIgA antibody titers in small intestinal washings were tested by using ELISA as described previously [19]. In brief, 96-well immunoplates were coated with 1 µg of VP2 and incubated overnight at 4℃, then blocked with blocking buffer (3% bovine serum albumin (BSA) in PBST) at 37℃ for 1 h. Serum samples and standards were then added. After incubation and washing, anti-mouse-HRP conjugate was added and additionally incubated with 0.02% o-phenylenediamine (OPD) and 0.015% H2O2 (Zymed, USA). The reaction proceeded at room temperature until it was terminated by the addition of a stop solution, 2 N H2SO4. The absorbance was measured at 492 nm by using an automated ELISA reader.
Statistical analysis
All data were expressed as mean ± SEM values. Statistical analysis was performed by applying two-tailed Student's t-tests via GraphPad Prism software (ver. 5.0; GrapPad Software, USA).
Results
Cloning of VP2 recombinant plasmid
A 1764 bp fragment was amplified from GPV DNA by using the primer pair P1 and P2. The results showed that the expected DNA band for the VP2 gene had been amplified; the PCR product was approximately 1764 bp in length (Fig. 1). The purified PCR product was ligated with the pGEM-T vector and transformed into E. coli JM 109 competent cells. A positive colony was extracted to detect whether the VP2 gene was inserted into pGEM-T. The segment and vector were checked by digestion using specific restriction endonucleases. Double restriction enzyme digestion of the recombinant plasmid using XbaI and KpnI led to the expected DNA fragments, which were 3000 bp and 1764 bp in size (Fig. 2). Separately, the gene that was detected by sequencing shared 99% identity with the original VP2 sequence according to GenBank (GI: 156124944). These results indicate that the VP2 gene was successfully amplified.
Fig. 1. Polymerase chain reaction (PCR) results for the goose parvovirus (GPV) VP2 gene. M, marker DL2000; Lanes 1 and 2, PCR products of the VP2 gene (1764 bp); Lane 3, negative control.
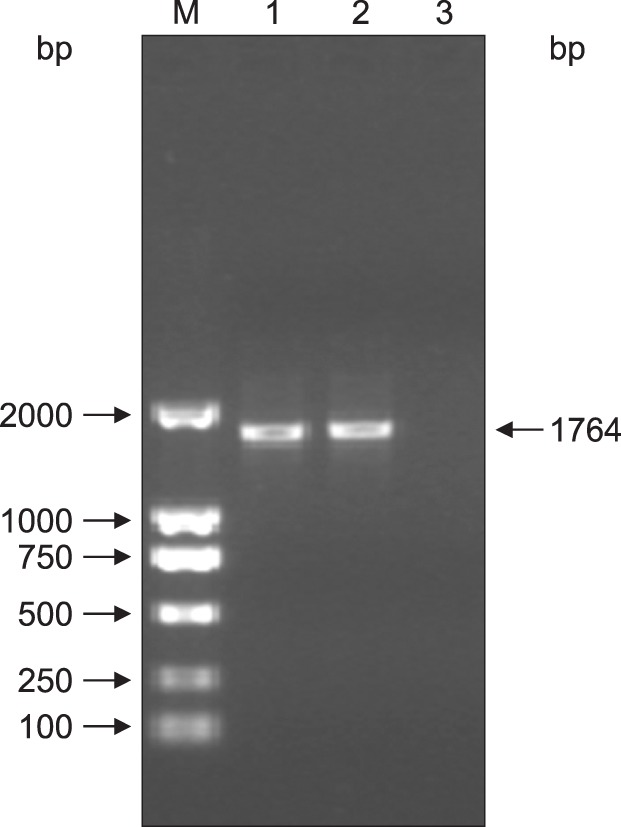
Fig. 2. Confirmation of the pGEM-T-VP2 recombinant plasmid obtained via restriction enzyme digestion. Lane 1–4, pGEM-T-VP2 digested with KpnI and XbaI (3000 bp and 1764 bp, respectively); M1, DL2000 marker; M2, λ-Hind III.
Identification of the recombinant plasmid pSIP-409-VP2
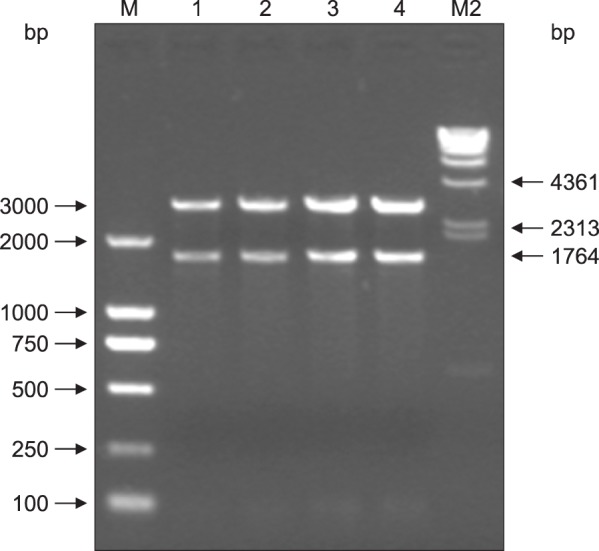
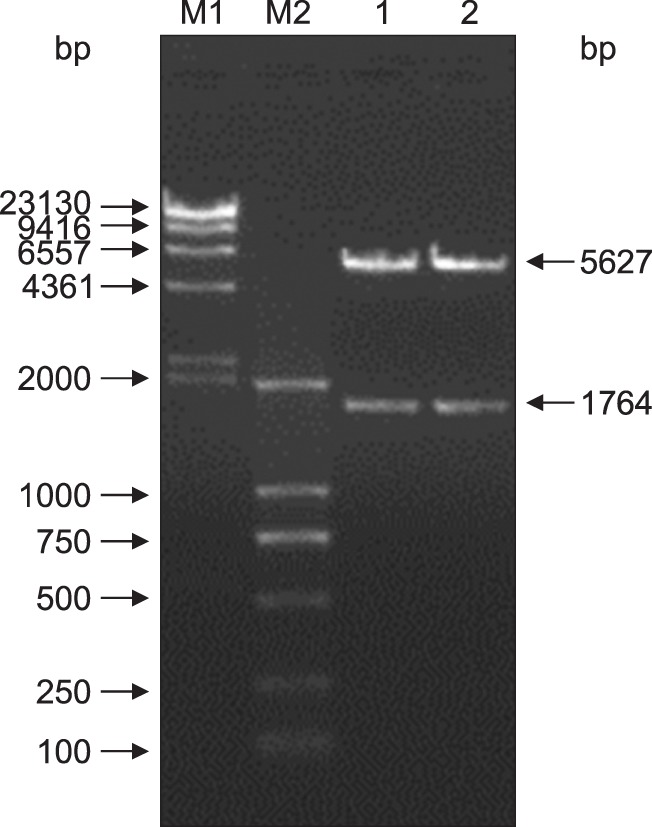
PCR screening was performed on the VP2 DNA of cells harboring pSIP-409-VP2. The results showed that the expected DNA band for the VP2 gene was amplified; the PCR product was approximately 1764 bp in length (Fig. 3). Amplified product was extracted, and double digestion with XbaI and KpnI was performed on the recombinant plasmid pSIP-409-VP2. The results showed that a 5627 bp vector band and a 1764 bp fragment were obtained. These results indicate that the target gene was successfully recombined into pSIP-409 according to the planned design (Fig. 4).
Fig. 3. Electropherogram of the VP2 gene amplified via polymerase chain reaction (PCR) from the pSIP-409-VP2 recombinant plasmid. Lane 1, negative control; Lanes 2 and 3, VP2 PCR product (1764 bp); M, DL2000 marker.
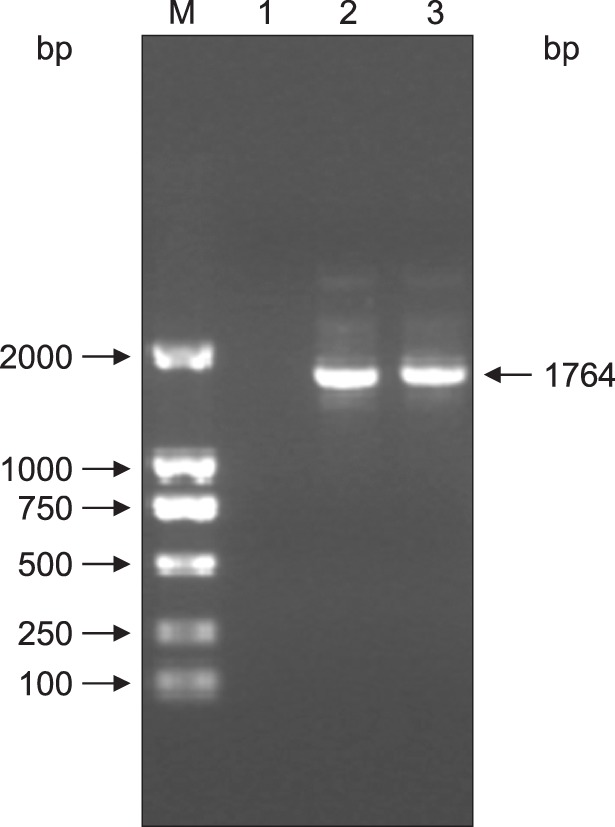
Fig. 4. Confirmation of the pSIP-409-VP2 recombinant plasmid obtained via restriction enzyme digestion. Lanes 1 and 2, pSIP409-VP2 digested with KpnI and XbaI (5627 bp and 1764 bp, respectively); M1, λ-Hind III; M2, DL2000 marker.
SDS-PAGE and western blotting analyses
After pSIP-409-VP2/NC8 was induced with SppIP, the recombinant protein was detected by 12% SDS-PAGE, and the expected bands for the recombinant proteins were visualized by using Coomassie brilliant blue staining. The results indicated that the molecular weight of the expressed recombinant protein was approximately 70 kDa (panel A in Fig. 5). The empty vector used in the control group did not produce the expected band.
Fig. 5. SDS-PAGE and western blotting analyses of the VP2 protein expressed in NC8. (A) All of the pSIP-409-VP2 protein bands at the induction times were 70 kDa in size. Lane M, protein marker; Lanes 1–6, induction of pSIP-409-VP2 with SppIP at 1 h, 2 h, 3 h, 4 h, 5 h, and 6 h, respectively; Lane C, empty pSIP-409 vector. (B) Western blotting analysis using an anti-GPV polyclonal antibody. The analysis demonstrates the specific reactivity of the VP2 protein expressed in NC8; the protein band was 70 kDa in size. Lane M, protein molecular mass standards; Lane 1, pSIP-409-VP2/NC8; Lane C, pSIP-409/NC8.
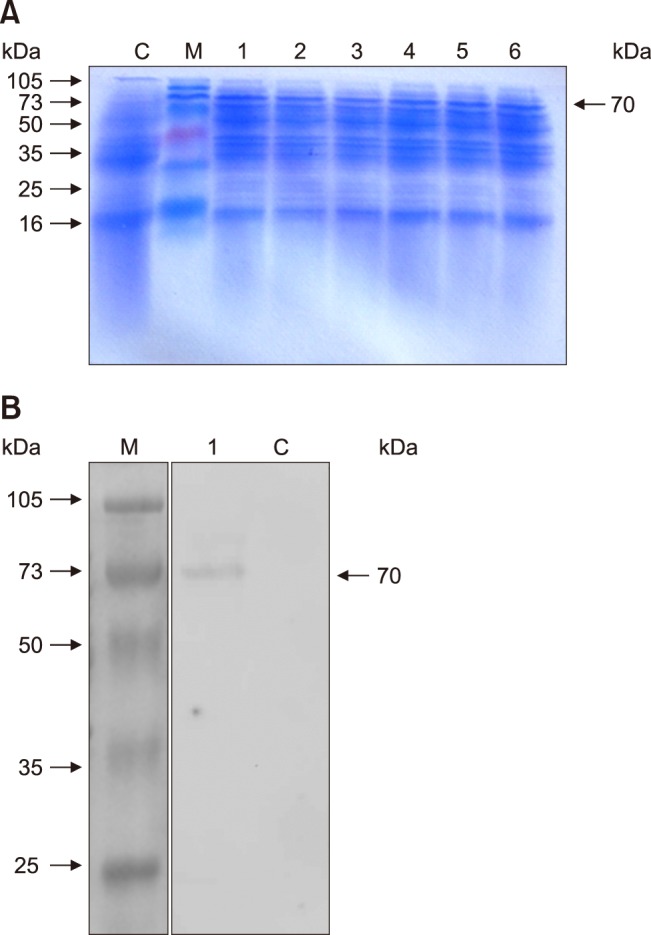
In addition, western blotting was performed to detect the recombinant VP2 protein, and it was clearly observed that the recombinant protein pSIP-409-VP2/NC8 reacted specifically with the primary antibody; the molecular weight of VP2 was 70 kDa. The pSIP409 vector used in the control group did not produce the expected band (panel B in Fig. 5). These results were in accordance with the SDS-PAGE analysis, and the molecular weights were consistent with the calculated values.
Changes in CD11c, CD4, and CD8 expression
To investigate the effect of recombinant L. plantarum NC8 on CD11c expression on the surfaces of dendritic cells (DCs), flow cytometric analysis was performed. As shown in panels A and B in Fig. 6, CD11c levels in the spleen showed significant differences between the pSIP409-VP2/NC8, pSIP409/NC8, and PBS groups, though there was no significant difference in CD11c expression between the two control groups. To evaluate further the effects of recombinant L. plantarum NC8 on T cell responses, the frequencies of CD3+CD4+ and CD3+CD8+ T cells in mouse spleen were examined (panels C and D in Fig. 6). There were higher percentages of CD3+CD4+ and CD3+CD8+ cells in the pSIP409-VP2/NC8 group than in the pSIP409/NC8 group. Taken together, these results suggest that a greater degree of cellular immunogenicity was induced by pSIP-409-VP2/NC8 than by the other constructs and conditions.
Fig. 6. CD11c, CD4, and CD8 expressions in immunized mice. Group A, pSIP409-VP2/NC8; Group B, pSIP409/NC8; Group C, phosphate-buffered saline (PBS). (A) Histograms show flow cytometry result (on isolated cells from spleen) from mice administered pSIP409-VP2/NC8 (thick red line) in comparison with the mice administered pSIP409/NC8 (thick green line) or PBS (filled histograms with gray color). (B) The CD11c+ levels of Group A were significantly different from those of Group B (*p < 0.05). (C) Gate strategy. (D) There were significantly different percentages of CD3+CD4+ and CD3+CD8+ cells between Groups A, B and C (**p < 0.01). On average, there were greater changes in CD11c+-, CD4+-, and CD8+-expressing spleen lymphocytes in Group A than in the other test groups. The data are expressed as mean ± SEM values from triplicate experiments. n = 3 mice per group.
Cytokine production
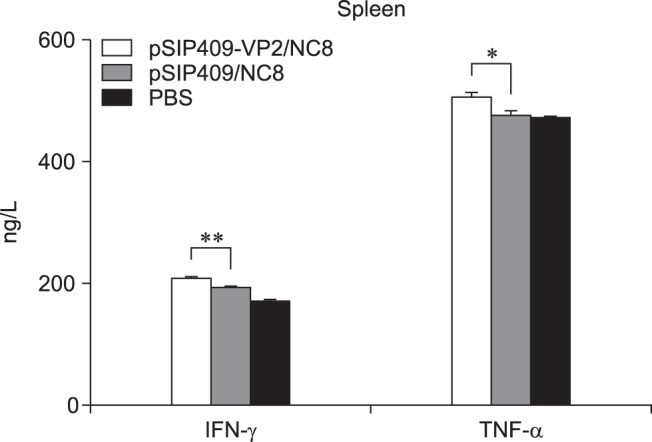
The TNF-α and IFN-γ levels were statistically significantly different among the groups. The TNF-α levels in the pSIP-409-VP2/NC8 group were significantly different from those in the pSIP-409/NC8 and PBS groups. In addition, the IFN-γ levels were highly significantly different between the pSIP-409-VP2/NC8 and the pSIP-409/NC8 groups (Fig. 7). These results again suggest that cellular immunogenicity induced by pSIP-409-VP2/NC8 was greater than that induced by the other constructs and conditions.
Fig. 7. Effect of recombinant Lactobacillus plantarum on cytokine production in mice. The levels of tumor necrosis factor (TNF)-α and interferon (IFN)-γ in the supernatants of spleen lymphocyte cultures were determined by enzyme-linked immunosorbent assay. Values are the means ± SEM for three mice. Asterisks represent statistically significant differences; *p < 0.05, **p < 0.01 compared with the control group.
Effects of recombinant L. plantarum on intestinal sIgA production
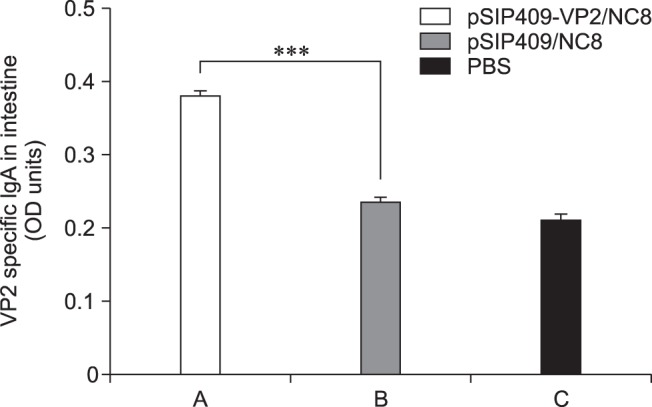
The intestinal content of each mouse was collected from similar sections of their small intestines, and the level of intestinal immunoglobulin A (sIgA) production was determined by ELISA (Fig. 8). Remarkably, the sIgA level in the pSIP409-VP2/NC8 group was highly significantly different from that in the pSIP409/NC8 group. Hence, recombinant L. plantarum NC8 has a relatively high capacity to induce sIgA production.
Fig. 8. Effect of administering recombinant Lactobacillus plantarum on sIgA production in mouse intestine. The levels of sIgA in the intestine were determined by enzyme-linked immunosorbent assay. Asterisks represent statistically significant differences; ***p < 0.001 compared with the control group. The data are expressed as mean ± SEM values of triplicate experiments. n = 3 mice per group.
Discussion
The livestock- and poultry-breeding industries have undergone rapid development in recent years. Nevertheless, infectious diseases remain the most significant challenge to the further progress of these industries. GPV infections have caused serious economic losses for many countries engaged in industrialized goose production. Preventive vaccination against GPV is an effective way for preventing disease in animals, and different vaccination strategies are normally applied against GPV infection. The first involves immunization of breeder geese prior to laying, which protects the goslings through maternally derived antibodies. The second involves vaccination of susceptible goslings with relatively low virulence vaccine strains [35]. Nevertheless, these two methods, which have varying levels of efficacy, lead to wasted financial resources. Hence, the development of a safe, effective, and low-cost vaccine is necessary. Based on these required characteristics, we chose L. plantarum as a candidate vaccine vehicle. L. plantarum strain NC8 has been considered as a delivery vehicle for the expression of integral membrane proteins and has been widely applied in both prokaryotes and eukaryotes. One of this strain's significant advantages is that it can induce relatively effective immunological responses and reduce the risk of eliciting immunological tolerance. Furthermore, NC8 has the potential for high expression of recombinant proteins, efficient secretion, and membrane protein expression, and it generates strong immunoprotection against influenza virus in BALB/c mice [23]. In addition, a study reported that Lactobacillus can be used as an expression vector due to its ability to modulate systemic and mucosal immune responses [22].
Furthermore, probiotic-based vaccines can regulate immunostimulatory responses and activate antigen-presenting cells (APCs), such as macrophages and DCs. DCs are seen as professional APCs that elicit a primary immune response against pathogens challenge [12]. Several investigations have indicated that immunostimulatory function showed tissue-specific differences among DCs in terms of inducing T helper (Th) cell responses [3]; generally, splenic DCs preferentially activate a Th1 responses, whereas DCs isolated from mucosal tissues are involved in the activation of Th2 responses [14]. Our study shows that recombinant L. plantarum NC8 can increase the activation of CD11c expression on the surfaces of splenic cells, thus enhancing regulation of the immune response of Th1 cells. Certain studies have also discussed the effects of probiotics on immune function, focusing on the Th1/Th2 or pro-inflammatory/anti-inflammatory cytokine balance [24,11,7]. Th1 cells can produce IFN-γ and TNF-α, which are able to stimulate Tc and macrophages to activate cellular immunity against intracellular pathogens such as viruses [9]. In the present study, we focused on changes in IFN-γ and TNF-α levels in the supernatants of splenic lymphocyte cultures. IFN-γ is produced by T lymphocytes and NK cells, and it plays a major role in enhancement of phagocytic activity and activation of Th1 responses against virus infection. TNF-α is produced by both T lymphocytes and monocytes, and it has an important function in inflammatory responses. Our study demonstrated that IFN-γ levels were significantly increased in the pSIP-409-VP2/NC8 group and that TNF-α levels differed significantly between the mice immunized with pSIP-409-VP2/NC8 and the control group, which is consistent with previous studies demonstrating that most probiotic strains are capable of inducing TNF-α [15]. In summary, our findings imply that recombinant L. plantarum NC8 may increase the activation of helper CD4+ T cells and cytotoxic CD8+ T cells and modulate adaptive immunity.
It is well known that the mucosal immune system has a crucial role in the first line of defense against pathogens infection, such as bacteria and viruses [22]. The gastrointestinal tract is the largest of these barriers and is especially suitable for colonization by probiotics. Natural sIgA is induced through constant antigenic stimulation by intestinal microflora, and live probiotics can reach the intestinal epithelial surface or interact with the underlying mucosal immune system, where they can aid digestion and significantly impact mucosal immunity. Additionally, a study has reported that sIgA was associated with the health of patients with inflammatory bowel disease [16]. Based on these reports, we performed additional experiments and showed increased small intestinal sIgA production in BALB/c mice following the administration of recombinant L. plantarum NC8. There is increasing evidence to indicate that the specific lesions observed in GPV-infected geese are predominantly characterized by severe enteritis, along with small intestine mucous membrane shedding and necrosis [18]. Hence, it is important to strengthen local immunity to decrease the risk of GPV infection.
In conclusion, vectors based on lactic acid bacteria have been widely used as live vaccine vehicles against diverse foreign microbes. We generated a recombinant GPV VP2 protein using L. plantarum NC8 as an avirulent, convenient, and effective vaccine vector to express GPV VP2 and evaluated its potential as a candidate vaccine against GP in goslings. The results of western blotting showed that pSIP-409-VP2/NC8 could induce strong favorable immunogenic responses to GPV. We did not evaluate the immune responses in geese due to many external factors such as the season and the environment. There is an accumulation of studies demonstrating that structural proteins play a vital function in viral infection [20], replication, and pathogenicity, which suggests that the VP2 structural protein is the main GPV functional immunological area that serves as a viral protective antigen and provides beneficial antigenicity [5]. Additionally, this study showed the practicality of developing genetically engineered L. plantarum for use as a mucosal vaccine and elucidated the immune response induced by L. plantarum. Further studies into memory B cell responses to pSIP-409-VP2/NC8 and natural geese GPV infections are needed.
Acknowledgments
This work was supported by the National High-Tech R&D Program of China (863 Program) (2013AA102806 and 2011AA10A215), National Natural Science Foundation of China (31272541 and 31272552), and Science and Technology Development Program of Jilin Province (20160519011JH), special funds for Industrial Innovation of Jilin Province (2016C063) and the Doctoral Project sponsored by the Scientific Research Foundation of Jilin Agricultural University of China (201601).
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Axelsson L, Lindstad G, Naterstad K. Development of an inducible gene expression system for Lactobacillus sakei . Lett Appl Microbiol. 2003;37:115–120. doi: 10.1046/j.1472-765x.2003.01360.x. [DOI] [PubMed] [Google Scholar]
- 2.Axelsson L, Rud I, Naterstad K, Blom H, Renckens B, Boekhorst J, Kleerebezem M, van Hijum S, Siezen RJ. Genome sequence of the naturally plasmid-free Lactobacillus plantarum strain NC8 (CCUG 61730) J Bacteriol. 2012;194:2391–2392. doi: 10.1128/JB.00141-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Brown KE, Green SW, Young NS. Goose parvovirus—an autonomous member of the dependovirus genus. Virology. 1995;210:283–291. doi: 10.1006/viro.1995.1345. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Li C, Zhu Y, Wang B, Meng C, Liu G. Immunogenicity of virus-like particles containing modified goose parvovirus VP2 protein. Virus Res. 2012;169:306–309. doi: 10.1016/j.virusres.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Cho HJ, Kim JY, Lee Y, Kim JM, Kim YB, Chun T, Oh YK. Enhanced humoral and cellular immune responses after sublingual immunization against human papillomavirus 16 L1 protein with adjuvants. Vaccine. 2010;28:2598–2606. doi: 10.1016/j.vaccine.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 7.de Roock S, van Elk M, van Dijk MEA, Timmerman HM, Rijkers GT, Prakken BJ, Hoekstra MO, de Kleer IM. Lactic acid bacteria differ in their ability to induce functional regulatory T cells in humans. Clin Exp Allergy. 2010;40:103–110. doi: 10.1111/j.1365-2222.2009.03344.x. [DOI] [PubMed] [Google Scholar]
- 8.Dicks LM, Botes M. Probiotic lactic acid bacteria in the gastro-intestinal tract: health benefits, safety and mode of action. Benef Microbes. 2010;1:11–29. doi: 10.3920/BM2009.0012. [DOI] [PubMed] [Google Scholar]
- 9.Dong H, Rowland I, Yaqoob P. Comparative effects of six probiotic strains on immune function in vitro . Br J Nutr. 2012;108:459–470. doi: 10.1017/S0007114511005824. [DOI] [PubMed] [Google Scholar]
- 10.Doron S, Gorbach SL. Probiotics: their role in the treatment and prevention of disease. Expert Rev Anti Infect Ther. 2006;4:261–275. doi: 10.1586/14787210.4.2.261. [DOI] [PubMed] [Google Scholar]
- 11.Drago L, Nicola L, Iemoli E, Banfi G, De Vecchi E. Strain-dependent release of cytokines modulated by Lactobacillus salivarius human isolates in an in vitro model. BMC Res Notes. 2010;3:44. doi: 10.1186/1756-0500-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72:3299–3309. doi: 10.1128/IAI.72.6.3299-3309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eijsink VG, Brurberg MB, Middelhoven PH, Nes IF. Induction of bacteriocin production in Lactobacillus sake by a secreted peptide. J Bacteriol. 1996;178:2232–2237. doi: 10.1128/jb.178.8.2232-2237.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everson MP, Lemak DG, McDuffie DS, Koopman WJ, McGhee JR, Beagley KW. Dendritic cells from Peyer's patch and spleen induce different T helper cell responses. J Interferon Cytokine Res. 1998;18:103–115. doi: 10.1089/jir.1998.18.103. [DOI] [PubMed] [Google Scholar]
- 15.Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007;13:236–243. doi: 10.3748/wjg.v13.i2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukushima Y, Kawata Y, Hara H, Terada A, Mitsuoka T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol. 1998;42:39–44. doi: 10.1016/s0168-1605(98)00056-7. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert C, Robinson K, Le Page RW, Wells JM. Heterologous expression of an immunogenic pneumococcal type 3 capsular polysaccharide in Lactococcus lactis . Infect Immun. 2000;68:3251–3260. doi: 10.1128/iai.68.6.3251-3260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glávits R, Zolnai A, Szabó E, Ivanics E, Zarka P, Mató T, Palya V. Comparative pathological studies on domestic geese (Anser anser domestics) and Muscovy ducks (Cairina moschata) experimentally infected with parvovirus strains of goose and Muscovy duck origin. Acta Vet Hung. 2005;531:73–89. doi: 10.1556/AVet.53.2005.1.8. [DOI] [PubMed] [Google Scholar]
- 19.Haan L, Verweij WR, Holtrop M, Brands R, van Scharrenburg GJM, Palache AM, Agsteribbe E, Wilschut J. Nasal or intramuscular immunization of mice with influenza subunit antigen and the B subunit of Escherichia coli heat-labile toxin induces IgA- or IgG-mediated protective mucosal immunity. Vaccine. 2001;19:2898–2907. doi: 10.1016/s0264-410x(00)00556-9. [DOI] [PubMed] [Google Scholar]
- 20.Huang Z, Elankumaran S, Yunus AS, Samal SK. A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J Virol. 2004;78:10054–10063. doi: 10.1128/JVI.78.18.10054-10063.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansson DS, Feinstein R, Kardi V, Mató T, Palya V. Epidemiologic investigation of an outbreak of goose parvovirus infection in Sweden. Avian Dis. 2007;51:609–613. doi: 10.1637/0005-2086(2007)51[609:EIOAOO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi Y, Kunitoh-Asari A, Hayakawa K, Imai S, Kasuya K, Abe K, Adachi Y, Fukudome S, Takahashi Y, Hachimura S. Oral administration of Lactobacillus plantarum strain AYA enhances IgA secretion and provides survival protection against influenza virus infection in mice. PLoS One. 2014;9:e86416. doi: 10.1371/journal.pone.0086416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D, Lu H, Shi K, Su F, Li J, Du R. Immunogenicity of recombinant BCGs expressing predicted antigenic epitopes of bovine viral diarrhea virus E2 gene. Res Vet Sci. 2014;97:430–438. doi: 10.1016/j.rvsc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 24.López P, Gueimonde M, Margolles A, Suárez A. Distinct Bifidobacterium strains drive different immune responses in vitro. Int J Food Microbiol. 2010;138:157–165. doi: 10.1016/j.ijfoodmicro.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 25.Maldonado A, Ruiz-Barba JL, Jiménez-Díaz R. Purification and genetic characterization of plantaricin NC8, a novel coculture-inducible two-peptide bacteriocin from Lactobacillus plantarum NC8. Appl Environ Microbiol. 2003;69:383–389. doi: 10.1128/AEM.69.1.383-389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercenier A, Pavan S, Pot B. Probiotics as biotherapeutic agents: present knowledge and future prospects. Curr Pharm Des. 2003;9:175–191. doi: 10.2174/1381612033392224. [DOI] [PubMed] [Google Scholar]
- 27.Park MJ, General T, Lee SP. Physicochemical properties of roasted soybean flour bioconverted by solid-state fermentation using Bacillus subtilis and Lactobacillus plantarum . Prev Nutr Food Sci. 2012;17:36–45. doi: 10.3746/pnf.2012.17.1.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poonia B, Dunn PA, Lu H, Jarosinski KW, Schat KA. Isolation and molecular characterization of a new Muscovy duck parvovirus from Muscovy ducks in the USA. Avian Pathol. 2006;35:435–441. doi: 10.1080/03079450601009563. [DOI] [PubMed] [Google Scholar]
- 29.Schultz M, Veltkamp C, Dieleman LA, Grenther WB, Wyrick PB, Tonkonogy SL, Sartor RB. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm Bowel Dis. 2002;8:71–80. doi: 10.1097/00054725-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Shi SH, Yang WT, Yang GL, Cong YL, Huang HB, Wang Q, Cai RP, Ye LP, Hu JT, Zhou JY, Wang CF, Li Y. Immunoprotection against influenza virus H9N2 by the oral administration of recombinant Lactobacillus plantarum NC8 expressing hemagglutinin in BALB/c mice. Virology. 2014;464-465:166–176. doi: 10.1016/j.virol.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Sørvig E, Grönqvist S, Naterstad K, Mathiesen G, Eijsink VGH, Axelsson L. Construction of vectors for inducible gene expression in Lactobacillus sakei and L plantarum. FEMS Microbiol Lett. 2003;229:119–126. doi: 10.1016/S0378-1097(03)00798-5. [DOI] [PubMed] [Google Scholar]
- 32.Sørvig E, Mathiesen G, Naterstad K, Eijsink VGH, Axelsson L. High-level, inducible gene expression in Lactobacillus sakei and Lactobacillus plantarum using versatile expression vectors. Microbiology. 2005;151:2439–2449. doi: 10.1099/mic.0.28084-0. [DOI] [PubMed] [Google Scholar]
- 33.Spath K, Heinl S, Grabherr R. Direct cloning in Lactobacillus plantarum: electroporation with non-methylated plasmid DNA enhances transformation efficiency and makes shuttle vectors obsolete. Microb Cell Fact. 2012;11:141. doi: 10.1186/1475-2859-11-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tannock GW. A special fondness for lactobacilli. Appl Environ Microbiol. 2004;70:3189–3194. doi: 10.1128/AEM.70.6.3189-3194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Cong Y, Yin R, Feng N, Yang S, Xia X, Xiao Y, Wang W, Liu X, Hu S, Ding C, Yu S, Wang C, Ding Z. Generation and evaluation of a recombinant genotype VII Newcastle disease virus expressing VP3 protein of goose parvovirus as a bivalent vaccine in goslings. Virus Res. 2015;203:77–83. doi: 10.1016/j.virusres.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Cheng XX, Chen SY, Zhu XL, Chen SL, Lin FQ, Li ZL. Genetic characterization of a potentially novel goose parvovirus circulating in Muscovy duck flocks in Fujian province, China. J Vet Med Sci. 2013;75:1127–1130. doi: 10.1292/jvms.12-0527. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Yu Q, Gao J, Yang Q. Mucosal and systemic immune responses induced by recombinant Lactobacillus spp. expressing the hemagglutinin of the avian influenza virus H5N1. Clin Vaccine Immunol. 2012;19:174–179. doi: 10.1128/CVI.05618-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xin KQ, Hoshino Y, Toda Y, Igimi S, Kojima Y, Jounai N, Ohba K, Kushiro A, Kiwaki M, Hamajima K, Klinman D, Okuda K. Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood. 2003;102:223–228. doi: 10.1182/blood-2003-01-0110. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Li Y, Liu M, Zhang D, Guo D, Liu C, Zhi H, Wang X, Li G, Li N, Liu S, Xiang W, Tong G. Development and evaluation of a VP3-ELISA for the detection of goose and Muscovy duck parvovirus antibodies. J Virol Methods. 2010;163:405–409. doi: 10.1016/j.jviromet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Zhou Z, Huang Y, Yu R, Dong S, Li Z, Zhang Y. Identification of a recombinant Muscovy duck parvovirus (MDPV) in Shanghai, China. Vet Microbiol. 2014;174:560–564. doi: 10.1016/j.vetmic.2014.10.032. [DOI] [PubMed] [Google Scholar]