Abstract
Porcine alveolar macrophages (PAMs) represent the first line of defense in the porcine lung after infection with porcine circovirus type 2 (PCV2) via the respiratory tract. However, PCV2 infection impairs the microbicidal capability of PAMs and alters cytokine production and/or secretion. At present, the reason for the imbalance of cytokines has not been fully elucidated, and the regulatory mechanisms involved are unclear. In this study, we investigated the expression levels and regulation of interleukin-1beta (IL-1β) and IL-10 in PAMs following incubation with PCV2 in vitro. Levels of IL-1β and IL-10 increased in PAM supernatants, and the distribution of nuclear factor kappa B (NF-κB) p65 staining in nucleus, expression of MyD88 and p-IκB in cytoplasm, and DNA-binding activity of NF-κB increased after incubation with PCV2, while p65 expression in PAM cytoplasm decreased. However, when PAMs were co-incubated with PCV2 and small interfering RNA targeting MyD88, those effects were reversed. Additionally, mRNA expression levels of Toll-like receptors (TLR)-2, -3, -4, -7, -8, and -9 increased when PAMs were incubated with PCV2. These results show that PCV2 induces increased IL-1β and IL-10 production in PAMs, and these changes in expression are related to the TLR–MyD88–NF-κB signaling pathway.
Keywords: NF-kappa B, interleukin-10, interleukin-1beta, porcine alveolar macrophage, porcine circovirus type 2
Introduction
Porcine circovirus 2 (PCV2) is a small, single-stranded, non-enveloped DNA virus that is thought to be the primary cause of postweaning multisystemic wasting syndrome (PMWS), which mainly affects weaning piglets 3 to 15 weeks old [1,6]. The disorder is characterized by cytokine production and the depletion of lymphocytes in lymphoid organs [5,15,17,22], which can result in immunosuppression and lead to secondary infection or co-infection with other pathogens, such as porcine reproductive and respiratory syndrome virus (PRRSV), porcine torque teno virus, or Mycoplasma hyopneumoniae [8,12,20].
The primary target cells of PCV2 are monocyte/macrophage lineage cells (MLCs), including porcine alveolar macrophages (PAMs). However, PCV2 cannot replicate in MLCs, and these cells only act as carriers of PCV2 [4,9,25]. Most importantly, PAMs are the initial targets of PCV2, which is transmitted via the respiratory and digestive tracts, and are the primary responders in the pulmonary innate immune system that defends against invasion by foreign organisms. However, the phagocytosis, microbial killing, and endogenous antigen presentation capabilities of PCV2-infected PAMs are impaired [3,13]. Cytokines secreted by PAMs also have a critical role in pulmonary defense against foreign organisms and regulating the immune response. However, few studies have characterized the cytokine changes in PAMs. It has been reported that tumor necrosis factor alpha (TNF-α) and interleukin-8 (IL-8) levels are significantly increased after infection of PAMs with PCV2, and the mRNA expression levels of IFN-γ, IL-1α, and IL-8 are increased in the lungs of pigs with PCV2-associated respiratory disease [2,3]. Therefore, studies are urgently needed to investigate changes in the cytokines produced by PAMs and to elucidate the underlying regulatory mechanisms.
The proinflammatory cytokine IL-1 is mainly produced by macrophages. IL-10 can also be produced by macrophages and is an anti-inflammatory cytokine that has potent immunosuppressive properties [18]. Changes in IL-1 and IL-10 levels can reflect the immune status of PAMs after infection with PCV2. Additionally, it has been reported that Toll-like receptors (TLRs) and nuclear factor kappa B (NF-κB) participate in the innate immune response to PCV2 infection [7,23]. In the present study, we investigated the altered patterns of IL-1β and IL-10 expression and the regulatory mechanisms involved in piglet PAMs after PCV2 infection. The PCV2 infection induced increased IL-1β and IL-10 levels, marked nuclear NF-κB p65 localization, high cytoplasmic expression of MyD88 and p-IκB, low cytoplasmic expression of p65, activation of NF-κB DNA-binding activity, and increased TLR-2, -3, -4, -7, -8, and -9 mRNA expression levels. Knockdown of MyD88 by small interfering (si)RNA reversed the changes in IL-1β and IL-10 levels, distribution of NF-κB p65, expression of p65 and p-IκB, and the DNA-binding activity of NF-κB. The results show that production levels of IL-1β and IL-10 are regulated by the MyD88–NF-κB signaling pathway.
Materials and Methods
Viruses
The virus isolate PCV2-SH (GenBank accession No. GQ845027) was obtained from the Key Laboratory of Animal Disease Diagnosis and Immunology at Nanjing Agricultural University. The virus stock titers were 5 × 106.5 TCID50 (50% tissue culture infective dose)/mL, as determined by titration on PK-15 cells using an immunofluorescence assay (IFA).
Preparation of porcine alveolar macrophages (PAMs)
All animal procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. The specific protocol of this study was reviewed and approved (project 31172292). The euthanasia and sampling procedures strictly followed the “Guidelines on Ethical Treatment of Experimental Animals” (2006) No. 398 established by the Ministry of Science and Technology, China, and the “Regulation Regarding the Management and Treatment of Experimental Animals” (2008) No. 45 established by the Jiangsu Provincial People's Government. Six 30-day-old weaned piglets without antibodies against PCV2 and PRRSV were obtained from a commercial pig farm. To confirm further that the pigs were free of PCV2 and PRRSV infection, PCR or RT-PCR was performed to measure viral nucleic acid level. Pigs were euthanized by jugular injection with 10% mg/kg body weight of 3% sodium pentobarbital (Sinopharm Chemical Reagent, China). PAMs were collected according to the method described in previous studies [14,19]. Briefly, the lungs were removed immediately after the pigs were euthanized and washed three times with cold, sterile calcium and magnesium-free Dulbecco's phosphate-buffered saline (D-PBS) supplemented with 0.2% EDTA. The wash media were collected and poured through a layer of gauze into a sterile bottle to remove mucus. The collected liquid was then centrifuged at 300 × g at 4℃ for 10 min. Cell pellets were resuspended in RPMI-1640 containing 10% fetal bovine serum, 100 units/mL penicillin, and 100 µg/mL streptomycin at a density of 5 × 106 cells/mL. The viability of PAMs, as determined by trypan blue exclusion, was greater than 95%, and the purity of PAMs was more than 96%, based on flow cytometric detection of porcine CD169.
Small interfering RNA and transfection
We designed the siRNA to target myeloid differentiation primary response gene 88 (MyD88). The MyD88 siRNA and a non-specific control siRNA were chemically synthesized by Invitrogen (China). The siRNA sequences used were as follows: MyD88, 5'-AUGCCUGAGCAUUUUGAUGTT-3' (sense) and 5'-CAUCAAAAUGCUCAGGCAUTT-3' (antisense); and non-specific control siRNA, 5'-CUGCCCCAGCGAUAUCCAGTT-3' (sense) and 5'-CUGGAUAUCGCUGGGGCAGTT-3' (antisense). PAMs were transfected with 10 nM siRNA using Lipofectamine 2000 (Invitrogen, China) for 24 h and were then inoculated with PCV2 virus after washing twice with PBS buffer. The siRNA dose was optimized in pilot experiments, and no appreciable cellular toxicity was observed.
Experimental design
The PAMs were divided into four groups as follows: Group 1, inoculation with PCV2 virus at 1 × 106.5 TCID50; Group 2, inoculation with MyD88 siRNA and PCV2 virus at 1 × 106.5 TCID50; Group 3, inoculation with MyD88 siRNA alone; and Group 4, untreated and used as a negative control. PAMs from each group were collected at 6, 12, 24, and 48 h for further experiments.
Indirect immunofluorescence assay
Infected cells were washed with PBS, fixed with cold acetone/methanol (1/1 v/v) for 20 min and permeabilized with 3% TritonX-100 for 10 min in room temperature and then allowed to air-dry. The fixed cells were incubated for 1 h with polyclonal anti-PCV2 antibody (Veterinary Medical Research & Development, USA) or a mouse monoclonal antibody specific for p65 (Cell Signaling Technology, USA) at 37℃ and then washed with PBS. Next, PAMs were incubated with FITC-conjugated goat anti-swine IgG antibody (Sigma-Aldrich, USA) or TRITC-conjugated goat anti-mouse IgG antibody (Beijing Dingguo Changsheng Biotechnology, China). After washing with PBS, 5 µg/mL DAPI (Sigma-Aldrich) was used to counterstain the nuclei for 10 min. Finally, cells were washed and examined via fluorescence microscopy (Zeiss, Germany).
Measurement of IL-1β and IL-10 levels
Levels of IL-1β and IL-10 in cultured PAM supernatants were measured by using porcine IL-1β and IL-10 ELISA kits (both from R&D Systems, USA) according to the manufacturer's instructions.
Detection of NF-κB p65, p-IκB, and MyD88 by western blotting
Nuclear and cytoplasmic proteins were extracted from PAMs by using a NE-PER Nuclear and Cytoplasmic Extraction Reagent Kit (Pierce, USA) according to the manufacturer's instructions. Protein concentrations were determined by using a BCA Protein Assay Kit (Pierce). The quantities of p65, p-IκB, and MyD88 in cytoplasmic protein extracts were measured by western blotting as we described previously [7,16]. Monoclonal antibodies against NF-κB p65 (Enzo Life Sciences, USA), p-IκB (Enzo Life Sciences), MyD88 (Abcam, UK), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Abcam) or β–actin (Cell Signaling Technology) were used.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assays (EMSAs) were used to detect the DNA-binding activity of NF-κB. The assays were performed by using a commercial kit (Pierce) according to the manufacturer's instructions, as we described previously [7,16].
Quantitative real-time RT-PCR detection of TLRs
Total RNA was extracted from PAMs by using RNAiso Plus (Takara Bio, Japan). RNA samples were then reverse-transcribed into cDNA by using the PrimeScript RT Master Mix Perfect Real-Time Kit (Takara Bio). The cDNA products were amplified in a 20 µL reaction mixture containing SYBR Green Real-Time PCR Master Mix-Plus (Takara Bio). We used gene-specific primers for real-time RT-PCR that we designed and described previously [7]. Beta-actin was used as the housekeeping gene. Quantitative PCR was performed by using the Bio-Rad IQ5 qPCR thermocycler (Bio-Rad Laboratories, USA). Relative quantity of target gene mRNA = 2−ΔΔCt, where ΔΔCt = (Ct target mRNA − Ct β-actin mRNA)test group − (Ct target mRNA − Ct β-actin mRNA)control group.
Statistical analysis
Statistical analyses of experimental data were performed by using SPSS Statistics software (ver. 18.0, SPSS, USA). Results are presented as mean ± standard deviation values. One-way ANOVA was used to determine the significance of differences between groups. P values < 0.05 or < 0.01 were considered to represent statistically significant differences, and are represented by asterisks (* or **, respectively) on each figure.
Results
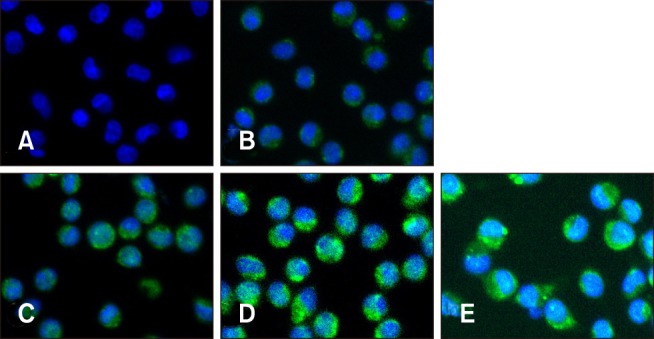
Confirmation of PCV2 infection in PAMs
The IFA was used to detect PCV2 infection after incubation of virus with PAMs for 6, 12, 24, and 48 h (Fig. 1). Green fluorescence was detected in PAMs incubated with PCV2, whereas no green fluorescence was observed in the control group, indicating that the PAMs had been infected with PCV2. Green fluorescence was observed in the cytoplasm of PAMs, while no fluorescence was observed in PAM nucleus. Moreover, it appeared that there was no difference in the number of green fluorescent PAMs among groups incubated for 6, 12, 24, or 48 h (at all times, more than 98% of PAMs exhibited green fluorescence), but the intensity of green fluorescence in PAMs noticeably increased with the increase in incubation times, indicating that the amount of PCV2 in PAMs can increase with an extended incubation time.
Fig. 1. Porcine circovirus type 2 (PCV2) infection in piglet porcine alveolar macrophages (PAMs). Control PAM cultures (A) and PAMs incubated with PCV2 for 6, 12, 24, and 48 h (B–E, respectively) were stained with anti-PCV2 antibody (green) and visualized by indirect immunofluorescence. DAPI was used to stain nuclei (blue).
Effect of PCV2 on IL-1β and IL-10 production
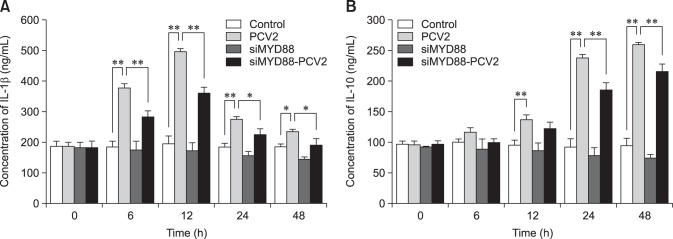
Levels of IL-1β and IL-10 in PAM culture supernatants were detected by using ELISA kits (Fig. 2). The levels of IL-1β in the PCV2-infected group at 6, 12, 24, and 48 h post-infection (hpi) were higher than those in the control group (p < 0.05). Moreover, the levels of IL-1β reached a peak at 12 hpi and then decreased in the PCV-2-infected groups. The levels of IL-10 in the PCV2-infected group at 12, 24, and 48 hpi were also significantly higher than those in the control group (p < 0.01), and the levels of IL-10 increased with the increase in incubation times in the PCV2-infected groups. These results suggest that PCV2 infection induced an increase in cytokine secretion by PAMs. The levels of IL-1β in the group co-incubated with MyD88 siRNA and PCV2 at 6, 12, 24, and 48 hpi were lower than those in the PCV2-infected group (p < 0.01), and the levels of IL-10 in the group co-incubated with MyD88 siRNA and PCV2 at 24 and 48 hpi were also lower than in the PCV2-infected group, indicating that MyD88 knockdown leads to the reduced secretion of IL-1β and IL-10.
Fig. 2. Changes in interleukin-1beta (IL-1β) and IL-10 levels after porcine circovirus type 2 (PCV2) infection. Supernatants from control cultures and porcine alveolar macrophages cultured with PCV2, MyD88 small interfering (si)RNA, or a combination of both were sampled after 6, 12, 24, and 48 h and were underwent enzyme-linked immunosorbent assay to measure the concentrations of IL-1β and IL-10; *p < 0.05, **p < 0.01.
Effect of PCV2 on MyD88 expression
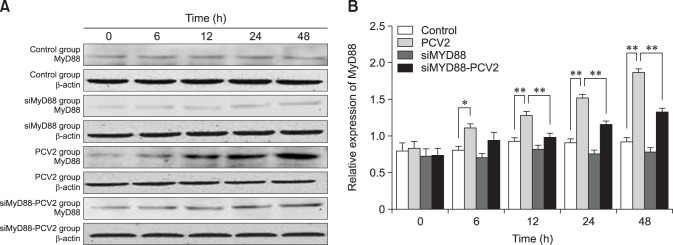
MyD88 is involved in orchestrating the inflammatory response provoked by bacterial infections. To determine the effect of PCV2 on MyD88 expression, the protein levels of MyD88 were assessed by western blotting (Fig. 3). The relative expression levels of MyD88 in the PCV2-infected group at 6, 12, 24, and 48 hpi were higher than those in the control group (p < 0.05), demonstrating that PCV2 activated MyD88 expression. The relative expression of MyD88 in the group co-incubated with PCV2 and MyD88 siRNA was lower than that in the PCV2-infected group (p < 0.05), indicating that the MyD88 siRNA used in this study can inhibit MyD88 expression.
Fig. 3. Changes in MyD88 protein expression after porcine circovirus type 2 (PCV2) infection. Western blotting was used to measure the expression of MyD88 protein in the cytoplasm at 6, 12, 24, and 48 h in control, PCV2, MyD88 siRNA, and PCV2 +MyD88 small interfering (si)RNA groups. Expression of β-actin was used as a positive control; *p < 0.05, **p < 0.01.
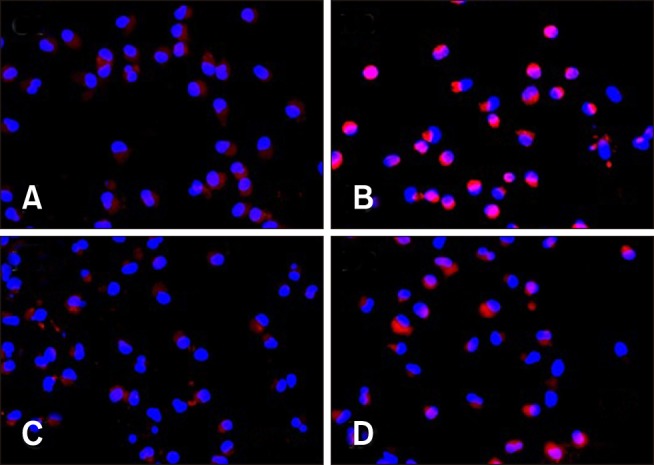
Effect of PCV2 on NF-κB p65 nuclear translocation
To explore whether NF-κB was activated after infection of PAMs with PCV2, IFA was used to detect the nuclear translocation of NF-κB p65 after incubation with virus (Fig. 4). After 24 h incubation, we observed that NF-κB p65 was predominately found in the cytoplasm in the control group (panel A in Fig. 4), whereas there was pronounced NF-κB p65 staining in the nucleus in the PCV2-infected group (panel B in Fig. 4), indicating that PCV2 infection promoted NF-κB p65 translocation to the nucleus. The distribution of NF-κB p65 staining in the nucleus of the group co-incubated with MyD88 siRNA and PCV2 (panel D in Fig. 4) decreased compared to that in the PCV2-infected group, suggesting that MyD88 siRNA inhibited NF-κB p65 translocation to the nucleus.
Fig. 4. Localization of nuclear factor kappa B p65 in the nucleus of porcine alveolar macrophages (PAMs) after porcine circovirus type 2 (PCV2) infection for 24 h. Control PAM cultures (A) and PAMs incubated with PCV2, MyD88 small interfering (si)RNA, and PCV2 + MyD88 siRNA (B–D, respectively) for 24 h were stained with a mouse monoclonal antibody against p65 (red) and visualized by indirect immunofluorescence. DAPI was used to stain nuclei (blue).
Effect of PCV2 on NF-κB p65 and p-IκB expression
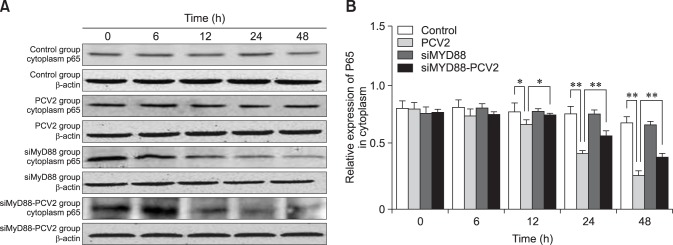
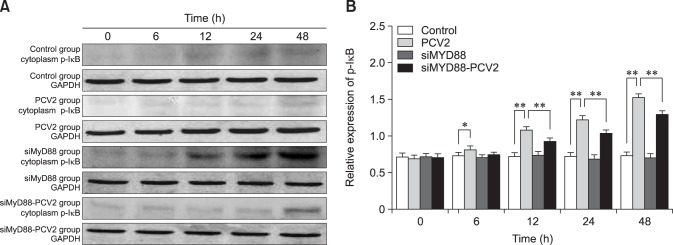
To confirm the effect of PCV2 on NF-κB activation, the protein expression levels of NF-κB p65 and p-IκB in the cytoplasm were detected by western blotting. The relative expression levels of p65 in the PCV2-infected group at 12, 24, and 48 hpi were lower than those in the control group (panel B in Fig. 5; p < 0.05), which is in accordance with the observation of nuclear translocation of NF-κB p65. The relative expression levels of p-IκB in the PCV2-infected group at 6, 12, 24, and 48 hpi were higher than those in the control group (panel B in Fig. 6; p < 0.05). These results indicate that the NF-κB signaling pathway was activated in PAMs after incubation with PCV2. The relative expression levels of p65 in the group co-incubated with MyD88 siRNA and PCV2 at 12, 24, and 48 hpi were higher than those in the PCV2-infected group (panel B in Fig. 5; p < 0.05), whereas the relative expression of p-IκB was reduced (panel B in Fig. 6; p < 0.01), suggesting that MyD88 siRNA inhibited activation of the NF-κB pathway.
Fig. 5. Changes in nuclear factor kappa B (NF-κB) p65 protein expression in the cytoplasm after porcine circovirus type 2 (PCV2) infection. Western blotting was used to measure cytoplasmic expression of NF-κB p65 at 6, 12, 24, and 48 h in the control, PCV2, MyD88 small interfering (si)RNA, and PCV2 + MyD88 siRNA groups. Expression of β-actin was used as a positive control; *p < 0.05, **p < 0.01.
Fig. 6. Changes in nuclear factor KappaB inhibitor alpha (p-IκBα) protein expression after porcine circovirus type 2 (PCV2) infection. Western blotting was used to measure the cytoplasmic expression of p-IκBα protein at 6, 12, 24, and 48 h in the control, PCV2, MyD88 small interfering (si)RNA, and PCV2 + MyD88 siRNA groups. Expression of GADPH was used as a positive control; *p < 0.05, **p < 0.01.
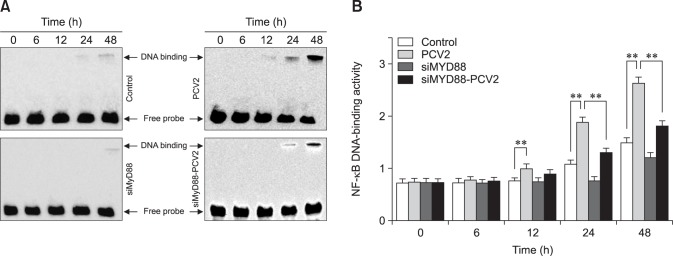
Effect of PCV2 on DNA-binding activity of NF-κB
To confirm the activation of NF-κB, the ability of NF-κB to bind to DNA was measured by performing EMSAs (Fig. 7). The binding activity of NF-κB in the PCV2-infected group were notably greater than that in the control group at 12, 24, and 48 hpi (p < 0.01), suggesting that NF-κB signaling was activated. In contrast, the binding activity of NF-κB in the group co-incubated with MyD88 siRNA and PCV2 was significantly lower than that in the PCV2-infected group at 24 and 48 hpi (p < 0.01), indicating that NF-κB activation was inhibited by MyD88 siRNA.
Fig. 7. Changes in nuclear factor kappa B (NF-κB) DNA-binding activity after porcine circovirus type 2 (PCV2) infection. An electrophoretic mobility shift assay was used to measure NF-κB DNA-binding activity after 6, 12, 24, and 48 h in the control, PCV2, MyD88 small interfering (si)RNA, and PVC2 + MyD88 siRNA groups; *p < 0.05, **p < 0.01.
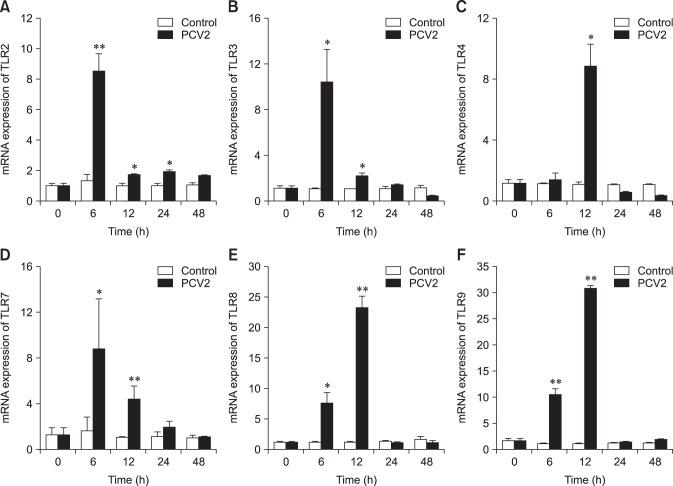
Effect of PCV2 on the mRNA expression levels of TLRs
To determine whether TLRs were involved in the infection of PAMs by PCV2, the mRNA expression levels of TLRs were measured by using real-time RT-PCR (Fig. 8). The mRNA expression levels of TLR2 in the PCV2-infected group at 6, 12, and 24 hpi were higher than those in the control group (p < 0.05). Similarly, the mRNA expression level of TLR4 in the PCV2-infected group at 12 hpi was higher than that in the control group (p < 0.05), and the mRNA expression levels of TLR-3, -7, -8, and -9 in the PCV2-infected group at 6 and 12 hpi were higher than those in the control group (p < 0.05). These results suggest that TLR expression levels are increased after PAM incubation with PCV2.
Fig. 8. Changes in mRNA expression levels of various Toll-like receptors (TLRs) after porcine circovirus type 2 (PCV2) infection. Real-time RT-PCR was used to assess the mRNA expression levels of TLR-2, -3, -4, -7, -8, and -9 in porcine alveolar macrophages incubated with PCV2 and controls after 6, 12, 24, and 48 h; *p 0.05, **p < 0.01.
Discussion
PAMs represent the first line of the pulmonary defense system against various pathogens. In this study, it was observed that PCV2 entered PAMs, and the abundance of PCV2 in PAMs increased with incubation time, indicating that PAMs were infected by PCV2 and their microbial killing activity was decreased, in agreement with a previous study [3]. Moreover, PCV2 was observed in the cytoplasm of PAMs rather than in the nucleus, demonstrating that PCV2 could not replicate in PAMs, but suggesting that PAMs might act as a carrier to spread PCV2 in infected pigs. Infection of PAMs may be one factor that allows PCV2 to establish persistent porcine infections.
Cytokines secreted by PAMs play an important role in immune responses and pulmonary defenses against foreign organisms. In this study, the levels of IL-1β and IL-10 increased after incubating PAMs with PCV2, indicating that PCV2 can produce a change in cytokine production. IL-1β is a proinflammatory cytokine that affects the differentiation of T cells and enables the transmigration of immunocompetent cells to infection sites. The increased levels of IL-1β may initially help inhibit pulmonary PCV2 infection, but excessive IL-1β secretion may also result in damage to the lung. It has been reported that increased levels of IL-1β may contribute to immunopathology in the lungs of PCV2-infected pigs [2]. The level of IL-1β in culture supernatants increased after PAMs were incubated with PCV2 for 6 and 12 h, indicating that PAMs release proinflammatory cytokines to resist the invasion of PCV2 at the beginning of a PCV2 infection. The level of IL-1β decreased after 24 and 48 h, demonstrating that the production of proinflammatory cytokines was disrupted, thus indicating that the virus might have created a suitable living environment for itself. IL-10 is an anti-inflammatory cytokine that has an inhibitory function on cell-mediated immune responses and antigen presentation. Increased levels of IL-10 may contribute to the immunosuppressive state, which can predispose the host to pulmonary infection by opportunistic pathogens. It has been reported that levels of IL-10 in the serum and lymphoid organs of PCV2-infected pigs were elevated [5,7,22]. The level of IL-10 gradually increased after incubation of PAMS with PCV2 for 24 and 48 h, indicating that the immunity activities of PAMs were impaired, and the abilities of PAMs to eliminate virus were reduced. Moreover, the levels of IL-1β decreased after 12 h of incubation, whereas levels of IL-10 increased with incubation time, suggesting the functions of the two cytokines are different. The increased production of IL-1β at the beginning of PCV2 infection might be related to restriction of PCV2 invasion, while the decreased production of IL-1β and increased production of IL-10 occurring later in the infection might related to immunosuppression; thus, the patterns of the two cytokines are different. Considering our results together with those of previous studies [11,21] indicates that IL-10 inhibits the expression of proinflammatory cytokines to terminate inflammatory responses, which might induce an immunosuppressive state in pigs.
TLRs are the major pattern recognition receptors (PRRs) that can induce cytokine production to resist a viral infection. All TLRs utilize the MyD88 adaptor to mediate downstream signaling, except TLR3, which relays signals via an adaptor molecule known as TRIF or Ticam-1 [10,24]. In this study, the expression of MyD88 in the PCV2-infected group was higher than that in the control group, indicating the MyD88 was activated in PAMs after incubation with PCV2. The MyD88 siRNA abrogated the changes in IL-1β and IL-10 levels, demonstrating that both genes were regulated by MyD88. MyD88 can lead to the induction of inflammatory cytokine genes by activating NF-κB, mitogen-activated protein kinases, and interferon regulatory factors [16]. NF-κB is a common transcription factor that controls the expression of cytokines, and previous studies have demonstrated that PCV2 infection can activate NF-κB signaling [7,23]. In this study, nuclear translocation of NF-κB p65, reduced cytoplasmic expression of p65, increased expression of pIκB, and increased DNA-binding activity of NF-κB in the PCV2 group, indicating that the NF-κB signaling pathway was activated. However, MyD88 siRNA reversed the changes in cytoplasmic p65 and pIκB expressions and the DNA-binding activity of NF-κB, demonstrating that the NF-γB signaling pathway was regulated by MyD88. Against this background, it can be inferred that PCV2 activates the MyD88-NF-κB signaling pathway, which then regulates the production of IL-1β and IL-10. TLRs are upstream receptors that signal through MyD88, and, to date, at least 10 porcine TLRs have been identified. In this study, the mRNA expression levels of TLR-3, -4, -7, -8, and -9 at 6 and 12 hpi, as well as the mRNA expression level of TLR-2 at 6, 12, and 24 hpi in the PCV2-infected group were higher than those in the control group, suggesting that a TLR signaling pathway was involved in PCV2 infection. However, the mRNA expression of TLRs decreased at 24 hpi, suggesting that TLR expression was related to the location of the TLR. TLR-2, -4, -5, and -6 are located at the plasma membrane and TLR-3, -7, -8, and -9 are at the endosome. When the virus first enters a PAM, the virus nucleic acid might be initially recognized by PRRs located at the plasma membrane; hence, the mRNA expressions of TLRs increased at 6 and 12 hpi. After PCV2 entry into PAMs, the membrane PRRs would no longer be activated, thus the mRNA expressions of TLRs would decrease. The details of this mechanism need to be investigated further.
In conclusion, we observed that PCV2 infection of PAMs induced increases in IL-1β and IL-10 levels, increased the expression of MyD88 and TLRs, and activated NF-κB signaling. Knockdown of MyD88 expression inhibited the NF-κB signaling pathway and abrogated the effects of PCV2 on cytokine production, resulting in decreased IL-1β and IL-10 levels. These results indicate that a TLR–MyD88–NF-κB signaling pathway contributed to the production of IL-1β and IL-10.
Acknowledgments
This study was supported by a grant (KYZ201629) from the Fundamental Research Funds for the Central Universities, by grants (31172292 and 31101786) from the Natural Science Foundation of China, and by a project funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, China.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Allan GM, Ellis JA. Porcine circoviruses: a review. J Vet Diagn Invest. 2000;12:3–14. doi: 10.1177/104063870001200102. [DOI] [PubMed] [Google Scholar]
- 2.Chae JS, Choi KS. Proinflammatory cytokine expression in the lung of pigs with porcine circovirus type 2-associated respiratory disease. Res Vet Sci. 2011;90:321–323. doi: 10.1016/j.rvsc.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Chang HW, Jeng CR, Lin TL, Liu JJ, Chiou MT, Tsai YC, Chia MY, Jan TR, Pang VF. Immunopathological effects of porcine circovirus type 2 (PCV2) on swine alvelolar macrophages by in vitro inoculation. Vet Immunol Immunopathol. 2006;110:207–219. doi: 10.1016/j.vetimm.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Chang HW, Pang VF, Chen LJ, Chia MY, Tsai YC, Jeng CR. Bacterial lipopolysaccharide induces porcine circovirus type 2 replication in swine alveolar macrophages. Vet Microbiol. 2006;115:311–319. doi: 10.1016/j.vetmic.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Darwich L, Pié S, Rovira A, Segalés J, Domingo M, Oswald IP, Mateu E. Cytokine mRNA expression profiles in lymphoid tissues of pigs naturally affected by postweaning multisytemic wasting syndrome. J Gen Virol. 2003;84:2117–2125. doi: 10.1099/vir.0.19124-0. [DOI] [PubMed] [Google Scholar]
- 6.Darwich L, Segalés J, Mateu E. Pathogenesis of postweaning multisystemic wasting syndrome caused by Porcine circovirus 2: an immune riddle. Arch Virol. 2004;149:857–874. doi: 10.1007/s00705-003-0280-9. [DOI] [PubMed] [Google Scholar]
- 7.Duan D, Zhang S, Li X, Guo H, Chen M, Zhang Y, Han J, Lv Y. Activation of the TLR/MyD88/NF-κB signal pathway contributes to changes in IL-4 and IL-12 production in piglet lymphocytes infected with porcine circovirus type 2 in vitro. PloS One. 2014;9:e97653. doi: 10.1371/journal.pone.0097653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis JA, Allan G, Krakowka S. Effect of coinfection with genogroup 1 porcine torque teno virus on porcine circovirus type 2-associated postweaning multisystemic wasting syndrome in gnotobiotic pigs. Am J Vet Res. 2008;69:1608–1614. doi: 10.2460/ajvr.69.12.1608. [DOI] [PubMed] [Google Scholar]
- 9.Gilpin DF, McCullough K, Meehan BM, McNeilly F, McNair I, Stevenson LS, Foster JC, Ellis JA, Krakowka S, Adair BM, Allan GM. In vitro studies on the infection and replication of porcine circovirus type 2 in cells of the porcine immune system. Vet Immunol Immunopathol. 2003;94:149–161. doi: 10.1016/s0165-2427(03)00087-4. [DOI] [PubMed] [Google Scholar]
- 10.Hertzog PJ, O'Neill LA, Hamilton JA. The interferon in TLR signaling: more than just antiviral. Trends Immunol. 2003;24:534–539. doi: 10.1016/j.it.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Kekarainen T, Montoya M, Mateu E, Segalés J. Porcine circovirus type 2-induced interleukin-10 modulates recall antigen responses. J Gen Virol. 2008;89:760–765. doi: 10.1099/vir.0.83354-0. [DOI] [PubMed] [Google Scholar]
- 12.Krakowka S, Ellis J, McNeilly F, Waldner C, Rings DM, Allan G. Mycoplasma hyopneumoniae bacterins and porcine circovirus type 2 (PCV2) infection: induction of postweaning multisystemic wasting syndrome (PMWS) in the gnotobiotic swine model of PCV2-associated disease. Can Vet J. 2007;48:716–724. [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Yu Q, Nie X, Guo X, Song Q, Li H. Effects of porcine circovirus type 2 on expression of mRNA associated with endogenous antigen processing and presentation in pulmonary alveolar macrophages and circulating T lymphocytes in piglets. Vet J. 2012;193:199–205. doi: 10.1016/j.tvjl.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Bai J, Lu Q, Zhang L, Jiang Z, Michal JJ, He Q, Jiang P. Two-dimensional liquid chromatography-tandem mass spectrometry coupled with isobaric tags for relative and absolute quantification (iTRAQ) labeling approach revealed first proteome profiles of pulmonary alveolar macrophages infected with porcine circovirus type 2. J Proteomics. 2013;79:72–86. doi: 10.1016/j.jprot.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Lv Y, Dai L, Han H, Zhang S. PCV2 induces apoptosis and modulates calcium homeostasis in piglet lymphocytes in vitro. Res Vet Sci. 2012;93:1525–1530. doi: 10.1016/j.rvsc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Lv Y, Zhang X, Sun Y, Zhang S. Activation of NF-κB contributes to production of pig-major acute protein and serum amyloid A in pigs experimentally infected with porcine circovirus type 2. Res Vet Sci. 2013;95:1235–1240. doi: 10.1016/j.rvsc.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Mandrioli L, Sarli G, Panarese S, Baldoni S, Marcato PS. Apoptosis and proliferative activity in lymph node reaction in postweaning multisystemic wasting syndrome (PMWS) Vet Immunol Immunopathol. 2004;97:25–37. doi: 10.1016/j.vetimm.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang VF, Lambert RJ, Felsburg PJ, Beasley VR, Buck WB, Haschek WM. Experimental T-2 toxicosis in swine following inhalation exposure: effects on pulmonary and systemic immunity, and morphological changes. Toxicol Pathol. 1987;15:308–319. doi: 10.1177/019262338701500309. [DOI] [PubMed] [Google Scholar]
- 20.Rovira A, Balasch M, Segalés J, García L, Plana-Durán J, Rosell C, Ellerbrok H, Mankertz A, Domingo M. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J Virol. 2002;76:3232–3239. doi: 10.1128/JVI.76.7.3232-3239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21:315–324. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Shi KC, Guo X, Ge XN, Liu Q, Yang HC. Cytokine mRNA expression profiles in peripheral blood mononuclear cells from piglets experimentally co-infected with porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Vet Microbiol. 2010;140:155–160. doi: 10.1016/j.vetmic.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Wei L, Kwang J, Wang J, Shi L, Yang B, Li Y, Liu J. Porcine circovirus type 2 induces the activation of nuclear factors kappa B by IκBα degradation. Virology. 2008;378:177–184. doi: 10.1016/j.virol.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi Q, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 25.Yu S, Opriessnig T, Kitikoon P, Nilubol D, Halbur PG, Thacker E. Porcine circovirus type 2 (PCV2) distribution and replication in tissues and immune cells in early infected pigs. Vet Immunol Immunopathol. 2007;115:261–272. doi: 10.1016/j.vetimm.2006.11.006. [DOI] [PubMed] [Google Scholar]