Abstract
To investigate the effects of molybdenum (Mo) and/or cadmium (Cd) on antioxidant function and the apoptosis-related genes in duck spleens. Sixty healthy 11-day-old ducks were randomly divided into six groups of 10 ducks (control, low Mo group, high Mo, Cd, low Mo + Cd, and high Mo + Cd groups). All were fed a basal diet containing low or high dietary doses of Mo and/or Cd. Relative spleen weight, antioxidant indices, apoptosis-related gene mRNA expression levels, and ultrastructural changes were evaluated after 120 days. The results showed that the relative spleen weight decreased significantly in the high Mo + Cd treatment group which compared with control group. Malondialdehyde levels increased and xanthine oxidase and catalase activities decreased in the Mo and/or Cd groups compared with levels in the control group. Bak-1 and Caspase-3 expressions were upregulated in the high Mo + Cd group, while Bcl-2 was downregulated. In addition, mitochondrial crest fracture, swelling, vacuolation, deformed nuclei, and karyopyknosis in both Mo + Cd treated groups were more severe than in the other groups. The results suggest that Mo and/or Cd can induce oxidative stress and apoptosis of spleen via effects on the mitochondrial intrinsic pathway. Moreover, the results indicate the two elements have a possible synergistic relationship.
Keywords: apoptosis, cadmium, duck, molybdenum, oxidative stress
Introduction
Molybdenum (Mo) is an essential element, with an important role in the biological functions of most organisms [1]. However, the bodies of humans and other animals can be damaged by a high dietary intake of Mo. With increasing development of the mining industry, there are concerns associated with the toxicity of Mo discharges to aquatic and terrestrial organisms [6]. Mo contamination of agricultural soils as a result of industrial discharge is a serious threat to human and animal health through bioaccumulation in the food chain. High dietary Mo intake can cause spleen damages and lymphocyte apoptosis, which can inhibit spleen development of spleen and impair immune function in young chickens [40]. Mo nanoparticles have induced intracellular reactive oxygen species (ROS) generation, mitochondrial membrane potential (MMP) changes, cell cycle arrest, and DNA damage [30]. Additionally, high levels of Mo can cause renal tubule degeneration, glomerular atrophy, nuclei deformation, and fracture in goat kidney [12].
Cadmium (Cd) is a highly toxic heavy metal that arises primarily from various industries and waste sources and is deemed an environmental pollutant [2]. Cd generates damages in humans via complex mechanisms involving interactions with other metals, induction of oxidative stress and apoptosis [24]. Following exposure to Cd, mitochondrial oxidative damage occurs via inhibition of Bcl-2 and Caspase-3 activation, leading to nuclear chromatin condensation, DNA fragmentation, and cell death [13]. Typical morphological changes associated with apoptosis were observed in chicken splenic lymphocytes treated with Cd, including membrane bleeds and overall cell shrinkages [17]. Oxidative stress and Ca2+ signaling pathways have been also suggested as having a significant role in Cd-induced apoptosis [35].
A large number of tungsten ore resources are distributed in Jiangxi Province of China, particularly in the southern areas. Large amounts of tailings that contain Mo and Cd have resulted through long-term accumulation from mining and screening processes; accumulations that can affect the livestock within a large area. Until now, many studies on Mo or Cd have been reported, but there are few studies into the toxicity of Mo + Cd co-exposure, especially in waterfowl. In this study, the toxicity of Mo and/or Cd on duck spleen was investigated by determining their effects on antioxidant indices, mRNA expression levels of apoptotic genes, and ultrastructural changes.
Materials and Methods
Animals and treatments
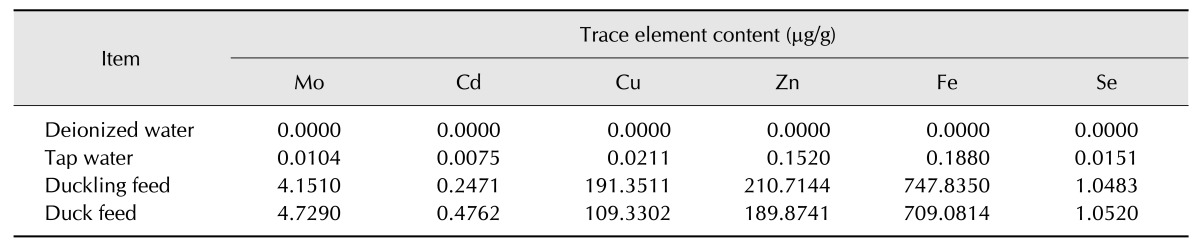
Ducks were treated according to the provisions of the National Institution of Health for experimental care and use of Laboratory animals (NIH Publication 8023, revised 1978). The duck model used to examine excessive exposure to Mo and/or Cd was as described in our previous publication [38]. Sixty healthy 11-day-old ducks were randomly divided into six groups of 10 ducks. The ducks in each group were fed a basal diet with different levels of Mo and/or Cd: Control group (0 mg/kg Mo, 0 mg/kg Cd), low dietary Mo group (LMo group, 15 mg/kg Mo), high dietary Mo group (HMo group, 100 mg/kg Mo), Cd group (4 mg/kg Cd), LMo + Cd group (15 mg/kg Mo, 4 mg/kg Cd) and HMo + Cd group (100 mg/kg Mo, 4 mg/kg Cd). Hexaammonium molybdate (Shanghai Chaoyan Biological Technology, China) and Cadmium sulfate (Shanghai Aiyan Biological Technology) were used as Mo and Cd sources, respectively. Ducklings were provided a duckling basal diet and a duck basal diet before and after being 21-days-old, respectively. The dietary treatments lasted for 120 days. The levels of Mo, Cd, Copper (Cu), Zinc (Zn), Iron (Fe), and selenium (Se) in the basal diet and water are shown in Table 1.
Table 1. Molybdenum (Mo), cadmium (Cd), copper (Cu), zinc (Zn), iron (Fe), and selenium (Se) content in the duck's basal diet and water.
Relative spleen weight
After being treated for 120 days, the duck body was weighed before being euthanized. During necropsy of each duck, the spleen was removed and weighed. Relative spleen weight was calculated according to the following formula [40]:
Acquisition and processing of samples
At the 120th day of dietary treatment, after the body weight of each duck was weighed, and a blood sample was collected via venous puncture of the wing and allowed to clot. Subsequently, serum was obtained by centrifugation at 1,100 × g for 10 min at 4℃ and stored at −20℃ until analyzed to determine antioxidant levels. The ducks were then euthanized via an overdose intravenous injection of sodium pentobarbital (Nembuta, l50 mg/kg; Abbot Labs, USA), and the spleen was immediately excised. Portions of each spleen were homogenized by using cold 0.9% NaCl solution, then centrifuged at 1,100 × g for 10 min at 4℃ by using a refrigerated centrifuge (Heal Force, China). The supernatant was collected and stored at −20℃ prior to antioxidant analysis. In addition, portions of the spleen samples were stored at −80℃ until used for apoptosis-related mRNA analysis. The remaining portion of each spleen specimen was routinely processed for ultrastructural examination.
Determination of trace elements
Trace element levels, including those for Cd, Mo, Cu, Fe, Zn, and Se, in dietary water and feed were measured by using an Agilent 240 Series AA atomic absorption spectrophotometer (Agilent, USA). All analyses were carried out according to the manufacturer's instructions.
Determination of antioxidant levels
Xanthine oxidase (XOD) can catalyze hypoxanthine to produce simultaneously xanthine and free radical superoxide anions, and when an electron acceptor and a color agent are present, an amaranth conjugate will be formed. Based on the absorbance at 530 nm, determined by a spectrophotometer (Nanjing Jiancheng Bioengineering Institute, China), XOD activity was calculated. The activity of catalase (CAT) on decomposing H2O2 can be immediately stopped by adding ammonium molybdate. The surplus H2O2 combines with ammonium molybdate to form a faint yellow clathrate. Based on absorbance at 405 nm, determined by a spectrophotometer (Nanjing Jiancheng Bioengineering Institute), the level of CAT activity was determined. Malondialdehyde (MDA) can condense with thiobarbituric acid (TBA) to form a red compound. Base on variations in the color, absorbance at 532 nm was determined by spectrophotometer (Nanjing Jiancheng Bioengineering Institute), and the MDA content determined.
Ultrastructural examination
Transmission electron microscopy (TEM) was carried out according to a previously reported protocol [29]. Spleen samples obtained at day 120 were processed and observed carefully via TEM; in addition, photographs were taken.
RNA Isolation and primer designing
Total RNA was purified from spleen samples by using Trizol reagents (Takara Bio, China) according to the manufacturer's instructions and was then reverse-transcribed into cDNA in accordance with instructions for the PrimeScript RT reagent Kit with gDNA Eraser (Takara Bio). The reverse transcription reaction was conducted in a 20 µL mixture containing 2 µL of 5× DNA eraser buffer, 1 µL of gDNA eraser, 1 µL of total RNA, and 6 µL of RNase-free dH2O. The mixture was incubated for 2 min at 42℃. Next, 4 µL of 5× prime script buffer 2, 1 µL of prime script RT enzyme mix I, 1 µL of RT primer mix, and 5 µL of RNase-free dH2O were added to the reaction solution, and the reaction was run at 37℃ for 15 min, 85℃ for 5 seconds, and 4℃ for 10 min. The cDNA samples were stored at −20℃ prior to use in TaqMan RT-PCR.
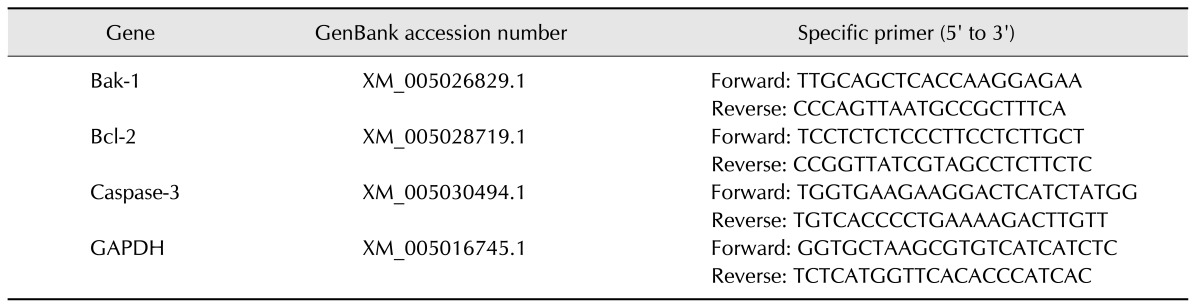
The primers for the amplification of genes Bak-1, Bcl-2, Caspase-3, and GAPDH were designed by using Primer Express 3.0 software and were synthesized by Invitrogen (China). Duck GAPDH was employed as the housekeeping gene. The primer sequences and GenBank accession numbers are presented in Table 2.
Table 2. Gene-specific primers used in the study.
GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
TaqMan real-time quantitative PCR
Gene expression levels were measured by performing real-time quantitative polymerase chain reactions (RT-PCR). The PCR profiles were as follows: Stage 1, 1 cycle at 50℃ for 2 min; Stage 2, 1 cycle at 95℃ for 5 min; Stage 3, 40 cycles at 95℃ for 15 seconds; and Stage 4, 1 cycle at 60℃ for 1 min. Following RT-PCR completion, melt curve analyses were performed for all genes. All reactions were carried out by using the ABI Prism 7900 real-time PCR system (Applied Biosystems, China).
Statistical analysis
Statistical analysis was performed by using GraphPad Prism 5 (GraphPad, USA) and SPSS for Windows (ver. 13, SPSS, USA). When a significant value was obtained by one-way analysis of variance, further analysis was carried out. Duncan's multiple range tests were used to detect statistical significance (p < 0.05) between treatment groups. All values are expressed as means ± SEM.
Results
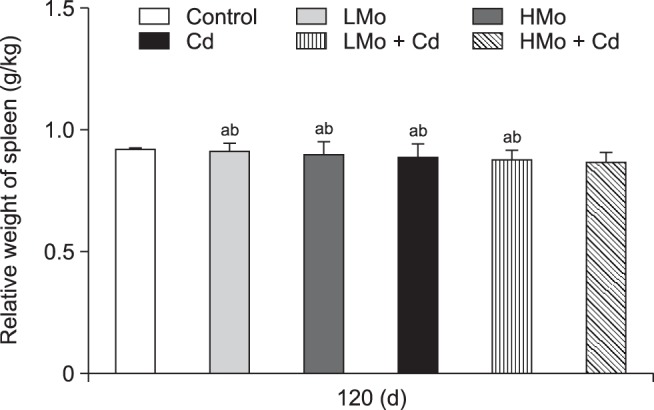
Changes in relative spleen weight
As shown in Fig. 1, the overall trend was a decrease in relative spleen weight from that in the control group in all five treatment groups. The relative spleen weight was significantly lower in the HMo + Cd group than in control group at 120 days (p < 0.05).
Fig. 1. Changes in the relative weight of duck spleen. Different lowercase letters are significantly different between groups (p < 0.05), and common lowercase or uppercase letters are not significantly different between groups (p > 0.05). Each value represents the mean ± SEM.
Serum MDA levels and XOD and CAT activities
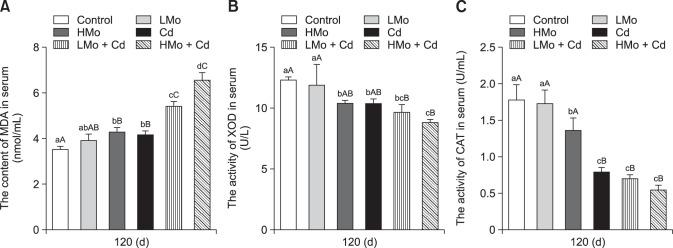
The effects of dietary Mo and/or Cd treatments on serum MDA levels as well as XOD and CAT activities are shown in panels A–C in Fig. 2, respectively. The MDA levels of the HMo and Cd groups were significantly higher than that in the control group (p < 0.01). The MDA levels in the LMo + Cd and HMo + Cd groups were significantly higher than in the other groups (p < 0.01). In addition, MDA concentration was higher in the HMo + Cd group than in the LMo + Cd group (p < 0.05). Decreased XOD activity was observed in the HMo and Cd groups compared to that in the control group (p < 0.05). The XOD activity levels in the LMo + Cd and HMo + Cd groups were significantly lower than those in the control and LMo groups (p < 0.01). In addition, the activity in the LMo group was higher than those in the HMo and Cd groups (p < 0.05). The CAT activity was lower in the HMo group than in the control and LMo groups (p < 0.05), and CAT activity level of the Cd group was significantly lower than that in the control group (p < 0.01). The CAT activity levels in the LMo + Cd and HMo + Cd groups were markedly lower than those in the control, LMo, and HMo groups (p < 0.01).
Fig. 2. Antioxidant index levels in duck serum. (A) Malondialdehyde (MDA) levels. (B) Xanthine oxidase (XOD) activities. (C) Catalase (CAT) activities. Different lowercase letters are significantly different between groups (p < 0.05), different uppercase letters are highly significantly different between groups (p < 0.01), and common lowercase or uppercase letters are not significantly different between groups (p > 0.05). Each value represents the mean ± SEM.
Spleen MDA levels and XOD and CAT activities
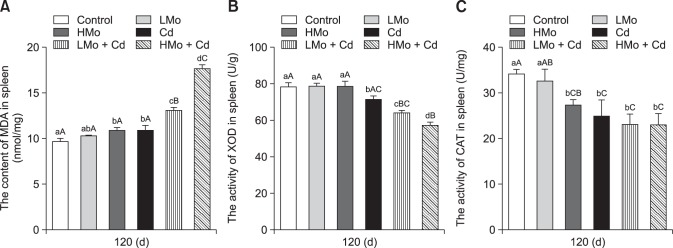
As shown in Fig. 3, MDA level was higher in the HMo and Cd groups than in the control group (p <0.05). MDA levels in the LMo + Cd and HMo + Cd groups were significantly higher than those in the other groups (p < 0.01), and the MDA concentration in the HMo + Cd group was significantly higher than that in the LMo + Cd group (p < 0.01). The XOD activity level was significantly lower in the LMo + Cd group than in the control, LMo, and HMo groups (p < 0.01), and the XOD activity of the HMo + Cd group was significantly lower than those in the other groups (p < 0.01). In addition, the XOD activity in the HMo + Cd group was lower than that in the LMo + Cd group (p < 0.05). The CAT activity levels in the HMo, Cd, LMo + Cd, and HMo + Cd groups were significantly lower than that in the control group (p < 0.01). The CAT activities of the Cd, LMo + Cd, and HMo + Cd groups were not significantly different (p > 0.05).
Fig. 3. Antioxidant index values in duck spleen. (A) Malondialdehyde (MDA) levels. (B) Xanthine oxidase (XOD) activities. (C) Catalase (CAT) activities. Different lowercase letters are significantly different between groups (p < 0.05), different uppercase letters are highly significantly different between groups (p < 0.01), and common lowercase or uppercase letters are not significantly different between groups (p > 0.05). Each value represents the mean ± SEM.
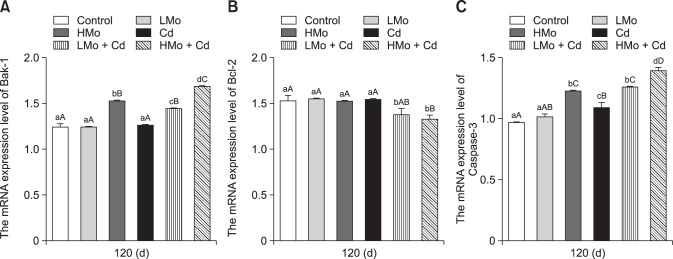
Expressions of Bak-1, Bcl-2, and Caspase-3 mRNA in spleen tissue
As shown in Fig. 4, the mRNA expression level of Bak-1 was significantly higher in the HMo group than in the control and LMo groups (p < 0.01). Moreover, Bak-1 mRNA expression was significantly higher in the LMo + Cd group than in the control, LMo, and Cd groups (p < 0.01). Bak-1 expression in HMo + Cd group was significantly higher than those in the control, HMo, and Cd groups (p < 0.01). In addition, mRNA expression of Bak-1 in the HMo + Cd group was significantly upregulated compared to that in the LMo + Cd group (p < 0.01). The mRNA expression level of Bcl-2 was lower in the LMo + Cd group than in the control, LMo, HMo, and Cd groups (p < 0.05). Moreover, Bcl-2 expression was significantly lower in the HMo + Cd group than in the control, LMo, HMo and Cd groups (p < 0.01). The mRNA expression level of Caspase-3 was significantly higher in the HMo group than in the control and LMo groups (p < 0.01) and significantly higher in the LMo + Cd group than in the control, LMo, and Cd groups (p < 0.01). Caspase-3 expression was significantly upregulated in the HMo + Cd group compared to the expression levels in the other groups (p < 0.01).
Fig. 4. Effects of molybdenum (Mo)- and/or cadmium (Cd)-induced changes in the mRNA levels of apoptosis-related genes. (A) Bak-1. (B) Bcl-2. (C) Caspase-3. Different lowercase letters are significantly different between groups (p < 0.05), different uppercase letters are highly significantly different between groups (p < 0.01), and common lowercase or uppercase letters are not significantly different between groups (p > 0.05). Each value represents the mean ± SEM.
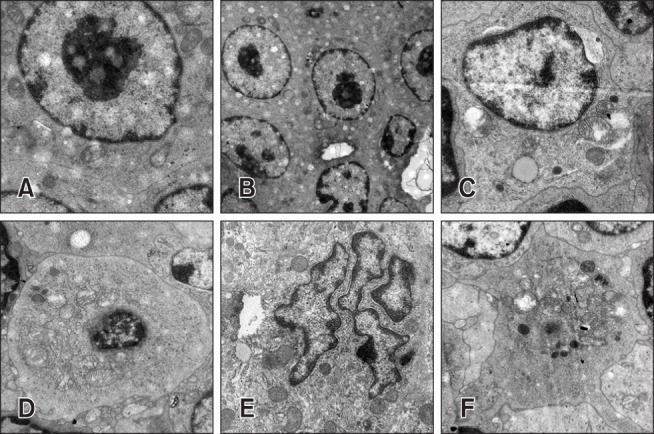
Ultrastructural changes to spleen cells
As shown in Fig. 5, TEM revealed normal spleen cells in the control group (panel A in Fig. 5). Panels C and D in Fig. 5 show ultrastructural changes of spleen cells, including mitochondrial crest fracture and vacuolation, in the HMo and Cd groups. The spleen cells in the LMo + Cd group exhibited severe nuclear deformation and chromatin marginalization (panel E in Fig. 5), and the HMo + Cd group showed typical characteristics of apoptotic karyopyknosis, mitochondrial crest fracture, and vacuolation (panel F in Fig. 5).
Fig. 5. Representative images from transmission electron microscopy of spleen tissue. (A) Normal spleen cell in control group. (B) No significant changes in spleen cells in the low dietary molybdenum (LMo) group. (C) Mitochondrial crest fracture and vacuolation of spleen cells in the high dietary molybdenum (HMo) group. (D) Vacuolation and crest fracture of spleen cells in the cadmium (Cd) group. (E) Nuclear deformation and chromatin marginalization of spleen cells in the LMo + Cd group. (F) Typical characteristics of apoptosis, including karyopyknosis, mitochondrial crest fracture, and vacuolation in the HMo + Cd group. 2,950× (A, C–E), 1,200× (B), 2,200× (F).
Discussion
The spleen is an organ involved in immune reactions against environmental stresses, and relative spleen weight is one of the indices used to estimate spleen injury. The results of the present study indicated that dietary Mo and/or Cd decreased the relative spleen weight in ducks. As a stressor, heavy metals can upset the balance of the oxidant/antioxidant system by affecting the regulation of enzymatic oxidation, protein oxidation, and lipid peroxidation activities, and cause tissue damage [28]. Liu et al. [20] reported that Mn exposure caused oxidative damage to the immune system of birds by altering the antioxidant defense enzyme systems and lipid peroxidation. CAT and superoxide dismutase (SOD) are active in antioxidant defense systems, and CAT can catalyze the decomposition of H2O2 to H2O [21]. XOD participates in a great part of free radical generation and catalyzes the conversion of hypoxanthine to xanthine, uric acid, and superoxide [27]. MDA is an oxidized lipid metabolite and can be used to measure the level of oxidative stress in an organism [9]. Cd is a pollutant that has caused poisoning in humans in many regions of the world [26], and it takes several weeks to eliminate Cd via kidney. Previous studies have connected Cd with oxidative stress because Cd is able to alter the antioxidant defense system [16,37]. Some studies have reported that Cd accumulation in the body results in a decrease in SOD and CAT activities and an increase in MDA and XOD levels in both plasma and tissues [7,41]. In the body, a high dosage of Mo can result in decreased activity of antioxidant enzymes and a decline in the antioxidant capacity of the organism [23]. A few studies in rabbits have shown that high Mo accumulation generates free radical processes or reactive intermediates, resulting in alteration of the levels of MDA and GSH-Px [3,14]. In the present study, a duck-based model for exposure to Mo and/or Cd was created. Mo and Cd co-induction increased MDA levels and decreased XOD and CAT activities in serum and spleen tissue, changes that were more obvious than those from single Cd or Mo treatments but similar to our previous findings [38]. This study demonstrated that both Mo and Cd accumulate in duck body during long-term exposure to the metals, and such accumulations induce oxidative stress and upset the balance of the antioxidant defense system resulting in a decrease in antioxidant function. In this study, Mo and Cd induced variable degrees of ultrastructural changes including karyopyknosis, karyotype irregular, chromatin marginalization, mitochondrial swelling, shrinkage, vacuolization, mitochondrial crest fracture, and vacuolation in duck spleen. In addition, the results indicated that Mo and Cd co-exposure may have a synergistic effect that can lead to more severe oxidative stress and damage to spleen tissue morphology. The decrease in MMP induced by Mo confirmed the resultant impairment of the mitochondrial membrane [30]. High Mo accumulation in the body decreases B-cell and T-cell population, but increases spleen cells apoptosis, leading to an impaired immune function [40]. Recent studies have indicated that dysfunctional mitochondria have a key role in the formation of excess ROS, and mitochondria are considered intracellular targets for cadmium. When mitochondria become dysfunctional through long-term exposure to environmental toxins, such as Cd, they produce less cell energy and more ROS, and the imbalance between the ROS and the natural antioxidants results in oxidative stress [5,6,11]. Our results also suggested that Mo and Cd exposures induce oxidative stress by accelerating lipid peroxidation and inhibiting antioxidant enzyme activities in duck spleen.
Apoptosis maintains an appropriate cell number by balancing cell division and cell death in the body [18]. It is a strictly controlled by several genes. There are two major pathways leading to apoptosis: Extrinsic and intrinsic. The former involves death receptors and the latter involves mitochondria. The Bcl-2 family of proteins has a crucial role in intracellular apoptosis signal transduction [31]. While Bcl-2 is an anti-apoptotic family member, Bak-1 is a pro-apoptotic protein mainly expressed in the mitochondrial membrane [8,34]. Caspases, a family of cysteine proteases, are reported to be an integral part of the apoptosis pathway [33]. Caspase-3 has a critical role in mediating apoptosis in both death receptor and mitochondrial pathways [36]. It was previously reported that Cd could trigger liver cell apoptosis through activation of Caspase-3A [10], and Cd can induce apoptosis of chicken splenic lymphocytes by increasing mRNA levels of Bak, Caspase-3, and Caspase-9 and by decreasing Bcl-2 [19]. Additionally, high levels of Mo can result in unregulated expression of Bax, CytC, and Caspase-3, whereas it downregulates Bcl-2 in goat kidney [12]. Mo administration has induced an increase in Bax expression and a decrease in Bcl-2 expression in chicken kidney cells [39]. In this study, Mo and/or Cd treatment resulted in downregulation of Bcl-2 mRNA expression but upregulation of Bak-1 and Caspase-3 mRNA expression in duck spleen tissue. Moreover, the combination of Mo and Cd was more powerful in regulating the mRNA expressions than single Mo or Cd treatments. Spleen is an important immune organ and is sensitive to environmental stress or stimuli. It has been suggested that splenic cells appear more susceptible than thymus cells to the adverse effects of Cd, and exposure to Cd can induce potentiation of oxidative stress followed by activation of the mitochondrial caspase-dependent apoptotic pathway [25]. That mechanism might indicate that Mo and Cd are sources of stress, stimulating the organism to activate apoptosis pathways, leading to the occurrence of apoptosis. Mo and/or Cd induce mitochondrial injury including lipid peroxidation, resulting in the accumulation of free radicals. Bax and Bak are essential components of the mitochondrial apoptosis pathway that can increase endoplasmic reticulum (ER) Ca2+ load and release and inhibit ER-localized Bcl-2. Bcl-2 can prevent entry of cytoplasmic Ca2+ into the mitochondria, thereby regulating the release of mitochondrial CytC and blocking caspase-mediated apoptosis. When CytC is released into the cytoplasm, caspase activation is initiated, leading to cell structure damage [15,22,32] and may result in spleen damage. In this study, co-treatment of Mo and Cd produced a synergistic effect on spleen toxicity. However, further research is needed to provide specific details of the mechanism involved in the synergistic toxic effects of Mo and/or Cd.
The results of this study have two important aspects. First, variable doses of Mo and/or Cd produced a decrease in relative spleen weight, significant expression of apoptosis-related genes, alteration of antioxidant indices, and ultrastructural changes in spleen tissue. Second, co-exposure to Mo and Cd produced a synergistic effect on spleen toxicity. A possible mechanism involves Mo and Cd stimulation of the organism to produce oxidative stress and activate apoptosis pathways, leading to apoptosis.
Acknowledgments
This work was supported by the National Science Foundation of China (31260625) and the Technology R&D Program of Jiangxi Province (20122BBF60078). All authors thank all members of the team for their help in the experimental process in the Clinical Veterinary Medicine Laboratory in the College of Animal Science and Technology, Jiangxi Agricultural University. The authors thank Bunlue Kornmatitsuk for correcting some flaws in the manuscript.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Barceloux DG. Molybdenum. J Toxicol Clin Toxicol. 1999;37:231–237. doi: 10.1081/clt-100102422. [DOI] [PubMed] [Google Scholar]
- 2.Bekheet SHM, Awadalla EA, Salman MM, Hassan MK. Bradykinin potentiating factor isolated from Buthus occitanus has a protective effect against cadmium-induced rat liver and kidney damage. Tissue Cell. 2011;43:337–343. doi: 10.1016/j.tice.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Bersényi A, Berta E, Kádár I, Glávits R, Szilágyi M, Fekete SG. Effects of high dietary molybdenum in rabbits. Acta Vet Hung. 2008;56:41–55. doi: 10.1556/AVet.56.2008.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Cannino G, Ferruggia E, Luparello C, Rinaldi AM. Cadmium and mitochondria. Mitochondrion. 2009;9:377–384. doi: 10.1016/j.mito.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K. Cadmium stress: an oxidative challenge. Biometals. 2010;23:927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- 6.Davies TD, Pickard J, Hall KJ. Acute molybdenum toxicity to rainbow trout and other fish. J Environ Eng Sci. 2005;4:481–485. [Google Scholar]
- 7.Dwivedi VK, Bhatanagar A, Chaudhary M. Protective role of ceftriaxone plus sulbactam with VRP1034 on oxidative stress, hematological and enzymatic parameters in cadmium toxicity induced rat model. Interdiscip Toxicol. 2012;5:192–200. doi: 10.2478/v10102-012-0032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37:719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 9.Fu J, Liu CP, Zhang ZW, Xing MW, Xu SW. Influence of inflammatory pathway markers on oxidative stress induced by cold stress in intestine of quails. Res Vet Sci. 2013;95:495–501. doi: 10.1016/j.rvsc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Gao D, Xu Z, Qiao P, Liu S, Zhang L, He P, Zhang X, Wang Y, Min W. Cadmium induces liver cell apoptosis through caspase-3A activation in purse red common carp (Cyprinus carpio) PLoS One. 2013;8:e83423. doi: 10.1371/journal.pone.0083423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gobe G, Crane D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol Lett. 2010;198:49–55. doi: 10.1016/j.toxlet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Gu X, Ali T, Chen R, Hu G, Zhuang Y, Luo J, Cao H, Han B. In vivo studies of molybdenum-induced apoptosis in kidney cells of caprine. Biol Trace Elem Res. 2015;165:51–58. doi: 10.1007/s12011-015-0238-2. [DOI] [PubMed] [Google Scholar]
- 13.Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998;19:529–535. [PubMed] [Google Scholar]
- 14.Kiersztan A, Winiarska K, Drozak J, Przedlacka M, Wegrzynowicz M, Fraczyk T, Bryla J. Differential effects of vanadium, tungsten and molybdenum on inhibition of glucose formation in renal tubules and hepatocytes of control and diabetic rabbits: beneficial action of melatonin and N-acetylcysteine. Mol Cell Biochem. 2004;261:9–21. doi: 10.1023/b:mcbi.0000028733.88718.c3. [DOI] [PubMed] [Google Scholar]
- 15.Larsen BD, Rampalli S, Burns LE, Brunette S, Dilworth FJ, Megeney LA. Caspase 3/caspase-activated DNase promote cell differentiation by inducing DNA strand breaks. Proc Natl Acad Sci U S A. 2010;107:4230–4235. doi: 10.1073/pnas.0913089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li JL, Jiang CY, Li S, Xu SW. Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium. Ecotoxicol Environ Saf. 2013;96:103–109. doi: 10.1016/j.ecoenv.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Li JL, Li HX, Li S, Tang ZX, Xu SW, Wang XL. Oxidative stress-mediated cytotoxicity of cadmium in chicken splenic lymphocytes. Polish J Environ Stud. 2010;19:947–956. [Google Scholar]
- 18.Liu J, Yao Y, Ding H, Chen R. Oxymatrine triggers apoptosis by regulating Bcl-2 family proteins and activating caspase-3/caspase-9 pathway in human leukemia HL-60 cells. Tumour Biol. 2014;35:5409–5415. doi: 10.1007/s13277-014-1705-7. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Xu FP, Yang ZJ, Li M, Min YH, Li S. Cadmium-induced injury and the ameliorative effects of selenium on chicken splenic lymphocytes: mechanisms of oxidative stress and apoptosis. Biol Trace Elem Res. 2014;160:340–351. doi: 10.1007/s12011-014-0070-0. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Li Z, Tie F, Liu N, Zhang Z, Xu S. Effects of manganese-toxicity on immune-related organs of cocks. Chemosphere. 2013;90:2085–2100. doi: 10.1016/j.chemosphere.2012.10.072. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Zhang L, Guan H, Zhang Z, Xu S. Effects of oxidative stress on apoptosis in manganese-induced testicular toxicity in cocks. Food Chem Toxicol. 2013;60:168–176. doi: 10.1016/j.fct.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 22.Llambi F, Moldoveanu T, Tait SWG, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann Neurol. 2005;58:730–735. doi: 10.1002/ana.20629. [DOI] [PubMed] [Google Scholar]
- 24.Nemmiche S, Chabane-Sari D, Kadri M, Guiraud P. Cadmium chloride-induced oxidative stress and DNA damage in the human Jurkat T cell line is not linked to intracellular trace elements depletion. Toxicology In Vitro. 2011;25:191–198. doi: 10.1016/j.tiv.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Pathak N, Khandelwal S. Role of oxidative stress and apoptosis in cadmium induced thymic atrophy and splenomegaly in mice. Toxicol Lett. 2007;169:95–108. doi: 10.1016/j.toxlet.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Prozialeck WC, Edwards JR. Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. J Pharmacol Exp Ther. 2012;343:2–12. doi: 10.1124/jpet.110.166769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sagor MAT, Tabassum N, Potol MA, Alam MA. Xanthine oxidase inhibitor, allopurinol, prevented oxidative stress, fibrosis, and myocardial damage in isoproterenol induced aged rats. Oxid Med Cell Longev. 2015;2015:478039. doi: 10.1155/2015/478039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahin E, Gümüşlü S. Cold-stress-induced modulation of antioxidant defence: role of stressed conditions in tissue injury followed by protein oxidation and lipid peroxidation. Int J Biometeorol. 2004;48:165–171. doi: 10.1007/s00484-004-0205-7. [DOI] [PubMed] [Google Scholar]
- 29.Shao JJ, Yao HD, Zhang ZW, Li S, Xu SW. The disruption of mitochondrial metabolism and ion homeostasis in chicken hearts exposed to manganese. Toxicol Lett. 2012;214:99–108. doi: 10.1016/j.toxlet.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui MA, Saquib Q, Ahamed M, Farshori NN, Ahmad J, Wahab R, Khan ST, Alhadlaq HA, Musarrat J, Al-Khedhairy AA, Pant AB. Molybdenum nanoparticles-induced cytotoxicity, oxidative stress, G2/M arrest, and DNA damage in mouse skin fibroblast cells (L929) Colloids Surf B Biointerfaces. 2015;125:73–81. doi: 10.1016/j.colsurfb.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 32.Szegezdi E, MacDonald DC, Ni Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296:C941–C953. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- 33.Tayarani-Najaran Z, Mousavi SH, Vahdati-Mashhadian N, Emami SA, Parsaee H. Scutellaria litwinowii induces apoptosis through both extrinsic and intrinsic apoptotic pathways in human promyelocytic leukemia cells. Nutr Cancer. 2012;64:80–88. doi: 10.1080/01635581.2012.630162. [DOI] [PubMed] [Google Scholar]
- 34.Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Zhu H, Liu X, Liu Z. Oxidative stress and Ca2+ signals involved on cadmium-induced apoptosis in rat hepatocyte. Biol Trace Elem Res. 2014;161:180–189. doi: 10.1007/s12011-014-0105-6. [DOI] [PubMed] [Google Scholar]
- 36.Wang JY, Luo ZG. Non-apoptotic role of caspase-3 in synapse refinement. Neurosci Bull. 2014;30:667–670. doi: 10.1007/s12264-014-1454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Fang J, Leonard SS, Rao KM. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med. 2004;36:1434–1443. doi: 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Xia B, Cao H, Luo J, Liu P, Guo X, Hu G, Zhang C. The co-induced effects of molybdenum and cadmium on antioxidants and heat shock proteins in duck kidneys. Biol Trace Elem Res. 2015;168:261–268. doi: 10.1007/s12011-015-0348-x. [DOI] [PubMed] [Google Scholar]
- 39.Xiao J, Cui HM, Yang F, Peng X, Cui Y. Effect of dietary high molybdenum on the cell cycle and apoptosis of kidney in broilers. Biol Trace Elem Res. 2011;142:523–531. doi: 10.1007/s12011-010-8772-4. [DOI] [PubMed] [Google Scholar]
- 40.Yang F, Cui H, Xiao J, Peng X, Deng J, Zuo Z. Increased apoptotic lymphocyte population in the spleen of young chickens fed on diets high in molybdenum. Biol Trace Elem Res. 2011;140:308–316. doi: 10.1007/s12011-010-8697-y. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, He Z, Wen L, Wu J, Yuan L, Lu Y, Guo C, Zhu L, Deng S, Yuan H. Cadmium suppresses the proliferation of piglet Sertoli cells and causes their DNA damage, cell apoptosis and aberrant ultrastructure. Reprod Biol Endocrinol. 2010;8:97. doi: 10.1186/1477-7827-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]