Abstract
Background
The aim of this study was to investigate the glucose-lowering efficacy of antidiabetic treatments in patients with type 2 diabetes mellitus (T2DM) uncontrolled by sulfonylurea plus metformin.
Methods
This open-label, multicenter, prospective, observational study was conducted in 144 centers in Korea, from June 2008 to July 2010, and included patients with T2DM who had received sulfonylurea and metformin for at least 3 months and had levels of glycosylated hemoglobin (HbA1c) >7.0% in the last month. Data of clinical and biochemical characteristics were collected at baseline and 6 months after treatment. The treatment option was decided at the physician's discretion. Subjects were classified into the following three groups: intensifying oral hypoglycemic agents (group A), adding basal insulin (group B), or starting intensified insulin therapy (group C).
Results
Of 2,995 patients enrolled, 2,901 patients were evaluated, and 504 (17.4%), 2,316 (79.8%), and 81 patients (2.8%) were classified into groups A, B, and C, respectively. Subjects in group C showed relatively higher baseline levels of HbA1c and longer duration of diabetes. The mean decrease in HbA1c level was higher in the insulin treated groups (−0.9%±1.3%, −1.6%±1.3%, and −2.4%±2.3% in groups A, B, and C, respectively, P=0.042). The proportion of patients who achieved target HbA1c <7.0% was comparable among the groups; however, intensified insulin therapy seemed to be the most effective in achieving the target HbA1c of 6.5%.
Conclusion
These findings suggest that insulin-based therapy will be an important option in the improved management of Korean patients with T2DM whose glycemic control is not sufficient with sulfonylurea and metformin.
Keywords: Diabetes mellitus, type 2; Insulin therapy; Oral hypoglycemic agent
INTRODUCTION
Globally, an estimated 382 million people have diabetes, a number that is expected to rise to 392 million within a generation [1]. In Korea, more than 3 million people have diabetes; the number is increasing rapidly and is expected to reach 6 million by 2050 [2]. Rapid economic development, aging populations, and Westernized lifestyle are factors that contribute to the rise of diabetes in this area [3]. Despite the growing diabetes epidemic, the current treatment of diabetes is not optimal. Only 43.4% of patients with diabetes have been reported to achieve a glycosylated hemoglobin (HbA1c) <7.0% [4], which is similar to data from other Asian countries [5]. In fact, hyperglycemia generally worsens over time primarily due to the progression of β-cell dysfunction [6]. Therefore, treatment strategies are needed to overcome this lack of treatment efficacy.
The current treatment algorithm for type 2 diabetes mellitus (T2DM) recommends initial therapy with lifestyle modifications and metformin administration [7,8]. The guideline recommends the combination of two or more treatment options if a previous single or combined regimen fails to achieve glycemic goals [7,8]. Insulin therapy is recommended as the initial treatment if initial HbA1c >9.0% or 10.0% with hyperglycemic symptoms or after metformin failure [9]. However, physicians usually start insulin treatment after two or more oral hypoglycemic agents (OHA) and their combinations have failed [10,11]. The main reason for delayed insulin use is that it is an injectable drug that patients often refuse, and it can induce hypoglycemia and weight gain more than other OHAs [12]. However, early insulin therapy could be beneficial in considering the prevention of β-cell dysfunction [13].
The early initiation of insulin therapy has been suggested specifically in patients who were not achieving the glycemic target using combined OHA because OHA showed repeat failure and weak evidence in treatment durability [14,15,16]. The important treatment rationale is that insulin reduces glucotoxicity and helps preserve pancreatic β-cell function for a longer time period than OHA, either alone or in combination [13]. A large meta-analysis of randomized controlled trials in patients with T2DM has shown that ≥50% of patients who had previously uncontrolled disease on zero, one, or two OHAs (baseline HbA1c, 8.7% to 9.1%), achieved HbA1c ≤7.0% after 24 weeks of treatment following the addition of basal insulin [17].
According to the data from clinical trials, individual treatment options, such as maximizing OHAs, adding basal insulin, or initiation of an intensified insulin regimen showed an advantage by lowering glucose; however, real-world data are scarce in patients treated with combination OHAs such as sulfonylurea plus metformin. Hence, this observational study was conducted to evaluate the efficacy of prescribed therapeutic options in patients with T2DM uncontrolled by sulfonylurea plus metformin in actual clinical practice.
METHODS
This open-label, multicenter, non-interventional, prospective, observational disease registry was conducted in 144 non-tertiary hospitals in various districts in Korea from June 2008 to July 2010. This study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki and in compliance with the International Conference on Harmonization-Good Clinical Practice guidelines. Before the start of the study, written informed consent was obtained by the investigators from each patient. The study protocol was approved by the Institutional Review Board of each site (IRB No. B-0808-060-002).
This study included patients with uncontrolled T2DM who had been prescribed sulfonylurea and metformin for at least 3 months and who had HbA1c >7.0% in the last month. Patients currently involved in another trial were excluded from the study. The antidiabetic medication was decided according to the physician's discretion. In the final analysis, subjects were classified into the following three groups according to treatment regimen: intensifying OHAs (group A), adding basal insulin (group B), or starting intensified insulin therapy (group C). The intensified insulin therapy included basal bolus, pre-mixed insulin, and continuous subcutaneous insulin infusion.
This study was scheduled for a period of 6 months after a change in the patients' treatment regimen. The follow-up period was 6±1 months for each patient. The following three prospective visits were planned: visit 1 (recruitment date), visit 2 (3±1 months), and visit 3 (6±1 months). Any additional visits during the 6-month period were adjusted to the closest visit in the three-visit schedule. For patients who did not attend visit 3, visit 2 was recorded as the last visit.
Data were recorded by investigators for variables including patient baseline demographics, HbA1c, body weight, and fasting plasma glucose (FPG) levels at all visits. The number of patients by type of prescription was also recorded at all visits. Because this study was a non-interventional, observational registry, safety data were spontaneously and voluntarily collected based on investigator judgment. For analysis of safety data, we reviewed medical chart notes.
Statistical analysis
Descriptive statistics were provided for all collected variables. Categorical data were summarized as frequency and percentage, and quantitative data were summarized as mean±standard deviation. Baseline characteristics were analyzed with analysis of variance and post hoc analysis was performed using Scheffe's test. The change in the HbA1c, FPG, and body weight from baseline to the end of the study according to group was analyzed by analysis of covariance with adjusted baseline values. The glycemic control rate was defined as the proportion of patients who achieved target HbA1c <7.0% or <6.5% and was calculated using chi-square test. The target FPG was <130 mg/dL according to the treatment guideline of the Korean Diabetes Association [8]. All statistical analyses were performed with SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). For patients whose data at 6 months post-baseline were not collected, visit 2 was used as the last visit.
RESULTS
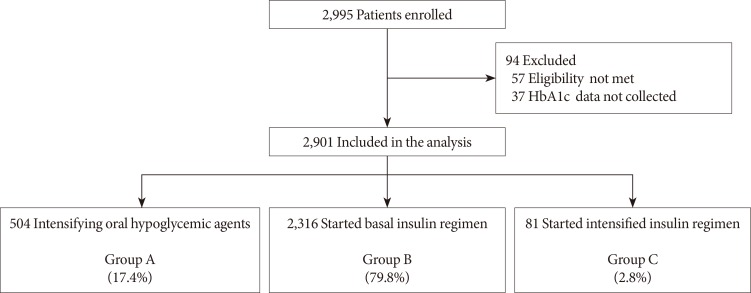
Of the total 2,995 patients enrolled, 94 patients were excluded from the analysis due to a violation of inclusion criteria (57 patients) and HbA1c data not being collected (37 patients). Finally, data from 2,901 patients were analyzed (Fig. 1). There were 504 (17.4%), 2,316 (79.8%), and 81 patients (2.8%) in groups A, B, and C, respectively. The mean age of the study population was 58.8±10.9 years, and the mean duration of diabetes was 7.8±5.5 years (Table 1). Patients in group C showed higher levels of HbA1c, lower body mass index (BMI), and longer duration of diabetes at baseline. The mean dose of metformin was approximately 1,200 mg per day, but the dose prescribed to group C was relatively lower than other groups. Glimepiride was the most commonly prescribed sulfonylurea followed by gliclazide and glibenclamide. The starting daily insulin doses were 15.4±6.7 IU in group B and 37.6±11.4 IU in group C (P<0.001). In group C, pre-mixed insulin, basal plus rapid-acting insulin, and other insulin regimens including continuous insulin treatment or basal bolus insulin were prescribed in 8, 50, and 23 patients, respectively.
Fig. 1. Study flow chart. Group A: intensifying oral hypoglycemic agents (OHAs; sulfonylurea+metformin dose titration or fixed-dose combination added to other OHAs). Group B: basal insulin alone or added to OHA mono/combination therapy. Group C: basal bolus, premixed insulin, and continuous subcutaneous insulin infusion alone or added to OHA mono/combination therapy. HbA1c, glycosylated hemoglobin.
Table 1. Baseline clinical and biochemical characteristics by prescription group.
| Characteristic | Group A | Group B | Group C | Total | P value |
|---|---|---|---|---|---|
| Number | 0504 | 2,316 | 81 | 2,901 | |
| Male sex | 284 (56.4) | 1,245 (53.8) | 32 (39.5) | 1,561 (53.8) | 0.020 |
| Age, yr | 58.3±10.8 | 58.8±10.9 | 59.4±11.3 | 58.8±10.9 | 0.508 |
| Body weight, kg | 68.1±11.2a | 65.4±10.6b | 60.2±12.2c | 65.7±10.8 | <0.001 |
| BMI, kg/m2 | 25.4±3.1a | 24.3±3.0b | 23.4±3.9c | 24.4±3.0 | <0.001 |
| Diabetes duration, yr | 7.3±5.1a | 7.9±5.5a | 9.7±5.5b | 7.8±5.5 | 0.005 |
| HbA1c, % | 8.4±1.1a | 9.1±1.4b | 10.3±2.3c | 9.0±1.4 | <0.001 |
| FPG, mg/dL | 178±61a | 200±61b | 193±74a,b | 196±61 | <0.001 |
| Metformin dose, mg | 1,242±521a | 1,234±513a | 1,078±489b | 1,231±514 | 0.024 |
| Sulfonyluread | |||||
| Glimepiride | 370 (73.3) | 2,093 (90.2) | 75 (91.5) | 2,538 (87.3) | <0.001 |
| Gliclazide | 104 (20.6) | 150 (6.5) | 3 (3.7) | 257 (8.8) | <0.001 |
| Glibenclamide | 31 (6.1) | 78 (3.4) | 4 (4.9) | 113 (3.9) | 0.014 |
Values are presented as number (%) or mean±standard deviation. P values were calculated using chi-square test and analysis of variance.
BMI, body mass index; HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose.
a,b,cThe data with different superscript letters represent significant difference according to Scheffe's post hoc test, dSome patients were taking more than two types of sulfonylurea.
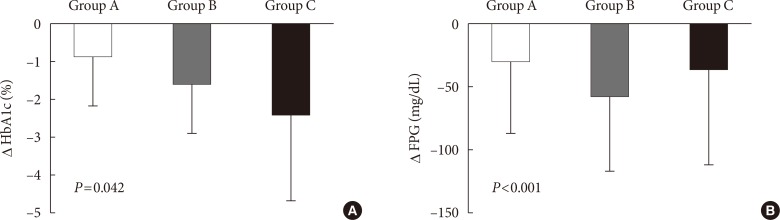
The mean follow-up period from baseline to study end was 6.1±1.0 months. At the second visit (3 months), 2,228 patients were followed, and among them, 24 patients did not attend the 6-month visit. In the final analysis, we used the final data collected at visit 2 in 15 (3.0%), 48 (2.1%), and three subjects (3.7%) in group A, B, and C respectively. Changes in HbA1c during the study period in groups A, B, and C were −0.9%±1.3%, −1.6%±1.3%, and −2.4%±2.3%, respectively (P=0.042) (Fig. 2A). Group C was further divided into three groups of practical relevance: pre-mixed insulin (n=8); basal plus rapidacting insulin (n=50); and other treatments, including continuous insulin treatment and basal bolus injection of rapid-acting insulin (n=23). The changes in HbA1c were −1.5%±1.4%, −2.6%±2.3%, and −2.1%±2.6%, respectively (P<0.001). The changes in FPG were −28.8±57.6, −57.6±59.4, and −36.0±75.6 mg/dL in groups A, B, and C, respectively (P<0.001) (Fig. 2B). Overall, 39.7% of patients reached the target HbA1c (<7.0%) and 10.4% of patients reached target HbA1c (<6.5%) after 6 months of treatment. After 3 months of treatment, more patients in group C achieved the target HbA1c <7.0% (30% of patients) and FPG <130 mg/dL (18.5% of patients) (Table 2). The percentages of patients attaining HbA1c <7.0% at 6 months were 40.9%, 39.6%, and 35.8% in groups A, B, and C, respectively (P=0.666) (Table 2). Additionally, the percentages of patients with HbA1c <6.5% were 17.3%, 8.7%, and 18.5% in groups A, B, and C, respectively, at 6 months (P<0.001) (Table 2). There was no significant difference in body weight changes between groups (P=0.606) (Table 2). Three episodes of symptomatic hypoglycemia in one patient in group B were reported during the study period.
Fig. 2. Change in (A) glycosylated hemoglobin (HbA1c) and (B) fasting plasma glucose (FPG). Group A: oral hypoglycemic agents (OHAs; sulfonylurea+metformin dose titration or fixed-dose combination added to other OHAs). Group B: basal insulin alone or added to OHA mono/combination therapy. Group C: basal insulin plus rapid acting insulin combination therapy alone or added to OHA mono/combination therapy. P value was calculated by analysis of covariance with adjustment of baseline HbA1c.
Table 2. Percentage of patients who achieved target HbA1ca, target fasting plasma glucoseb, and change in body weight from baseline to each visit by prescription group.
| Variable | Group A | Group B | Group C | P value |
|---|---|---|---|---|
| Number | 0504 | 2,316 | 81 | |
| Proportion of patients, % (HbA1c <7.0%) | ||||
| At 3 months | 17.1 | 10.2 | 30 | 0.001 |
| At 6 months | 40.9 | 39.6 | 35.8 | 0.666 |
| Proportion of patients, % (HbA1c <6.5%) | ||||
| At 3 months | 7.1 | 3.6 | 5.0 | 0.535 |
| At 6 months | 17.3 | 8.7 | 18.5 | <0.001 |
| Proportion of patients, % (FPG <130 mg/dL) | ||||
| At 3 months | 25.3 | 29.7 | 46.2 | 0.004 |
| At 6 months | 30.5 | 56.1 | 53.9 | <0.001 |
| ∆ Body weight, kg | ||||
| At 6 months | 0.1±2.6 | 0.3±1.9 | 0.6±2.5 | 0.606 |
| ∆ BMI, kg/m2 | ||||
| At 6 months | 0.08±1.18 | 0.11±0.79 | 0.39±1.18 | 0.113 |
Values are presented as mean±standard deviation. P values were calculated using chi-square test and analysis of variance.
HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose; BMI, body mass index.
a<7.0% or <6.5%, b<130 mg/dL.
DISCUSSION
In this observational study, the majority of patients received initiating basal insulin therapy (group B) after treatment failure with sulfonylurea and metformin, followed by intensifying OHAs (group A), and the initiation of intensified insulin regimens (group C). The FPG-lowering efficacy seemed to be superior in group B, but the mean change in HbA1c level was greatest in group C, followed by group B and then group A (Fig. 2). The control rate of HbA1c <7.0% was similar among groups whereas the control rate of HbA1c <6.5% was superior in group C compared to group B (Table 2).
The United Kingdom Prospective Diabetes Study (UKPDS) showed benefits in microvascular and macrovascular complications with strict glycemic control [18,19], and the “treat to target” approach has emerged. According to this goal-oriented approach, the importance of early insulin therapy has been adopted in many treatment guidelines for T2DM, and the intensification strategy using combined treatment was recommended as a priority if the first or second drug therapy failed. However, our data indicated that insulin therapy was likely to be started only after the patient's HbA1c level was relatively high enough in an actual clinical practice, because baseline HbA1c levels were higher in groups B and C than group A. Additionally, the duration of diabetes was the longest in group C, and this finding suggested that there is a clinical inertia in initiating insulin therapy. According to studies of other ethnic groups, there was also a barrier in starting insulin therapy [20,21]. Continuous efforts are needed to educate patients and physicians to overcome this gap between guidelines and actual clinical practice. In this context, the results from this study will give us a good evidence for the importance of initiating insulin therapy.
In the current study, the achievement rates of HbA1c target <7.0% were similar among the groups, whereas the percentage of patients in group C achieving a HbA1c target <6.5% was higher compared to that of the other groups (Table 2). We inferred that a significantly higher starting dose of insulin in group C compared to group B might have influenced this difference. Although data are lacking about the types and dosages of insulin, patients in group C might have received a sufficient dose of prandial insulin to control postprandial hyperglycemia. However, because the nature of this study design was that of an observational study, there were significant differences in baseline glucose levels and key clinical parameters among groups. To overcome this limitation, we investigated the difference in glucose-lowering efficacy after adjusting baseline HbA1c and clinical parameters such as gender, body weight (or BMI), and diabetes duration. In this analysis, the significant difference of glucose-lowering efficacy remained. This result may suggest that intensified insulin treatment could be useful for individuals whose HbA1c target is low, such as younger age patients without prior history of cardiovascular disease.
This research was not a randomized clinical trial but an observational study; therefore, a treat-to-target algorithm was not used. Previous studies have explained that the fear of hypoglycemia by both physicians and patients is the main reason for under-titration [22,23]. Physicians or patients might not increase the dose of insulin optimally, and this factor could result in low target achievement rates together with few reports of hypoglycemia in our study. However, the glucose-lowering effect was not likely to be inferior compared to the effect in a recently published study that compared treatment with pre-mixed insulin and basal bolus insulin in an Asian population [24]. Although the clinical trial followed study-specific titration algorithms and our study did not, our study showed numerically better glycemic control rates than the above-mentioned study [24]. Therefore, even if insulin treatment was not intensified according to treat-to-target algorithm, initiating insulin treatment might be beneficial in terms of reducing hyperglycemia in actual clinical practice.
Other important points regarding the efficacy of insulin therapy are patient-related factors. Previous observation studies conducted in Korea showed that the greatest glucose-lowering effect was observed using the basal bolus regimen [25,26] especially in patients with a high baseline HbA1c (≥9.0%) [25]. In our study, we also observed the greatest glucose-lowering efficacy using intensified insulin regimens (group C), and the patients in group C showed relatively higher baseline HbA1c levels as well as longer duration of diabetes. Even though this study did not compare the efficacy of insulin therapy according to a patient's clinical characteristics, we could speculate that patients with long-lasting diabetes having high HbA1c would be a good candidate for intensified insulin therapy. However, in addition to a greater reduction of hyperglycemia, which was usually followed by reduction of glycosuria [27], there was a trend towards increasing BMI in group C.
In this study, we restricted participants to those receiving metformin and sulfonylurea; whereas other actual clinical studies included patients receiving diverse OHAs [25,26]. Therefore, we could minimize the bias driven by variety in OHAs. In addition, the high follow-up rate (97.7%) is another important strength of this study.
The data from the current study should be understood based on a few limitations. First, this study was an observational study. Therefore, the effectiveness between treatment regimens cannot be compared. Instead, this study described the real clinical situation of delayed intensification of antidiabetic treatment and the gap between routine clinical practice and treatment guidelines. Second, there were few adverse events reported, probably due to under-reporting from patients or incomplete medical records. A modest adjustment of insulin dose in actual practice to avoid hypoglycemia might be another reason. Third, we did not collect the data about the final dosage of insulin in detail. Fourth, as various emerging treatments for T2DM have been introduced, other combination treatments with OHAs such as metformin plus a dipeptidyl peptidase-4 inhibitor are widely used. Therefore, further study is needed to evaluate the efficacy and safety of insulin treatment strategies in addition to various combinations of OHAs and intensifying OHAs.
In summary, this study revealed actual clinical practice in which physicians initiated insulin therapy if patients' hyperglycemia reached a relatively high level and the duration of diabetes was relatively long. Finally, intensified insulin therapy might be the most effective treatment for T2DM that does not cause a significant increase in body weight and hypoglycemia compared to combination treatments of OHAs or basal insulin therapy.
ACKNOWLEDGMENTS
The authors also acknowledge all of the following investigators of the MOHAS study: Bong-Ki Jung (Choonchun Kangnam Hospital), Bong-Nam Che (Chae Bong-Nam's Internal Medicine Clinic), Byung-Jun Yoo (Yoo Byung Jun's Internal Medicine Clinic), Chang-Min Woo (Gimchun Medical Center), Chang-Ok Yoon (Yoon Chang Ok's Internal Medicine Clinic), Chang-Rae Cho (Jinhae Yonsei Hospital), Chang-Woo Yoo (Jeonju St. Mary's Hospital), Chang-Yung Ha (Myongji St. Mary's Hospital), Chan-Woo Lee (Pohang St. Mary's Hospital), Chul-Hee Park (Echon Medical Center), Chul-Min Kang (Kang Chul-Min's Internal Medicine Clinic), Do-Hyun Jang (Jang Pyunhan Internal Medicine Clinic), Dong-Chae Lee (Seoul Litz Internal Medicine Clinic), Dong-Seop Choi (Korea University Anam Hospital), Dong-Wan Kim (Gimhae Centum Hospital), Duk-Yung Lee (Hayang Joongang Internal Medicine Clinic), Ee-Chul Shin (21st Century Internal Medicine Clinic), Eun-Hee Cho (Kangwon National University Hospital), Eun-Kyung Byun (SAM Medical Center), Eun-Yung Lee (Na Eun Hospital), Gyu-Yeop Hwang (Gongju Hyundae Hospital), Hae-Dong Park (Geoje Baik Hospital), Hee-Kwon Ahn (Ahn Hee-Kwon's Internal Medicine Clinic), Heung-Sun Yoo (Gimhae Samsung Hospital), Ho-Joon Jo (J Internal Medicine Clinic), Hong-Joon Ahn (Ahn Hong-Joon's Internal Medicine Clinic), Hong-Suk Kim (Seran Sungshim Clinic), Hong-Yul Kim (Kim Hong-Yul's Internal Medicine Clinic), Hun-Kwan Lim (Woori Internal Medicine Clinic), Hwa-Jong Park (Espero Internal Medicine Clinic), Hwan-Suk Choi (Choi Hwan-Suk's Internal Medicine Clinic), Hyo-I Jun (Seoul Family Medicine Clinic), Hyo-Suk Kim (Daegu Medical Center), Hyuk-Soo Sohn (Sungju Hyeseong Hospital), Hyun Choi (Yulin Internal Medicine Clinic), Hyun-Dae Cho (Hwamyeong Hansol Hospital), Hyung-Han Moon (Moon Hyung-Han's Internal Medicine Clinic), Hyun-Joo Jang (Hyundai Hospital), Hyun-Seung Kim (Medi Hill Hospital), Ie-Byung Park (Gachon University Gil Medical Center), In-Hwan Yoo (Yoo's Internal Medicine Clinic), In-Won Kim (Sejin Internal Medicine Clinic), Jae-Hong Kim (Haedong Internal Medicine Clinic), Jae-Hoon Jun (Jun Jae-Hoon's Internal Medicine Clinic), Jae-Il Lee (Lee Jae-Il's Internal Medicine Clinic), Jae-Myung Yu (Hallym University Hangang Sacred Heart Hospital), Jee-Young Oh (Ewha Womans University Mokdong Hospital), Je-Ryong Lee (Lee Je-Ryong's Family Medicine Clinic), Jin-Ah Park (Metro Hospital), Jin-Hyun Choi (Choi Jin-Hyun's Internal Medicine Clinic), Jin-Sung Kim (Seoul Internal Medicine Clinic), Ji-Oh Mok (Soon Chun Hyang University Bucheon Hospital), Jong-Ho Park (Bangbae Jeil Hospital), Jong-Hoon Kim (Dangjin St. Mary's Internal Medicine Clinic), Jong-Hyung Kim (Cheongshim International Medical Center), Jong-Ryeal Hahm (Gyeongsang National University Hospital), Joo-Chul Kim (Dongsuwon Hospital), Joo-Ho Kim (Kim Joo-Ho's Internal Medicine Clinic), Joon-Ki Yeo (Yeo Joon-Ki's Internal Medicine Clinic), Joon-Sang Yoo (Yoo Joon-Sang's Family Medicine Clinic), Jung-Eun Seo (Yujin Internal Medicine Clinic), Jung-Han Kim (Sungae Hospital), Jung-Hoon Sung (Semyung Internal Medicine Clinic), Jung-Ik Woo (Yonsei Home Clinic), Jung-Pil Park (e-Joeun Joongang Hospital), Ki-Duk Kim (Chungkoo Jeil Internal Medicine Clinic), Ki-Young Kim (Kim Ki-Young's Internal Medicine Clinic), Kwang-Jae Lee (Daedong Hospital), Kwang-Min Pyo (Pyo Kwang-Min's Internal Medicine Clinic), Kwang-Soo Cha (Cha Kwang-Soo's Internal Medicine Clinic), Kwan-Hyung Lee (Hyundai Yunhap Internal Medicine Clinic), Kyo-Sun Kim (Kim Kyo-Sun's Internal Medicine Clinic), Kyoung-Ah Kim (Dongguk University Ilsan Hospital), Mi-Ae Cho (Dong Rae Bong Seng Hospital), Mi-Jung Kim (Pureun Mirae Internal Medicine Clinic), Mi-Kyung Kim (Baptist Hospital), Min-Ah Nah (Busan Medical Center), Min-Seop Song (Seoul Song Internal Medicine Clinic), Myung-Choon Lee (Lee's Family Medicine Clinic), Nam-Jin Yoo (Gunsan Medical Center), Nam-Yung Kang (Kang Nam-Yung's Internal Medicine Clinic), O-Yoon Kwon (Kwon O-Yoon's Internal Medicine Clinic), Sang-Ho Jang (Yonsei Internal Medicine Clinic), Sang-Ho Lee (Daejeon Hankook Hospital), Sang-Hyun Joo (Boomin Hospital), Sang-Yong Lee (Heerak Seoul Family Medicine Clinic), Se-Chang Oh (Songdo Hospital), Se-Hee Kim (Jungdong Hospital), Se-In Hong (Kwangjoo Bohoon Hospital), Seok-O Park (Kwangmyung Sungae Hospital), Seok-Woo Kang (Seoul Red Cross Hospital), Seung-Hoon Lee (Dongjak Kyunghee Hospital), Seung-Hyun Lee (Yeungnam University Yeongcheon Hospital), Shin-Eung Kim (Kim Shin-Eung's Internal Medicine Clinic), Soo-Min Nam (Daejeon Sun Hospital), Suk-Joo Ahn (Ahn Suk-Joo's Internal Medicine Clinic), Sung-Chil Kim (Kim's Internal Medicine Clinic), Sung-Ho Kwon (Gangnam Donggang Hospital), Sung-Koo Kang (Suwon Hankook Hospital), Sung-Kwan Hong (Seoul Endo Internal Medicine Clinic), Sung-Soo Park (Park Sung-Soo's Internal Medicine Clinic), Sun-Hwa Lee (Incheon Christian Hospital), Tae-Kyung Kwon (Kijang Hospital), Tae-Wook Park (Chunan Choongmoo Hospital), Won-Shik Shin (Kangseo Jeil Hospital), Won-Taek Jung (Kijang Korea Clinic), Yoon-Ho Kim (Kim Yoon-Ho's Internal Medicine Clinic), Yoon-Ja Kim (Hyundai Internal Medicine Clinic), Yoon-Jeong Do (Raphael Internal Medicine Clinic), Yun Lee (Seoul Metropolitan Bukbu Geriatric Hospital), Yung-Chan Kang (Kang Yung-Chan's Family Medicine Clinic), Yung-Chan Kim (Chungdam Anse Hospital), Yung-Chul Cho (Patima Yunhap Internal Medicine Clinic), Yung-Do Suh (Suh Yung-Do's Internal Medicine Clinic), Yung-Geun Choi (Joeun Samsun Hospital), Yung-Jae Ko (Good Morning Internal Medicine Clinic), Yung-Joo Choi (Huh's Internal Medicine Clinic), Yung-Joon Kim (Kim Yung-Joon's Internal Medicine Clinic), Keun-Young Park, Dong-Mee Lim (Konyang University Hospital), Ki-Rak Park, Choon-Shik Lee (Gyeongjoo Internal Medicine Clinic), Ho-Yeon Chung, Kyu-Jeung Ahn, Gyu-Cheol Hwang (Kyung Hee University Hospital at Gangdong), Keun-Gyu Park, Hye-Soon Kim (Keimyung University Dongsan Medical Center), Duk-Kyu Kim, Mi-Kyoung Park, Ja-Won Kim (Dong-A University Hospital), Mi-Kyung Kim, Ji-Hye Suk (Maryknoll Hospital), Jong-Eun Park, Chan-Gyu Park (Park's Internal Medicine Clinic), Jeong-Hyun Park, Min-Jeong Kwon (Inje University Busan Paik Hospital), Yong-Wook Cho, Seok-Won Park, Soo-Kyoung Kim (CHA Bundang Medical Center, CHA University), Kyung-Soo Ko, Byung-Doo Lee (Inje University Sanggye Paik Hospital), Kun-Ho Yoon, Hun-Sung Kim, Seung-Hwan Lee, Yoon-Hee Choi (Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea), Yung-Sook Nah, Ji-Ho Noh (Sokcho Medical Center), Yoon-Ee Kim, Bang-Hoon Lee (Seoul Metropolitan Dongbu Hospital), Hee-Kyung Kim, Sung-Won Park (Andong Hospital), Ji-Hye Kim, Sun-Kyung Song (Yesu Hospital), Young-Il Kim, Ilsung Nam-Goong, Eun-Sook Kim (Ulsan University Hospital), Kwang-Seop Lee, Sang-Woon Lee (Lee's Internal Medicine Clinic), Sung-Dae Moon, Je-Ho Han (Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea), Dong-Jun Kim, Jung-Hyun Noh (Inje University Ilsan Paik Hospital), Sung-Rae Cho, Gwi-Hwa Jung (Changwon Fatima Hospital), Dong-Sun Kim, Tae-Wha Kim (Hanyang University Seoul Hospital), and Myung Bae, Dong-Hoon Shin (Hanil Hospital).
Footnotes
CONFLICTS OF INTEREST: This study was sponsored by Sanofi-Aventis Korea. The authors acknowledge Jeevan Scientific Technology Limited (Hyderabad, India) and Anahita Gouri (Sanofi, Mumbai, India) for providing writing and editing assistance and Jee Hyun Lee and Kyoungsoo Ha (Sanofi-Aventis Korea) for providing statistical analysis in preparing this manuscript.
References
- 1.International Diabetes Federation. IDF diabetes atlas. 6th ed. Brussels: International Diabetes Federation; 2013. [Google Scholar]
- 2.Korean Diabetes Association. Diabetes fact sheet in Korea 2012. Seoul: Korean Diabetes Association/Korea Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 3.Kim DJ. The epidemiology of diabetes in Korea. Diabetes Metab J. 2011;35:303–308. doi: 10.4093/dmj.2011.35.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korean Diabetes Association. Diabetes fact sheet in Korea 2013. Seoul: Korean Diabetes Association; 2013. [Google Scholar]
- 5.Yeung RO, Zhang Y, Luk A, Yang W, Sobrepena L, Yoon KH, Aravind SR, Sheu W, Nguyen TK, Ozaki R, Deerochanawong C, Tsang CC, Chan WB, Hong EG, Do TQ, Cheung Y, Brown N, Goh SY, Ma RC, Mukhopadhyay M, Ojha AK, Chakraborty S, Kong AP, Lau W, Jia W, Li W, Guo X, Bian R, Weng J, Ji L, Reyes-dela Rosa M, Toledo RM, Himathongkam T, Yoo SJ, Chow CC, Ho LL, Chuang LM, Tutino G, Tong PC, So WY, Wolthers T, Ko G, Lyubomirsky G, Chan JC. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014;2:935–943. doi: 10.1016/S2213-8587(14)70137-8. [DOI] [PubMed] [Google Scholar]
- 6.Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl 3):S16–S21. doi: 10.2337/diabetes.53.suppl_3.s16. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. 7 Approaches to glycemic treatment. Diabetes Care. 2016;39(Suppl 1):S52–S59. doi: 10.2337/dc16-S010. [DOI] [PubMed] [Google Scholar]
- 8.Korean Diabetes Association. Treatment guideline for diabetes. 5th ed. Seoul: Gold' Planning and Development; 2015. [Google Scholar]
- 9.Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez G, Davidson MH. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21:438–447. doi: 10.4158/EP15693.CS. [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki T, Ohtani T, Naito Y, Odawara M. Potential formula for the calculation of starting and incremental insulin glargine doses: ALOHA subanalysis. PLoS One. 2012;7:e41358. doi: 10.1371/journal.pone.0041358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kostev K, Dippel FW. Predictors for the initiation of a basal supported oral therapy (BOT) in type 2 diabetic patients under real-life conditions in Germany. Prim Care Diabetes. 2012;6:329–335. doi: 10.1016/j.pcd.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Ratanawongsa N, Crosson JC, Schillinger D, Karter AJ, Saha CK, Marrero DG. Getting under the skin of clinical inertia in insulin initiation: the Translating Research Into Action for Diabetes (TRIAD) Insulin Starts Project. Diabetes Educ. 2012;38:94–100. doi: 10.1177/0145721711432649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, Hu Y, Zhou Z, Yan X, Tian H, Ran X, Luo Z, Xian J, Yan L, Li F, Zeng L, Chen Y, Yang L, Yan S, Liu J, Li M, Fu Z, Cheng H. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 14.TODAY Study Group. Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, Arslanian S, Cuttler L, Nathan DM, Tollefsen S, Wilfley D, Kaufman F. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riedel AA, Heien H, Wogen J, Plauschinat CA. Loss of glycemic control in patients with type 2 diabetes mellitus who were receiving initial metformin, sulfonylurea, or thiazolidinedione monotherapy. Pharmacotherapy. 2007;27:1102–1110. doi: 10.1592/phco.27.8.1102. [DOI] [PubMed] [Google Scholar]
- 16.Riedel AA, Heien H, Wogen J, Plauschinat CA. Secondary failure of glycemic control for patients adding thiazolidinedione or sulfonylurea therapy to a metformin regimen. Am J Manag Care. 2007;13:457–463. [PubMed] [Google Scholar]
- 17.Fonseca V, Gill J, Zhou R, Leahy J. An analysis of early insulin glargine added to metformin with or without sulfonylurea: impact on glycaemic control and hypoglycaemia. Diabetes Obes Metab. 2011;13:814–822. doi: 10.1111/j.1463-1326.2011.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 20.Li C, Ford ES, Zhao G, Tsai J, Balluz LS, Giles WH. Trends of insulin use among US adults with type 2 diabetes: the Behavioral Risk Factor Surveillance System, 1995-2007. J Diabetes Complications. 2012;26:17–22. doi: 10.1016/j.jdiacomp.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Khunti K, Damci T, Meneghini L, Pan CY, Yale JF, Group SS. Study of Once Daily Levemir (SOLVE): insights into the timing of insulin initiation in people with poorly controlled type 2 diabetes in routine clinical practice. Diabetes Obes Metab. 2012;14:654–661. doi: 10.1111/j.1463-1326.2012.01602.x. [DOI] [PubMed] [Google Scholar]
- 22.Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Metab Res Rev. 1999;15:42–46. doi: 10.1002/(sici)1520-7560(199901/02)15:1<42::aid-dmrr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 23.Baser O, Tangirala K, Wei W, Xie L. Real-world outcomes of initiating insulin glargine-based treatment versus premixed analog insulins among US patients with type 2 diabetes failing oral antidiabetic drugs. Clinicoecon Outcomes Res. 2013;5:497–505. doi: 10.2147/CEOR.S49279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia W, Xiao X, Ji Q, Ahn KJ, Chuang LM, Bao Y, Pang C, Chen L, Gao F, Tu Y, Li P, Yang J. Comparison of thrice-daily premixed insulin (insulin lispro premix) with basal-bolus (insulin glargine once-daily plus thrice-daily prandial insulin lispro) therapy in east Asian patients with type 2 diabetes insufficiently controlled with twice-daily premixed insulin: an open-label, randomised, controlled trial. Lancet Diabetes Endocrinol. 2015;3:254–262. doi: 10.1016/S2213-8587(15)00041-8. [DOI] [PubMed] [Google Scholar]
- 25.Hwang YC, Kang JG, Ahn KJ, Cha BS, Ihm SH, Lee S, Kim M, Lee BW. The glycemic efficacies of insulin analogue regimens according to baseline glycemic status in Korean patients with type 2 diabetes: sub-analysis from the A(1)chieve((R)) study. Int J Clin Pract. 2014;68:1338–1344. doi: 10.1111/ijcp.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SS, Kim IJ, Kim YK, Yoon KH, Son HY, Park SW, Sung YA, Baek HS. Insulin initiation in insulin-naive Korean type 2 diabetic patients inadequately controlled on oral antidiabetic drugs in real-world practice: the modality of insulin treatment evaluation study. Diabetes Metab J. 2015;39:481–488. doi: 10.4093/dmj.2015.39.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makimattila S, Nikkila K, Yki-Jarvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia. 1999;42:406–412. doi: 10.1007/s001250051172. [DOI] [PubMed] [Google Scholar]