Abstract
Metagenomic analysis based on the 16S rRNA gene is generally performed to examine the diversity and abundance of commensal bacteria in feces, which is now recognized to be associated with human health and diseases. Guanidine thiocyanate (GuSCN) solution is used as a less onerous way compared with a frozen method to transport and stock fecal samples at room temperature for DNA analysis; however, optimal methods to measure fecal bacterial composition in GuSCN solution remain to be investigated. Here, we examined the influence of various factors such as pretreatment (e.g., removing GuSCN solution and washing feces with phosphate-buffered saline (PBS) before mechanical lysis), fecal concentration in the GuSCN solution, storage time, and position of fecal subsampling on the 16S rRNA-based analysis of fecal bacteria in GuSCN solution. We found that pretreatment and fecal concentration affected the bacterial composition, and a little change was noted with subsampling position. Based on these results, we propose a basic protocol, including fecal sampling, sample storage, and DNA extraction, for the 16S rRNA-based analysis of bacterial composition in feces suspended in GuSCN solution.
Introduction
Gut commensal bacteria are now recognized as an important factor in the maintenance of health because they facilitate food digestion and concomitant metabolite generation1, regulate the immune system2, and inhibit infection by potentially pathogenic microorganisms by competing for niches3. Technical advances in the deep sequencing of highly conserved regions in the 16S ribosomal RNA (rRNA) gene provide a convenient platform for the comprehensive and comparative analysis of the composition of commensal bacteria, including those that cannot currently be cultured4, 5. The results of such sequencing analyses reveal that altered composition of commensal bacteria frequently associates with the development and/or status of inflammatory immune diseases (e.g., Crohn’s disease and celiac disease) and allergies6, 7 as well as non-immune diseases such as obesity8, irritable bowel syndrome9, and diabetes10.
The bacterial composition in the gut has been evaluated mainly by using fecal samples, which are easy and non-invasive to obtain. Many factors during analysis affect the calculated composition of gut microbiota, including sample storage (frozen or room temperature; solution composition), homogenization method for DNA extraction (e.g., mechanical or enzymatic)11, 12, and choice of PCR primers13. For fecal storage, guanidine thiocyanate (GuSCN) is frequently used as a simple and safe means of storing feces at room temperature because GuSCN inactivates various nucleases14–16. The content of GuSCN solution is 100 mM Tris–HCl (pH 9), 40 mM EDTA, 4 M guanidine thiocyanate, and 0.001% bromothymol17. Generally, the use of GuSCN solution is combined with mechanical lysis using beads, followed by silica column purification. Although the DNA extraction method18 and design of PCR primers19, 20 has been extensively evaluated, optimization of the pre-treatment of fecal samples in GuSCN solution before mechanical lysis has not been well examined. In addition, procedures for the optimal storage of fecal samples in GuSCN solution remain to be examined. Here, we addressed these issues by comparing the data on the bacterial composition in fecal samples treated by various procedures, and validated whether the methods were truly optimized.
Results and Discussion
Effects of supernatant removal and washing of pellets on the microbial composition in GuSCN solution
GuSCN solution is used as a simple way to transport and stock fecal samples at room temperature for DNA analysis; however, optimal methods for extraction of DNA from fecal samples in GuSCN solution to measure fecal bacterial composition remain to be investigated. In this study, we initially investigated whether pretreatment or no treatment (conventional method) before mechanical lysis affected microbial composition in feces suspended in GuSCN solution. To address this issue, we obtained fecal samples from 18 healthy individuals. Following a previous report21, the samples were divided into groups 1 to 3 based on the abundant bacteria at the phylum level: i.e., Actinobacteria, Firmicutes, and Bacteroidetes, respectively (Fig. 1a). Proteobacteria was a very minor component of the samples in all groups (Fig. 1a).
Figure 1.
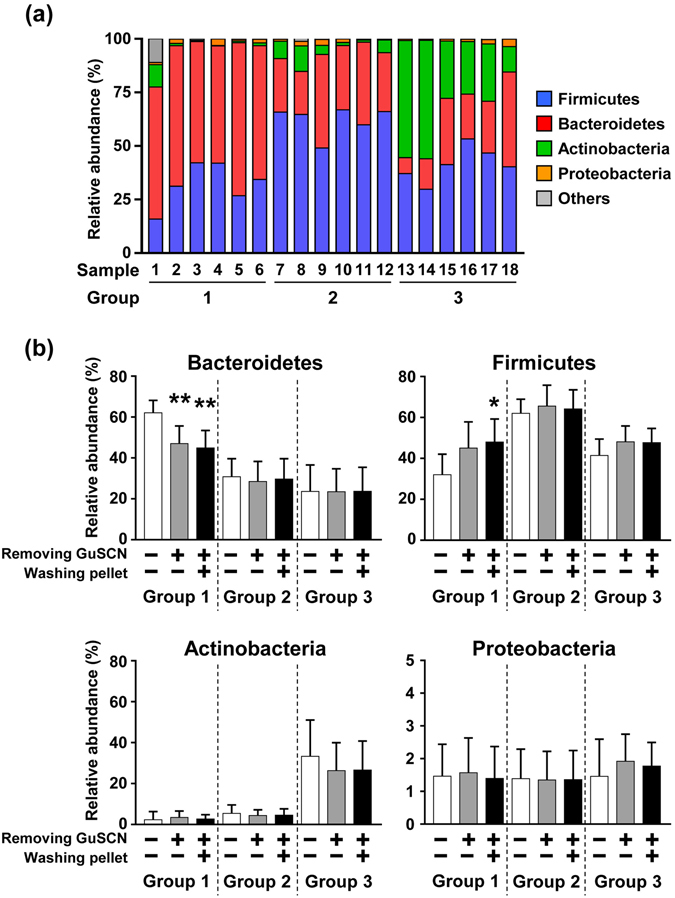
Effects of pretreatment on microbial composition in fecal samples stored in GuSCN solution. (a) Microbial composition of fecal samples from 18 healthy individuals at the phylum level. DNA was extracted directly from fecal samples in GuSCN solution by the bead beating method and sequenced. (b) Relative abundance of Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria before and after pre-treatment in the DNA extraction procedure is shown. Data are presented as means ± SD (n = 6). An asterisk means statistically significant difference as calculated by using Student’s t test (*p < 0.05, **p < 0.01).
To address the effect of removal of GuSCN solution before mechanical lysis, GuSCN solution was removed by centrifugation and then DNA was extracted from the fecal pellets. Although this treatment did not affect the total number of operational taxonomic units (OTUs) when all groups were combined (Supplementary Fig. 1), the microbial composition appeared to be changed (Fig. 1b). Removing the GuSCN solution decreased the relative abundance of the predominant phylum Bacteroidetes and tended to increase the relative abundance of Firmicutes (p = 0.078) in group 1 (Fig. 1b and Supplementary Fig. 2). Group 2 (predominantly Firmicutes) did not show any marked changes in bacterial composition (Fig. 1b and Supplementary Fig. 2). Group 3 (abundantly Actinobacteria) did not show any marked changes as average (Fig. 1b), but some changes were observed in each individual; decrease of Actinobacteria in three individuals, decrease of Bacteroides in two individuals, and decrease in both Actinobacteria and Bacteroides in one individual were observed (Supplementary Fig. 2). Similar results were obtained when fecal pellets were further washed with PBS after removal of the GuSCN solution (Fig. 1b and Supplementary Fig. 2).
We next performed a detailed analysis at the genus level. As mentioned above, in group 1, the dominant phylum Bacteroidetes decreased after pretreatment (Fig. 1b and Supplementary Fig. 2); at the level of genera, Bacteroides and Prevotella (in phylum Bacteroidetes) were reciprocally dominant in different individuals and tend to be decreased similarly after pretreatment with different significance among individuals (Fig. 2; Group 1). Although no marked differences at the phylum level between before and after pretreatment were noted in group 2 (Fig. 1b and Supplementary Fig. 2), there was a slight trend toward alteration of the bacterial composition of some genera in Firmicutes: with pretreatment there was a decrease in Faecalibacterium in some individuals, a marginal increase in Blautia, but no apparent change in Ruminococcus (Faecalibacterium, p = 0.421; Blautia, p = 0.661; Ruminococcus, p = 0.839) (Fig. 2; Group 2). Bifidobacterium was the predominant genus in the Actinobacteria phylum. Consistent with phylum data (Fig. 1 and Supplementary Fig. 2), some individuals with decreased ratio of Actinobacteria (#13–15) exhibited the decrease of Bifidobacterium (Fig. 2).
Figure 2.
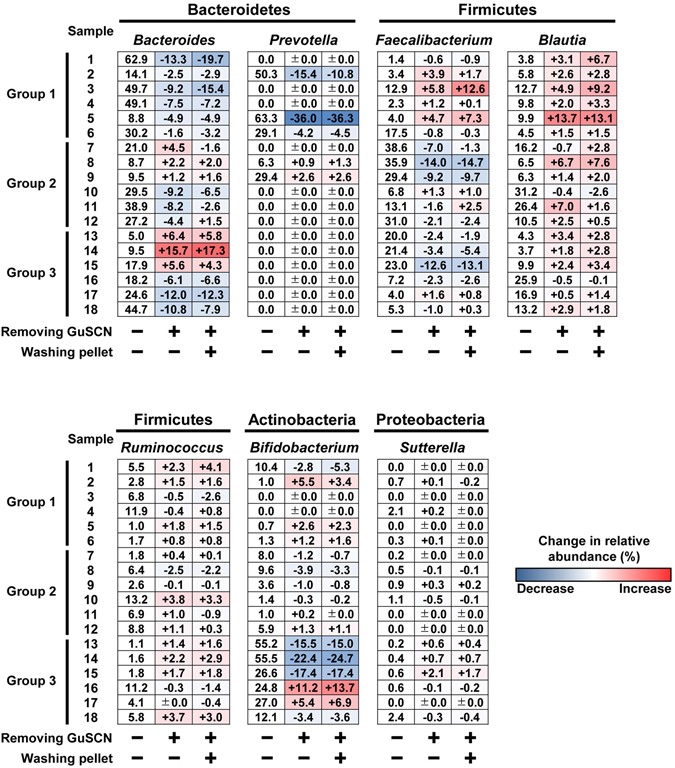
Effects of pretreatment on microbial composition in fecal samples at the genus level. The relative abundance (%) of Bacteroides, Prevotella, Faecalibacterium, Blautia, Ruminococcus, Bifidobacterium, and Sutterella in fecal samples collected from 18 individuals was shown. DNA was directly extracted from fecal samples in GuSCN solution (left column). The heat map (middle and right columns) shows the change in relative abundance (%) after pre-treatment in DNA extraction procedure.
Because GuSCN is a chaotropic agent that denatures proteins (e.g., nucleases) and peptidoglycan, and lyses cells22, 23, each bacterium’s sensitivity to GuSCN may differ due to its cellular structure (e.g., cell morphology and size) and the components of its cell membrane and cell wall24. Indeed, in previous studies a different efficacy of DNA extraction was noted between gram-negative and -positive bacteria12, 25, 26. Thus, it is plausible that genomic DNA was released into the GuSCN solution from some bacteria (e.g., Bacteroides and Bifidobacterium) and discarded by pre-treatment procedures such as centrifugation and washing. To confirm this hypothesis, we examine whether genomic DNA of Bacteroides and Bifidobacterium was present in GuSCN solution by PCR using specific primers to each bacterial taxon. Genomic DNA of Bacteroides was detected in all samples of all groups (Supplementary Fig. 3a) and genomic DNA of Bifidobacterium was detected in some samples of groups 1 and 2, and in all samples of group 3 (Supplementary Fig. 3b). Therefore, we propose that DNA extraction should be performed directly from human fecal samples in GuSCN solution without pre-treatment. Expectedly, similar alteration may occur in shot-gun sequencing analysis of fecal samples in GuSCN solution.
Changes in the bacterial composition in GuSCN solutions containing highly concentrated feces
We next compared bacterial composition in GuSCN solutions with various concentrations of feces. At the phylum level, the fecal concentration did not affect the bacterial composition if the samples were analyzed within 3 days of storage (Fig. 3a and Supplementary Table 1). In contrast, when the samples were stored for 1 month, solutions with highly concentrated feces (0.3 g/ml) exhibited an increased relative abundance of Proteobacteria at the phylum level and Gammaproteobacteria at the class level in all three groups (Fig. 3a and b and Supplementary Table 1). When we performed a detailed analysis of Gammaproteobacteria at the genus level in each sample, we found that the proportions of several genera, such as Citrobacter, Escherichia, Enterobacter, and Trabulsiella, were moderately to highly increase in some samples, but not in others (Supplementary Fig. 4).
Figure 3.
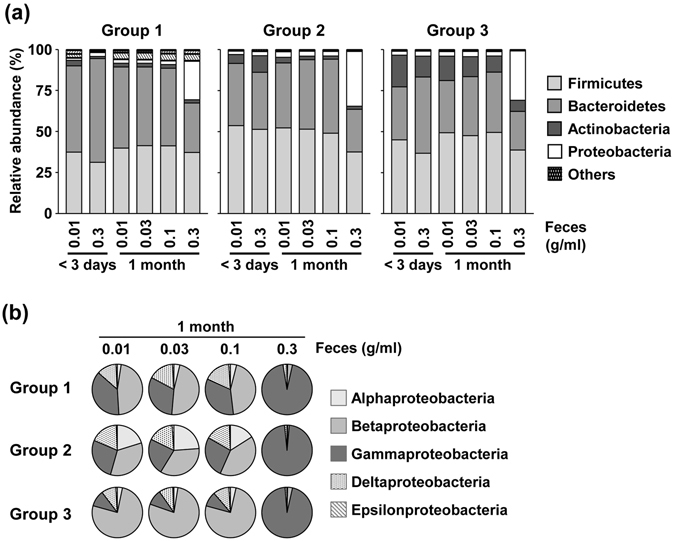
Effects of fecal concentration on microbial composition in fecal samples stored in GuSCN solution. (a) Microbial composition at the phylum level in GuSCN solutions containing different concentrations of feces (0.01–0.3 g/ml); Aliquots (0.01–0.3 g) of fecal samples were in 15 ml vials containing 1 ml of GuSCN solution. DNA was extracted within 3 days or after storage for one month at room temperature. (b) Class abundance of Proteobacteria after storage for one month at room temperature, graphed according to fecal concentration.
The main populations of commensal bacteria in feces are obligate anaerobes, which are primarily composed of the genera Bacteroides, Eubacterium, and Clostridium 27; obligate anaerobes cannot grow in aerobic conditions because they cannot use oxygen to generate ATP. Feces also contain facultative anaerobes as a minor population; these bacteria, which include members of Gammaproteobacteria, such as the orders Enterobacteriales, Vibrionales, and Pasteurellales28, can grow in both aerobic and anaerobic conditions due to their diverse capabilities of aerobic respiration, fermentation, and anaerobic respiration. Therefore, it is plausible that bacterial cells in highly concentrated feces (0.3 g/ml) are not inactivated sufficiently in GuSCN solution, leading to the observed increase in Gammaproteobacteria when samples were stored for one month under aerobic conditions. This hypothesis was also supported by PCR analysis using bacterium-specific primers; genomic DNA of Citrobacter was detected in highly concentrated samples stored for one month but not within 3 days (Supplementary Fig. 5).
Additionally, we noticed that the increase in the relative proportion of Gammaproteobacteria in the samples with high fecal concentrations compared with low fecal concentrations occurred in all groups, but not in all individuals within each group (Supplementary Fig. 4). We therefore aimed to identify an individual-specific factor that determines the increase in Gammaproteobacteria. It was previously reported that the water content of a normal fecal sample is about 75%, while that of a diarrhoeic fecal sample is >85%, and that this difference affects the bacterial component29. In our experimental samples, the water content ranged between 64.9% and 85.6% (Supplementary Fig. 6). We found that the water content in feces was inversely correlated to the proportion of Proteobacteria at the phylum level, and the proportion of Gammaproteobacteria at the class level (Fig. 4a,b and Supplementary Fig. 6). These results suggest that highly concentrated feces (0.3 g/ml) with low water content were incompletely suspended in GuSCN solution, and therefore bacterial growth was not sufficiently inhibited.
Figure 4.
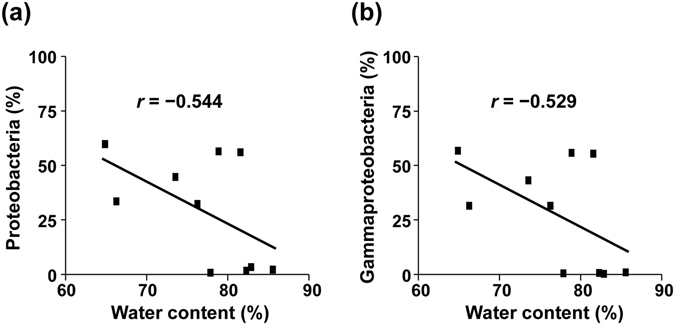
Relationship between water content in feces and the proportion of Proteobacteria and Gammaproteobacteria. The proportion of Proteobacteria (a) and Gammaproteobacteria (b) is shown for highly concentrated fecal samples (0.3 g/ml) in GuSCN solution after storage for one month at room temperature. r, Pearson correlation coefficient.
Dilution of GuSCN solution interferes with the maintenance of bacterial composition in feces
To explore this proposition of sample storage by using GuSCN solution further, we then compared fecal bacterial composition in GuSCN solutions diluted with various volumes of PBS. We used 0.1 g/ml as an experimental fecal concentration that has little effect on bacterial composition in the conventional method (Fig. 3a). The proportion of Proteobacteria increased at the phylum level when samples were diluted with 1.0 ml of PBS and stored for 1 month at room temperature (Fig. 5a and Supplementary Table 2). As was observed for GuSCN solutions with highly concentrated feces (0.3 g/ml) (Fig. 3), an increase in Proteobacteria was observed in all groups with sample dilution (Fig. 5a and Supplementary Table 2) and was coincident with the increase of the proportion of Gammaproteobacteria (Fig. 5b). Thus, we propose that insufficient bacteria killing by the diluted GuSCN solution led to the growth of Gammaproteobacteria in aerobic conditions.
Figure 5.
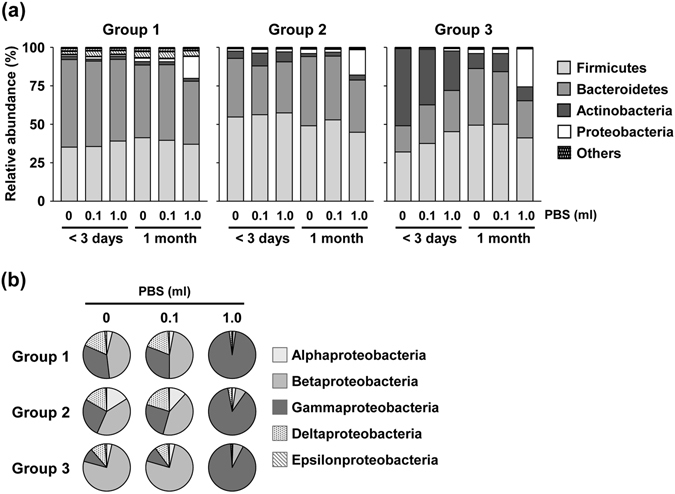
Effect of dilution of GuSCN solution on microbial composition in fecal samples. (a) Microbial composition at the phylum level for feces in GuSCN solution diluted with PBS; Aliquots (0.1 g) of fecal samples were in 15 ml vials containing 1 ml of GuSCN solution with 0, 0.1, or 1.0 ml of PBS. DNA was extracted within 3 days or after storage for one month at room temperature. (b) Abundance of various Proteobacteria at the class level in fecal samples after storage for one month at room temperature.
We then performed a detailed analysis of each sample at the genus level and found that an increased proportion of several Gammaproteobacteria genera, such as Citrobacter, Enterobacter, and Klebsiella, in the samples diluted with PBS (Supplementary Fig. 7). Genomic DNA of Citrobacter was detected in the diluted samples stored for one month but not within 3 days by Citrobacter-specific PCR (Supplementary Fig. 8). As we discussed above, differences in bacterial structure may explain the differing effects on the inhibition of bacterial growth for different genera in the diluted GuSCN solution24.
In addition, the other conditions (e.g., dilution with 0.1 or 1.0 ml of PBS and storage for less than 3 days, and dilution with 0.1 ml of PBS for 1 month) affected the bacterial compositions in group 3, but not in group 1 or 2. Even when samples were stored for less than 3 days, dilution of samples decreased the relative proportion of Actinobacteria and simultaneously increased the relative proportions of Firmicutes and Bacteroidetes; similar but larger changes were observed with higher dilution (i.e., 1 ml of PBS) (Fig. 5a and Supplementary Table 2). A decrease in Actinobacteria and increase in Firmicutes and Bacteroidetes was also observed when non-diluted samples were stored for 1 month rather than less than 3 days (Fig. 5a and Supplementary Table 2). Therefore, we preformed further experiment to examine effect of storage term on bacterial composition of fecal samples in GuSCN solution. Consistently, the relative abundance of Bifidobacterium (predominant genus in Actinobacteria) appeared to decrease as time passes (Supplementary Fig. 9). These results suggest that the Actinobacteria DNA signal is easily lost when samples are stored for 2 to 4 weeks; it is possible that the release of DNA from Actinobacteria into GuSCN solution could lead to structural and/or biochemical changes that could affect PCR amplification and/or DNA extraction efficiency. Taken together, these results indicate that storage for one month at any temperature might lead to inaccurate bacterial composition data, especially for Actinobacteria-rich samples.
Fecal sampling from several portions of same feces
Finally, we examined whether bacterial composition was altered when we took subsamples from different positions (e.g., outer or inner; front, middle or back) within the feces (Fig. 6a). Overall, sampling position had little effect on the bacterial composition at the phylum level (Supplementary Fig. 10); however, phylogenetic analysis at the genus level indicated that subsamples from some individuals (e.g., #2, #8, and #14) showed differences between the outer layer at the front (indicated as “a” in Fig. 6a) and the other positions (Fig. 6b, indicated by asterisks). Additionally, a previous study by using more sensitive PCR analysis had shown that bacterial taxa abundance (e.g., Firmicutes and Bifidobacteria spp.) was different between the inside and outside of feces30. Because the bacterial composition obtained from a mixture of three positions (outer: “a”, “b”, “c” in Fig. 6a) appeared representative bacterial composition for each individual (Fig. 6b and c and Supplementary Fig. 10), fecal samples should be collected from several positions and mixed well before DNA extraction.
Figure 6.
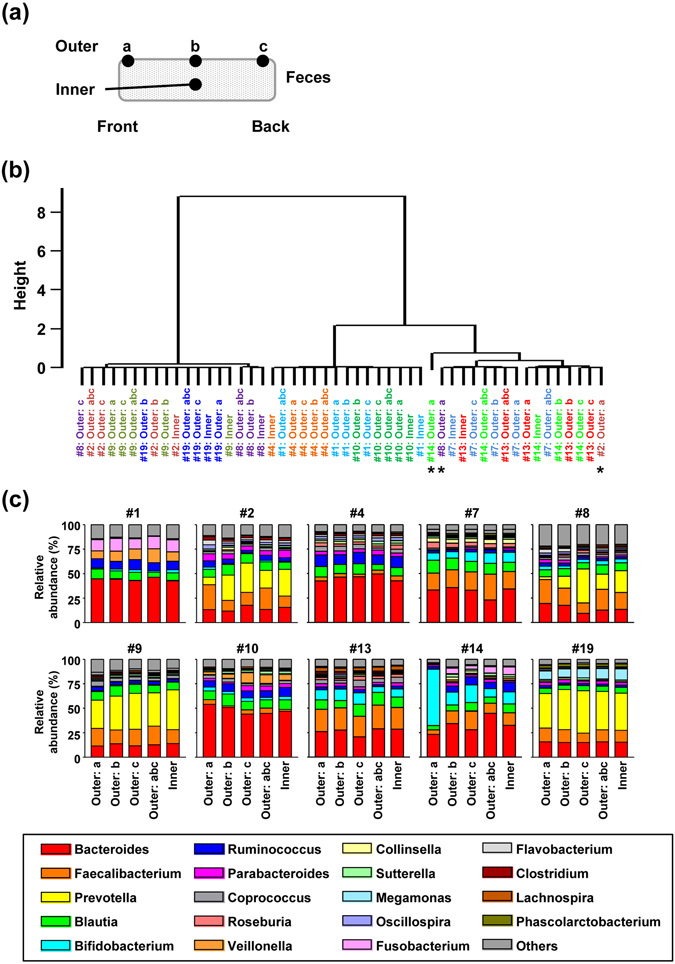
Comparison of the microbial composition at different positions in fecal samples. (a) Schematic diagram showing fecal sampling position. Subsamples were collected from different positions (outer or inner layer; front, middle, or back) in the feces. (b) Phylogenetic tree based on the microbial composition at the genus level. Samples collected from the same individual are indicated by the same color. The height represents relative genetic distance. The asterisks indicate samples that were clustered far from other samples collected from the same individual. (c) Microbial composition at genus level of different positions in feces from 10 individuals.
Representative methods from fecal sampling to DNA extraction
Our current findings suggest that the method using GuSCN solution is not a great solution for microbiome analysis if sampling and storage are not appropriately operated. Hence, the other methods such as liquid nitrogen homogenization technique can considered to be additional option. But, if researchers use GuSCN solution, we suggest the following protocol for representative extraction of DNA from bacterial populations in feces (Fig. 7):
Collect and pool subsamples from several positions on feces
To avoid growth of bacteria (especially Proteobacteria) during storage,
-
(i)
make sure that the fecal concentration in the GuSCN solution is not too high;
-
(ii)
suspend fecal samples, especially those with low water content, completely in the GuSCN solution
-
(3)
Avoid long-term storage of feces to prevent reduction in the calculated proportion of Actinobacteria (ideally extract DNA within 1 week).
-
(4)
Directly extract bacterial DNA from feces in GuSCN solution without any pretreatment.
Figure 7.
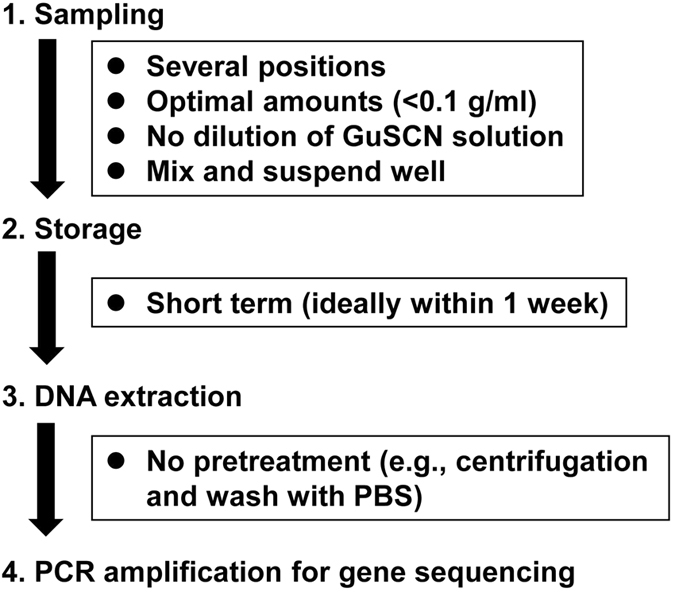
Schematic description of the proposed optimal protocol for 16S rRNA-based analysis of bacterial composition in fecal samples stored in GuSCN solution.
Materials and Methods
Sample collection
Fecal samples were collected from totally 25 healthy adult volunteers (12 males: 22–55 years old; 13 females: 23–52 years old) at the National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN), Osaka and Tokyo, Japan. All experiments were approved by the Committee of NIBIOHN and were conducted in accordance with their guidelines (approval numbers: kenei-14-1 and kenei-3) together with informed consent from all participants. Fecal samples from 10 individuals were used for the analyses in Figs 1–5, Supplementary Figs 1–9, and Supplementary Table 1, 2. Because two individuals withdrew from the study, an additional 2 individuals were recruited for the analyses in Fig. 6 and Supplementary Fig. 10. Furthermore, to generalize our results, additional 8 individuals were recruited for the analyses in Figs 1 and 2 and Supplementary Figs 1–3 and an additional 6 individuals were recruited for the analyses in Supplementary Fig. 9. Each fecal sample was mixed well and divided as follows: (1) some aliquots were placed in 15 ml vials containing 3 ml GuSCN solution (TechnoSuruga Laboratory Co., Ltd, Shizuoka, Japan)17, mixed by vortexing, and stored at room temperature (25 °C), (2) the other aliquots were placed in the vials containing GuSCN solution, phosphate-buffered saline (PBS) was added in some experiments, and the samples were mixed by vortexing, and stored at room temperature.
DNA extraction
DNA was extracted from human fecal samples in GuSCN solution by the bead beating method. To remove GuSCN solution before mechanical lysis, 0.5 ml of fecal sample in GuSCN solution was centrifuged at 13,000 × g for 5 min at room temperature, the supernatant was removed, and the pellet was suspended in 0.5 ml of PBS. To wash the pellet, the suspension was centrifuged as above, the supernatant was removed, and the pellet was re-suspended in 0.5 ml of PBS. An aliquot (0.2 ml) of sample in GuSCN solution, the suspension, and the re-suspension were placed in 2-ml vials (WAKENBTECH Co., Ltd., Tokyo, Japan) containing 0.3 ml of lysis buffer (No. 10, Kurabo Industries Ltd., Osaka, Japan) and 0.5 g of 0.1-mm glass beads. The mixture was mechanically disrupted by bead beating using a Cell destroyer PS1000 (Bio Medical Science, Tokyo, Japan) at 4,260 rpm for 50 s at room temperature. The homogenized sample was centrifuged at 13,000 × g for 5 min at room temperature and 0.2 ml of the supernatant was collected, mixed with 0.15 ml of lysis buffer and 0.15 ml of proteinase K buffer (No. 2, Kurabo Industries Ltd) containing 0.4 mg/ml proteinase K, and DNA was extracted by using a Gene Prep Star PI-80X device (Kurabo Industries Ltd). The concentration of the extracted DNA was determined by using a NanoDrop Spectrophotometer ND-1000 (Thermo Fisher Scientific Inc., DE, USA), and samples were stored at −30 °C until use.
16S rRNA gene amplification and sequencing
The V3–V4 region of the 16S rRNA gene was amplified from the fecal DNA samples by using the following primers: forward: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGCGACAGCCTACGGGNGGCWGCAG-3′, and reverse: 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′20. Reactions were carried out in 25-µl solutions containing 0.2 mM dNTPs, 1 mM MgSO4, 0.2 µM of each primer, 1 U KOD-Plus-v2 (Toyobo Co., Ltd., Osaka, Japan), 1 × PCR buffer for KOD-Plus, and 12.5 ng of sample DNA. The following thermal cycling conditions were used: initial denaturation at 95 °C for 3 min, followed by 25 cycles consisting of denaturation (95 °C for 30 s), annealing (55 °C for 30 s), and extension (68 °C for 1 min), and a final extension step at 68 °C for 5 min. PCR products were purified with 20 µl of Agecourt AMPure XP (Beckman Coulter, Inc., CA, USA) in accordance with the manufacturer’s protocol and eluted into 50 µl of 10 mM Tris-HCl, pH 8.5.
To prepare a DNA library for Illumina MiSeq using Nextera kit set A (Illumina Inc., CA, USA), PCR reactions were then performed in a 50 µl solution containing 0.2 mM dNTPs, 1 mM MgSO4, 5 µl of each primer, 1 U KOD-Plus v2, 1 × PCR buffer for KOD-plus, and 5 µl of the eluted DNA. The following thermal cycling conditions were used: initial denaturation at 95 °C for 3 min, followed by 8 cycles consisting of denaturation (95 °C for 30 s), annealing (55 °C for 30 s), and extension (68 °C for 1 min), and a final extension step at 68 °C for 5 min. PCR products were purified with 56 µl of Agecourt AMPure XP in accordance with the manufacturer’s protocol and eluted into 25 µl of 10 mM Tris-HCl, pH 8.5. The concentration of each PCR product was determined by using the QuantiFluor dsDNA System (Promega, Co., MI, USA); samples were diluted to a final concentration of 4 nM and pooled. The quality of the PCR products was analyzed by agarose gel electrophoresis. 16S rRNA gene sequencing of the PCR products was performed by using Illumina MiSeq (Illumina) in accordance with the manufacturer’s instructions.
16S rRNA sequence data analysis
Sequence reads from Illumina MiSeq were analyzed by using the Quantitative Insights Into Microbial Ecology (QIIME) software package31, 32. Twenty thousand paired-end reads (Phred quality score, >Q30) were selected from each sample. The selected reads were assembled with fastq-join and filtered by using split-libraries-fastq.py, and chimeric sequences were removed by using USEARCH v6.1. The sequences were clustered into OTUs on the basis of sequence similarity (>97%) using open reference OTU picking with UCLUST software. Taxonomy (phylum, class, order, family, genus, and species) and relative abundance were calculated by using the BaseSpace application 16S Metagenomics provided by Illumina and were based on the Greengenes database33, 34. Phylogenetic analysis was performed by using the R package with pvclust based on Pearson correlation coefficient and ward.D2 method (nboost = 10,000)35.
Bacterium specific PCR
Bacterium specific PCR was performed by using primers specific for Bacteroides 36 (forward: 5′-AACGCTAGCTACAGGCTT-3′ and reverse: 5′-CAATCGGAGTTCTTCGTG-3′, annealing at 53 °C), Bifidobacterium 37 (forward: 5′-GGGTGGTAATGCCGGATG-3′ and reverse: 5′-CCACCGTTACACCGGGAA-3′, annealing at 62 °C), and Citrobacter 38 (forward: 5′-TTGGCGTCCAGCGCATTCA-3′ and reverse: 5′-AATTCCAGCCTTCGGCAAACG-3′, annealing at 60 °C). Reactions were carried out in 20-µl solutions containing 0.2 mM dNTPs, 1 mM MgSO4, 0.2 µM of each primer, 1 U KOD-Plus-v2 (Toyobo Co., Ltd.), 1 × PCR buffer for KOD-Plus, and 0.01 ng of sample DNA. The following thermal cycling conditions were used: initial denaturation at 94 °C for 2 min, followed by 35 (for Bacteroides) and 40 (for Bifidobacterium and Citrobacter) cycles consisting of denaturation (94 °C for 15 s), annealing (30 s), and extension (68 °C for 1 min), and a final extension step at 68 °C for 5 min. PCR product was detected by electrophoresis in a 1.5% agarose gel, stained with ethidium bromide, and visualized by transillumination by UV light. DNA molecular weight standard (Nacalai tesque, Inc., Kyoto, Japan) was analyzed along with the samples in each electrophoresis run.
Electronic supplementary material
Acknowledgements
This work was supported by grants from the Ministry of Health, Labour and Welfare of Japan; and the Japan Agency for Medical Research and Development (16817372, 16768433, 16727634 and 17933231); the Ministry of Education, Culture, Sports, Science and Technology of Japan [grant numbers 26293111, 16H01373, 23229004, 15K18950, 17918005 and 15638619]; the Astellas Foundation for Research on Metabolic Disorders; the Terumo Foundation for Life Sciences and Arts; the Suzuken Memorial Foundation; Nipponham Foundation for the Future of Food; and the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry.
Author Contributions
K. Hosomi and J. Kunisawa designed the study and wrote the main manuscript text. H. Ohno, H. Murakami, Y. Natsume-Kitatani, K. Tanisawa, K. Mizuguchi, and M. Miyachi contributed to data collection, analysis and interpretation of data and assisted in the preparation of the manuscript. S. Hirata, H. Suzuki, T. Nagatake, and T. Nishino contributed to data collection and interpretation of data. All authors reviewed the manuscript and the final version of the manuscript was approved by all authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04511-0
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Motohiko Miyachi, Email: miyachi@nibiohn.go.jp.
Jun Kunisawa, Email: kunisawa@nibiohn.go.jp.
References
- 1.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attar N. Bacterial toxins: Strain competition keeps a lid on gut pathogens. Nat. Rev. Microbiol. 2016;14:546. doi: 10.1038/nrmicro.2016.121. [DOI] [PubMed] [Google Scholar]
- 4.Clarridge JE., 3rd Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 2004;17:840–862. doi: 10.1128/CMR.17.4.840-862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiergeist A, Glasner J, Reischl U, Gessner A. Analyses of intestinal microbiota: Culture versus sequencing. ILAR J. 2015;56:228–240. doi: 10.1093/ilar/ilv017. [DOI] [PubMed] [Google Scholar]
- 6.McLoughlin RM, Mills KH. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. J. Allergy Clin. Immunol. 2011;127:1097–1107. doi: 10.1016/j.jaci.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuno MI. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front. Microbiol. 2014;5:190. doi: 10.3389/fmicb.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simren M, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010;33:2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swidsinski A, Loening-Baucke V, Verstraelen H, Osowska S, Doerffel Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology. 2008;135:568–579. doi: 10.1053/j.gastro.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Salonen A, et al. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J. Microbiol. Methods. 2010;81:127–134. doi: 10.1016/j.mimet.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Kuczynski J, et al. Experimental and analytical tools for studying the human microbiome. Nat. Rev. Genet. 2011;13:47–58. doi: 10.1038/nrg3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 15.Ciulla TA, Sklar RM, Hauser SL. A simple method for DNA purification from peripheral blood. Anal. Biochem. 1988;174:485–488. doi: 10.1016/0003-2697(88)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Nishimoto Y, et al. High stability of faecal microbiome composition in guanidine thiocyanate solution at room temperature and robustness during colonoscopy. Gut. 2016;65:1574–1575. doi: 10.1136/gutjnl-2016-311937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hisada T, Endoh K, Kuriki K. Inter- and intra-individual variations in seasonal and daily stabilities of the human gut microbiota in Japanese. Arch. Microbiol. 2015;197:919–934. doi: 10.1007/s00203-015-1125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan S, Cohen DB, Ravel J, Abdo Z, Forney LJ. Evaluation of methods for the extraction and purification of DNA from the human microbiome. PloS One. 2012;7:e33865. doi: 10.1371/journal.pone.0033865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PloS One. 2011;6:e27310. doi: 10.1371/journal.pone.0027310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klindworth A, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishijima S, et al. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016;23:125–133. doi: 10.1093/dnares/dsw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingram LO. Mechanism of lysis of Escherichia coli by ethanol and other chaotropic agents. J. Bacteriol. 1981;146:331–336. doi: 10.1128/jb.146.1.331-336.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason PE, Neilson GW, Dempsey CE, Barnes AC, Cruickshank JM. The hydration structure of guanidinium and thiocyanate ions: implications for protein stability in aqueous solution. Proc. Natl. Acad. Sci. USA. 2003;100:4557–4561. doi: 10.1073/pnas.0735920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 25.von Wintzingerode F, Gobel UB, Stackebrandt E. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 1997;21:213–229. doi: 10.1111/j.1574-6976.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 26.Carbonero F, Nava GM, Benefiel AC, Greenberg E, Gaskins HR. Microbial DNA extraction from intestinal biopsies is improved by avoiding mechanical cell disruption. J. Microbiol. Methods. 2011;87:125–127. doi: 10.1016/j.mimet.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canny GO, McCormick BA. Bacteria in the intestine, helpful residents or enemies from within? Infect. Immun. 2008;76:3360–3373. doi: 10.1128/IAI.00187-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joubert WA, Britz TJ. Characterization of aerobic, facultative anaerobic, and anaerobic bacteria in an acidogenic phase reactor and their metabolite formation. Microb. Ecol. 1987;13:159–168. doi: 10.1007/BF02011251. [DOI] [PubMed] [Google Scholar]
- 29.Fischer B, Hoh S, Wehler M, Hahn EG, Schneider HT. Faecal elastase-1: lyophilization of stool samples prevents false low results in diarrhoea. Scand. J. Gastroenterol. 2001;36:771–774. doi: 10.1080/003655201300192058. [DOI] [PubMed] [Google Scholar]
- 30.Gorzelak MA, et al. Methods for improving human gut microbiome data by reducing variability through sample processing and storage of stool. Plos one. 2015;10:e0134802. doi: 10.1371/journal.pone.0134802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokulich NA, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeSantis TZ, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki R, Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 36.Bernhard AE, Field KG. Identification of nonpoint sources of fecal pollution in coastal waters by using host-specific 16S ribosomal DNA genetic markers from fecal anaerobes. Appl. Environ. Microbiol. 2000;66:1587–1594. doi: 10.1128/AEM.66.4.1587-1594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuki T, Watanabe K, Tanaka R. Genus- and species-specific PCR primers for the detection and identification of Bifidobacteria. Curr. Issues Intest. Microbiol. 2003;4:61–69. [PubMed] [Google Scholar]
- 38.Kaclíková E, Krascsenicsová K, Pangallo D, Kuchta T. Detection and quantification of Citrobacter freundii and C. braakii by 5′-nuclease polymerase chain reaction. Curr. Microbiol. 2005;51:229–232. doi: 10.1007/s00284-005-4528-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


