Abstract
While exposure to nicotine during developmental periods can significantly affect brain development, studies examining the association between maternal smoking and autism spectrum disorder (ASD) in offspring have produced conflicting findings, and prior meta-analyses have found no significant association. Our meta-analysis used a novel approach of investigating population-level smoking metrics as moderators. The main meta-analysis, with 22 observational studies comprising 795,632 cases and 1,829,256 control participants, used a random-effects model to find no significant association between maternal smoking during pregnancy and ASD in offspring (pooled odds ratio (OR) = 1.16, 95% CI: 0.97–1.40). However, meta-regression analyses with moderators were significant when we matched pooled ORs with adult male smoking prevalence (z = 2.55, p = 0.01) in each country, using World Health Organization data. Our study shows that using population-level smoking metrics uncovers significant relationships between maternal smoking and ASD risk. Correlational analyses show that male smoking prevalence approximates secondhand smoke exposure. While we cannot exclude the possibility that our findings reflect the role of paternal or postnatal nicotine exposure, as opposed to maternal or in utero nicotine exposure, this study underlines the importance of investigating paternal and secondhand smoking in addition to maternal smoking in ASD.
Introduction
Autism is a neurodevelopmental disorder defined by persistent difficulties in reciprocal social interaction and in verbal and nonverbal communication skills. Restrictive and repetitive behavioral symptoms and stereotyped patterns of interest are characterized to varying degrees1. The Centers for Disease Control and Prevention estimate that 1 in 68 children have been diagnosed with autism spectrum disorders (ASDs), accounting for more than 3.5 million disability-adjusted life years in America2.
ASDs are considered multifactorial disorders that arise from an interaction between genetics and environmental factors. Several genomics studies have revealed single nucleotide polymorphisms, copy-number variations, and de novo mutations that are associated with ASD3–6. However, other studies highlight the fact that most genetic risk factors, while able to influence the ASD phenotype, cannot independently produce ASD7, 8. Current studies have been exploring environmental factors (ex: viral infection, environmental toxins, gestational diabetes) possibly involved in ASD etiology9. Identifying controllable environmental risk factors will be important for preventing ASD.
One such environmental risk factor that has garnered interest is smoking tobacco. A combination of animal studies and human epidemiological studies point to a plausible relationship between maternal smoking and ASD in offspring. Nicotine, among the thousands of ingredients of tobacco smoke, is the chemical with the highest likelihood of adversely affecting brain development10. Nicotine’s adverse effects are thought to occur via its actions at nicotinic acetylcholine receptors, which mediate neural structural changes11, 12, that in turn can have significant consequences for the offspring in later life. In mice, developmental nicotine exposure in utero and up to postnatal day 21 significantly affected neuronal dendritic morphology and behavior in a passive avoidance paradigm11. Human studies have shown that socioeconomic status (SES) is significantly associated with ASD13, and that women with lower SES have a higher tendency to smoke during pregnancy14. Additionally, smoking is associated with adverse birth outcomes, such as fetal growth restriction and low birth weight15, which are in turn associated with increased risk of ASD16. Smoking during pregnancy has also been associated with neurodevelopmental disorders such as attention deficit hyperactivity disorder, conduct disorder, and antisocial behavior17.
Epidemiological studies exploring the relationship of maternal smoking during pregnancy and ASD have produced contradictory results. For example, in Swedish populations, Lee et al.18 found no evidence of an association between maternal smoking and ASD risk, while Larsson et al.19 found a positive association (OR = 2.09, 95% CI: 1.08–4.03), and Haglund et al.20 found a negative association (OR = 0.7, 95% CI: 0.5–1.0). Similarly, positive21, 22, negative23, 24, and null25–28 associations have been observed in United States study populations. Three meta-analyses have also been conducted, each finding no evidence for a significant association between maternal smoking and ASD risk in offspring. Gardener et al.29 included 5 studies and reported an overall OR of 1.00 (95% CI: 0.75–1.36). Rosen et al.30 included 15 studies and found a summary OR of 1.02 (95% CI: 0.93–1.12), while Tang et al.31 analyzed a partially different set of 15 studies and found a summary OR of 1.02 (95% CI: 0.93–1.13). However, these prior meta-analyses failed to account for study-level and population-level factors regarding smoke exposure that may moderate the relationship between maternal smoking and ASD risk. Specifically, the studies do not systematically account for the possible role of indirect, secondhand smoke exposure; most of the observational studies included in these meta-analyses only reported active maternal smoking. This assessment alone may inadequately reflect total in utero nicotine exposure.
Here, we analyze population smoking prevalence in our meta-regression to investigate how these measures, as potential indicators of secondhand smoke exposure, might moderate the relationship between maternal smoking and ASD. Additionally, two studies22, 32 supporting an association between maternal smoking and ASD risk have been published after the most recent meta-analysis. One of the two studies32 is the first to report extractable data regarding maternal smoking and ASD in an Asian population. Previous meta-analyses have only included studies conducted in European or North American populations. Therefore, the current meta-analysis also included a subgroup analysis by study location. This study was conducted to assess the association between maternal smoking and ASD risk comprehensively, and to examine study location and population smoking metrics as important study-level and population-level moderators of this relationship.
Results
Literature Search and Selection
Our search strategy returned a total of 2,417 publications from PubMed, EMBASE, Web of Science, and Cochrane Library databases (Fig. 1). All references were imported into EndNote X7.6 (Thomson Reuters), which automatically removed 638 duplicates. An additional 129 duplicates were removed manually, leaving 1650 articles that were screened by titles and abstracts for relevance to maternal smoking and ASD. Studies included in previously published meta-analyses30, 31 were also evaluated for inclusion criteria. After screening, 26 studies remained for detailed evaluation of the full texts. Four articles33–36 were then excluded for lacking sufficient data to fill the contingency table of binary maternal smoking and ASD variables. Consequently, 22 articles17–28, 32, 37–45 were included for meta-analysis.
Figure 1.
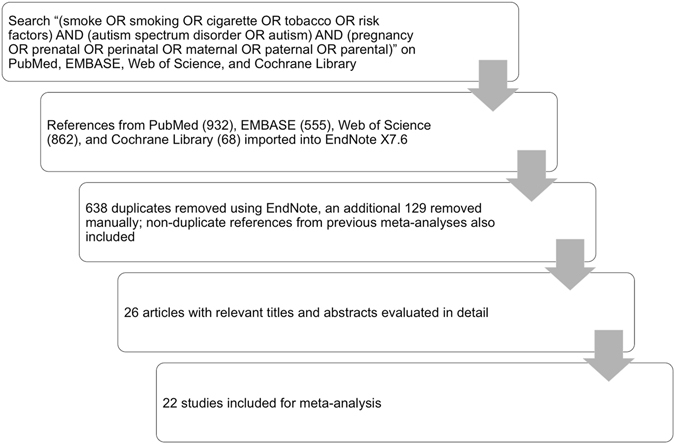
Study selection strategy.
Study Characteristics
The 22 studies analyzed include seven cohort studies19, 23, 39, 40, 43–45 and fifteen case-control studies17, 18, 20–22, 24–28, 32, 37, 38, 41, 42, with a total of 795,632 cases and 1,829,256 control participants. Nine studies17–20, 37, 38, 41, 42, 45 were from Europe (Denmark, Sweden, Finland, The Netherlands, and Poland), twelve studies21–28, 39, 40, 43, 44 were from North America (Canada and U.S.) and one study32 was from Asia (China). Information on smoking during pregnancy was collected during a prenatal visit or at birth in 11/22 studies, and ASD diagnosis was ascertained by medical records in 16/22 studies (Supplementary Table S1).
Quality Assessment and Publication Bias
Two authors independently assigned Newcastle-Ottawa Scale (NOS) scores to each study, with high correlation between scorers (r(20) = 0.81, p < 0.00001) (Fig. 2a). Overall, the studies had moderate methodological quality as scored on a conservatively-interpreted NOS, with an average score of 6.5 and a range of 4–9. Based on thirds of the range of scores, four studies were considered “high” quality with NOS scores of 8 or 917, 22, 25, 37, ten studies were considered “medium” quality with NOS scores of 6 or 718, 21, 23, 26, 38–40, 42, 44, 45, and eight studies were considered “low” quality with NOS scores of 4 to 5.519, 20, 24, 27, 28, 32, 41, 43 (Supplementary Tables S1 and S2).
Figure 2.
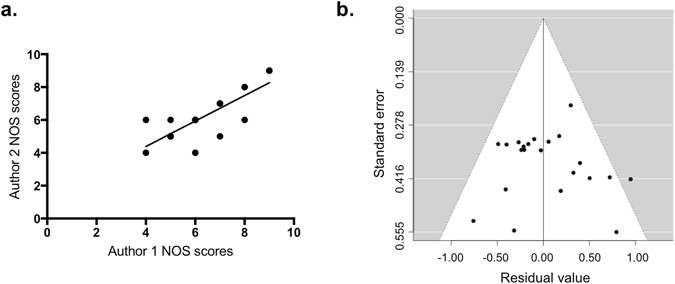
Distribution of studies used in the meta-analysis by (a) study quality, as assessed on the NOS by two independent raters with high correlation (r(20) = 0.81, p < 0.00001), and by (b) funnel plot, to diagnose publication bias.
The shape and symmetry of the funnel plot of the log ORs from the 22 studies are illustrated in Fig. 2b. Begg’s rank correlation test (p = 0.27) and Egger’s regression test (p = 0.54) suggest no significant publication bias46, 47.
Main Analysis
The pooled OR estimates showed that maternal smoking during pregnancy was not associated with a significantly increased risk of ASDs (OR = 1.16; 95% CI: 0.97–1.40) (Fig. 3). A statistically significant level of heterogeneity was found across studies (I 2 = 94.08%, Q(21) = 156.94, p < 0.0001).
Figure 3.
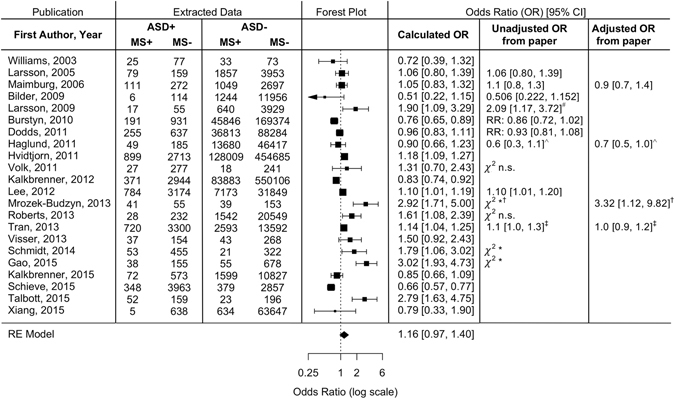
Meta-analysis of the association between maternal smoking during pregnancy and ASD risk based on 22 observational studies. The pooled OR using a random-effects model was found to be 1.16 (95% CI: 0.97–1.40). Data extracted from the papers and ORs calculated from this data are shown, as well as unadjusted and adjusted ORs reported in the observational study publications, or an alternate statistic reported if not ORs. ASD = autism spectrum disorder; MS = maternal smoking during pregnancy; RR = relative risk; *p ≤ 0.05; n.s. = p > 0.05. #As reported for “yes” in response to maternal smoking query. Data extracted for meta-analysis combined “yes” and “yes, but stopped” responses. ∧As reported for ASD and smoking ≥10 cigarettes/day. Data extracted for meta-analysis combined ASD and Asperger’s subgroups, and smoking 1–9 and smoking ≥10 cigarettes/day. †As reported for active maternal smoking. Data extracted for meta-analysis combined active and passive maternal smoking. ‡As reported for smoking in all pregnancy. Data extracted for meta-analysis combined only first trimester and all pregnancy smoking.
Subgroup and Sensitivity Analyses, and Moderator Analysis with Meta-regression
To identify potential causes of the high heterogeneity in our main analysis, we performed subgroup and sensitivity analyses. Subgroup analyses were stratified by study design, location, sample size, quality, adjustment for confounders, maternal smoking assessment, and ASD status ascertainment (Table 1, Supplementary Tables S1 and S2). Maternal smoking during pregnancy was associated with significantly elevated risk of ASD in European populations (OR = 1.14, 95% CI: 1.08–1.19) and in the single study conducted in Asia (OR = 3.02, 95% CI: 1.93–4.73), but not in North American populations (OR = 1.00, 95% CI: 0.77–1.28). Maternal smoking was also associated with elevated ASD risk in studies with the lowest quartile of sample sizes (OR = 1.61, 95% CI: 1.12–2.33). Subgroup analyses by study design, study quality, and adjustment for confounders showed no significant differences in pooled ORs across strata. Assessment of maternal smoking at a prenatal visit (OR = 1.10, 95% CI: 1.03–1.17) or after birth (OR = 1.82, 95% CI: 1.37–2.42) showed significant associations with ASD in offspring, whereas assessment at birth (OR = 0.75, 95% CI: 0.65–0.85) had an apparent protective effect (Table 1). For the sensitivity analysis, the pooled ORs varied moderately, ranging from 1.08 (95% CI: 0.92–1.29) when Gao et al.32 was excluded to 1.17 (95% CI: 0.98–1.47) when Bilder et al.25 was excluded. Case deletion diagnostics identified Talbott et al.22 as a potential outlier. The main meta-analysis without Talbott et al. resulted in a similar pooled OR (OR = 1.12, 95% CI: 0.94–1.33) as the original analysis (OR = 1.16; 95% CI: 0.97–1.40).
Table 1.
Summary of results from subgroup analyses.
| Variable | No. of Studies | I2% | OR (95% CI) |
|---|---|---|---|
| All Studies | 22 | 94.05% | 1.14 (0.96–1.36) |
| Location | |||
| Europe | 9 | 0.02% | 1.14 (1.08–1.19) |
| North America | 12 | 91.69% | 1.00 (0.77–1.28) |
| Asia | 1 | 3.02 (1.93–4.73) | |
| Design | |||
| Case control | 15 | 95.91% | 1.22 (0.93–1.60) |
| Cohort | 7 | 85.04% | 1.04 (0.77–1.39) |
| Adjusted Analysis | |||
| Yes | 11 | 96.20% | 1.09 (0.83–1.45) |
| No | 11 | 91.15% | 1.24 (0.96–1.60) |
| Quality a | |||
| High | 4 | 92.90% | 1.19 (0.65–2.15) |
| Medium | 10 | 84.15% | 1.00 (0.88–1.14) |
| Low | 8 | 91.19% | 1.32 (0.86–2.02) |
| Sample Size b | |||
| 1Q | 6 | 62.87% | 1.61 (1.12–2.33) |
| 2Q | 5 | 94.17% | 1.24 (0.88–1.73) |
| 3Q | 5 | 81.20% | 1.04 (0.74–1.46) |
| 4Q | 6 | 83.63% | 0.37 (0.67–1.22) |
| Maternal Smoking Assessment | |||
| Prenatal | 7 | 35.97% | 1.10 (1.03–1.17) |
| At birth | 4 | 53.26% | 0.75 (0.65–0.85) |
| After birth | 9 | 63.77% | 1.82 (1.37–2.42) |
| ASD Diagnosis | |||
| Direct evaluation | 4 | 43.61% | 1.29 (0.89–1.87) |
| Medical/Developmental record | 16 | 96.55% | 1.09 (0.88–1.37) |
| Parental report | 2 | 0.00% | 1.70 (1.23–2.35) |
aAveraged NOS scores of 8–9 (high), 6–7 (medium), and 4–5.5 (low).
bSample sizes of 208–870 (1Q), 871–10,309 (2Q), 10,310–55,993 (3Q), and 55,994–637,304 (4Q).
Interestingly, subgroup analysis using meta-regression with a continuous moderator found that smoking prevalence in males in the country of each study had a significant influence on ASD risk after maternal smoking (z = 2.33, p < 0.05). The results also showed that the OR increased with increasing smoking prevalance percentage. Therefore, we used the prediction function to obtain the average log OR values for the smoking prevalence percentages while holding the year constant. Smoking prevalence in males by country showed a significant correlation with ASD risk (z = 2.55, p = 0.011) (Fig. 4a). In contrast, smoking prevalence in females by country did not have a statistically significant correlation, (z = −1.13, p = 0.26) (Fig. 4b).
Figure 4.
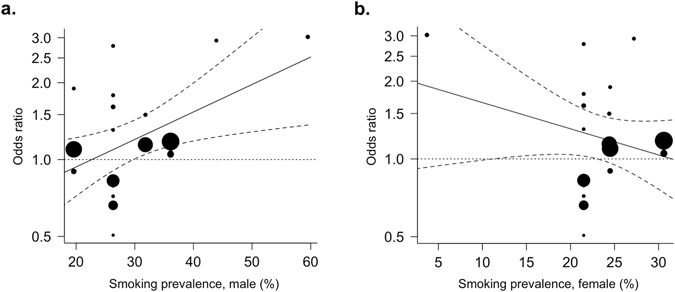
Meta-regression analysis using population smoking metrics as moderators. The OR of ASD with maternal smoking during pregnancy as moderated by (a) smoking prevalence in adult males (%) (z = 2.55, p = 0.011), and (b) smoking prevalence in adult females (%) (z = −1.13, p = 0.26) in the country of each study population. The diameters of the circles are proportional to study population size.
Correlations between population smoking metrics and measures of secondhand smoke exposure
Reports of secondhand smoke exposure in either youth48 or in female adults49 were only available for three (U.S., China, Poland) of the eight countries represented in our study, precluding direct use of secondhand smoke measures in our meta-analyses. Therefore, we performed linear regression analyses to test the relationship between the population smoking metrics used in our analysis and the secondhand smoke exposure data we extracted from global survey reports. These analyses showed significant positive correlations between smoking prevalence in adult males with youth reports of smoke exposure in the home (r(108) = 0.63, p < 0.0001) and outside the home (r(106) = 0.61, p < 0.001) (Fig. 5a). Smoking prevalence in adult females also had significant correlations with youth reports of smoke exposure in the home (r(108) = 0.58, p < 0.0001) and outside the home (r(106) = 0.55, p < 0.0001) (Fig. 5b). Similar results were found when comparing male smoking prevalence to adult female reports of secondhand smoke exposure at home (r(18) = 0.70, p = 0.0006) and at work (r(21) = 0.64, p = 0.0011) (Fig. 5c). There was no significant association between female smoking prevalence and adult female reports of secondhand smoke exposure at home (r(18) = −0.011, p = 0.96) or at work (r(21) = −0.17, p = 0.44) (Fig. 5d).
Figure 5.
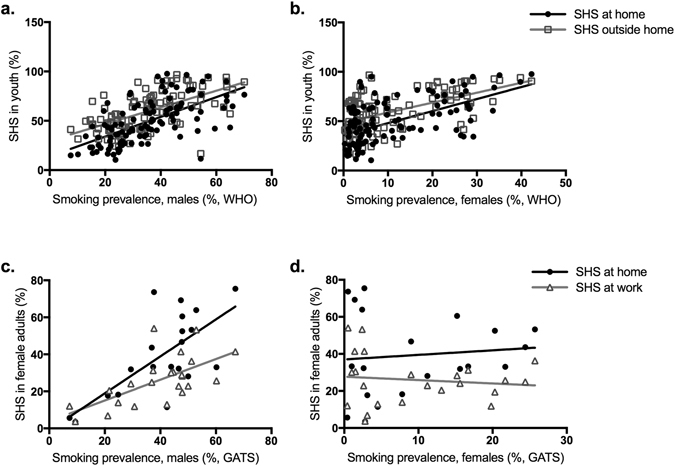
Correlation analyses of population smoking metrics and reports of secondhand smoke exposure. (a) Male and (b) female smoking prevalence percentages compared to secondhand smoke exposure reported by youths 13–15 years old for exposures at home and outside the home, all extracted from WHO’s 2008 report on the global tobacco epidemic. (c) Male and (d) female smoking prevalence percentages compared to secondhand smoke exposure reported by female adults for exposures at home and at work, according to the Global Adult Tobacco Survey accessed through CDC databases. SHS = secondhand smoke.
Discussion
The present study used a comprehensive search strategy and explored the relationship between maternal smoking during pregnancy and ASD in offspring. Three previously published meta-analyses have shown no significant association between maternal smoking and ASD in offspring, analyzing 5 or 15 individual studies29–31. Our main analysis, which included 22 studies and is the first to include an observational study in an Asian population, also showed no significant association between maternal smoking during pregnancy and ASD (Fig. 3). To determine the source of the substantial heterogeneity in our main analysis, we performed subgroup analyses using meta-regression with various modalities. We produced novel findings by comparing results by study location and by using population-level measures of tobacco consumption. In subgroup analyses, a significant positive association between maternal smoking and ASD in the offspring was observed in studies conducted in China and Europe, but not those in North America. Correlating with these relative rankings, the smoking population of adult males in China and the European countries represented in this analysis, except Sweden, are higher than the adult male smoking populations in the U.S. and Canada (Table S2). Our meta-regression analysis with adult male smoking prevalence in each country as a continuous moderator also indicated a significant correlation with ASD risk (Fig. 4a). Furthermore, adult male smoking prevalence was strongly correlated with rates of secondhand smoke exposure reported by youth and adult women (Fig. 5). These results suggest that maternal tobacco exposure may indeed increase ASD risk in offspring, but that exclusively assessing active maternal smoking underestimates total exposure, which includes secondhand smoke, before or after birth. Measures of smoking in the overall population may provide this missing measure of secondhand smoke exposure, thereby modulating the relationship between maternal smoking and ASD in offspring to produce significant correlations.
Two important limitations of using the smoking prevalence data should be noted. First, the 2008 smoking prevalence data were used for all subjects included in the meta-analysis, who were born between 1980–2012. The 2008 report was chosen because it was the earliest report to comprehensively and systematically measure smoking prevalence in member countries. However, our meta-analytic approach and the wide range of birth years represented makes it impossible to precisely match population smoking indices to the subjects’ gestation. Indeed, we cannot exclude the possibility that male smoking may contribute to exposure after birth, not during gestation. Future studies could more accurately track this information by collecting information regarding secondhand smoke exposure directly from the mothers in observational studies. Secondly, the trend towards a negative correlation between OR of ASD risk and smoking prevalence in women, although not statistically significant, seems incongruent with the idea that this metric captures an important measure of secondhand smoke exposure. However, we found that female smoking prevalence, compared to male smoking prevalence, was less strongly correlated with secondhand smoke exposure (Fig. 5). It is also possible that these results may be skewed by the extremely low smoking prevalence in females in China, where only 3.7% of women reported current smoking, compared to 21.5–30.6% of women in other countries48. Such low smoking rates in women have also been observed in other Asian countries such as South Korea, where 7.1% of women smoked according to in-person self-report surveys, but 18.2% of women smoked according to urine cotinine content50. In comparison, 47.8% of men smoked according to self-reports, and 55.1% of men smoked according to urine cotinine content. There may be a similarly disproportionate degree of underreporting in Chinese women, especially around pregnancy51, thereby skewing the data towards a negative trend.
An alternate explanation is that the significant correlations between OR and male smoking prevalence indicate a primary effect of paternal smoking on ASD in offspring, rather than through secondhand smoke exposure in the mother. There is evidence that smoking induces significant changes of DNA methylation in sperm52, 53. DNA methylation is associated with changes in the gene expression profile. In utero nicotine exposure in mice has been shown to induce such changes in gene expression through epigenetic modification11. Therefore, it would not be surprising if both maternal and paternal smoking contribute to changes in gene expression underlying brain development. However, three studies included in our meta-analysis examined secondhand smoke exposure during pregnancy, and their findings suggest that smoking increases ASD risk in offspring primarily through in utero exposure19, 32, 41. Furthermore, two other studies conducted in Chinese populations reported positive associations between passive maternal smoke exposure and ASD54, 55. The passive smoke exposure in these studies was not exclusive to paternal smoking and included, for example, exposure from smoking colleagues; active maternal smoking data was not reported. Together, these results suggest that paternal smoking may be an important factor for its impact on maternal smoke exposure during pregnancy, rather than for direct effects on the father’s role in reproduction.
Importantly, population-level smoking is one of many possible risk factors for ASD. Results of our subgroup and moderator analyses indicated that population-level tobacco consumption, study location, and study sample size could only account for 14% of the total heterogeneity found in this analysis. As noted in the introduction, there are several adverse outcomes, such as low birth weight, linked to both maternal smoking and to ASD, that not only suggest maternal smoking is a plausible environmental cause of ASD, but also pose potential confounds for studying the direct association between ASD and maternal smoking. Several observational studies included in our meta-analysis adjusted for potential confounding factors, either by matching controls to cases on characteristics of interest (e.g., birth year and gender)25, 32, or by adjusting their statistical analysis for potential confounds (e.g., parental age, parental education, parental occupational class, family income, maternal origin of birth, and Apgar score)18, 41. Our study examined adjustment for confounding factors as a potential moderator and, similar to others30, 31, found no differences in our subgroup analyses. However, contributions of these factors cannot be entirely ruled out.
One significant result from our subgroup analyses was that maternal smoking was associated with elevated ASD risk in studies with the lowest quartile of sample sizes (OR = 1.61, 95% CI: 1.12–2.33) (Table 1). Interestingly, all six studies in the lowest quartile of sample sizes assessed maternal smoking exposure after birth; subgroup analyses showed that this method of maternal smoking assessment, used in 9/22 studies, was also associated with an increased risk of ASD (OR = 1.82, 95% CI: 1.37–2.42) (Table 1). Additionally, all 4/22 studies that diagnosed ASD by direct evaluation are represented in the lowest quartile of samples sizes. The significance of this observation is unclear; it may be that studies directly evaluating ASD had small sample sizes necessitated by limited resources, and that the significant effect of sample size in our subgroup analysis is due to sampling bias. Overall, there is still a large portion of heterogeneity in our analyses that is not accounted for.
There are also general limitations to consider regarding ascertainment of exposure and case definition. First, half of the studies (11/22) relied on retrospective reporting, which many believe may be more vulnerable to faulty recall and bias than prospective reporting, especially if reported after an ASD diagnosis30. In both this and a previous meta-analysis30, subgroup analyses showed that assessing maternal smoking at birth had an apparent protective effect, while assessment after birth produced the highest OR for ASD risk (Table 1). Interestingly, whereas Rosen et al.30 found that prenatal assessment resulted in no significant relationship between maternal smoking and ASD, our study found a small but significant effect in the prenatally assessed subgroup of studies (OR = 1.10, 95% CI: 1.03–1.17). Empirically, studies have shown that retrospective reporting is overall accurate and reliable56, 57. One study found that retrospective recall of any smoking in pregnancy, an average of 14.5 years later, had 95.6% and 80.3% sensitivity when compared to positive urine cotinine tests during pregnancy and prospective reporting at the first antenatal visit, respectively, and 93.2% and 92.9% specificity, respectively, when including only women who recalled any smoking after learning of pregnancy58. Importantly, the study also found that there were no differences in child behavior problems, specifically conduct disorder symptoms and antisocial behaviors, between mothers’ whose retrospective recall of smoking status was or was not congruent with prospective reporting or cotinine levels. Therefore, the number of studies relying on retrospective reporting in our meta-analysis is likely not a significant issue, and the results of the subgroup analyses in prenatally and postnatally assessed mothers may accurately reflect a positive association. Second, while retrospective timing of reporting may not be uniquely susceptible to bias, self-reporting of maternal smoking may be particularly underreported if women are aware of its adverse health consequences and/or perceive social disapproval51.
More importantly, self-reporting of active smoking alone likely misrepresents total nicotine exposure. In China, 57% of women reported exposure to secondhand smoke59. In New York City, 46.8% (102/218) of pregnant women who were nonsmokers were found to have serum cotinine levels indicating secondhand smoke exposure60. The results of our moderator analyses indicate a significant relationship between the risk of ASD in children whose mothers’ smoked during pregnancy and male population smoking prevalence, which in turn was significantly correlated with reports of secondhand smoke exposure in youth and female adults. Together, these data may suggest that reports of active smoking alone neglects to account for nicotine exposure from secondhand smoking, which when accounted for may provide new insight into relationships between maternal exposures and subsequent child outcomes.
Another potential limitation is our broad definition of maternal smoking as any smoking during pregnancy, which may have skewed our main analysis towards a null association if in utero nicotine exposure must meet some threshold to increase ASD risk. Studies of attention-deficit/hyperactivity disorder risk after exposure to maternal smoking in pregnancy suggest there may be such a dose effect61; if also true for ASD, it would align with our interpretation that population smoking metrics capture secondhand smoke exposure, which increases total in utero nicotine dose and therefore significantly predicts ASD risk. In the observational studies included in our meta-analysis, only Haglund et al.20 reported smoking dosage20.
Limitations are also inherent in determining case or control status. Our subgroup analyses showed no differences in ORs when ASD status was determined by direct evaluation or by medical or developmental records; parental reporting of ASD status, in the only two studies using this method, resulted in a positive association (OR = 1.70, 95% CI: 1.23–2.35) (Table 1). Diagnoses were variably based on DSM-III, DSM-IV, DSM-IV-TR, or DSM-V criteria. Our definition for positive ASD status was also broad and included all subtypes, such as Asperger’s and Persistent Developmental Disorder, if reported separately. Additionally, in one study, we used a group with intellectual disability (ID) but without an ASD diagnosis as the control group, and ASD with or without ID as the case group24. It is unclear whether the various ASD subtypes have different etiologies, either in general or specifically in relation to maternal smoking62. Future studies that more rigorously and consistently distinguish between subtypes may yield more information.
In summary, our study included 22 observational studies and is the first to include one conducted in an Asian population32. While our main meta-analysis is consistent with previous meta-analyses in finding no significant association between maternal smoking and ASD in offspring, our subgroup and moderator analyses with population smoking metrics suggest that a more nuanced interpretation of this null association is warranted. In future studies, more consistent and systematic examination of non-maternal sources of in utero or developmental nicotine exposure, such as smoking in the fathers, household, or general population, may yield important results.
Methods
Data Sources and Searches
We followed PRIMSA-P guidelines63 for meta-analytic studies. Literature searches were conducted on PubMed, EMBASE, Web of Science, and Cochrane Library to identify peer-reviewed studies published through May 2016 with the following search term: [(smoke ∪ smoking ∪ cigarette ∪ tobacco ∪ risk factors) ∩ (autism spectrum disorder ∪ autism) ∩ (pregnancy ∪ prenatal ∪ perinatal ∪ Maternal ∪ Paternal ∪ Parental)]. No language restrictions were applied. Additionally, studies used in previously published meta-analyses were evaluated for inclusion. Country-specific data on population smoking metrics were extracted from World Health Organization (WHO) reports of adult smoking prevalence for the year 2008, separated by sex48. Smoking was defined as any current smoking at the time of data collection, and age-standardized estimates of smoking prevalence were extracted to allow comparison between countries. Data on secondhand smoke exposure were extracted from the Global Youth Tobacco Survey as reported in the same 2008 WHO report from which adult smoking prevalence data were extracted. Exposure to smoke was defined as “during the last seven days prior to the survey, people smoked at least once in the presence of the interviewee,” and was reported by youths 13–15 years old for exposure inside the home and outside the home48. The Center for Disease Control and Prevention’s (CDC) Global Tobacco Surveillance System (GTSS) data portal was used to access additional data from the Global Adult Tobacco Survey (GATS), from which data on adult reports of secondhand smoke exposure and prevalence of any current smoking in males and females were extracted by country49. Since our study focuses on maternal smoking, we extracted data on female adults’ reports of exposure to secondhand smoking at home (any smoking in home) and at work (in the past 30 days).
Study Selection
Full texts of potentially relevant reports were examined for the following inclusion criteria: (i) a cohort or case-control design and (ii) data reported in such a way that the authors could extract or calculate the number of ASD cases with mothers who did and did not smoke during pregnancy, and control subjects with mothers who did and did not smoke during pregnancy. Two authors (YJ, AML) independently confirmed fulfillment of inclusion criteria.
Data Extraction and Quality Assessment
Information was extracted from each study as a contingency table using a binary maternal smoking variable and a binary ASD variable. Any maternal smoke exposure during pregnancy was considered positive for maternal smoking, and any ASD in offspring, regardless of subtype, was considered positive for ASD. Two authors (YJ, AML) independently extracted data and resolved any disagreements by discussion. Additionally, the following data were extracted: last name of the first author, publication year, study design, study location, maternal smoking assessment method, ASD status assessment method, and unadjusted and adjusted odds ratios (ORs) with 95% confidence interval (CI), or alternate statistic if reported, and whether any adjustments for confounders were made in either participant selection or statistical analysis.
Two authors (YJ, AML) used the Newcastle-Ottawa Scale (NOS) to assess methodological quality64. A maximum of nine points was assigned for eight items in three categories, Selection, Comparability, and Exposure. First, each author independently determined preliminary NOS scores for each study, which were discussed to arrive at a consensus definition of the eight items to be scored. Subsequently, the authors independently re-scored each study according to the agreed-upon criteria, and averaged their scores.
Data Analysis
Unadjusted ORs were calculated from the contingency tables abstracted from each study and combined to produce a measure of the association between maternal smoking and ASD risk. Cochrane Q statistic (significance level at p < 0.10) and the I 2 statistic were used to assess the heterogeneity of studies65, 66. A random-effects model was used to calculate the pooled OR in the context of a general linear model. Smoking prevalence in adult males or in adult females was used as a continuous moderator in the mixed-effects model to examine to what extent these moderators influence the strength of the relationship between maternal smoking and autism. A sensitivity analysis was performed to assess whether any individual study significantly affected pooled estimates by omitting one study in each turn using meta-regression fitting. Outliers or influencers in the studies were diagnosed by examining studentized deleted residuals (rstudent), difference of predicted average effect (DIFFITS), and total changes in parameter estimate (Cook’s distance) in meta-regression fitting. Additionally, subgroup analyses were performed to examine study design (case-control or cohort), location (by continent), sample size (by quartile), quality (NOS score high, medium, or low), adjustment for confounders (yes/no), timing of maternal smoking assessment (prenatal visit, at birth, after birth), and method of ascertaining ASD diagnosis (direct evaluation, medical or developmental record, parental report). Begg’s rank correlation and Egger’s regression tests were used to detect publication bias46. Metafor67 R package was used for all meta-analyses. GraphPad Prism version 7.0 was used to calculate Pearson’s correlation coefficient, r, to test for correlation between two authors’ independent NOS ratings, and to test for correlations between population smoking metrics and measures of secondhand smoke exposure. The correlation between female smoking prevalence and female adult smoke exposure based on GATS data was computed as a nonparametric Spearman correlation due to the non-Gaussian distribution of data. All statistical tests had a significance level of p ≤ 0.05, unless otherwise specified.
Statistics
To determine the effect of moderators, a mixed-effects model was used, in which the unknown true effect, y i, is given by y i = β 0 + β 1 x i1 + … + β p x ip + u i + e i, where x ip denotes the value of the pth moderator for the ith study, and β p denotes the change in the average true effect for a one unit increase in x ip. The Metafor package is advantageous because it allows such mixed-effects models to be fitted with continuous or categorical x moderator variables, compared to previous packages that only allow categorical moderator variables to be fitted. Additionally, u i ~ N(0, τ2) where τ2 denotes the residual heterogeneity not accounted for by the moderators included in the model, and e i ~ N(0, v i) where v i denotes sampling variances. Some assumptions underlying the meta-analysis conducted by Metafor in general include (1) treating sampling variances as if they were known constants, (2) treating τ2 as a constant after it has been estimated and (3) assuming effect sizes are normally distributed67. Further explanation of this model can be found in the Metafor package manual68 and clinical vignette67.
Electronic supplementary material
Acknowledgements
This work was supported by the National Institutes of Health grants DA033945 to S.A.M. and M.R.P., DA014241 and MH077681 to M.R.P., P50DA033945 to S.A.M. and NIH Medical Scientist Training Program Training Grant T32GM007205 to A.M.L.
Author Contributions
Y.J. designed the study, Y.J. and A.M.L. performed data collection, data analyses, wrote and edited the manuscript. S.A.M. provided crucial suggestions for the analysis and edited the manuscript. M.R.P. assisted in interpretation of all studies and edited the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Yonwoo Jung and Angela M. Lee contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04413-1
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders V. 5th edn, (American Psychiatric Press, 2013).
- 2.Centers for Disease Control and Prevention. CDC estimates 1 in 68 children has been identified with autism spectrum disorder. (https://www.cdc.gov/media/releases/2014/p0327-autism-spectrum-disorder.html, 2014).
- 3.Gaugler T, et al. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46:881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Rubeis S, Buxbaum JD. Genetics and genomics of autism spectrum disorder: embracing complexity. Hum Mol Genet. 2015;24:R24–31. doi: 10.1093/hmg/ddv273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry. 2015;20:640–646. doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- 6.Shishido E, Aleksic B, Ozaki N. Copy-number variation in the pathogenesis of autism spectrum disorder. Psychiatry Clin Neurosci. 2014;68:85–95. doi: 10.1111/pcn.12128. [DOI] [PubMed] [Google Scholar]
- 7.Clarke TK, et al. Common polygenic risk for autism spectrum disorder (ASD) is associated with cognitive ability in the general population. Mol Psychiatry. 2016;21:419–425. doi: 10.1038/mp.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson EB, et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 2016;48:552–555. doi: 10.1038/ng.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabrucker AM. Environmental factors in autism. Front Psychiatry. 2012;3:118. doi: 10.3389/fpsyt.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiesler CM, Heinrich J. Prenatal nicotine exposure and child behavioural problems. Eur Child Adolesc Psychiatry. 2014;23:913–929. doi: 10.1007/s00787-014-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung Y, et al. An epigenetic mechanism mediates developmental nicotine effects on neuronal structure and behavior. Nat Neurosci. 2016;19:905–914. doi: 10.1038/nn.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Onofrio BM, et al. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: a population-based study in Sweden. Arch Gen Psychiatry. 2010;67:529–538. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekblad M, Gissler M, Korkeila J, Lehtonen L. Trends and risk groups for smoking during pregnancy in Finland and other Nordic countries. Eur J Public Health. 2014;24:544–551. doi: 10.1093/eurpub/ckt128. [DOI] [PubMed] [Google Scholar]
- 15.Banderali G, et al. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: a descriptive review. J Transl Med. 2015;13:327. doi: 10.1186/s12967-015-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128:344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran PL, et al. Smoking during pregnancy and risk of autism spectrum disorder in a Finnish National Birth Cohort. Paediatr Perinat Epidemiol. 2013;27:266–274. doi: 10.1111/ppe.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BK, et al. Brief report: maternal smoking during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2012;42:2000–2005. doi: 10.1007/s10803-011-1425-4. [DOI] [PubMed] [Google Scholar]
- 19.Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6–8 years of age. Neurotoxicology. 2009;30:822–831. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haglund NG, Kallen KB. Risk factors for autism and Asperger syndrome. Perinatal factors and migration. Autism. 2011;15:163–183. doi: 10.1177/1362361309353614. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt RJ, Tancredi DJ, Krakowiak P, Hansen RL, Ozonoff S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am J Epidemiol. 2014;180:890–900. doi: 10.1093/aje/kwu208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talbott EO, et al. Fine particulate matter and the risk of autism spectrum disorder. Environ Res. 2015;140:414–420. doi: 10.1016/j.envres.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Kalkbrenner AE, et al. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology. 2015;26:30–42. doi: 10.1097/EDE.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 24.Schieve LA, Clayton HB, Durkin MS, Wingate MS, Drews-Botsch C. Comparison of Perinatal Risk Factors Associated with Autism Spectrum Disorder (ASD), Intellectual Disability (ID), and Co-occurring ASD and ID. J Autism Dev Disord. 2015;45:2361–2372. doi: 10.1007/s10803-015-2402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics. 2009;123:1293–1300. doi: 10.1542/peds.2008-0927. [DOI] [PubMed] [Google Scholar]
- 26.Kalkbrenner AE, et al. Maternal smoking during pregnancy and the prevalence of autism spectrum disorders, using data from the autism and developmental disabilities monitoring network. Environ Health Perspect. 2012;120:1042–1048. doi: 10.1289/ehp.1104556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119:873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams G, Oliver JM, Allard AM, Sears L. Autism and associated medical and familial factors: A case control study. J Dev Phys Disabil. 2003;15:335–349. doi: 10.1023/A:1026310216069. [DOI] [Google Scholar]
- 29.Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. 2009;195:7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen BN, Lee BK, Lee NL, Yang Y, Burstyn I. Maternal Smoking and Autism Spectrum Disorder: A Meta-analysis. J Autism Dev Disord. 2015;45:1689–1698. doi: 10.1007/s10803-014-2327-z. [DOI] [PubMed] [Google Scholar]
- 31.Tang S, Wang Y, Gong X, Wang G. A Meta-Analysis of Maternal Smoking during Pregnancy and Autism Spectrum Disorder Risk in Offspring. Int J Environ Res Public Health. 2015;12:10418–10431. doi: 10.3390/ijerph120910418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao L, et al. Association between Prenatal Environmental Factors and Child Autism: A Case Control Study in Tianjin, China. Biomed Environ Sci. 2015;28:642–650. doi: 10.3967/bes2015.090. [DOI] [PubMed] [Google Scholar]
- 33.Hultman CM, Sparen P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology. 2002;13:417–423. doi: 10.1097/00001648-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Juul-Dam N, Townsend J, Courchesne E. Prenatal, perinatal, and neonatal factors in autism, pervasive developmental disorder-not otherwise specified, and the general population. Pediatrics. 2001;107:E63. doi: 10.1542/peds.107.4.e63. [DOI] [PubMed] [Google Scholar]
- 35.Lehti V, et al. Parental migration and Asperger’s syndrome. Eur Child Adolesc Psychiatry. 2015;24:941–948. doi: 10.1007/s00787-014-0643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nilsen RM, et al. Analysis of self-selection bias in a population-based cohort study of autism spectrum disorders. Paediatr Perinat Epidemiol. 2013;27:553–563. doi: 10.1111/ppe.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson, H. J. et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol161, 916–925; discussion 926–918, doi:10.1093/aje/kwi123 (2005). [DOI] [PubMed]
- 38.Maimburg RD, Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatr Scand. 2006;114:257–264. doi: 10.1111/j.1600-0447.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- 39.Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can. 2010;30:125–134. [PubMed] [Google Scholar]
- 40.Dodds L, et al. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord. 2011;41:891–902. doi: 10.1007/s10803-010-1114-8. [DOI] [PubMed] [Google Scholar]
- 41.Mrozek-Budzyn, D., Majewska, R. & Kieltyka, A. Prenatal, perinatal and neonatal risk factors for autism - study in Poland. Open Medicine8, 10.2478/s11536-013-0174-5 (2013).
- 42.Visser JC, et al. Narrowly versus broadly defined autism spectrum disorders: differences in pre- and perinatal risk factors. J Autism Dev Disord. 2013;43:1505–1516. doi: 10.1007/s10803-012-1678-6. [DOI] [PubMed] [Google Scholar]
- 43.Roberts AL, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect. 2013;121:978–984. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang AH, et al. Association of maternal diabetes with autism in offspring. JAMA. 2015;313:1425–1434. doi: 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]
- 45.Hvidtjorn D, et al. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. J Epidemiol Community Health. 2011;65:497–502. doi: 10.1136/jech.2009.093823. [DOI] [PubMed] [Google Scholar]
- 46.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 47.Egger M, Davey Smith G, Schneider M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. WHO REPORT on the global TOBACCO epidemic. (2008).
- 49.Centers for Disease Control and Prevention. Global Tobacco Surveillance System Data. (https://www.cdc.gov/tobacco/global/gtss/gtssdata/index.html, 2016).
- 50.Park MB, Kim CB, Nam EW, Hong KS. Does South Korea have hidden female smokers: discrepancies in smoking rates between self-reports and urinary cotinine level. BMC Womens Health. 2014;14:156. doi: 10.1186/s12905-014-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong M, Koren G. Bias in maternal reports of smoking during pregnancy associated with fetal distress. Can J Public Health. 2001;92:109–112. doi: 10.1007/BF03404942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai J, et al. Nicotine elevates sperm motility and induces Pfn1 promoter hypomethylation in mouse testis. Andrology. 2015;3:967–978. doi: 10.1111/andr.12072. [DOI] [PubMed] [Google Scholar]
- 53.Kim SK, Jee BC, Kim SH. Histone methylation and acetylation in ejaculated human sperm: effects of swim-up and smoking. Fertil Steril. 2015;103:1425–1431. doi: 10.1016/j.fertnstert.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Duan G, Yao M, Ma Y, Zhang W. Perinatal and background risk factors for childhood autism in central China. Psychiatry Res. 2014;220:410–417. doi: 10.1016/j.psychres.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, et al. Prenatal and perinatal risk factors for autism in China. J Autism Dev Disord. 2010;40:1311–1321. doi: 10.1007/s10803-010-0992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heath AC, et al. Accuracy of mothers’ retrospective reports of smoking during pregnancy: comparison with twin sister informant ratings. Twin Res. 2003;6:297–301. doi: 10.1375/136905203322296656. [DOI] [PubMed] [Google Scholar]
- 57.Tomeo CA, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10:774–777. doi: 10.1097/00001648-199911000-00022. [DOI] [PubMed] [Google Scholar]
- 58.Pickett KE, Kasza K, Biesecker G, Wright RJ, Wakschlag LS. Women who remember, women who do not: a methodological study of maternal recall of smoking in pregnancy. Nicotine Tob Res. 2009;11:1166–1174. doi: 10.1093/ntr/ntp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdullah AS. Changing the tobacco trend in China. Lancet. 2000;356:432–433. doi: 10.1016/S0140-6736(05)73581-9. [DOI] [PubMed] [Google Scholar]
- 60.Hawkins SS, et al. Secondhand smoke exposure among nonsmoking pregnant women in New York City. Nicotine Tob Res. 2014;16:1079–1084. doi: 10.1093/ntr/ntu034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kotimaa AJ, et al. Maternal smoking and hyperactivity in 8-year-old children. J Am Acad Child Adolesc Psychiatry. 2003;42:826–833. doi: 10.1097/01.CHI.0000046866.56865.A2. [DOI] [PubMed] [Google Scholar]
- 62.RK, C. Y. et al. Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat Neurosci, doi:10.1038/nn.4524 (2017). [DOI] [PMC free article] [PubMed]
- 63.Shamseer L, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 64.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 65.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 66.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 68.Viechtbauer, W. Meta-Analysis Package for R. CRAN. https://cran.r-project.org/web/packages/metafor/metafor.pdf (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


