Abstract
Purpose
Previous studies indicated conflicting results regarding the prognosis of gastric cancer with a family history (FHX). This study aimed to determine the clinicopathological features and survival of patients with gastric cancer with a FHX.
Materials and Methods
We reviewed 2,736 patients with gastric cancer who underwent surgery between 2003 and 2009. The prognostic value of a FHX was determined in the multivariate model after adjusting for variables in the Asian and internationally validated prognostic models.
Results
Of the patients, 413 (15.1%) had a FHX of gastric cancer. The patients with a FHX were younger (58.1 vs. 60.4 years; P<0.001) than the patients without a FHX. There were no significant differences in the histopathological characteristics between the 2 groups. A FHX was associated with a better overall survival (OS) rate only in the stage I group (5-year survival rate, 95% vs. 92%; P=0.006). However, the disease-specific survival (DSS) rate was not significantly different between the 2 groups in all stages. The multivariate model adjusted for the variables in the Asian and internationally validated prognostic models revealed that FHX has no significant prognostic value for OS and DSS.
Conclusions
The clinicopathological features and survival of the patients with gastric cancer with a FHX did not significantly differ from those of the patients without a FHX.
Keywords: Stomach neoplasms, Prognosis, Clinicopathological features, Family history, Survival
INTRODUCTION
Despite its decreasing global incidence, gastric cancer is one of the most common malignant diseases and a leading cause of cancer mortality in East Asian countries [1]. Most gastric cancers develop in response to the cumulative effect of multiple environmental factors, including Helicobacter pylori infection, smoking, and dietary factors, such as high consumption of salty foods and nitrite compounds [2,3]. On the contrary, genetic factors, such as the CDH1 germline mutation and other hereditary genetic defects, account for a very small percentage of gastric cancer cases [4]. The marked geographical variation, decreasing time trend, and migratory effect on gastric cancer incidence suggest that environmental factors are probably major contributors to gastric carcinogenesis [5].
A family history (FHX) is one of the major risk factors for gastric cancer. A FHX exists in approximately 10% to 20% of patients with gastric cancer [6]. The association of a FHX with an increasing gastric cancer risk may suggest the role of genetic predisposition in gastric carcinogenesis [7]. However, many suggest that sharing of non-genetic environmental risk factors is a more important cause for increasing gastric cancer risk within family members of patients with gastric cancer [8,9].
Besides the pathogenesis, the biological behavior of gastric cancers with a FHX is also a matter of debate. Previous studies have shown conflicting results regarding the clinicopathological features and prognosis of gastric cancer with a FHX [10,11,12,13]. In this study, we compared the clinicopathological features and survival between patients with gastric cancer with and without a FHX.
MATERIALS AND METHODS
Patients and data
From a gastric cancer database in our institution (Chonnam National University Hwasun Hospital [CNUHH]), we selected 2,736 consecutive patients who underwent surgery between 2003 and 2009 and compared the clinicopathological features and survivals between the patients with and without a FHX of gastric cancer. Patients who had 1) a bypass or exploratory laparotomy only, 2) surgery for remnant gastric cancer, 3) combined other organ malignancies, or 4) incomplete medical records were excluded from the study.
Patient data and details of the FHX of gastric cancer were obtained from the prospectively constructed database. Demographic data included age, sex, body mass index (BMI), and underlying comorbidities. Clinicopathological data included operative results, pathological reports, hospital course, recurrence, and survival. Comorbidity was defined as a pre-existing medical illness that requires medication or treatment. Pathological staging was based on the 7th edition of the Union for International Cancer Control's tumor, node, and metastasis (TNM) classification [14].
FHX of gastric cancer, including the number and the relation of relatives who had gastric cancer, was investigated during patient interviews. A positive FHX was defined as the presence of first- or second-degree relatives with gastric cancer. If the patients had a FHX involving both first- and second-degree relatives, they were regarded as having a first-degree FHX for purposes of this study.
The primary outcomes were overall survival (OS) and disease-specific survival (DSS). OS was defined as the time from surgery to death from any cause after surgery. DSS was defined as the time from surgery to death from gastric cancer. For DSS, any deaths from other causes were censored at the last documented date. The patients were followed up until December 2014. This study was approved by the Institutional Review Board of CNUHH, Korea, which waived the requirement for obtaining an informed consent.
All surgeries were performed by 4 experienced gastric surgeons. Surgical procedures of gastric resection and lymph node (LN) dissection predominately followed the Japanese gastric cancer treatment guideline [15]. For instance, a gastric resection with D2 LN dissection was performed as a standard procedure for the majority of patients, with the exception of cT1N0 gastric cancer. After surgery, the patients with stage ≥II disease routinely received adjuvant chemotherapy with 5-fluorouracil/cisplatin or oral S-1. The patients were regularly followed up every 6 to 12 months for up to 5 postoperative years with abdominal computed tomography (CT) and endoscopy. Chest CT, magnetic resonance imaging, or positron emission tomography (PET)/CT was selectively performed when an additional work-up was indicated.
Statistical analysis
Continuous data were compared using the t-test or Mann-Whitney U test. Categorical data were compared using the χ2 test or Fisher's exact test, as appropriate. Survival function was calculated using the Kaplan-Meier method and compared with the log-rank test. The Cox proportional hazards model was used for the multivariate analyses of survival. To assess the prognostic value of FHX, hazard ratios (HRs) of FHX for OS and DSS were calculated after adjusting for the variables in the Asian [16] and internationally validated prognostic models [17]. All statistical analyses were performed using the SPSS version 23.0 (IBM Corp., Armonk, NY, USA), and 2-sided P-values of <0.05 were considered statistically significant.
RESULTS
Of the 2,736 patients analyzed, 413 (15.1%) had a FHX of gastric cancer: 353 patients had a first-degree relative, 49 had a second-degree relative, and 11 had both. With respect to the patients with a FHX, 363 (13.3%) had only one patient with gastric cancer in their family, and 50 (1.8%) had 2 or more. Among them, 151 (5.5%) met the diagnostic criteria for familial gastric cancer according to the International Gastric Cancer Linkage Consortium (IGCLC): 1) gastric cancer in 2 or more first- or second-degree relatives, with at least 1 diagnosis at <50 years of age, and 2) gastric cancer in 3 or more first- or second-degree relatives, independent of their age [4].
The patients with a FHX had a younger age (58.1 vs. 60.4 years; P<0.001) than the patients without a FHX (Table 1). However, there were no significant differences in sex, BMI, and comorbidity between the 2 groups. Pathological examinations showed no significant differences in the histopathological characteristics between the 2 groups, such as tumor size and location, histological types, differentiation, Lauren classification, macroscopic type, tumor depth (pT), nodal metastasis (pN), and distant metastasis (M).
Table 1. Clinicopathological characteristics of the patients with and without a FHX of gastric cancer.
| Characteristics | FHX of gastric cancer | P | ||
|---|---|---|---|---|
| Absent (n=2,323) | Present (n=413) | |||
| Age (yr) | 60.4±11.9 | 58.1±11.9 | <0.001 | |
| Sex (Male) | 1,549 (66.7) | 291 (70.5) | 0.132 | |
| BMI (kg/m2) | 22.9±3.0 | 23.2±2.9 | 0.132 | |
| Comorbidity | 1,150 (49.5) | 207 (50.1) | 0.818 | |
| Tumor markers | ||||
| CEA (elevated) | 304 (13.1) | 58 (14.0) | 0.628 | |
| CA19-9 (elevated) | 142 (6.1) | 23 (5.6) | 0.538 | |
| Tumor location | 0.965 | |||
| Lower third | 1,356 (58.4) | 246 (59.6) | ||
| Middle third | 622 (26.8) | 109 (26.4) | ||
| Upper third | 317 (13.6) | 53 (12.8) | ||
| Entire stomach | 28 (1.2) | 5 (1.2) | ||
| Gastric resection | 0.694 | |||
| Distal gastrectomy | 1,736 (75.9) | 316 (76.5) | ||
| Total gastrectomy | 552 (23.8) | 97 (23.5) | ||
| Others | 8 (0.3) | 0 | ||
| No. of harvested LNs | 37±16 | 35±15 | 0.132 | |
| No. of metastatic LNs | 2.8±7.5 | 2.4±5.6 | 0.257 | |
| Differentiation | 0.556 | |||
| Differentiated | 998 (43.0) | 171 (41.4) | ||
| Undifferentiated | 1,325 (57.0) | 242 (58.6) | ||
| Lauren classification | 0.816 | |||
| Intestinal | 1,339 (57.6) | 246 (59.6) | ||
| Diffuse | 466 (20.1) | 82 (19.9) | ||
| Intermediate | 28 (1.2) | 3 (0.7) | ||
| Mixed | 281 (12.1) | 50 (12.1) | ||
| Undetermined | 209 (9.0) | 32 (7.7) | ||
| Macroscopic type | 0.921 | |||
| Superficial | 1,142 (49.2) | 213 (51.6) | ||
| Borrmann I | 73 (3.1) | 12 (2.9) | ||
| Borrmann II | 325 (14.0) | 54 (13.1) | ||
| Borrmann III | 683 (29.4) | 118 (28.6) | ||
| Borrmann IV | 100 (4.3) | 16 (3.9) | ||
| Lymphovascular invasion | 755 (32.5) | 148 (35.8) | 0.184 | |
| Perineural invasion | 785 (33.8) | 146 (35.4) | 0.538 | |
| Tumor size (mm) | 37±26 | 37±25 | 0.997 | |
| Tumor depth | 0.865 | |||
| pT1 | 1,299 (55.9) | 235 (56.9) | ||
| pT2 | 249 (10.7) | 39 (9.4) | ||
| pT3 | 350 (15.1) | 85 (15.7) | ||
| pT4 | 425 (18.3) | 74 (17.9) | ||
| Nodal metastasis | 0.845 | |||
| pN0 | 1,509 (65.0) | 272 (65.9) | ||
| pN1 | 269 (11.6) | 45 (10.9) | ||
| pN2 | 235 (10.1) | 47 (11.4) | ||
| pN3 | 310 (13.4) | 49 (11.9) | ||
| Distant metastasis | 171 (7.4) | 21 (5.1) | 0.095 | |
Variables are expressed as means±standard deviation or number (%).
FHX = family history; BMI = body mass index; CEA = carcinoembryonic antigen; CA19-9 = carbohydrate antigen 19-9; LN = lymph node; pT = tumor depth; pN = nodal metastasis.
Survival of the patients with a FHX
During the follow-up period, 751 patients had died: 557 due to gastric cancer-related causes and 194 due to other causes. The median follow-up time was 77 months (range, 1–140 months). The 3- and 5-year OS rates were 83% and 77%, respectively. The 3- and 5-year DSS rates were 85% and 81%, respectively.
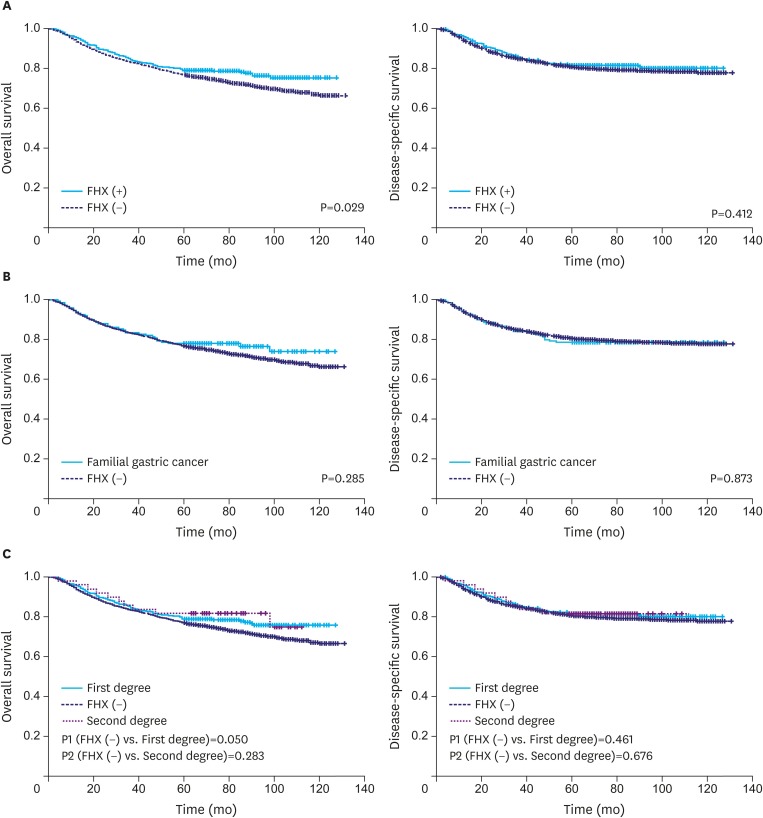
Fig. 1A shows the OS and DSS rates of the patients with and without a FHX. The patients with a FHX showed a better OS rate (5-year survival rate, 79% vs. 77%; P=0.029) than those without, while the DSS rates were not significantly different between the 2 groups. Fig. 1B shows the survival rates of the patients who met the criteria for familial gastric cancer (151 patients), and their OS and DSS rates did not significantly differ from those of the patients without a FHX. Survival according to the degree of FHX revealed that the patients with a first-degree FHX showed a better OS rate than the patients without a FHX (5-year survival rate, 79% vs. 77%; P=0.050). However, the DSS rates of the patients with a first- and second-degree FHX were not significantly different from those of the patients without a FHX (Fig. 1C).
Fig. 1.
(A) Survival of the patients with and without a FHX. (B) Survival of familial gastric cancer and gastric cancer without a FHX. (C) Survival according to the degree of FHX.
FHX = family history.
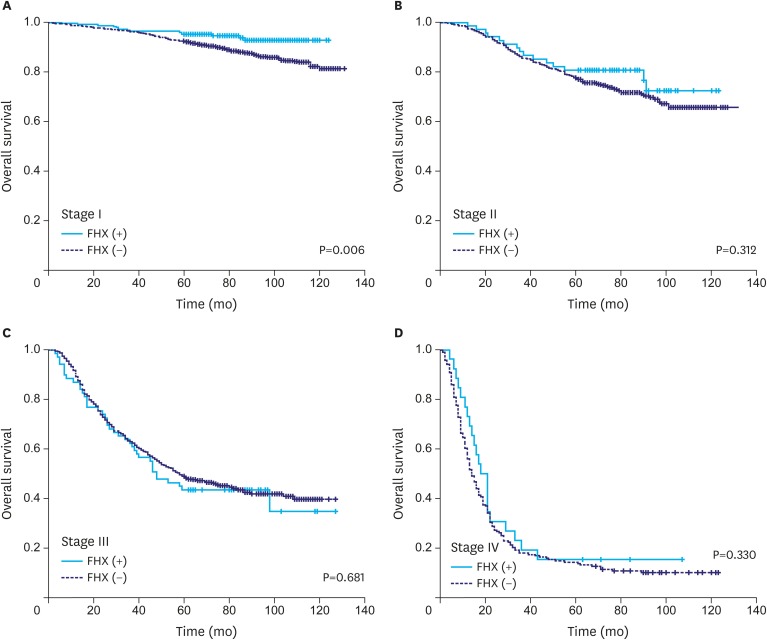
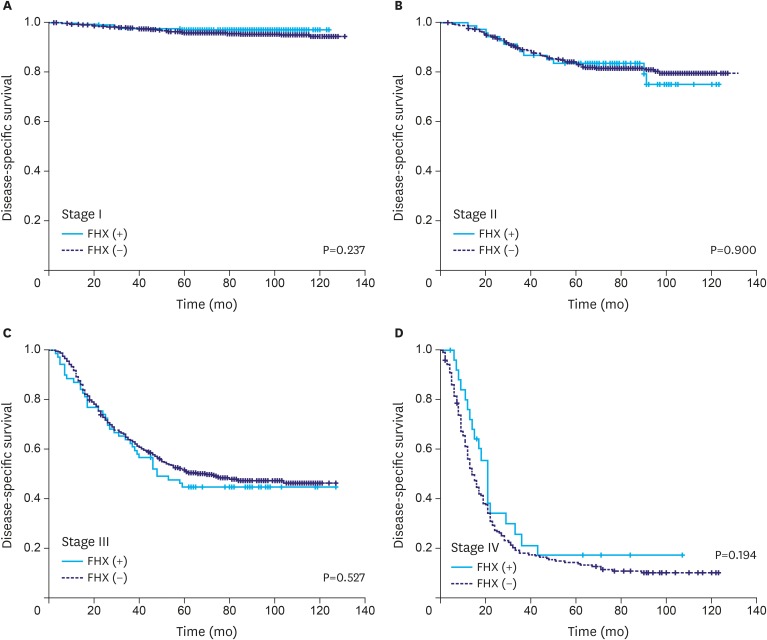
When the OS rates of the patients with and without a FHX were compared with respect to different tumor stages, a difference in the OS rate was only found in the stage I group (5-year survival rate, 95% vs. 92%; P=0.006) (Fig. 2). Meanwhile, there were no significant differences in the DSS rate between the groups at any stage (Fig. 3).
Fig. 2.
OS of the patients with and without a FHX of gastric cancer in different tumor stages. A difference in the OS is only found in the stage I group.
OS = overall survival; FHX = family history.
Fig. 3.
DSS of the patients with and without a FHX of gastric cancer in different tumor stages. The DSS rates are not significantly different between the groups at any disease stage.
DSS = disease-specific survival; FHX = family history.
Prognostic value of FHX in the multivariate prognostic model
To determine the prognostic value of FHX, HRs of FHX were calculated after adjusting for the prognostic variables in the Asian and internationally validated multivariate prognostic models (Table 2). After adjusting for the variables in the Asian prognostic model, the HRs of FHX for OS and DSS were 0.842 (95% confidence interval [CI], 0.676–1.049) and 0.958 (95% CI, 0.752–1.220), respectively. After adjusting for the variables in the internationally validated model, the HRs of FHX for OS and DSS were 0.840 (95% CI, 0.673–1.047) and 0.963 (95% CI, 0.755–1.228), respectively.
Table 2. Prognostic value of a FHX of gastric cancer in the Asian and internationally validated multivariate prognostic models.
| Variables in model | OS | DSS | |||
|---|---|---|---|---|---|
| Adjusted HR for FHX of gastric cancer (95% CI) | P | Adjusted HR for FHX of gastric cancer (95% CI) | P | ||
| Asian validated prognostic model [18] | FHX+age+tumor depth+No. of metastatic LN+No. of harvested LN+distant metastasis+extent of resection | 0.842 (0.676–1.049) | 0.125 | 0.958 (0.752–1.220) | 0.727 |
| Internationally validated prognostic model [19] | FHX+age+sex+Lauren type+tumor location+tumor size+No. of metastatic LN+No. of harvested LN | 0.840 (0.673–1.047) | 0.121 | 0.963 (0.755–1.228) | 0.762 |
FHX = family history; OS = overall survival; DSS = disease-specific survival; HR = hazard ratio; CI = confidence interval; LN = lymph node.
DISCUSSION
FHX is a major risk factor for gastric cancer. However, the biological behavior of gastric cancers with a FHX is largely unknown. In particular, previous studies have shown conflicting results regarding the association of FHX with cancer survival in patients with gastric cancer [10,11,12,13]. The present study is the largest study to investigate the clinicopathological features and survival of patients with gastric cancer with a FHX. We found no significant differences in the tumor characteristics between the patients with and without a FHX. Notably, a FHX had no prognostic impact in the multivariate model adjusted for the variables in the validated prognostic models. Therefore, our results suggest that the biological behavior of gastric cancers with a FHX does not significantly differ from that of gastric cancers without a FHX.
In the present study, the patients with a FHX showed a marginally superior OS rate only in the stage I group (5-year survival rate, 95% vs. 92%; P=0.006). Interestingly, we found that the survival difference became more pronounced as the follow-up time increased over 5 years. Meanwhile, the DSS rate was not significantly different between the groups. This implies that deaths from causes other than gastric cancer were more common in the patients without a FHX. This is probably because of the significant age difference between the patients with and without a FHX. With regard to OS, the HR of FHX was not significant when age was adjusted with other prognostic factors in the multivariate model. Similarly, Fang et al. [18] showed that patients with a FHX had a better survival as they were younger and had a less advanced tumor stage, and a FHX had no direct effect on the gastric cancer survival.
There are a limited number of studies investigating the association of FHX with survival for gastric cancer. In a small Italian study, a first-degree FHX showed no significant association with survival of gastric cancer when adjusted for age, sex, and tumor stage [10]. Similarly, a Chinese study of 914 patients with gastric cancer reported no significant association between FHX and survival in both patients with cardiac and non-cardiac gastric cancer [11]. By contrast, a large Korean study of 1,273 patients with gastric cancer showed that a first-degree FHX was associated with a reduced risk of recurrence and death in patients with stage III–IV gastric cancer [13]. However, this study is limited by the small number of patients in the stage III–IV group (only 48 cases with a FHX) and the unusually young age of the patients without a FHX [19].
The molecular pathogenesis associated with an increasing gastric cancer risk with a FHX has yet to be elucidated. Some researchers reported a higher prevalence of microsatellite instability in patients with a FHX than in those without [20,21]. Genetic polymorphisms of pro-inflammatory cytokines, combined with familial clustering of H. pylori infection, have been reported to be associated with an increased risk of gastric cancer with a FHX [22,23]. Ebert et al. [24] reported an overexpression of transforming growth factor (TGF)-β1 in the gastric mucosa of first-degree relatives of patients with gastric cancer, which suggests an indirect role for TGF-β1 in the genetic predisposition of gastric cancer. A better understanding on the molecular pathogenesis associated with an increasing gastric cancer risk with a FHX would facilitate early identification and intervention, thereby improving life expectancy.
The IGCLC defined “familial gastric cancer” as 1) having 2 or more first- or second-degree relatives with at least one gastric tumor before the age of 50 years, or 2) 3 or more first- or second-degree relatives with gastric cancer regardless of age [25]. Patients with familial gastric cancer are more likely to possess a genetic predisposition and may require genetic examination to determine genetic defects and to provide tailored care plans for patients and family members. Previous studies showed that 0.9% to 3.1% of patients with gastric cancer met the diagnostic criteria for familial gastric cancer; however, in the majority of the patients, no causal genetic defects could be identified [26,27]. In our study, 5.5% (151 patients) of the patients were found to meet these criteria, and an analysis of their survival showed that the OS and DSS rates were not significantly different from those of the patients without a FHX. Additionally, their clinicopathological characteristics did not significantly differ from those of the patients without a FHX (data not shown). Considering a low incidence, large nationwide multicenter studies will be helpful in determining the epidemiological and biological behaviors of familial gastric cancer.
This study has some limitations. First, misreporting of the FHX status was a possibility because we relied on self-reporting. However, previous studies have shown that self-reporting of FHX with respect to cancer is reasonably accurate, especially for first-degree relatives [28]. Second, our study did not collect information on the age and tumor type of the affected family members. In addition, we could not identify the cohabitation status of the patients with their affected family members; this could provide further insight into the association between FHX and survival. Finally, our study population was confined to a single institution in Korea. Considering the wide geographical variation in the epidemiological and biological behaviors of gastric cancer, the association of FHX with survival may differ in areas with a low prevalence of the disease.
In conclusion, the present study analyzed the largest available dataset to investigate the clinicopathological features and survival rates of patients with gastric cancer with a FHX of the disease. We found no significant clinicopathological characteristic or survival differences between the patients with and without a FHX. Although negative, our results may give a significant insight into the biological behavior of gastric cancer with a FHX.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Parkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR, et al. Fifty years of cancer incidence: CI5 I–IX. Int J Cancer. 2010;127:2918–2927. doi: 10.1002/ijc.25517. [DOI] [PubMed] [Google Scholar]
- 2.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 3.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 4.Setia N, Clark JW, Duda DG, Hong TS, Kwak EL, Mullen JT, et al. Familial gastric cancers. Oncologist. 2015;20:1365–1377. doi: 10.1634/theoncologist.2015-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yatsuya H, Toyoshima H, Mizoue T, Kondo T, Tamakoshi K, Hori Y, et al. Family history and the risk of stomach cancer death in Japan: differences by age and gender. Int J Cancer. 2002;97:688–694. doi: 10.1002/ijc.10101. [DOI] [PubMed] [Google Scholar]
- 7.Yaghoobi M, Bijarchi R, Narod SA. Family history and the risk of gastric cancer. Br J Cancer. 2010;102:237–242. doi: 10.1038/sj.bjc.6605380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin CM, Kim N, Lee HS, Lee DH, Kim JS, Jung HC, et al. Intrafamilial aggregation of gastric cancer: a comprehensive approach including environmental factors, Helicobacter pylori virulence, and genetic susceptibility. Eur J Gastroenterol Hepatol. 2011;23:411–417. doi: 10.1097/MEG.0b013e328343b7f5. [DOI] [PubMed] [Google Scholar]
- 9.Ebert MP, Malfertheiner P. Pathogenesis of sporadic and familial gastric cancer--implications for clinical management and cancer prevention. Aliment Pharmacol Ther. 2002;16:1059–1066. doi: 10.1046/j.1365-2036.2002.01288.x. [DOI] [PubMed] [Google Scholar]
- 10.Palli D, Russo A, Saieva C, Salvini S, Amorosi A, Decarli A. Dietary and familial determinants of 10-year survival among patients with gastric carcinoma. Cancer. 2000;89:1205–1213. doi: 10.1002/1097-0142(20000915)89:6<1205::aid-cncr3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Hu N, Han X, Giffen C, Ding T, Goldstein A, et al. Family history of cancer and risk for esophageal and gastric cancer in Shanxi, China. BMC Cancer. 2009;9:269–279. doi: 10.1186/1471-2407-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee WJ, Hong RL, Lai IR, Chen CN, Lee PH, Huang MT. Clinicopathologic characteristics and prognoses of gastric cancer in patients with a positive familial history of cancer. J Clin Gastroenterol. 2003;36:30–33. doi: 10.1097/00004836-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Han MA, Oh MG, Choi IJ, Park SR, Ryu KW, Nam BH, et al. Association of family history with cancer recurrence and survival in patients with gastric cancer. J Clin Oncol. 2012;30:701–708. doi: 10.1200/JCO.2011.35.3078. [DOI] [PubMed] [Google Scholar]
- 14.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. New York (NY): John Wiley & Sons; 2011. [Google Scholar]
- 15.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 16.Woo Y, Son T, Song K, Okumura N, Hu Y, Cho GS, et al. A novel prediction model of prognosis after gastrectomy for gastric carcinoma: development and validation using Asian databases. Ann Surg. 2016;264:114–120. doi: 10.1097/SLA.0000000000001523. [DOI] [PubMed] [Google Scholar]
- 17.Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003;21:3647–3650. doi: 10.1200/JCO.2003.01.240. [DOI] [PubMed] [Google Scholar]
- 18.Fang WL, Chang SC, Lan YT, Huang KH, Lo SS, Li AF, et al. Molecular and survival differences between familial and sporadic gastric cancers. Biomed Res Int. 2013;2013:396272. doi: 10.1155/2013/396272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemminki K, Sundquist J, Ji J. Is family history associated with improved survival in patients with gastric cancer? J Clin Oncol. 2012;30:3150–3151. doi: 10.1200/JCO.2012.42.1149. [DOI] [PubMed] [Google Scholar]
- 20.Kanemitsu K, Kawasaki K, Nakamura M, Li D, Yasuda T, Kuroda D, et al. MSI is frequently recognized among gastric cancer patients with a family history of cancer. Hepatogastroenterology. 2007;54:2410–2414. [PubMed] [Google Scholar]
- 21.Pedrazzani C, Corso G, Velho S, Leite M, Pascale V, Bettarini F, et al. Evidence of tumor microsatellite instability in gastric cancer with familial aggregation. Fam Cancer. 2009;8:215–220. doi: 10.1007/s10689-008-9231-7. [DOI] [PubMed] [Google Scholar]
- 22.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 23.Brenner H, Bode G, Boeing H. Helicobacter pylori infection among offspring of patients with stomach cancer. Gastroenterology. 2000;118:31–35. doi: 10.1016/s0016-5085(00)70411-2. [DOI] [PubMed] [Google Scholar]
- 24.Ebert MP, Yu J, Miehlke S, Fei G, Lendeckel U, Ridwelski K, et al. Expression of transforming growth factor beta-1 in gastric cancer and in the gastric mucosa of first-degree relatives of patients with gastric cancer. Br J Cancer. 2000;82:1795–1800. doi: 10.1054/bjoc.1999.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kluijt I, Sijmons RH, Hoogerbrugge N, Plukker JT, de Jong D, van Krieken JH, et al. Familial gastric cancer: guidelines for diagnosis, treatment and periodic surveillance. Fam Cancer. 2012;11:363–369. doi: 10.1007/s10689-012-9521-y. [DOI] [PubMed] [Google Scholar]
- 26.Shinmura K, Kohno T, Takahashi M, Sasaki A, Ochiai A, Guilford P, et al. Familial gastric cancer: clinicopathological characteristics, RER phenotype and germline p53 and E-cadherin mutations. Carcinogenesis. 1999;20:1127–1131. doi: 10.1093/carcin/20.6.1127. [DOI] [PubMed] [Google Scholar]
- 27.Kim SJ, Cho SJ, Heo SC, Yang HK, Kim WH, Park JG, et al. The incidence of hereditary gastric cancer in Korean. J Korean Cancer Assoc. 2000;32:1–6. [Google Scholar]
- 28.Kerber RA, Slattery ML. Comparison of self-reported and database-linked family history of cancer data in a case-control study. Am J Epidemiol. 1997;146:244–248. doi: 10.1093/oxfordjournals.aje.a009259. [DOI] [PubMed] [Google Scholar]