Abstract
The continuing emergence of multi-drug resistant pathogens has sparked an interest in seeking alternative therapeutic options. Antimicrobial combinatorial therapy is one such avenue. A number of studies have been conducted, involving combinations of bacteriocins with other antimicrobials, to circumvent the development of antimicrobial resistance and/or increase antimicrobial potency. Such bacteriocin-antimicrobial combinations could have tremendous value, in terms of reducing the likelihood of resistance development due to the involvement of two distinct mechanisms of antimicrobial action. Furthermore, antimicrobial synergistic interactions may also have potential financial implications in terms of decreasing the costs of treatment by reducing the concentration of an expensive antimicrobial and utilizing it in combination with an inexpensive one. In addition, combinatorial therapies with bacteriocins can broaden antimicrobial spectra and/or result in a reduction in the concentration of an antibiotic required for effective treatments to the extent that potentially toxic or adverse side effects can be reduced or eliminated. Here, we review studies in which bacteriocins were found to be effective in combination with other antimicrobials, with a view to targeting clinical and/or food-borne pathogens. Furthermore, we discuss some of the bottlenecks which are currently hindering the development of bacteriocins as viable therapeutic options, as well as addressing the need to exercise caution when attempting to predict clinical outcomes of bacteriocin-antimicrobial combinations.
Keywords: bacteriocins, antibiotic resistance, synergy, stressors, pathogens, antimicrobials, combinations
Introduction
The recent increase in the number of cases of antibiotic resistance has encouraged scientists to reassess alternative therapeutic options (Michael et al., 2014; Holmes et al., 2016). The rate of antibiotic resistance is worrying since progress in the discovery of novel antibiotics with different modes of action has slowed significantly. There are some exceptions, such as the relatively recent discovery of teixobactin, which exhibits activity against Gram positive pathogens including methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pneumoniae and mycobacteria, and which has a distinct mechanism of action involving the inhibition of peptidoglycan synthesis (Ling et al., 2015). Encouragingly, teixobactin proved to be effective in mouse trials in decreasing the load of S. pneumoniae and MRSA. Furthermore, S. aureus and M. tuberculosis isolates displaying resistance to teixobactin could not be isolated under laboratory conditions (Ling et al., 2015). In general, however, the widespread discovery of novel antibiotics remains largely uncommon (Cooper and Shlaes, 2011).
One option to compensate for the dearth of novel antibiotics is to introduce bacteriocins as therapeutic options in clinical settings. Bacteriocins are ribosomally-synthesized antimicrobial peptides produced by bacteria and can exhibit narrow spectra of activity (targeting members of the same species), whereas others display broader activity spectra (targeting other species and genera) (Cotter et al., 2013). Bacteriocins are broadly classified into class I (post-translationally modified) and class II (unmodified) groups. The most extensively studied subclass of bacteriocins is the lantibiotics, which includes nisin, lacticin 3147, mersacidin, lacticin 481 and staphylococcin C55, amongst others (Brötz et al., 1995; McAuliffe et al., 1998; Navaratna et al., 1998; Xie et al., 2004; Field et al., 2008). Several lantibiotics exhibit potent activity against clinically relevant and food-borne pathogens (Mota-Meira et al., 2000; Kruszewska et al., 2004; Cotter et al., 2005, 2013; Rea et al., 2007; de Kwaadsteniet et al., 2009; Piper et al., 2009; Jabes et al., 2011; Field et al., 2012, 2015). Significantly, some lantibiotics have been shown to possess activity against antibiotic-resistant targets such as vancomycin-resistant enterococci (VRE) and MRSA (Chatterjee et al., 1992; Kruszewska et al., 2004; Piper et al., 2009). In a number of instances, the receptor for the lantibiotic subclass of bacteriocins is the peptidoglycan precursor lipid II, which is also the binding site for vancomycin, albeit at a different site within the precursor (Breukink and de Kruijff, 2006). The class II bacteriocins, which are unmodified or cyclic in nature, are further divided into class IIa-IIe (Cotter et al., 2013). The class IIa subgroup of bacteriocins generally have strong activity against the food-borne pathogen, Listeria monocytogenes (Pucci et al., 1988; Eijsink et al., 1998; Gravesen et al., 2002; Héchard and Sahl, 2002; Dabour et al., 2009). In contrast to class I bacteriocins, several class IIa bacteriocins bind the mannose phosphotransferase receptor (Man-PTS; Oppegård et al., 2007). Overall, bacteriocins exhibit strong activity against their target strains, often in the nanomolar range, rendering them more potent than their antibiotic counterparts in certain cases (Mathur et al., 2013; Ming et al., 2015). Thus, bacteriocins on their own have potential for use in clinical applications.
However, perhaps an even better option is to combine bacteriocins with other existing antibiotics/antimicrobials. It is plausible that using antimicrobials that function synergistically with bacteriocins may expedite each other's killing effects, thereby possibly reducing the likelihood of resistance development to either the bacteriocin or the antimicrobial stressor. Furthermore, combinations of bacteriocins with antibiotics can decrease the concentration of antibiotics required to kill a target pathogen, thereby diminishing the likelihood of adverse side effects associated with the antibiotic. An example of such undesirable effects is the nephrotoxicity associated with the polymyxin group of antibiotics (Mendes et al., 2009; Abdelraouf et al., 2012). Synergistic combinations of bacteriocins with antibiotics can also reduce the financial burden associated with the synthesis and administration of the more expensive antibiotics. Finally, successful synergistic interactions between bacteriocins and other antimicrobials can broaden the spectrum of activity, which may be useful in treating clinical infections of unknown etiology.
A variety of different methods assessing antimicrobial synergy in laboratory conditions have been described in the literature. Examples of such tests include the broth-based checkerboard assay, as well as agar-based screens such as E-tests (bioMérieux) to evaluate synergy (Orhan et al., 2005; Tsuji and Rybak, 2006; Foweraker et al., 2009; Sopirala et al., 2010; Soltani et al., 2012). However, there is a general consensus that agar-based screens are relatively crude compared to the more accurate broth-based methods. The checkerboard method permits the determination of the fractional inhibitory concentration (FIC) index. Although some researchers disagree about the interpretation of results obtained with checkerboard assays, there appear to be five different effects on which the majority of researchers have reached a consensus. These five effects are (i) full synergy (FIC ≤ 0.5), (ii) partial synergy (0.5 ≤ FIC ≤ 0.75), (iii) additive effects (0.75 ≤ FIC ≤ 1.0), (iv) indifferent effects (1.0 ≤ FIC ≤ 2.0) and (v) antagonistic effects (FIC ≥ 2.0) (Bacon et al., 1991; Orhan et al., 2005). The main disadvantage of the checkerboard assay is that it can only examine two antimicrobials at a time. On the other hand, an assay known as the multiple combination bactericidal test (MCBT) has the potential to evaluate combinations of up to four antimicrobials concurrently. While checkerboard assays evaluate different antimicrobial concentrations, MCBT assays only evaluate defined set concentrations of antimicrobials. MCBT assays are based on the premise that 99.9% of the bacterial population is killed after 24 h. Synergy can also be assessed by combining antimicrobials together and conducting time-kill assays, whereby samples are taken at defined time points to evaluate the effects of two or more antimicrobials together (Doern, 2014). Finally, it must be emphasized that there can be variations in terms of the types of interactions obtained between different methods. Variations frequently observed between different methods can be ascribed to slight differences in the end-points used in the assays. It should also be noted that in vitro results do not necessarily predict the success of different combinations in vivo.
In this review, we outline a selection of studies conducted whereby bacteriocins were combined successfully with other bacteriocins, antibiotics, phage lysins and other antimicrobials/stressors such as naturally-derived plant essential oils, with a view to targeting clinical/veterinary pathogens, pathogens involved in biofilms, and food-borne pathogens.
Effects of antimicrobial combinations involving bacteriocins against clinical and veterinary pathogens
Lantibiotics in combination with antibiotics against clinical and veterinary pathogens
The lantibiotics are the most extensively studied group of bacteriocins (Cotter et al., 2013). These bacteriocins are post-translationally modified antimicrobial peptides, characterized by the presence of unusual amino acids, and their structure involves lanthionine and β-methyl lanthionine intramolecular bridges. During the formation of these intramolecular bridges, serine and threonine residues are dehydrated to dehydroalanine and dehydrobutyrine respectively (Cotter et al., 2013). In one study, the most thoroughly investigated lantibiotic, nisin (at concentrations ranging from 1.5 to 16 μg/ml), was combined with the glycolipodepsipeptide ramoplanin (at concentrations ranging from 0.38 to 1.5 μg/ml), both of which target lipid II, and the combination resulted in synergistic interactions against 14 out of 20 MRSA strains assessed (Brumfitt et al., 2002). Resistance to methicillin in S. aureus is a significant problem and is mediated by the expression of PBP2a (penicillin binding protein 2a) instead of, or in addition to, the regular PBP (Hackbarth et al., 1995; Stapleton and Taylor, 2002; Chambers and Deleo, 2009). Furthermore, β-lactamase expression is also known to contribute to resistance to methicillin amongst S. aureus strains (Montanari et al., 1996), rendering such MRSA strains amongst the most challenging pathogens to target in clinical settings. In contrast to the effective nisin-rampolanin interactions however, the study by Brumfitt et al., showed that a combination of nisin with 3–12 μg/ml chloramphenicol (which inhibits protein synthesis in bacteria) yielded antagonistic effects against these MRSA strains (Brumfitt et al., 2002).
Enterococcus faecalis is another nosocomial pathogen and is an etiological agent of endocarditis, urinary tract infections and other systemic infections, and has exhibited resistance to several groups of antibiotics including aminoglycosides, daptomycin, quinolones, macrolides, rifampicin and β-lactams, amongst others (Murray, 1990; Johnston and Jaykus, 2004; Flores-Mireles et al., 2015). A study by Tong et al., demonstrated that the presence of 200 U/ml nisin led to a reduction in minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values of 18 different antibiotics against E. faecalis. The combinations of 200 U/ml nisin with 1–16 μg/ml chloramphenicol or with 2 μg/ml penicillin were especially effective, with statistically significant improvements in MIC values in the presence of nisin. Synergistic interactions between nisin in combination with either penicillin or chloramphenicol against three E. faecalis strains were also apparent with checkerboard assays. In addition, transmission electron microscopy (TEM) demonstrated that these combinations were highly effective at destroying E. faecalis cells (Tong et al., 2014a). In another study, the lipid II-binding lantibiotic, actagardine, was combined with the antibiotics metronidazole, vancomycin and ramoplanin against several Clostridium difficile isolates (Mathur et al., 2013). C. difficile is primarily a nosocomial pathogen and is the causative agent of C. difficile-associated diarrhea (CDAD), which often occurs due to perturbations of the gut microbiota resulting from broad-spectrum antibiotics (Rea et al., 2010; Leffler and Lamont, 2015). Interestingly, it was revealed that actagardine in combination with ramoplanin behaved in a partial synergistic/additive fashion against 61.5% of the target C. difficile strains assessed in the relatively recent study (Mathur et al., 2013). Actagardine-metronidazole and actagardine-vancomycin combinations were also effective with partial synergistic/additive effects obtained against 54% and 38% of C. difficile strains respectively (Mathur et al., 2013).
With respect to veterinary pathogens, LeBel et al., reported that nisin behaved in a synergistic manner when combined with the β-lactams amoxicillin, penicillin or ceftiofur, and also when combined with streptomycin or tetracycline against Streptococcus suis (LeBel et al., 2013). Although S. suis is primarily a porcine pathogen, it has also been described as a zoonotic pathogen with transmission to humans possible (Hughes et al., 2009; Goyette-Desjardins et al., 2014; Huong et al., 2014). The novel lantibiotic, suicin 3908, was also shown to interact in an additive manner with amoxicillin or penicillin against S. suis in a recent study (Vaillancourt et al., 2015).
Sactibiotics and other groups of bacteriocins, in combination with antimicrobials against clinical pathogens
The sactibiotics are a relatively newly-designated group of bacteriocins, characterized by post-translational modifications involving radical S-adenosyl methionine (SAM) methylases and the presence of sulfur to α-carbon linkages in their structure (Arnison et al., 2013; Mathur et al., 2015). In general, the sactibiotics tend to be relatively hydrophobic in nature with characteristic hairpin-shaped structures. The efficacy of the sactibiotic, thuricin CD, was assessed in combination with metronidazole, ramoplanin and vancomycin, with a view to targeting C. difficile strains. It was revealed that thuricin CD interacted in a partial synergistic manner when combined with ramoplanin against 31% of the target strains tested (Mathur et al., 2013). In contrast, combinations of thuricin CD-vancomycin and thuricin CD-metronidazole resulted in indifferent effects (1.0 ≤ FIC ≤ 2.0) against the majority of C. difficile isolates tested in the study (Mathur et al., 2013). Antimicrobial combination studies have also been conducted with the sactibiotic subtilosin A against Gardnerella vaginalis. This opportunistic pathogen is one of the predominant causative agents of bacterial vaginosis (Catlin, 1992). It was determined that subtilosin A exhibited Bliss synergy when combined with clindamycin phosphate, metronidazole, ε-Poly-L-Lysine (polylysine) and lauramide arginine ethyl ester (LAE) against the pathogen (Cavera et al., 2015). Bliss synergy is based on the principle that drug effects are outcomes of probabilistic processes and assumes that drugs act independently in such a manner that neither of them interferes with the other (different sites of action), but each contributes to a common result (Tang et al., 2015). However, only subtilosin A-metronidazole and subtilosin A-clindamycin combinations yielded synergistic effects against G. vaginalis when FIC indices were evaluated (Cavera et al., 2015). Subtilosin produced by Bacillus amyloliquefaciens was also shown to exhibit synergistic activity with lauric arginate, ε-poly-L-lysine and glycerol monolaurate against G. vaginalis in an earlier study (Noll et al., 2012). Finally, a recent study demonstrated that combinations of the class II bacteriocin, durancin 61A and the broad-spectrum antimicrobial reuterin yielded FIC indices of 0.2 against C. difficile, indicating highly synergistic activity (Schaefer et al., 2010; Hanchi et al., 2017). Interestingly, durancin 61A combinations with vancomycin were also synergistic against MRSA (S. aureus ATCC 700699) with FIC values of 0.3 obtained (Hanchi et al., 2017).
With respect to the treatment of oral pathogens, one study investigated the combination of the bacteriocin PsVP-10, a non-lantibiotic displaying characteristics akin to class II bacteriocins, with the antimicrobials triclosan and chlorhexidine in an effort to target Streptococcus mutans and Streptococcus sobrinus (Lobos et al., 2009). S. mutans is the predominant causative agent of dental caries (Loesche, 1986; Metwalli et al., 2013), while S. sobrinus has also been implicated in its causation, albeit to a lesser extent than S. mutans (Conrads et al., 2014). Synergistic effects were obtained when PsVP-10 was combined with chlorhexidine, whereas partial synergistic/additive effects were apparent with PsVP-10-triclosan combinations in the study by Lobos et al. (2009). Candida albicans is the etiological agent of many opportunistic yeast infections in the oral cavity as well as other parts of the body and it is noteworthy that the class II bacteriocins plantaricin E, F, J, and K have displayed activity against C. albicans when used in combination with several different antibiotics (Sharma and Srivastava, 2014). This opportunistic pathogen typically infects immunosuppressed individuals. Indeed, it is frequently implicated in oro-pharyngeal thrush in AIDS patients and can also be a causative agent of systemic infections such as vaginitis (Kim and Sudbery, 2011). Thus, effective combinations of therapeutics to target this pathogen are warranted.
Effective bacteriocin-antimicrobial combinations against biofilms
Bacteria present in a biofilm are inherently more resistant to antimicrobials than those present in a planktonic state predominantly due to the complex polymeric matrix which biofilms are composed of, and this matrix often impedes penetration of the antimicrobial to the deepest strata of the biofilm (Mah and O'Toole, 2001; Davies, 2003; Flemming and Wingender, 2010). Thus, there is an increased emphasis on trying to seek alternative therapeutic options and/or effective antimicrobial combinations to target biofilms. A recent study showed that nisin interacts synergistically with several antibiotics and such combinations were effective against Staphylococcus biofilms (Field et al., 2016a). In particular, the bioengineered variant of nisin, M21V, in combination with penicillin was effective at inhibiting biofilms of S. aureus SA113. In contrast to SA113 biofilms however, M21V was most effective in combination with chloramphenicol against SA113 planktonic cells (Figure 1C). Furthermore, another variant, nisin I4V, was reported to be highly effective in conjunction with chloramphenicol against Staphylococcus pseudintermedius DSM21284 biofilms in the same study, while combinations of I4V with penicillin were particularly potent against DSM21284 planktonic cells (Field et al., 2016a; Figures 1B, 2). S. pseudintermedius is an opportunistic pathogen, primarily affecting dogs (van Duijkeren et al., 2011) and an outbreak of methicillin-resistant S. pseudintermedius in Finland has been documented in recent years (Grönthal et al., 2014). Significantly, a case of S. pseudintermedius in a human was also described for the first time in 2006 (Van Hoovels et al., 2006). While the use of the colorimetric XTT assay revealed that nisin I4V-chloramphenicol combinations were effective at diminishing the viability of the biofilms of a S. pseudintermedius strain in the study by Field and co-workers, the authors found that there was no synergy with nisin-vancomycin combinations against Staphylococcus biofilms (Field et al., 2016a). Indeed, variations with respect to the nature of interactions between nisin and vancomycin when targeting MRSA or methicillin-sensitive S. aureus (MSSA) have also been reported in previous studies (Dosler and Gerceker, 2011, 2012). A separate study by Mataraci and Dosler also investigated the potency of several antibiotics combined with nisin against MRSA ATCC43300 biofilms and it was determined that antibiotic-nisin combinations were effective at preventing biofilm formation at 1x MIC. However, biofilm-associated bacteria were highly resistant to antibiotics or antibiotic combinations (Mataraci and Dosler, 2012). In another study, it emerged that nisin in combination with ciprofloxacin or daptomycin was effective against 24 h-old MRSA biofilms. The combination elicited a reduction in MRSA CFU counts by 3 logs, whereas the individual antimicrobials were unable to decrease the CFU to such an extent (Dosler and Mataraci, 2013). A recent interesting study evaluated the activity of nisin and lysostaphin against pre-formed biofilms of S. aureus involved in bovine mastitis (Ceotto-Vigoder et al., 2016). After treatment for 24 h with a combination of nisin and lysostaphin, the biofilm pre-formed by all eight strains tested in the study was reduced by >50%, as suggested by biofilm detachment from the microtiter plates. Although no remarkable detachment could be noticed by confocal laser scanning microscopy (CLSM) analysis after a 4 h treatment, when the biofilm matrix of S. aureus 4181 was assayed for cell viability, most cells were shown to be dead (Ceotto-Vigoder et al., 2016). In contrast to the synergistic activity between nisin and lysostaphin, significantly higher concentrations of lysostaphin used on its own, up to 50-fold higher, were required to cause the same level of biofilm detachment achieved by nisin-lysostaphin combinations. A separate study evaluated the antimicrobial effects of the class IIc bacteriocin enterocin AS-48 both independently and in combination with several biocides against three MRSA and three MSSA strains (Caballero Gómez et al., 2013). It was determined that the anti-biofilm activity of the biocides triclosan, benzalkonium chloride and polyhexamethylene guanidinium chloride (PHMG) were highly effective in combination with 50 μg/ml of AS-48 (Caballero Gómez et al., 2013). Thus, these combinations may prove to be useful therapeutic options for MRSA. Finally, Tong and co-workers also reported the efficacy of nisin in combination with chloramphenicol, ciprofloxacin or penicillin at targeting biofilms of the nosocomial pathogen, E. faecalis (Tong et al., 2014a).
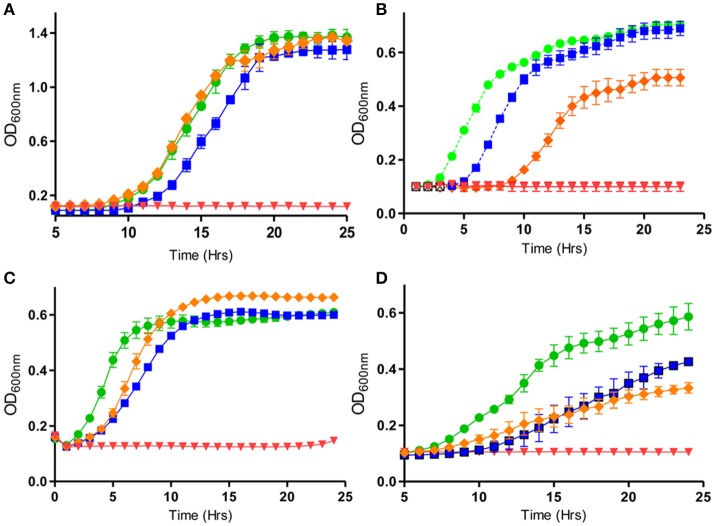
Figure 1.
Activity of nisin and bioengineered derivatives thereof, in combination with different antimicrobials against various pathogens: (A) Pseudomonas aeruginosa PA-01 in the presence of nisin A (50 μg/ml) (orange diamond), colistin (0.75 μg/ml) (blue square), in combination (red diamond) and untreated (green circle), (B) S. pseudintermedius DSM 21284 in the presence of nisin I4V peptide (0.932 μg/ml) (orange diamond) with penicillin (Pen) (0.8 μg/ml) (blue square), in combination (red diamond), and untreated (green circle). (C) S. aureus SA113 in the presence of nisin V (3.0 μg/ml), (orange diamond), 1.5 μg/ml chloramphenicol (Cm) (blue square), in combination (red diamond), and untreated (green circle) and (D) E. coli O157:H7 in the presence of nisin S29A (orange diamond), carvacrol (200 μg/ml) (blue square) and combinations of nisin S29A and carvacrol (red inverted triangle) and untreated (green circle). Adapted from Field et al. (2016a,b) and Campion et al. (2017). Rights and Permissions have been obtained from Copyright Clearance Center's RightsLink service.
Figure 2.
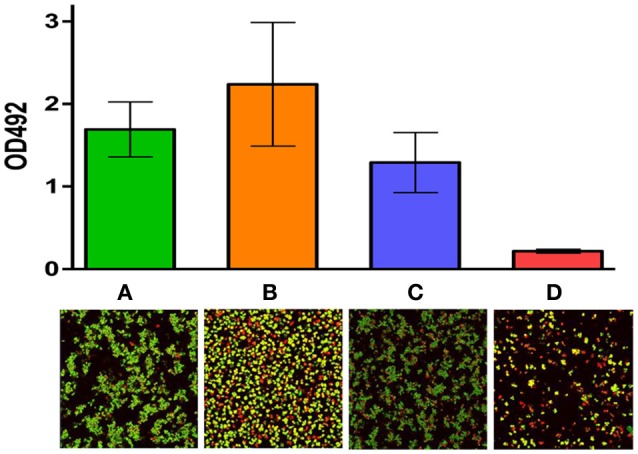
Activity of nisin I4V in combination with chloramphenicol against S. pseudintermedius DSM21284 biofilms: Viability of biofilms of S. pseudintermedius DSM 21284 when (A) untreated, (B) treated with 1X MIC chloramphenicol alone, (C) treated with 4X MIC nisin I4V peptide alone and (D) 1X MIC chloramphenicol and 4X MIC nisin I4V peptide in combination as evaluated by the colorimetric XTT assay and measured using a microtiter plate reader (top) and Live/dead staining confocal images (bottom). Adapted from Field et al. (2016a). Rights and Permissions have been obtained from Copyright Clearance Center's RightsLink service.
With a specific goal of finding therapeutics against oral biofilms, Tong and co-workers also examined the anti-biofilm activities of the lantibiotic nisin, independently and in combination with free amino acids against S. mutans biofilms. The results of crystal violet biofilm assays indicated that mixtures of either the L or D-enantiomers of Glu, Asp or Cys in combination with nisin could ameliorate the potency of the lantibiotic against biofilms of S. mutans (Tong et al., 2014b). In an earlier study, the same group found that nisin interacted synergistically with sodium fluoride against S. mutans biofilms, in that the combination was more effective than sodium fluoride used independently at inhibiting the formation of biofilms at 4 and 16 h (Tong et al., 2011). The same investigators also assessed the effect of adding nisin to the dental irrigant, MTAD, and its anti-biofilm activity against oral E. faecalis isolates. Importantly, it was found that nisin in combination with doxycycline successfully inhibited E. faecalis biofilms whereas MTAD on its own was ineffective against such biofilms (Tong et al., 2013). E. faecalis, best known as a notorious nosocomial pathogen causing systemic infections, is also implicated in root canal infections. These successful combinations mentioned above could be harnessed as effective antimicrobial combinations against E. faecalis and S. mutans biofilms or as part of an endodontic irrigant following root canal treatments to prevent post-operative infections.
With respect to Gram negative organisms, a recent study found that nisin was effective when used together with polymyxins against Pseudomonas aeruginosa biofilms (Field et al., 2016b). P. aeruginosa is an important opportunistic pathogen and its biofilm-forming abilities contribute to its pathogenicity (Gellatly and Hancock, 2013). It is particularly common in lungs of patients with cystic fibrosis and thus warrants further extensive research to target its biofilm-forming and consequent pathogenic properties (Reen et al., 2016). The study by Field and co-workers demonstrated that decreased concentrations of polymyxins (either 0.5x MIC or even 0.2x MIC) are required to prevent P. aeruginosa PA-01 biofilm formation, when combined with 0.25x MIC nisin. Combinations of 0.33x MIC of nisin with 0.5x MIC of polymyxin were also effective against PA-01 planktonic cells (Field et al., 2016b; Figure 1A). Nisin was also used in combination with other antimicrobial agents used in the clinic against planktonic cells of P. aeruginosa ATCC 27853, as well as 40 other strains of P. aeruginosa in a separate study (Giacometti et al., 1999). A synergistic interaction of this nature can be hugely beneficial as it has the potential to attenuate the undesired nephrotoxicity associated with polymyxins.
A recent study using the sactibiotic, subtilosin, in combination with LAE was undertaken with a view to targeting G. vaginalis biofilms (Algburi et al., 2015). A combination of two methods; ATP viability assays and resazurin assays, were conducted to show the efficacy of these combinations against biofilms of the pathogen. Encouragingly, this combination of subtilosin-LAE was ineffective against healthy vaginal Lactobacillus biofilms. This highlights the specific nature of antimicrobial combinations and such a combination could be harnessed as a targeted therapeutic option against bacterial vaginosis. Furthermore, bacteriocins can potentially be combined with agents that target biofilms through non-microbiocidal means such as by altering molecular pathways which are responsible for regulating biofilm formation. Examples of such agents include thiazolidinone derivatives and diterpenoids (Buommino et al., 2014).
A summary of studies involving bacteriocins in combination with other stressors with a view to targeting clinical and veterinary pathogens, as well as biofilms, is found in Tables 1, 2.
Table 1.
Bacteriocins in combination with other stressors against clinical and veterinary pathogens.
| Bacteriocin | Antimicrobial/Stressor | Target | Effect | References |
|---|---|---|---|---|
| Nisin | Ramoplanin or chloramphenicol | MRSA | Synergy with ramoplanin against 14/20 strains; antagonism with chloramphenicol | Brumfitt et al., 2002 |
| Polymyxin E or clarithromycin | P. aeruginosa | Synergy | Giacometti et al., 1999 | |
| Amoxicillin, penicillin, streptomycin ceftiofur, tetracycline | S. suis | Synergy with every combination | LeBel et al., 2013 | |
| Chloramphenicol or penicillin | E. faecalis | Synergy with the antibiotics | Tong et al., 2014a | |
| Actagardine | Ramoplanin | C. difficile | Partial synergy/additive against 8/13 strains | Mathur et al., 2013 |
| Metronidazole | C. difficile | Partial synergy/additive against 7/13 strains | Mathur et al., 2013 | |
| Vancomycin | C. difficile | Partial synergy/additive against 5/13 strains | Mathur et al., 2013 | |
| Lacticin 3147 | Polymyxin B or E | E. faecium DO | Indifference | Draper et al., 2013 |
| Polymyxin B | S. aureus 5247 | Partial synergy | Draper et al., 2013 | |
| Polymyxin E | S. aureus 5247 | Indifference | Draper et al., 2013 | |
| Suicin 3908 | Amoxicillin or penicillin | S. suis | Additive effects | Vaillancourt et al., 2015 |
| Thuricin CD | Ramoplanin | C. difficile | Partial synergy/additive against 4/13 strains | Mathur et al., 2013 |
| Metronidazole | C. difficile | Indifference against 13/13 strains | Mathur et al., 2013 | |
| Vancomycin | C. difficile | Partial synergy/additive against 2/13 strains | Mathur et al., 2013 | |
| Subtilosin A | Clindamycin phosphate or metronidazole | G. vaginalis | Synergy | Cavera et al., 2015 |
| Lauramide arginate or Ester poly-lysine | G. vaginalis | Bliss synergy | Cavera et al., 2015 | |
| Subtilosin | Lauric arginate, ϵ-poly-L-lysine, glycerol monolaurate | G. vaginalis | Synergy | Noll et al., 2012 |
| PsVP-10 | Triclosan | S. mutans and S. sobrinus | Partial Synergy | Lobos et al., 2009 |
| Chlorhexidine | S. mutans and S. sobrinus | Synergy | Lobos et al., 2009 | |
| Plantaricin E,F,J,K | Several antibiotics | Candida albicans | Synergy | Sharma and Srivastava, 2014 |
| Durancin 61A | Reuterin | C. difficile | FIC values of 0.2 against C. difficile | Hanchi et al., 2017 |
| Vancomycin | MRSA | FIC values of 0.3 against S. aureus ATCC 700699 | Hanchi et al., 2017 |
Table 2.
Bacteriocins in combination with other antimicrobials/stressors against biofilms.
| Bacteriocin | Antimicrobial/Stressor | Target biofilm | Effect | References |
|---|---|---|---|---|
| Nisin | Glu, Asp, Cys | S. mutans | Improved potency of nisin against biofilms | Tong et al., 2014b |
| Sodium fluoride | S. mutans | Synergy | Tong et al., 2011 | |
| Doxycycline | E. faecalis | Inhibition of biofilms | Tong et al., 2013 | |
| Several antibiotics | MRSA ATCC43300 | Synergy in preventing the formation of biofilms | Mataraci and Dosler, 2012 | |
| Ciprofloxacin or daptomycin | MRSA | Decrease in MRSA CFU counts by 3 logs | Dosler and Mataraci, 2013 | |
| Lysostaphin | S. aureus | Pre-formed biofilm reduced by >50% for 8 strains | Ceotto-Vigoder et al., 2016 | |
| Polymyxin | P. aeruginosa | Reduced concentrations of polymyxins needed to inhibit biofilm formation | Field et al., 2016b | |
| Nisin M21V | Penicillin | S. aureus SA113 | Biofilm inhibited | Field et al., 2016a |
| Nisin I4V | Chloramphenicol | S. pseudintermedius DSM21284 | Biofilm inhibited | Field et al., 2016a |
| Nisin M21A | Citric acid, cinnamaldehyde | L. monocytogenes | L. monocytogenes biofilm inhibited | Smith et al., 2016 |
| Subtilosin | Lauramide arginine ethyl ester (LAE) | G. vaginalis | Effective at inhibiting biofilm | Algburi et al., 2015. |
| Enterocin AS-48 | Triclosan, benzalkonium chloride, PHMG | MRSA | Effective when biocides were combined with 50 μg/ml AS-48 | Caballero Gómez et al., 2013 |
| Enterocin B3A-B3B | Nisin | L. monocytogenes | MIC needed to disrupt biofilm reduced | Al-Seraih et al., 2017 |
Effects of antimicrobial combinations involving bacteriocins against food-borne pathogens
With regards to the food industry, disease-causing and spoilage organisms can have tremendous implications in terms of morbidity/mortality, as well as financial implications. Several persistent pathogens exist in food systems, both in planktonic states as well as in biofilms. Furthermore, there is an emphasis on attempting to replace chemically-derived antimicrobials in food with more natural antimicrobials such as nisin and plant-derived essential oils. The increase in the extent of global food distribution, in conjunction with more frequent travel has elicited an increase in the dissemination of food-borne diseases and solutions are required to combat this trend (Hussain and Dawson, 2013). While the use of chemical preservatives as well as heat treatment have proven to be successful in the past in limiting food-borne pathogens as part of the hurdle effect, such treatments can have an impact on the organoleptic properties of food. In addition, increasing pressure from consumers for safe to eat food which is minimally processed has ignited an interest in the development of effective natural antimicrobials or antimicrobial combinations to control food-borne pathogens.
Combinations of bacteriocins with essential oils, naturally-derived compounds and preservatives against gram positive food-borne pathogens
The lantibiotic nisin has been the subject of several antimicrobial combination studies with a view to targeting food-borne pathogens. Indeed, it has been utilized for decades as a food preservative in more than 50 countries (Delves-Broughton, 1990). L. monocytogenes, the causative agent of listeriosis, is a notorious food-borne pathogen, and can cause opportunistic infections which can lead to meningitis and sepsis in severe cases (Low and Donachie, 1997; Schuppler and Loessner, 2010). In one study, it was found that 6,400 IU of nisin in combination with a green tea extract (GTE) or in combination with a grape seed extract (GSE) resulted in effective cell damage in a target L. monocytogenes strain (Sivarooban et al., 2008). The purified phenolic compounds present in GTE (0.02% epicatechin and 0.02% caffeic acid), as well as the pure phenolic compounds present in GSE (0.02% catechin and 0.02% epicatechin) were also used in the study (Sivarooban et al., 2008). When nisin was combined with GTE or GSE compounds, a compromised cell membrane and a condensed cytoplasm were apparent with TEM. With a starting inoculum of 106 CFU/ml, the combination of nisin with GSE resulted in Listeria levels which were undetectable after 24 h of incubation, whereas the combination of nisin and GTE led to a decrease in Listeria levels to a mere 3.76 CFU/ml (Sivarooban et al., 2008). In another study, it was shown that semi-purified preparations of nisin A and a bioengineered derivative nisin V, combined effectively with the essential oils carvacrol, thymol and trans-cinnamaldehyde to inhibit L. monocytogenes in a validated food model system, as well as in laboratory media. It was shown that a greatly delayed lag phase was apparent during growth curves when nisin V was combined with 0.02% of the above-mentioned essential oils, compared to the nisin A equivalent (Field et al., 2015). Additionally, in time-kill assays, a 2-log decrease in cell numbers over and above that achieved by the nisin A combination with carvacrol or cinnamaldehyde was observed against the target L. monocytogenes EGDe, which was also maintained in a number of food settings (Field et al., 2015). In a separate study, the antimicrobial activity of nisin Z was potentiated when combined with thymol at sub-inhibitory concentrations, against the target strain L. monocytogenes ATCC7644 (Ettayebi et al., 2000). Significantly, the study showed that 40 IU/ml nisin combined with 0.02% thymol was more effective at inhibiting ATCC7644 than either of the antimicrobials used on their own. A combination of this nature may also preclude the emergence of bacterial sub-populations displaying resistance to the lantibiotic (Ettayebi et al., 2000). Another study investigated the effects of combining nisin with carvacrol or mountain savory essential oils on the viability of target L. monocytogenes strains subsequent to γ-irradiation. There was an increase in the relative sensitivity of L. monocytogenes to γ-irradation by a factor of 6.31 when nisin and mountain savory essential oils were combined and a corresponding increase in sensitivity by a factor of 4.19 with nisin-carvacrol combinations (Ndoti-Nembe et al., 2013).
With regards to combinations of bacteriocins with preservatives, a study reported that combinations of nisin-potassium sorbate-ethylene diamine tetraacetic acid (EDTA), as well as combinations of nisin-sodium-diacetate-EDTA were effective at reducing the numbers of L. monocytogenes on shrimp inoculated with the pathogen (Wan Norhana et al., 2012). Synergistic interactions between nisin and low concentrations of EDTA in targeting L. monocytogenes were also described in a study by Branen and Davidson. In addition, the authors showed that the antimicrobial activity of nisin against L. monocytogenes was potentiated in the presence of lactoferrin in the same study (Branen and Davidson, 2004). Combinations of 10 IU/ml nisin with 250 μg/ml lactoferrin were also shown to perform synergistically to inhibit L. monocytogenes in a separate study, similar to Branen and Davidson's findings (Murdock et al., 2007).
With respect to other classes of bacteriocins, Grande and co-workers reported synergistic effects when the class IIc bacteriocin enterocin AS-48 was combined with the phenolic compound carvacrol (Grande et al., 2007). The authors conducted a food trial with vegetable sauces whereby they investigated the efficacy of AS-48 both independently and when combined with phenolic substances with a view to inhibiting S. aureus. The activity of AS-48 was significantly enhanced against S. aureus, when combined with eugenol, geraniol, terpineol, carvacrol, hydrocinnamic acid, caffeic acid, citral and p-coumaric acid. The levels of the pathogen were decreased to less than detectable levels when 80 μg/ml of AS-48 was mixed with 126 nM carvacrol or 20 mM hydrocinnamic acid, in carbonara sauce kept at 22°C. Overall, the degree of synergy obtained depended on the concentration of the antimicrobials and also the type of sauce used (Grande et al., 2007). Enterocin AS-48 was also shown to interact in a synergistic manner with lactic acid, p-hydroxybenzoic methylester acid (PHBME) and citric acid in a separate study (Antonio et al., 2009). In salads spiked with L. monocytogenes, the application of 30 μg/g of AS-48 in combination with PHBME, Nisaplin or lactic acid led to a significant reduction in L. monocytogenes numbers (Antonio et al., 2009). Amrouche and co-workers showed that the sactibiotic bacteriocin subtlosin was effective at inhibiting L. monocytogenes Scott A and NR30 when combined with ε-poly-L-lysine, zinc lactate or with curcumin (Amrouche et al., 2010). The strain L. monocytogenes Scott A was originally implicated in a listeriosis outbreak in Massachusetts in 1983 (Fleming et al., 1985; Briers et al., 2011) while the strain NR-30 has been reported to display resistance to nisin (Mazzotta and Montville, 1997). Partial synergy against L. monocytogenes Scott A was apparent when subtilosin was used in conjunction with an encapsulated form of curcumin in the study by Amrouche et al., while subtilosin-zinc lactate combinations also exhibited synergy against both NR30 and Scott A. However, when combined with non-encapsulated poly-L-lysine or curcumin, subtilosin displayed merely additive effects against the two L. monocytogenes strains (Amrouche et al., 2010). Finally, a separate study reported that the combination of a cell-bound bacteriocin produced by Lactobacillus curvatus CWBI-B28 with savory essential oil or oregano was effective at reducing L. monocytogenes to below detectable levels in pork meat spiked with the pathogen, after a week of storage (Ghalfi et al., 2007). The oregano essential oil combined with the cell-bound bacteriocin was still effective after 3 weeks of storage, whereas all other combinations led to an increase in L. monocytogenes levels after the same duration of time. Overall, this cell-bound bacteriocin combined with savory essential oil was found to retard the growth of L. monocytogenes by 2 weeks, relative to the use of the cell-bound bacteriocin on its own (Ghalfi et al., 2007).
L. monocytogenes has the ability to form biofilms in addition to growing in a planktonic state, and a recent study demonstrated the effectiveness of the bioengineered derivative of nisin, M21A, in combination with natural food-grade additives (cinnamaldehyde and citric acid) in targeting biofilms of strain F6854 (Smith et al., 2016). This strain has been associated with contaminated turkey frankfurters (Nelson et al., 2004). The study by Smith et al., showed that the bioengineered nisin derivative M21A (0.1 μg/ml) was more effective at inhibiting F6954 biofilms than wild-type nisin, when combined with citric acid (175 μg/ml) or cinnamaldehyde (35 μg/ml) (Smith et al., 2016). A separate study recently reported that the class IIb bacteriocin, enterocin B3A-B3B, was effective when used in combination with nisin at decreasing the MIC needed to disrupt the growth of L. monocytogenes in either a planktonic state or when present as a biofilm (Al-Seraih et al., 2017). Finally, the cell-free supernatant (CFS) containing a bacteriocin from Lb. curvatus ET31 was tested in combination with EDTA and the investigators noted that while the bacteriocin and the EDTA independently were ineffective against biofilms which had already formed, the CFS combined with EDTA was effective at reducing the viability of L. monocytogenes biofilms which had already formed, whilst not fully eliminating the biofilms (Camargo et al., 2016).
Bacteriocins in combination with other stressors against gram negative food-borne pathogens
Gram negative pathogens in particular have proven to be more problematic to tackle using bacteriocins than Gram positive pathogens primarily because of the outer membrane present in Gram negatives that limits access to the cell membrane. Importantly however, the bioengineered nisin variants S29A and S29G have been shown to display activity against Gram negatives (Field et al., 2012). While nisin A has been shown to be effective against Gram negatives when used in combination with chelating agents such as EDTA, perhaps a more attractive option is combining nisin with natural phenolic compounds such as thymol and carvacrol, which facilitate the permeabilization/disruption of the membrane (Stevens et al., 1991; Helander et al., 1998). However, as concentrations of essential oils which are needed to have antimicrobial activity can compromise the organoleptic qualities of foods, their use as preservatives in high concentrations have been rather limited thus far.
Nonetheless, a recent study utilized nisin A, and its bioengineered derivatives nisin S29A and nisin M21V independently and in combination with the essential oils carvacrol, trans-cinnamaldehyde, thymol, as well as the preservative citric acid, to evaluate the efficacy of such combinations against the Gram negative food-borne pathogens Escherichia coli O157: H7 and Cronobacter sakazakii (Campion et al., 2017). E. coli O157: H7 is an enterohaemorrhagic strain and has been associated with outbreaks in the US, Canada, UK and Japan (Besser et al., 1993; Bach et al., 2002; Vidovic and Korber, 2016). C. sakazakii has been associated with contaminated infant milk formula and is implicated in cases of enteritis, meningitis and septicaemia (Gurtler et al., 2005; Drudy et al., 2006; Iversen and Forsythe, 2007; Yan et al., 2012). Campion et al., noted that extended lag phases of C. sakazakii and O157: H7 were apparent when 30 μM of the bioengineered nisin variants were combined with 0.035% trans-cinnamaldehyde, 0.03% carvacrol and 0.015% thymol, when compared to corresponding combinations of nisin A-essential oils. In addition, a 4-log reduction of C. sakazakii and a 3-log reduction in viable counts of O157: H7 was particular noteworthy when 60 μM of the nisin variants were combined with 0.03% carvacrol, in comparison to corresponding nisin A-carvacrol combinations (Figure 1D). Importantly, the study also showed that when stored at room temperature, sub-lethal concentrations of nisin variants in combination with carvacrol were successful in fully inactivating O157: H7 in apple juice, again when compared to nisin A-carvacrol combinations. Similarly, the commercial product Nisaplin at concentrations of 10 mg/ml, in combination with 30 mM citric acid, elicited >3 log decreases in C. sakazakii viable counts in infant formula after 3 h of incubation (Campion et al., 2017). The increased stability of nisin at lower pH conditions compounded by the ameliorated diffusion properties of essential oils in acidic conditions could explain the effective combinations in low-pH drinks such as apple juice (Delves-Broughton, 1990; Burt, 2004; Campion et al., 2017). As thymol and carvacrol disrupt Gram negative outer membranes (which renders them more sensitive to nisin), while trans-cinnamaldehyde disrupts the transmembrane ATPase, combinations of such oils with nisin may prove to be the most effective option to control Gram negative food-borne pathogens (Helander et al., 1998; Gill and Holley, 2006a,b).
With regards to other studies investigating bacteriocin synergy against Gram negative food-borne pathogens, Moon et al., reported that a bacteriocin 4.5 kDa in size from Pediococcus acidilactici K10 in combination with the organic acids lactic acid, succinic acid and acetic acid interacted synergistically against E. coli O157: H7 both in vitro and in situ. This bacteriocin from P. acidilactici K10 in combination with 0.35% lactic acid or 0.25% acetic acid was evaluated in a ground beef sample at 4°C and it was noteworthy that a 2.8-log reduction of O157: H7 was observed with lactic acid combinations (Moon et al., 2002). Thus, P. acidilactici and organic acid combinations may have potential as food bio-preservatives. Branen and Davidson showed the efficacy of nisin combined with EDTA against enterohaemorrhagic E. coli strains (Branen and Davidson, 2004) while a separate study demonstrated the efficacy of 250 IU/ml of nisin combined with 500 μg/ml lactoferrin at preventing E. coli O157: H7 growth (Murdock et al., 2007). Finally, Ananou et al., observed synergistic effects when enterocin AS-48 was combined with agents that disrupted the outer membrane of a pathogenic E. coli O157: H7 isolate (Ananou et al., 2005).
A summary of studies involving bacteriocins in combination with various stressors with a view to targeting food-borne pathogens is found in Table 3.
Table 3.
Bacteriocins in combination with naturally-derived compounds against food-borne pathogens.
| Bacteriocin | Antimicrobial/Stressor | Target | Effect | Reference |
|---|---|---|---|---|
| Nisin | Green tea extract or grape seed extract | L. monocytogenes | Decrease in Listeria levels, compromised cell membrane and condensed cytoplasm | Sivarooban et al., 2008 |
| Carvacrol or mountain savory essential oils | L. monocytogenes | Increased sensitivity to γ-irradiation | Ndoti-Nembe et al., 2013 | |
| Cefotaxime or ceftriaxone | Salmonella Typhimurium | Synergy | Singh et al., 2013 | |
| EDTA | Salmonella Typhimurium | Additive | Singh et al., 2013 | |
| Pediocin PA1 | E. coli, L. monocytogenes | Synergy against L. monocytogenes; ineffective against E. coli | Naghmouchi et al., 2011 | |
| Nisin Z | Thymol | L. monocytogenes and B. cereus | Dose of nisin Z required reduced | Ettayebi et al., 2000 |
| Nisin V | Carvacrol, trans-cinnamadehyde or thymol | L. monocytogenes | Extended log phase | Field et al., 2015 |
| Nisin S29A or M21V | Carvacrol, trans-cinnamadehyde or thymol | E. coli O157: H7 and C. sakazakii | Extended lag phase of strains. Viable counts of strains decreased with carvacrol combinations. Also, O157: H7 inactivated in apple juice trial with carvacrol combinations | Campion et al., 2017 |
| Subtilosin | Encapsulated curcumin | L. monocytogenes Scott A | Partial synergy | Amrouche et al., 2010 |
| Zinc lactate | L. monocytogenes Scott A and NR30 | Synergy | Amrouche et al., 2010 | |
| Non-encapsulated ε-poly-L-lysine, curcumin | L. monocytogenes Scott A and NR30 | Additive | Amrouche et al., 2010 | |
| Enterocin AS-48 | Carvacrol or hydrocinnamic acid | S. aureus | Synergy | Grande et al., 2007 |
| Eugenol, geraniol, terpineol, carvacrol, hydrocinnamic acid, caffeic acid, citral and p-coumaric acid | S. aureus | Enhanced activity of enterocin As-48 | Grande et al., 2007 | |
| Lactic acid, PHBME or citric acid | L. monocytogenes | Synergy | Antonio et al., 2009 | |
| Pediocin PA1 | Polymyxin E | L. monocytogenes | Reduced growth of L. monocytogenes in log and stationary phases | Naghmouchi et al., 2011 |
| Bacteriocin from Lb. curvatus CWBI-B28 | Savory essential oil, oregano | L. monocytogenes | Growth of L. monocytogenes retarded by 2 weeks | Ghalfi et al., 2007 |
| Bacteriocin from P. acidilactici K10 | Lactic acid, succinic acid, aceric acid | E. coli O157: H7 | Synergy in vitro and in situ | Moon et al., 2002 |
Bacteriocins in combination with antibiotics against food-borne pathogens
While antibiotics are unlikely to be used in foods, several studies have nonetheless investigated their efficacies in combination with bacteriocins against food-borne pathogens in laboratory conditions. Studies of this nature can be useful with regards to providing insights into the mechanisms of synergistic interactions, especially when bacteriocins are combined with antibiotics with known modes of action. Bacillus cereus is an important food-borne pathogen and several strains have the ability to cause food poisoning, often resulting in vomiting and diarrhea (Schoeni and Wong, 2005). In a relatively recent study, it was shown that the two-component lantibiotic, lacticin 3147, interacted synergistically with the antibiotic polymyxin B against B. cereus 8079 and B. cereus 5247 (Draper et al., 2013). Interestingly however, lacticin 3147-polymyxin E combinations resulted in indifferent (1.0 < FIC < 2.0) and antagonistic effects (FIC > 2.0), in contrast to the above-mentioned synergistic interactions with polymyxin B against the same targets, showing that a single amino acid change in the polymyxin backbone can lead to profound differences in terms of interactions with lacticin 3147 against specific target strains. The lantibiotic nisin and class IIa bacteriocin, pediocin PA-1, have also been combined with the antibiotic polymyxin E to target L. monocytogenes and E. coli isolates which had exhibited resistance to pediocin and polymyxin E respectively (Naghmouchi et al., 2011). Polymyxin and nisin combinations at concentrations of 0.6 μg/ml and 15.6 μg/ml; 4.7 μg/ml and 62.5 μg/ml; and 9.3 μg/ml and 32 μg/ml, respectively, resulted in the inhibition of polymyxin-resistant E. coli, pediocin PA-1-resistant L. monocytogenes and nisin-resistant L. monocytogenes variants by 74, 97, and 94% respectively, relative to the controls. While nisin A and pediocin PA-1 combinations were synergistic against L. monocytogenes and its resistant variants, they were ineffective against E. coli or its resistant variants. Nonetheless, nisin-polymyxin combinations at concentrations of 7.8 μg/ml and 0.3 μg/ml, respectively, and polymyxin independently at a concentration of 0.21 μg/ml decreased the growth of log phase E. coli cells by approximately 94 and 31% respectively. Pediocin PA-1-polymyxin E combinations at concentrations of 25 μg/ml and 4.7 μg/ml, respectively, reduced the growth of L. monocytogenes in the exponential and stationary phases by 90% and 78%, respectively. Thus, the study indicated that resistant L. monocytogenes and E. coli isolates can be managed by using combinations of nisin/polymyxin E or pediocin PA-1/polymyxin E respectively (Naghmouchi et al., 2011). The same group also evaluated the activity of colistin in combination with pediocin PA-1/AcH or nisin with a view to targeting E. coli O157: H7, Yersinia enterocolitica ATCC 9610, P. aeruginosa ATCC 27853 and Salmonella choleraesuis ATCC 14028. Significantly, 1.56 μg/ml of pediocin PA-1/AcH or 1.7 μg/ml of nisin in combination with colistin elicited a marked reduction in the concentration of colistin needed to inhibit O157: H7 (Naghmouchi et al., 2013).
With respect to studies involving other Gram negative food-borne pathogens, lacticin 3147 was reported to exhibit synergy when combined with polymyxin B against C. sakazakii DPC6440 with FIC values of 0.25 (Draper et al., 2013). The lantibiotic also exhibited synergistic activity against the same strain when combined with polymyxin E with corresponding FIC values of 0.062 against DPC6440. Interestingly however, indifferent and antagonistic effects were obtained when lacticin 3147 was combined with polymyxin B or polymyxin E against the target strains Salmonella Typhimurium UK1 and LT2, with FIC values greater than 1.125 in all cases (Draper et al., 2013). A separate study by Rishi and co-workers demonstrated the efficacy of β-lactam antibiotics combined with nisin with a view to inhibiting the food-borne pathogen Salmonella enterica serovar Typhi (Rishi et al., 2014). Nisin-β-lactam synergistic combinations were assessed by conducting FIC and time-kill assays and with the exception of three strains, synergy was observed with all combinations against the clinical Salmonella strains in the in vitro study by Rishi and co-workers with nisin-cefotaxime and nisin-ceftriaxone proving to be the most effective combinations (Rishi et al., 2014). Singh et al., in a similar study, also evaluated the effectiveness of nisin in combination with standard antibiotics against multi-drug resistant strains of Salmonella and any such synergistic interactions were evaluated by FIC determinations using the checkerboard assay as well as time-kill assays and radial diffusion assays (Singh et al., 2013). Furthermore, scanning electron microscopy (SEM) and mouse trials assessing the combinatorial interactions were also conducted to validate synergistic effects observed with in vitro assays. Decreases in the numbers of Salmonella in various organs of infected mice were observed as a consequence of the antimicrobial combination. Using FIC values and time-kill assays, nisin-cefotaxime and nisin-ceftriaxone combinations yielded synergistic effects whereas nisin-EDTA and nisin-ampicillin combinations yielded additive effects against serovar Typhimurium. Significant alterations in the outer membrane of the target cells, elicited by the antimicrobial combinations were apparent and bacteriocin-β-lactam combinations caused greater log decreases of Salmonella in the spleen, intestine and liver of mice, relative to treatment with the antimicrobials independently (Singh et al., 2013). More specifically, the combination of nisin (at concentrations of 25 mg/Kg body weight and 50 mg/Kg body weight) with ceftriaxone (also at concentrations of 25 mg/Kg and 50 mg/Kg) resulted in 2.83, 3.11, 2.6, and 3.1-log decreases in Salmonella respectively in the spleen. Identical concentrations of nisin and cefotaxime were also combined, resulting in 2.06, 2.49, 2.11, and 2.44-log decreases respectively in the spleen as well. In contrast, 25 mg/Kg nisin and 50 mg/Kg nisin administered independently resulted in a mere 0.16 and 0.3-log decrease respectively in Salmonella in the spleen. Similarly, combinations of nisin (25 mg/Kg body weight and 50 mg/Kg body weight) combined with identical concentrations of ceftriaxone proved to be effective, resulting in decreases in Salmonella in the liver ranging from 2.75 to 3.5-log units. Corresponding combinations of nisin and cefotaxime resulted in reductions in Salmonella in the liver ranging from 2.27 to 3.26-log units. These values were higher than the 0.42 to 0.67-log unit reductions achieved by nisin independently. Following on from this study, the same group attempted to elucidate the mechanism of these synergistic interactions (Singh et al., 2014). Essentially, the ability of nisin and the β-lactams to target the cell membrane was evaluated by conducting membrane permeabilizing assays in combination with pulse labeling techniques. The results showed that the bacteriocin-β-lactam combination affected membrane permeability, as confirmed by the uptake of 1-N-phenylnapthylamine (NPN) by the treated cells. This uptake of NPN as a consequence of membrane permeabilization, as well as interference with DNA, RNA and protein synthesis was dependent on both the dose of the antimicrobials, as well as the duration of exposure to the antimicrobials in combination. Significantly, results of in vivo assays involving mouse trials corroborated synergistic effects seen in in vitro assays in the study (Singh et al., 2014). Thus, nisin-cefotaxime and nisin-ceftriaxone synergistic effects against Salmonella were predominantly due to permeabilization of the membrane, as well as DNA, RNA, protein synthesis inhibition, and immune-modulatory activity (Singh et al., 2014).
Other types of effective interactions against pathogens involving bacteriocins
A potential strategy with a view to combating recalcitrance to traditional antibiotics may be to combine bacteriocins with phages/endolysins and some studies have already investigated such prospects. For instance, a recent study evaluated the efficacy of combinations of the class II bacteriocin coagulin C23 with listeriaphages against L. monocytogenes and found that they act in a synergistic manner against the food-borne pathogen (Rodríguez-Rubio et al., 2015). More specifically, coagulin C23 was combined with the phages FWLLm1 or FWLLm3 and synergistic effects were apparent when the two antimicrobials were mixed in sub-inhibitory concentrations. Encouragingly, L. monocytogenes 2000/47 levels were lower than 10 CFU/ml after 96 h of storage at 4°C, when the bacteriocin was combined with the phage FWLLm1. However, the combination of coagulin C23 and FWLLm3 was not effective at inhibiting L. monocytogenes 2000/47 and this could be attributed to the emergence of mutants resistant to coagulin C23 and FWLLm3. Significantly, the authors in the study concluded that the rate of development of resistance was higher when the antimicrobials were used independently, relative to the combination of the two antimicrobials together. This phenomenon of delayed resistance development could explain the synergistic effects observed in the study (Rodríguez-Rubio et al., 2015).
Synergy was also observed when nisin was combined with the S. aureus lytic phages phiϕ35 and ϕ88 in another study (Martínez et al., 2008). Unfortunately, the use of this combination as a viable therapeutic option has been hindered due to the emergence of resistance to the two phages employed and adaptation to the lantibiotic nisin. With regards to other studies relating to phages combined with bacteriocin-like agents with a view to targeting S. aureus, the bacteriolysin lysostaphin exhibited synergistic effects in combination with the two endolysins (LysK) against MRSA (Dajcs et al., 2002; O'Flaherty et al., 2005; Becker et al., 2008). A deeper understanding of the mechanisms of synergistic interactions between bacteriocins and endolysins is essential if such an interaction is to be used in food/clinical settings. In the case of lysostaphin-LysK synergy, it may be that LysK by virtue of the fact that it has two lytic domains, has the ability to further potentiate the lytic nature of lysostaphin, which merely possesses one lytic domain (Becker et al., 2008). Finally, a study by Garcia et al., also reported synergistic interactions between nisin and the phage endolysin LysH5 (García et al., 2010). The activity of LysH5 may be enhanced by the ability of nisin to permeabilize the cell membrane of target S. aureus strains (Nascimento et al., 2008; Obeso et al., 2008).
The use of mathematical models to assess antimicrobial synergy and predict resistance development
While synergistic antimicrobial interactions are likely to be beneficial in clinical applications, the emergence of multi-drug resistance arising from such interactions remains unclear. Recently, mathematical modeling has enhanced the predictive capabilities of such antimicrobial interactions with regards to development of resistance (Ankomah and Levin, 2012; Chen et al., 2015). Indeed, Torella et al., addressed this phenomenon by optimizing a mathematical model to study infections in vivo and found that there were two opposite effects of synergy: (i) the synergistic interaction results in more rapid clearance of the infection and consequently fewer opportunities for the development of resistant derivatives/mutants and (ii) the selection of resistant isolates/derivatives is favored over wild-type cells associated with synergistic interactions (Torella et al., 2010). The authors found that when resources are abundant, the synergistic effects are more potent at eliminating the infection but conversely, when resources are limited, the potential for development of multi-drug resistance also increases. Above a certain critical level of drug interaction, the potential for emergence of multi-drug resistance is enhanced. Interestingly, the study suggested that to dampen down the emergence of multi-drug resistance, antimicrobial antagonism may on occasions actually be better than antimicrobial synergy (Torella et al., 2010).
In a similar study, Landersdorfer et al. devised a model for evaluating the synergistic interactions of antibiotic combinations using a sequential dosing design (Landersdorfer et al., 2013). The authors used nisin in combination with either linezolid or amikacin for the study. Sequential, as well as simultaneous, administration of the antimicrobials enabled the evaluation of the efficacy of linezolid or amikacin against populations of cells which were nisin-resistant or nisin-intermediate cells. Landersdorfer et al. used the software NONMEM and S-ADAPT to model the synergistic interactions. The study found that while bacterial replication was inhibited by linezolid in populations less sensitive to nisin, this population of cells was not efficiently killed. The combination of amikacin with the lantibiotic nisin resulted in sub-population synergy. Such models of simultaneous or sequential antimicrobial dosing models may enable scientists to devise effective antimicrobial combination strategies for clinical applications (Landersdorfer et al., 2013).
In a landmark study, the concept of “the smile-frown transition” with respect to antimicrobial synergy was introduced (Pena-Miller et al., 2013). The authors used mathematical modeling, whole genome sequencing, genetic manipulation of resistance mechanisms and evolution experiments to demonstrate that synergistic antimicrobial combinations can be ineffective unless the first round of treatment results in bacterial clearance. It was suggested that the potency of antimicrobial synergy decreases concurrently with the emergence of drug-resistant bacteria. Evolution experiments exhibited that the efficacy of the antibiotics used exponentially decreased over a 5 day period. The authors also found that the replication of drug-resistant bacteria was fastest when the drug-sensitive counterparts were killed by aggressive treatment strategies (Pena-Miller et al., 2013). The initial synergistic interaction created a selective pressure for the emergence of resistance causing antagonistic effects after day 1 of the 5-day experiment. The authors dubbed this phenomenon “the smile-frown transition.” Genome sequencing in the study showed that emergence of resistance to the antibiotics may be due to the amplification of genes involved in drug-resistance mechanisms such as the acrAB efflux operon (Pena-Miller et al., 2013). The deletion of this acrAB operon precluded the transition from antimicrobial synergy to antimicrobial antagonism within 5 days of the evolution experiments. Thus, evidence such as that outlined by Pena-Miller implies that super-inhibitory concentrations of the two antimicrobials in combination may need to be used until the pathogen is fully cleared. Conversely, in the presence of sub-inhibitory concentrations of the two antimicrobials, the “smile-frown transition” is likely to take precedence (Pena-Miller et al., 2013).
Conclusions
The 20th century was the golden era for the discovery of novel antibiotics and successful infection control strategies. However, the over-prescription of broad-spectrum antibiotics by clinicians worldwide in combination with overuse in animal applications has triggered an increase in antibiotic resistance and, in addition, has contributed to nosocomial infections such as C. difficile infection (CDI), due to perturbations of the gut microbiota. Furthermore, the broad-spectrum nature of several antibiotics as well as the negative links associated with the causation of autoimmune and atopic diseases with certain antibiotics renders them unattractive options (Blaser, 2011; Willing et al., 2011). The over-exposure to antibiotics in the environment, healthcare settings and in agriculture has contributed to this problem. Furthermore, the increase in global travel, compounded by poor infection control standards, has also exacerbated the crisis (Holmes et al., 2016). In particular, in hospital settings, the dissemination of antibiotic-resistant pathogens, especially in immunosuppressed patients is a cause for concern. A thorough understanding of the mechanisms of antibiotic resistance is urgently warranted to mitigate this global concern. Alternative therapeutic options, including bacteriocins used either independently or in combination with other stressors must also be thoroughly explored. Amongst the key advantages of bacteriocins include their ribosomally-synthesized nature, which renders them amenable to bioengineering strategies. Such bioengineered variants may possess enhanced bioactivity against certain clinical/food-borne pathogens or food spoilage organisms, as well as potentially possessing ameliorated physicochemical properties such as improved solubility, protease resistance and pH tolerance, further augmenting their value and effectiveness as antimicrobials. Other potential advantages of using bacteriocins include their high potency against target strains, their stability and their low toxicity. A potential disadvantage is that oral ingestion is complicated due to their proteolytic digestion in the gut. However, this may be overcome by advances in encapsulation technologies. Another means to circumvent proteolytic breakdown is to administer them parenterally for systemic applications.
Although there have already been studies conducted which have revealed potentially promising synergistic interactions between bacteriocins and other stressors, it must be highlighted that, since there are a large number of bacteriocin-antimicrobial combinations that have yet to be investigated, there could still be very useful combinations against targeted pathogens which are currently untapped. Thus, far, a precise understanding of the mechanism of synergistic interactions of antimicrobial combinations has hindered the progress of alternative therapeutic options of bacteriocin-antimicrobial combinations against target strains, particularly in clinical settings. Indeed, there has been a general reluctance in resorting to alternative therapeutic options and changing the status quo in the clinical arena. Elucidation of the mode of action of these synergistic interactions using a combination of genomic, transcriptomic and proteomic tools is likely to expedite the processes involved in the deployment of these antimicrobial combinations in clinical and/or food settings.
With respect to the clinical efficacy of bacteriocin-antimicrobial combinations, the precise nature of physicochemical interactions, such as hydrophobic-hydrophobic or cationic-anionic interactions, between a proteinaceous bacteriocin and an antibiotic are likely to be important considerations when optimizing effective combinatorial therapy for use in vivo. In this regard, it may also be the case that combinations of two bacteriocins that are of a similar molecular weight may be more effective in vivo than combining a high molecular weight bacteriocin with a low molecular weight antibiotic. In addition, the pharmacodynamic and pharmacokinetic traits of a bacteriocin are likely to be critical factors determining its success as a potential therapeutic agent in vivo. This becomes even more relevant when combined with antibiotics as physicochemical interactions of the bacteriocin with the antibiotic can interfere with the pharmacodynamic properties of both antimicrobials. With respect to the pharmacokinetic properties of bacteriocins in combination with antibiotics, optimization of the route of administration of the two antimicrobials to the localized site of infection is likely to be an important step in determining the success of the treatment. Indeed, localized cutaneous, intravaginal or inhaled routes of administration of bacteriocins may be effective due to the relatively low absorption rates, minimizing potentially undesired systemic side effects (Ghobrial et al., 2009, 2010a; van Heel et al., 2011). However, this may be complicated in the presence of an antibiotic used in combination. With respect to systemic applications however, the lantibiotic group of bacteriocins in particular could prove to be less efficacious due to their propensity to bind blood components (Ghobrial et al., 2010b). Thus, the distribution and consequent bioavailability of such bacteriocins in the desired target site can unfortunately be significantly attenuated. Aside from reducing the bioavailability, the binding of bacteriocins to plasma proteins can also reduce the specific activity of the bacteriocin against a target strain by potentially hindering access of the bacteriocin to its target receptor (Ghobrial et al., 2010b). This problem can be further exacerbated due to the instability of certain lantibiotics under physiological pH conditions. However, bioengineering strategies with a view to seeking derivatives with enhanced stability has the potential to somewhat mitigate this issue (Rollema et al., 1995; Yuan et al., 2004). It must also be highlighted that differences in the rates of metabolism and excretion of bacteriocins combined with antibiotics are likely to exist between animals and humans, and the half-life of each of the antimicrobials can have an impact on the propensity for development of resistance. In addition, further insights with respect to the effects of such antimicrobial combinations on eukaryotic cells are also essential in order to prevent any undesired side effects. Encouragingly, the evidence accumulated thus far has shown that bacteriocins in general tend to display low toxicity rates against epithelial cells and with the exception of cytolysin, generally tend to exhibit extremely low levels of hemolysis (Cox et al., 2005; Maher and McClean, 2006; Aranha et al., 2008). However, all these factors above can be further complicated and clinical outcomes difficult to predict when bacteriocins are combined with other antimicrobials. Overall, optimization of effective therapeutic concentrations of bacteriocins, either independently or in combination with other antimicrobials can only truly be achieved with the availability of more data concerning the pharmacokinetic properties of each of the antimicrobials in question. Therefore, a complex interplay of factors is bound to be crucial in governing the clinical efficacy of such potential combinatorial therapeutic options.
While bacteriocins interacting synergistically with other antimicrobials and stressors could have great potential in clinical and food settings, one must be aware that unfortunately, bacteriocins are by no means a “magic bullet” and are not exempt from development of resistance (Modi et al., 2000; Draper et al., 2015). Indeed, several different mechanisms of resistance to the lantibiotic subclass of bacteriocins have been described, which include cell-envelope altering mechanisms utilized by bacteria such as DltA or MprF (Peschel et al., 1999; Poyart et al., 2001; Abachin et al., 2002; Kovacs et al., 2006; Khattar et al., 2009; McBride and Sonenshein, 2011a,b), two component systems such as CprK in C. difficile (McBride and Sonenshein, 2011a,b; Suarez et al., 2013) and LisRK in L. monocytogenes (Cotter et al., 1999; Kallipolitis and Ingmer, 2001), as well as other mechanisms such as production of nisin resistance proteins (Chatterjee et al., 2005; O'Driscoll et al., 2006; Khosa et al., 2013) (for a comprehensive review on lantibiotic resistance, see Draper et al., 2015). Resistance to the class II group of bacteriocins have also been described in laboratory conditions and is likely to be mediated through decreased expression of Man-PTS receptors (Kjos et al., 2011). Ultimately, greater insights into the precise mechanisms of development of resistance to bacteriocins will facilitate their deployment in both clinical settings and as preservatives in food, either individually or in combination with other antimicrobials.
While it is plausible that the combination of two antimicrobials with two distinct modes of action attenuates the likelihood of resistance development, the emergence of sub-populations of target pathogens recalcitrant to both the bacteriocin as well as the other antimicrobial used in combination, remains a very realistic worst case scenario. It is interesting, in this regard, to note that the development of resistance to bacteriocins over the years has generally been associated with a slower growth rate and an associated fitness cost in resistant variants. Even though bacteriocins have been studied for several decades, the precise mechanisms of antimicrobial action of several of these peptides are still unknown. Knowledge of the exact modes of action of such bacteriocins would potentially help researchers to tailor-make “designer bacteriocins” which may act synergistically with other antimicrobials, with a view to targeting specific pathogens. By doing so, researchers could combine bacteriocins with certain antimicrobials whose modes of action are already known, in a target-specific manner. Finally, it must be highlighted that successful synergistic interactions between bacteriocins and other antimicrobials in vitro may not necessarily correlate with clinical efficacy. Nonetheless, optimization of a variety of complex factors including the pharmacodynamics/pharmacokinetic properties of the antimicrobials as well as antimicrobial concentrations and ratios at which the antimicrobials work in a synergistic fashion can lead to effective alternative therapeutic options with the ultimate view to confronting the increasingly worrying problem of antibiotic resistance.
Author contributions
HM, DF, MR, PC, CH and RP wrote the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
HM is a researcher in Teagasc Food Research Centre and the APC Microbiome Institute, funded by the Science Foundation of Ireland (SFI)-funded Centre for Science, Engineering and Technology and the APC Microbiome Institute Grant Number SFI/12/RC/2273. Research in PC, CH, MR, and RP laboratories is supported by the Science Foundation of Ireland (SFI)-funded Centre for Science, Engineering and Technology and the APC Microbiome Institute.
Glossary
Abbreviations
- MRSA
methicillin-resistant Staphylococcus aureus
- VRE
vancomycin-resistant enterococci
- Man-PTS
mannose phosphotransferase
- FIC
fractional inhibitory concentration
- MCBT
multiple combination bactericidal test
- PBP
penicillin binding protein
- MIC
minimum inhibitory concentration
- MBC
minimum bactericidal concentration
- TEM
transmission electron microscopy
- CDAD
Clostridium difficile-associated diarrhea
- SAM
S-adenosylmethionine
- LAE
lauramide arginine ethyl ester
- ATCC
American Type Culture Collection
- XTT
2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
- MSSA
methicillin-sensitive Staphylococcus aureus
- CFU
colony forming units
- CLSM
confocal laser scanning microscopy
- PHMG
polyhexamethylene guanidinium chloride
- MTAD
mixture of tetracycline isonomer, acid and detergent
- ATP
adenosine triphosphate
- GTE
green tea extract
- GSE
grape seed extract
- EDTA
ethylene diamine tetraacetic acid
- PHBME
p-hydroxybenzoic methylester acid
- CFS
cell-free supernatants
- DPC
Dairy Products Culture Collection
- SEM
scanning electron microscopy
- NPN
1-N-phenylnapthylamine
- DNA
deoxyribonucleic acid
- RNA
ribonucleic acid
- NONMEM
non-linear mixed effects modeling tool
- CDI
C. difficile infection.
References
- Abachin E., Poyart C., Pellegrini E., Milohanic E., Fiedler F., Berche P., et al. (2002). Formation of d-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43, 1–14. 10.1046/j.1365-2958.2002.02723.x [DOI] [PubMed] [Google Scholar]
- Abdelraouf K., Braggs K. H., Yin T., Truong L. D., Hu M., Tam V. H. (2012). Characterization of polymyxin B-induced nephrotoxicity: implications for dosing regimen design. Antimicrob. Agents Chemother. 56, 4625–4629. 10.1128/AAC.00280-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algburi A., Volski A., Chikindas M. L. (2015). Natural antimicrobials subtilosin and lauramide arginine ethyl ester synergize with conventional antibiotics clindamycin and metronidazole against biofilms of Gardnerella vaginalis but not against biofilms of healthy vaginal lactobacilli. Pathog. Dis. 73:ftv018 10.1093/femspd/ftv018 [DOI] [PubMed] [Google Scholar]
- Al-Seraih A., Belguesmia Y., Baah J., Szunerits S., Boukherroub R., Drider D. (2017). Enterocin B3A-B3B produced by LAB collected from infant faeces: potential utilization in the food industry for Listeria monocytogenes biofilm management. A. Van Leeuw, J. Microb. 110, 205–219. 10.1007/s10482-016-0791-5 [DOI] [PubMed] [Google Scholar]
- Amrouche T., Sutyak Noll K., Wang Y., Huang Q., Chikindas M. L. (2010). Antibacterial activity of subtilosin Alone and combined with curcumin, poly-Lysine and zinc lactate against Listeria monocytogenes strains. Probiot. Antimicrob. Proteins 2, 250–257. 10.1007/s12602-010-9042-7 [DOI] [PubMed] [Google Scholar]
- Ananou S., Gálvez A., Martínez-Bueno M., Maqueda M., Valdivia E. (2005). Synergistic effect of enterocin AS-48 in combination with outer membrane permeabilizing treatments against Escherichia coli O157:H7. J. Appl. Microbiol. 99, 1364–1372. 10.1111/j.1365-2672.2005.02733x [DOI] [PubMed] [Google Scholar]
- Ankomah P., Levin B. R. (2012). Two-Drug Antimicrobial Chemotherapy: a mathematical model and experiments with Mycobacterium marinum. PLoS Pathog. 8:e1002487. 10.1371/journal.ppat.1002487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio C. M., Abriouel H., López R. L., Omar N. B., Valdivia E., Gálvez A. (2009). Enhanced bactericidal activity of enterocin AS-48 in combination with essential oils, natural bioactive compounds and chemical preservatives against Listeria monocytogenes in ready-to-eat salad Food Chem. Toxicol. 47, 2216–2223. 10.1016/j.fct.2009.06.012 [DOI] [PubMed] [Google Scholar]
- Aranha C. C., Gupta S. M., Reddy K. V. (2008). Assessment of cervicovaginal cytokine levels following exposure to microbicide Nisin gel in rabbits. Cytokine 43, 63–70. 10.1016/j.cyto.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Arnison P. G., Bibb M. J., Bierbaum G., Bowers A. A., Bugni T. S., Bulaj G., et al. (2013). Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30, 108–160. 10.1039/c2np20085f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach S. J., McAliister T. A., Veira D. M., Gannon V. P. J., Holley R. A. (2002). Transmission and control of Escherichia coli O157:H7 - A review. Can. J. Anim. Sci. 82, 475–490. 10.4141/A02-021 [DOI] [Google Scholar]
- Bacon A. E., McGrath S., Fekety R., Holloway W. J. (1991). In vitro synergy studies with Clostridium difficile. Antimicrob. Agents Chemother. 35, 582–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S. C., Foster-Frey J., Donovan D. M. (2008). The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol. Lett. 287, 185–191. 10.1111/j.1574-6968.2008.01308.x [DOI] [PubMed] [Google Scholar]
- Besser R. E., Lett S. M., Weber J. T., Doyle M. P., Barrett T. J., Wells J. G., et al. (1993). An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 269, 2217–2220. 10.1001/jama.1993.03500170047032 [DOI] [PubMed] [Google Scholar]
- Blaser M. (2011). Antibiotic overuse: stop the killing of beneficial bacteria. Nature 476, 393–394. 10.1038/476393a [DOI] [PubMed] [Google Scholar]
- Branen J. K., Davidson P. M. (2004). Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int. J. Food Microbiol. 90, 63–74. 10.1016/S0168-1605(03)00172-7 [DOI] [PubMed] [Google Scholar]
- Breukink E., de Kruijff B. (2006). Lipid II as a target for antibiotics. Nat. Rev. Drug Discov. 5, 321–332. 10.1038/nrd2004 [DOI] [PubMed] [Google Scholar]
- Briers Y., Klumpp J., Schuppler M., Loessner M. (2011). Genome sequence of Listeria monocytogenes Scott, A., a clinical isolate from a food-borne listeriosis outbreak. J. Bacteriol. 193, 4284–4285. 10.1128/JB.05328-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brötz H., Bierbaum G., Markus A., Molitor E., Sahl H. G. (1995). Mode of action of the lantibiotic mersacidin: inhibition of peptidoglycan biosynthesis via a novel mechanism? Antimicrob. Agents Chemother. 39, 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfitt W., Salton M. R., Hamilton-Miller J. M. (2002). Nisin, alone and combined with peptidoglycan-modulating antibiotics: activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J. Antimicrob. Chemother. 50, 731–734. 10.1093/jac/dkf190 [DOI] [PubMed] [Google Scholar]
- Buommino E., Scognamiglio M., Donnarumma G., Fiorentino A., D'Abrosca B. (2014). Recent advances in natural product-based anti-biofilm approaches to control infections. Mini Rev. Med. Chem. 14, 1169–1182. 10.2174/1389557515666150101095853 [DOI] [PubMed] [Google Scholar]
- Burt S. (2004). Essential oils: their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 94, 223–253. 10.1016/j.ijfoodmicro.2004.03.022 [DOI] [PubMed] [Google Scholar]
- Caballero Gómez N., Abriouel H., Grande M. J., Pérez Pulido R., Gálvez A. (2013). Combined treatments of enterocin AS-48 with biocides to improve the inactivation of methicillin-sensitive and methicillin-resistant Staphylococcus aureus planktonic and sessile cells. Int. J. Food Microbiol. 163, 96–100. 10.1016/j.ijfoodmicro.2013.02.018 [DOI] [PubMed] [Google Scholar]
- Camargo A. C., de Paula O. A., Todorov S. D., Nero L. A. (2016). In vitro evaluation of bacteriocins activity against Listeria monocytogenes biofilm formation. Appl. Biochem. Biotechnol. 178, 1239–1251. 10.1007/s12010-015-1941-3 [DOI] [PubMed] [Google Scholar]
- Campion A., Morrissey R., Field D., Cotter P. D., Hill C., Ross R. P. (2017). Use of enhanced nisin derivatives in combination with food-grade oils or citric acid to control Cronobacter sakazakii and Escherichia coli O157:H7. Food Microbiol. 65, 254–263. 10.1016/j.fm.2017.01.020 [DOI] [PubMed] [Google Scholar]
- Catlin B. W. (1992). Gardnerella vaginalis: characteristics, clinical considerations, and controversies. Clin. Microbiol. Rev. 5, 213–237. 10.1128/CMR.5.3.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavera V. L., Volski A., Chikindas M. L. (2015). The natural antimicrobial Subtilosin A synergizes with lauramide arginine ethyl ester (LAE), ε-Poly-L-lysine (Polylysine), clindamycin phosphate and metronidazole, against the vaginal pathogen Gardnerella vaginalis. Probiotics. Antimicrob. Proteins. 7, 164–171. 10.1007/s12602-014-9183-1 [DOI] [PubMed] [Google Scholar]
- Ceotto-Vigoder H., Marques S. L., Santos I. N., Alves M. D., Barrias E. S., Potter A., et al. (2016). Nisin and lysostaphin activity against preformed biofilm of Staphylococcus aureus involved in bovine mastitis. J. Appl. Microbiol. 121, 101–114. 10.1111/jam.13136 [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Deleo F. R. (2009). Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7, 629–641. 10.1038/nrmicro2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee C., Paul M., Xie L., van der Donk W. A. (2005). Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105, 633–684. 10.1021/cr030105v [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Chatterjee D. K., Jani R. H., Blumbach J., Ganguli B. N., Klesel N., et al. (1992). Mersacidin, a new antibiotic from Bacillus: in vitro and in vivo antibacterial activity. J. Antibiot. 45, 839–845. 10.7164/antibiotics.45.839 [DOI] [PubMed] [Google Scholar]
- Chen D., Liu X., Yang Y., Yang H., Lu P. (2015). Systematic synergy modeling: understanding drug synergy from a systems biology perspective. BMC Syst. Biol. 9:56. 10.1186/s12918-015-0202-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrads G., de Soet J. J., Song L., Henne K., Sztajer H., Wagner-Döbler I., et al. (2014). Comparing the cariogenic species Streptococcus sobrinus and S. mutans on whole genome level. J. Oral Microbiol. 6:26189. 10.3402/jom.v6.26189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. A., Shlaes D. (2011). Fix the antibiotics pipeline. Nature 472:32. 10.1038/472032a [DOI] [PubMed] [Google Scholar]
- Cotter P. D., Emerson N., Gahan C. G., Hill C. (1999). Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J. Bacteriol. 181, 6840–6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P. D., Hill C., Ross R. P. (2005). Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3, 777–788. 10.1038/nrmicro1273 [DOI] [PubMed] [Google Scholar]
- Cotter P. D., Ross R. P., Hill C. (2013). Bacteriocins - a viable alternative to antibiotics? Nat. Rev. Microbiol. 11, 95–105. 10.1038/nrmicro2937 [DOI] [PubMed] [Google Scholar]
- Cox C. R., Coburn P. S., Gilmore M. S. (2005). Enterococcal cytolysin: a novel two component peptide system that serves as a bacterial defense against eukaryotic and prokaryotic cells. Curr. Protein Pept. Sci. 6, 77–84. 10.2174/1389203053027557 [DOI] [PubMed] [Google Scholar]
- Dabour N., Zihler A., Kheadr E., Lacroix C., Fliss I. (2009). In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int. J. Food Microbiol. 133, 225–233. 10.1016/j.ijfoodmicro.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Dajcs J. J., Thibodeaux B. A., Girgis D. O., Shaffer M. D., Delvisco S. M., O'Callaghan R. J. (2002). Immunity to lysostaphin and its therapeutic value for ocular MRSA infections in the rabbit. Investig. Ophthalmol. Vis. Sci. 43, 3712–3716. [PubMed] [Google Scholar]
- Davies D. (2003). Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2, 114–122. 10.1038/nrd1008 [DOI] [PubMed] [Google Scholar]
- de Kwaadsteniet M., Doeschate K. T., Dicks L. M. (2009). Nisin F in the treatment of respiratory tract infections caused by Staphylococcus aureus. Lett. Appl. Microbiol. 48, 65–70. 10.1111/j.1472-765X.2008.02488.x [DOI] [PubMed] [Google Scholar]
- Delves-Broughton J. (1990). Nisin and its application as a food preservative. Int. J. Dairy Technol. 43, 73–76. 10.1111/j.1471-0307.1990.tb02449.x [DOI] [Google Scholar]
- Doern C. D. (2014). When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 52, 4124–4128. 10.1128/JCM.01121-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosler S., Gerceker A. A. (2011). In vitro activities of nisin alone or in combination with vancomycin and ciprofloxacin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. Chemotherapy 57, 511–516. 10.1159/000335598 [DOI] [PubMed] [Google Scholar]
- Dosler S., Gerceker A. A. (2012). In vitro activities of antimicrobial cationic peptides; melittin and nisin, alone or in combination with antibiotics against Gram-positive bacteria. J. Chemother. 24, 137–143. 10.1179/1973947812Y.0000000007 [DOI] [PubMed] [Google Scholar]
- Dosler S., Mataraci E. (2013). In vitro pharmacokinetics of antimicrobial cationic peptides alone and in combination with antibiotics against methicillin resistant Staphylococcus aureus biofilms. Peptides 49, 53–58. 10.1016/j.peptides.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Draper L. A., Cotter P. D., Hill C., Ross R. P. (2013). The two peptide lantibiotic lacticin 3147 acts synergistically with polymyxin to inhibit Gram negative bacteria. BMC Microbiol. 13:212. 10.1186/1471-2180-13-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper L. A., Cotter P. D., Hill C., Ross R. P. (2015). Lantibiotic resistance. Microbiol. Mol. Biol. Rev. 79, 171–191. 10.1128/MMBR.00051-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drudy D., Mullane N. R., Quinn T., Wall P. G., Fanning S. (2006). Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Clin. Infect. Dis. 42, 996–1002. 10.1086/501019 [DOI] [PubMed] [Google Scholar]
- Eijsink V. G., Skeie M., Middelhoven P. H., Brurberg M. B., Nes I. F. (1998). Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl. Environ. Microbiol. 64, 3275–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettayebi K., El Yamani J., Rossi-Hassani B. (2000). Synergistic effects of nisin and thymol on antimicrobial activities in Listeria monocytogenes and Bacillus subtilis. FEMS Microbiol. Lett. 183, 191–195. 10.1111/j.1574-6968.2000.tb08956x [DOI] [PubMed] [Google Scholar]
- Field D., Begley M., O'Connor P. M., Daly K. M., Hugenholtz F., Cotter P. D., et al. (2012). Bioengineered nisin A derivatives with enhanced activity against both Gram positive and Gram negative pathogens. PLoS ONE 7:e46884. 10.1371/journal.pone.0046884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field D., Connor P. M., Cotter P. D., Hill C., Ross R. P. (2008). The generation of nisin variants with enhanced activity against specific Gram-positive pathogens. Mol. Microbiol. 69, 218–230. 10.1111/j.1365-2958.2008.06279.x [DOI] [PubMed] [Google Scholar]
- Field D., Daly K., O'Connor P. M., Cotter P. D., Hill C., Ross R. P. (2015). Efficacies of nisin A and nisin V semipurified preparations alone and in combination with plant essential oils for controlling Listeria monocytogenes. Appl. Environ. Microbiol. 81, 2762–2769. 10.1128/AEM.00070-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field D., O'Connor R., Cotter P. D., Ross R. P., Hill C. (2016a). In vitro activities of nisin and nisin derivatives alone and in combination with antibiotics against Staphylococcus biofilms. Front. Microbiol. 7:508. 10.3389/fmicb.2016.00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field D., Seisling N., Cotter P. D., Ross R. P., Hill C. (2016b). Synergistic Nisin-Polymyxin combinations for the control of Pseudomonas biofilm formation. Front. Microbiol. 7:1713. 10.3389/fmicb.2016.01713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., et al. (1985). Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N. Engl. J. Med. 312, 404–407. 10.1056/NEJM198502143120704 [DOI] [PubMed] [Google Scholar]
- Flemming H. C., Wingender J. (2010). The biofilm matrix. Nat. Rev. Micro. 8, 623–633. 10.1038/nrmicro2415 [DOI] [PubMed] [Google Scholar]
- Flores-Mireles A. L., Walker J. N., Caparon M., Hultgren S. J. (2015). Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13, 269–284. 10.1038/nrmicro3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foweraker J. E., Laughton C. R., Brown D. F., Bilton D. (2009). Comparison of methods to test antibiotic combinations against heterogeneous populations of multiresistant Pseudomonas aeruginosa from patients with acute infective exacerbations in cystic fibrosis. Antimicrob. Agents Chemother. 53, 4809–4815. 10.1128/AAC.00269-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García P., Martínez B., Rodríguez L., Rodríguez A. (2010). Synergy between the phage endolysin LysH5 and nisin to kill Staphylococcus aureus in pasteurized milk. Int. J. Food Microbiol. 141, 151–155. 10.1016/j.ijfoodmicro.2010.04.029 [DOI] [PubMed] [Google Scholar]
- Gellatly S. L., Hancock R. E. (2013). Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog. Dis. 67, 159–173. 10.1111/2049-632X.12033 [DOI] [PubMed] [Google Scholar]
- Ghalfi H., Benkerroum N., Doguiet D. D., Bensaid M., Thonart P. (2007). Effectiveness of cell-adsorbed bacteriocin produced by Lactobacillus curvatus CWBI-B28 and selected essential oils to control Listeria monocytogenes in pork meat during cold storage. Lett. Appl. Microbiol. 44, 268–273. 10.1111/j.1472-765X.2006.02077.x [DOI] [PubMed] [Google Scholar]
- Ghobrial O., Derendorf H., Hillman J. D. (2010a). Pharmacokinetic and pharmacodynamic evaluation of the lantibiotic MU1140. J. Pharm. Sci. 99, 2521–2528. 10.1002/jps.22015 [DOI] [PubMed] [Google Scholar]
- Ghobrial O., Derendorf H., Hillman J. D. (2010b). Human serum binding and its effect on the pharmacodynamics of the lantibiotic MU1140. Eur. J. Pharm. Sci. 41, 658–664. 10.1016/j.ejps.2010.09.005 [DOI] [PubMed] [Google Scholar]
- Ghobrial O. G., Derendorf H., Hillman J. D. (2009). Development and validation of a LC-MS quantification method for the lantibiotic MU1140 in rat plasma. J. Pharm. Biomed. Anal. 49, 970–975. 10.1016/j.jpba.2008.12.022 [DOI] [PubMed] [Google Scholar]
- Giacometti A., Cirioni O., Barchiesi F., Fortuna M., Scalise G. (1999). In vitro activity of cationic peptides alone and in combination with clinically used antimicrobial agents against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 44, 641–645. [DOI] [PubMed] [Google Scholar]
- Gill A. O., Holley R. A. (2006a). Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 108, 1–9. 10.1016/j.ijfoodmicro.2005.10.009 [DOI] [PubMed] [Google Scholar]
- Gill A. O., Holley R. A. (2006b). Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 111, 170–174. 10.1016/j.ijfoodmicro.2006.04.046 [DOI] [PubMed] [Google Scholar]
- Goyette-Desjardins G., Auger J. P., Xu J., Segura M., Gottschalk M. (2014). Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 3:e45. 10.1038/emi.2014.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande M. J., López R. L., Abriouel H., Valdivia E., Ben Omar N., Maqueda M., et al. (2007). Treatment of vegetable sauces with enterocin AS-48 alone or in combination with phenolic compounds to inhibit proliferation of Staphylococcus aureus. J. Food Prot. 70, 405–411. [DOI] [PubMed] [Google Scholar]
- Gravesen A., Ramnath M., Rechinger K. B., Andersen N., Jänsch L., Héchard Y., et al. (2002). High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology 48, 2361–2369. 10.1099/00221287-148-8-2361 [DOI] [PubMed] [Google Scholar]
- Grönthal T., Moodley A., Nykäsenoja S., Junnila J., Guardabassi L., Thomson K., et al. (2014). Large outbreak caused by methicillin resistant Staphylococcus pseudintermedius ST71 in a Finnish veterinary teaching hospital–from outbreak control to outbreak prevention. PLoS ONE 9:e110084. 10.1371/journal.pone.0110084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtler J. B., Kornacki J. L., Beuchat L. R. (2005). Enterobacter sakazakii: a coliform of increased concern to infant health. Int. J. Food Microbiol. 104, 1–34. 10.1016/j.ijfoodmicro.2005.02.013 [DOI] [PubMed] [Google Scholar]
- Hackbarth C. J., Kocagoz T., Kocagoz S., Chambers H. F. (1995). Point mutations in Staphylococcus aureus PBP 2 gene affect penicillin-binding kinetics and are associated with resistance. Antimicrob. Agents Chemother. 39, 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchi H., Hammami R., Gingras H., Kourda R., Bergeron M. G., Ben Hamida J., et al. (2017). Inhibition of MRSA and of Clostridium difficile by durancin 61A: synergy with bacteriocins and antibiotics. Future Microbiol. 12, 205–212. 10.2217/fmb-2016-0113 [DOI] [PubMed] [Google Scholar]
- Héchard Y., Sahl H. G. (2002). Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 84, 545–557. 10.1016/S0300-9084(02)01417-7 [DOI] [PubMed] [Google Scholar]
- Helander I. M., Alakomi H. L., Latva-Kala K., Mattila-Sandholm T., Pol I., Smid E. J., et al. (1998). Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 46, 3590–3595. 10.1021/jf980154m [DOI] [Google Scholar]
- Holmes A. H., Moore L. S., Sundsfjord A., Steinbakk M., Regmi S., Karkey A., et al. (2016). Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 387, 176–187. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Wilson M. E., Wertheim H. F. L., Trung Nghia H. D., Taylor W., Schultsz C. (2009). Streptococcus suis: an emerging human pathogen. Clin. Infect. Dis. 48, 617–625. 10.1086/596763 [DOI] [PubMed] [Google Scholar]
- Huong V. T., Ha N., Huy N. T., Horby P., Nghia H. D., Thiem V. D., et al. (2014). Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg. Infect. Dis. 20, 1105–1114. 10.3201/eid2007.131594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M. A., Dawson C. O. (2013). Economic impact of food safety outbreaks on food businesses. Foods 2, 585–589. 10.3390/foods2040585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen C., Forsythe S. J. (2007). Comparison of media for the isolation of Enterobacter sakazakii. Appl. Environ. Microbiol. 73, 48–52. 10.1128/AEM.01562-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabes D., Brunati C., Candiani G., Riva S., Romano G., Donadio S. (2011). Efficacy of the new lantibiotic NAI-107 in experimental infections induced by MDR Gram positive pathogens. Antimicrob. Agents Chemother. 55, 1671–1676. 10.1128/AAC.01288-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. M., Jaykus L.-A. (2004). Antimicrobial resistance of Enterococcus species isolated from produce. Appl. Environ. Microbiol. 70, 3133–3137. 10.1128/AEM.70.5.3133-3137.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallipolitis B. H., Ingmer H. (2001). Listeria monocytogenes response regulators important for stress tolerance and pathogenesis. FEMS Microbiol. Lett. 204, 111–115. 10.1111/j.1574-6968.2001.tb10872.x [DOI] [PubMed] [Google Scholar]
- Khattar Z. A., Rejasse A., Destoumieux-Garzón D., Escoubas J., Sanchis V., Lereclus D., et al. (2009). The dlt operon of Bacillus cereus is required for resistance to cationic antimicrobial peptides and for virulence in insects. J. Bacteriol. 191, 7063–7073. 10.1128/JB.00892-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosa S., Alkhatib Z., Smits S. H. (2013). NSR from Streptococcus agalactiae confers resistance against nisin and is encoded by a conserved nsr operon. Biol. Chem. 394, 1543–1549. 10.1515/hsz-2013-0167 [DOI] [PubMed] [Google Scholar]
- Kim J., Sudbery P. (2011). Candida albicans, a major human fungal pathogen. J. Microbiol. 49, 171–177. 10.1007/s12275-011-1064-7 [DOI] [PubMed] [Google Scholar]
- Kjos M., Nes I. F., Diep D. B. (2011). Mechanisms of resistance to bacteriocins targeting the mannose phosphotransferase system. Appl. Environ. Microbiol. 77, 3335–3342. 10.1128/AEM.02602-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M., Halfmann A., Fedtke I., Heintz M., Peschel A., Vollmer W., et al. (2006). A functional dlt operon, encoding proteins required for incorporation of d-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J. Bacteriol. 188, 5797–5805. 10.1128/JB.00336-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszewska D., Sahl H. G., Bierbaum G., Pag U., Hynes S. O., Ljungh A. (2004). Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model. J. Antimicrob. Chemother. 54, 648–653. 10.1093/jac/dkh387 [DOI] [PubMed] [Google Scholar]
- Landersdorfer C. B., Ly N. S., Xu H., Tsuji B. T., Bulitta J. B. (2013).Quantifying subpopulation synergy for antibiotic combinations via mechanism-based modeling and a sequential dosing design. Antimicrob. Agents Chemother. 57, 2343–2351. 10.1128/AAC.00092-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBel G., Piché F., Frenette M., Gottschalk M., Grenier D. (2013). Antimicrobial activity of nisin against the swine pathogen Streptococcus suis and its synergistic interaction with antibiotics. Peptides 50, 19–23. 10.1016/j.peptides.2013.09.014 [DOI] [PubMed] [Google Scholar]
- Leffler D. A., Lamont J. T. (2015). Clostridium difficile infection. N. Engl. J. Med. 73, 287–288. 10.1056/NEJMc1506004 [DOI] [PubMed] [Google Scholar]
- Ling L. L., Schneider T., Peoples A. J., Spoering A. L., Engels I., Conlon B. P., et al. (2015). A new antibiotic kills pathogens without detectable resistance. Nature 517, 455–459. 10.1038/nature14098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobos O., Padilla A., Padilla C. (2009). In vitro antimicrobial effect of bacteriocin PsVP-10 in combination with chlorhexidine and triclosan against Streptococcus mutans and Streptococcus sobrinus strains. Arch. Oral Biol. 54, 230–234. 10.1016/j.archoralbio.2008.11.007 [DOI] [PubMed] [Google Scholar]
- Loesche W. J. (1986). Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50, 353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low J. C., Donachie W. (1997). A review of Listeria monocytogenes and listeriosis. Vet. J. 153, 9–29. 10.1016/S1090-0233(97)80005-6 [DOI] [PubMed] [Google Scholar]
- Mah T. F., O'Toole G. A. (2001). Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9, 34–39. 10.1016/S0966-842X(00)01913-2 [DOI] [PubMed] [Google Scholar]
- Maher S., McClean S. (2006). Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem. Pharmacol. 71, 1289–1298. 10.1016/j.bcp.2006.01.012 [DOI] [PubMed] [Google Scholar]
- Martínez B., Obeso J. M., Rodríguez A., García P. (2008). Nisin-bacteriophage cross resistance in Staphylococcus aureus. Int. J. Food Microbiol. 122, 253–258. 10.1016/j.ijfoodmicro.2008.01.011 [DOI] [PubMed] [Google Scholar]
- Mataraci E., Dosler S. (2012). In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 56, 6366–6371. 10.1128/AAC.01180-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur H., O'Connor P. M., Hill C., Cotter P. D., Ross R. P. (2013). Analysis of anti-Clostridium difficile activity of thuricin CD, vancomycin, metronidazole, ramoplanin, and actagardine, both singly and in paired combinations. Antimicrob. Agents Chemother. 57, 2882–2886. 10.1128/AAC.00261-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur H., Rea M. C., Cotter P. D., Hill C., Ross R. P. (2015). The sactibiotic subclass of bacteriocins: an update. Curr. Protein Pept. Sci. 16, 549–558. 10.2174/1389203716666150515124831 [DOI] [PubMed] [Google Scholar]
- Mazzotta A. S., Montville T. J. (1997). Nisin induces changes in membrane fatty acid composition of Listeria monocytogenes nisin-resistant strains at 10°C and 30°C. J. Appl. Microbiol. 82, 32–38. 10.1111/j.1365-2672.1997.tb03294.x [DOI] [PubMed] [Google Scholar]
- McAuliffe O., Ryan M. P., Ross R. P., Hill C., Breeuwer P., Abee T. (1998). Lacticin 3147, a broad-spectrum bacteriocin which selectively dissipates the membrane potential. Appl. Environ. Microbiol. 64, 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride S. M., Sonenshein A. L. (2011a). The dlt operon confers resistance to cationic antimicrobial peptides in Clostridium difficile. Microbiology 157, 1457–1465. 10.1099/mic.0.045997-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride S. M., Sonenshein A. L. (2011b). Identification of a genetic locus responsible for antimicrobial peptide resistance in Clostridium difficile. Infect. Immun. 79, 167–176. 10.1128/IAI.00731-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes C. A., Cordeiro J. A., Burdmann E. A. (2009). Prevalence and risk factors for acute kidney injury associated with parenteral polymyxin B use. Ann. Pharmacother. 43, 1948–1955. 10.1345/aph.1M277 [DOI] [PubMed] [Google Scholar]
- Metwalli K. H., Khan S. A., Krom B. P., Jabra-Rizk M. A. (2013). Streptococcus mutans, Candida albicans, and the human mouth: a sticky situation. PLoS Pathog. 9:e1003616. 10.1371/journal.ppat.1003616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael C. A., Dominey-Howes D., Labbate M. (2014). The antimicrobial resistance crisis: causes, consequences, and management. Front. Public Health. 2:145. 10.3389/fpubh.2014.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming L., Zhang Q., Yang L., Huang J. A. (2015). Comparison of antibacterial effects between antimicrobial peptide and bacteriocins isolated from Lactobacillus plantarum on three common pathogenic bacteria. Int. J. Clin. Exp. Med. 8, 5806–5811. [PMC free article] [PubMed] [Google Scholar]
- Modi K. D., Chikindas M. L., Montville T. J. (2000). Sensitivity of nisin-resistant Listeria monocytogenes to heat and the synergistic action of heat and nisin. Lett. Appl. Microbiol. 30, 249–253. 10.1046/j.1472-765x.2000.00708.x [DOI] [PubMed] [Google Scholar]
- Montanari M. P., Massidda O., Mingoia M., Varaldo P. E. (1996). Borderline susceptibility to methicillin in Staphylococcus aureus: a new mechanism of resistance? Microb. Drug Resist. 2, 257–260. 10.1089/mdr.1996.2.257 [DOI] [PubMed] [Google Scholar]
- Moon G. S., Kim W. J., Kim M. (2002). Synergistic effects of bacteriocin-producing Pediococcus acidilactici K10 and organic acids on inhibiting Escherichia coli O157:H7 and applications in ground beef. J. Microbiol. Biotechnol. 12, 936–942. [Google Scholar]
- Mota-Meira M., LaPointe G., Lacroix C., Lavoie M. C. (2000). MICs of mutacin B-Ny266, nisin A, vancomycin, and oxacillin against bacterial pathogens. Antimicrob. Agents Chemother. 44, 24–29. 10.1128/AAC.44.1.24-29.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock C. A., Cleveland J., Matthews K. R., Chikindas M. L. (2007). The synergistic effect of nisin and lactoferrin on the inhibition of Listeria monocytogenes and Escherichia coli O157:H7. Lett. Appl. Microbiol. 44, 255–261. 10.1111/j.1472-765X.2006.02076.x [DOI] [PubMed] [Google Scholar]
- Murray B. E. (1990). The life and times of the Enterococcus. Clin. Microbiol. Rev. 3, 46–65. 10.1128/CMR.3.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghmouchi K., Baah J., Hober D., Jouy E., Rubrecht C., Sané F., et al. (2013). Synergistic effect between colistin and bacteriocins in controlling Gram-negative pathogens and their potential to reduce antibiotic toxicity in mammalian epithelial cells. Antimicrob. Agents Chemother. 57, 2719–2725. 10.1128/AAC.02328-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghmouchi K., Belguesmia Y., Baah J., Teather R., Drider D. (2011). Antibacterial activity of class I and IIa bacteriocins combined with polymyxin E against resistant variants of Listeria monocytogenes and Escherichia coli. Res. Microbiol. 162, 99–107. 10.1016/j.resmic.2010.09.014 [DOI] [PubMed] [Google Scholar]
- Nascimento J. G., Guerreiro-Pereira M. D., Costa S. F., São-José C., Santos M. A. (2008). Nisin-triggered activity of Lys44, the secreted endolysin from Oenococcus oeni phage fOg44. J. Bacteriol. 190, 457–461. 10.1128/JB.01195-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratna M. A., Sahl H. G., Tagg J. R. (1998). Two-component anti-Staphylococcus aureus lantibiotic activity produced by Staphylococcus aureus C55. Appl. Environ. Microbiol. 64, 4803–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndoti-Nembe A., Vu K. D., Doucet N., Lacroix M. (2013). Effect of combination of essential oils and bacteriocins on the efficacy of gamma radiation against Salmonella Typhimurium and Listeria monocytogenes. Int. J. Radiat. Biol. 89, 794–800. 10.3109/09553002.2013.797621 [DOI] [PubMed] [Google Scholar]
- Nelson K., Fouts D., Mongodin E., Ravel J., DeBoy R., Kolonay J., et al. (2004). Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 32, 2386–2395. 10.1093/nar/gkh562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll K. S., Prichard M. N., Khaykin A., Sinko P. J., Chikindas M. L. (2012). The natural antimicrobial peptide subtilosin acts synergistically with glycerol monolaurate, lauric arginate, and ε-poly-L-lysine against bacterial vaginosis-associated pathogens but not human lactobacilli. Antimicrob. Agents Chemother. 56, 1756–1761. 10.1128/AAC.05861-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso J. M., Martínez B., Rodríguez A., García P. (2008). Lytic activity of the recombinant staphylococcal bacteriophage phiH5 endolysin active against Staphylococcus aureus in milk. Int. J. Food Microbiol. 128, 212–218. 10.1016/j.ijfoodmicro.2008.08.010 [DOI] [PubMed] [Google Scholar]
- O'Driscoll J., Glynn F., Fitzgerald G. F., van Sinderen D. (2006). Sequence analysis of the lactococcal plasmid pNP40: a mobile replicon for coping with environmental hazards. J. Bacteriol. 188, 6629–6639. 10.1128/JB.00672-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty S., Coffey A., Meaney W., Fitzgerald G. F., Ross R. P. (2005). The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 187, 7161–7164. 10.1128/JB.187.20.7161-7164.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppegård C., Rogne P., Emanuelsen L., Kristiansen P. E., Fimland G., Nissen-Meyer J. (2007). The two-peptide class II bacteriocins: structure, production, and mode of action. J. Mol. Microbiol. Biotechnol. 13, 210–219. 10.1159/000104750 [DOI] [PubMed] [Google Scholar]
- Orhan G., Bayram A., Zer Y., Balci I. (2005). Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 43, 140–143. 10.1128/JCM.43.1.140-143.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Miller R., Laehnemann D., Jansen G., Fuentes-Hernandez A., Rosenstiel P., Schulenburg H., et al. (2013). When the most potent combination of antibiotics selects for the greatest bacterial load: the smile-frown transition. PLoS Biol. 11:e1001540. 10.1371/journal.pbio.1001540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel A., Otto M., Jack R. W., Kalbacher H., Jung G., Gotz F. (1999). Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274, 8405–8410. 10.1074/jbc.274.13.8405 [DOI] [PubMed] [Google Scholar]
- Piper C., Draper L. A., Cotter P. D., Ross R. P., Hill C. (2009). A comparison of the activities of lacticin 3147 and nisin against drug-resistant Staphylococcus aureus and Enterococcus species. J. Antimicrob. Chemother. 64, 546–551. 10.1093/jac/dkp221 [DOI] [PubMed] [Google Scholar]
- Poyart C., Lamy M. C., Boumaila C., Fiedler F., Trieu-Cuot P. (2001). Regulation of d-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J. Bacteriol. 183, 6324–6334. 10.1128/JB.183.21.6324-6334.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M. J., Vedamuthu E. R., Kunka B. S., Vandenbergh P. A. (1988). Inhibition of Listeria monocytogenes by using bacteriocin PA-1 produced by Pediococcus acidilactici PAC 1.0. Appl. Environ. Microbiol. 54, 2349–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea M. C., Clayton E., O'Connor P. M., Shanahan F., Kiely B., Ross R. P., et al. (2007). Antimicrobial activity of lacticin 3147 against clinical Clostridium difficile strains. J. Med. Microbiol. 56, 940–946. 10.1099/jmm.0.47085-0 [DOI] [PubMed] [Google Scholar]
- Rea M. C., Sit C. S., Clayton E., O'Connor P. M., Whittal R. M., Zheng J., et al. (2010). Thuricin, C. D., a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl. Acad. Sci. U.S.A. 107, 9352–9357. 10.1073/pnas.0913554107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reen F. J., Flynn S., Woods D. F., Dunphy N., Chróinín M. N., Mullane D., et al. (2016). Bile signalling promotes chronic respiratory infections and antibiotic tolerance. Sci. Rep. 6:29768. 10.1038/srep29768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishi P., Preet Singh A., Garg N., Rishi M. (2014). Evaluation of nisin-β-lactam antibiotics against clinical strains of Salmonella enterica serovar Typhi. J. Antibiot. (Tokyo) 67, 807–811. 10.1038/ja.2014.75 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rubio L., García P., Rodríguez A., Billington C., Hudson J. A., Martínez B. (2015). Listeriaphages and coagulin C23 act synergistically to kill Listeria monocytogenes in milk under refrigeration conditions. Int. J. Food. Microbiol. 205, 68–72. 10.1016/j.ijfoodmicro.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Rollema H. S., Kuipers O. P., Both P., de Vos W. M., Siezen R. J. (1995). Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl. Environ. Microbiol. 61, 2873–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L., Auchtung T. A., Hermans K. E., Whitehead D., Borhan B., Britton R. A. (2010). The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 156, 1589–1599. 10.1099/mic.0.035642-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeni J. L., Wong A. C. (2005). Bacillus cereus food poisoning and its toxins. J. Food Prot. 68, 636–648. 10.4315/0362-028X-68.3.636 [DOI] [PubMed] [Google Scholar]
- Schuppler M., Loessner M. J. (2010). The opportunistic pathogen Listeria monocytogenes: pathogenicity and interaction with the mucosal immune system. Int. J. Inflam. 2010:704321. 10.4061/2010/704321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Srivastava S. (2014). Anti-Candida activity of two-peptide bacteriocins, plantaricins (Pln E/F and J/K) and their mode of action. Fungal Biol. 118, 264–275. 10.1016/j.funbio.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Singh A. P., Prabha V., Rishi P. (2013). Value addition in the efficacy of conventional antibiotics by Nisin against Salmonella. PLoS ONE 8:e76844. 10.1371/journal.pone.0076844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. P., Preet S., Rishi P. (2014). Nisin/β-lactam adjunct therapy against Salmonella enterica serovar Typhimurium: a mechanistic approach. J. Antimicrob. Chemother. 69, 1877–1887. 10.1093/jac/dku049 [DOI] [PubMed] [Google Scholar]
- Sivarooban T., Hettiarachchy N. S., Johnson M. G. (2008). Transmission electron microscopy study of Listeria monocytogenes treated with nisin in combination with either grape seed or green tea extract. J. Food Prot. 71, 2105–2109. [DOI] [PubMed] [Google Scholar]
- Smith M. K., Draper L. A., Hazelhoff P. J., Cotter P. D., Ross R. P., Hill C. (2016). A Bioengineered Nisin derivative, M21A, in combination with good grade additives eradicates biofilms of Listeria monocytogenes. Front. Microbiol. 7:1939. 10.3389/fmicb.2016.01939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani R., Khalili H., Shafiee F. (2012). Double-disk synergy test for detection of synergistic effect between antibiotics against nosocomial strains of Staphylococcus aureus. J. Res. Pharm Pract. 1, 21–24. 10.4103/2279-042X.99673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopirala M. M., Mangino J. E., Gebreyes W. A., Biller B., Bannerman T., Balada-Llasat J. M., et al. (2010). Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 54, 4678–4683. 10.1128/AAC.00497-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton P. D., Taylor P. W. (2002). Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci. Prog. 85, 57–72. 10.3184/003685002783238870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens K. A., Sheldon B. W., Klapes N. A., Klaenhammer T. R. (1991). Nisin treatment for inactivation of Salmonella species and other Gram-negative bacteria. Appl. Environ. Microbiol. 57, 3613–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez J. M., Edwards A. N., McBride S. M. (2013). The Clostridium difficile cpr locus is regulated by a noncontiguous two-component system in response to type A and B lantibiotics. J. Bacteriol. 195, 2621–2631. 10.1128/JB.00166-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Wennerberg K., Aittokallio T. (2015). What is synergy? The Saariselkä agreement revisited. Front. Pharmacol. 6:181. 10.3389/fphar.2015.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z., Ling J., Lin Z., Li X., Mu Y. (2013). The effect of MTADN on 10 Enterococcus faecalis isolates and biofilm: an in vitro study. J. Endod. 39, 674–678. 10.1016/j.joen.2012.12.010 [DOI] [PubMed] [Google Scholar]
- Tong Z., Zhang L., Ling J., Jian Y., Huang L., Deng D. (2014b). An in vitro study on the effect of free amino acids alone or in combination with nisin on biofilms as well as on planktonic bacteria of Streptococcus mutans. PLoS ONE 9:e99513. 10.1371/journal.pone.0099513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z., Zhang Y., Ling J., Ma J., Huang L., Zhang L. (2014a). In vitro study on the effects of Nisin on the antibacterial activities of 18 antibiotics against Enterococcus faecalis. PLoS ONE 9:e89209. 10.1371/journal.pone.0089209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z., Zhou L., Jiang W., Kuang R., Li J., Tao R., et al. (2011). An in vitro synergetic evaluation of the use of nisin and sodium fluoride or chlorhexidine against Streptococcus mutans. Peptides. 32, 2021–2026. 10.1016/j.peptides.2011.09.002 [DOI] [PubMed] [Google Scholar]
- Torella J. P., Chait R., Kishony R. (2010). Optimal drug synergy in antimicrobial treatments. PLoS Comput. Biol. 6:e1000796. 10.1371/journal.pcbi.1000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji B. T., Rybak M. J. (2006). Etest synergy testing of clinical isolates of Staphylococcus aureus demonstrating heterogeneous resistance to vancomycin. Diagn. Microbiol. Infect. Dis. 54, 73–77. 10.1016/j.diagmicrobio.2005.08.014 [DOI] [PubMed] [Google Scholar]
- Vaillancourt K., LeBel G., Frenette M., Gottschalk M., Grenier D. (2015). Suicin 3908, a new lantibiotic produced by a strain of Streptococcus suis serotype 2 isolated from a healthy carrier pig. PLoS ONE 10:e0117245. 10.1371/journal.pone.0117245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijkeren E., Catry B., Greko C., Moreno M. A., Pomba M. C., Pyörälä S., et al. (2011). Review on methicillin-resistant Staphylococcus pseudintermedius. J. Antimicrob. Chemother. 66, 2705–2714. 10.1093/jac/dkr367 [DOI] [PubMed] [Google Scholar]
- van Heel A. J., Montalban-Lopez M., Kuipers O. P. (2011). Evaluating the feasibility of lantibiotics as an alternative therapy against bacterial infections in humans. Expert Opin. Drug Metab. Toxicol. 7, 675–680. 10.1517/17425255.2011.573478 [DOI] [PubMed] [Google Scholar]
- Van Hoovels L., Vankeerberghen A., Boel A., Van Vaerenbergh K., De Beenhouwer H. (2006). First case of Staphylococcus pseudintermedius infection in a human. J. Clin. Microbiol. 44, 4609–4612. 10.1128/JCM.01308-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidovic S., Korber D. R. (2016). Escherichia coli O157: Insights into the adaptive stress physiology and the influence of stressors on epidemiology and ecology of this human pathogen. Crit. Rev. Microbiol. 42, 83–93. 10.3109/1040841X.2014.889654 [DOI] [PubMed] [Google Scholar]
- Wan Norhana M. N., Poole S. E., Deeth H. C., Dykes G. A. (2012). Effects of nisin, EDTA and salts of organic acids on Listeria monocytogenes, Salmonella and native microflora on fresh vacuum packaged shrimps stored at 4°C. Food Microbiol. 31, 43–50. 10.1016/j.fm.2012.01.007 [DOI] [PubMed] [Google Scholar]
- Willing B. P., Russell S. L., Finlay B. B. (2011). Shifting the balance: antibiotic effects on host–microbiota mutualism. Nat. Rev. Microbiol. 9, 233–243. 10.1038/nrmicro2536 [DOI] [PubMed] [Google Scholar]
- Xie L., Miller L. M., Chatterjee C., Averin O., Kelleher N. L., van der Donk W. A. (2004). Lacticin 481: in vitro reconstitution of lantibiotic synthetase activity. Science 303, 679–681. 10.1126/science.1092600 [DOI] [PubMed] [Google Scholar]
- Yan Q. Q., Condell O., Power K., Butler F., Tall B. D., Fanning S. (2012). Cronobacter species (formerly known as Enterobacter sakazakii) in powdered infant formula: a review of our current understanding of the biology of this bacterium. J. Appl. Microbiol. 113, 1–15. 10.1111/j.1365-2672.2012.05281.x [DOI] [PubMed] [Google Scholar]
- Yuan J., Zhang Z. Z., Chen X. Z., Yang W., Huan L. D. (2004). Site-directed mutagenesis of the hinge region of nisinZ and properties of nisinZ mutants. Appl. Microbiol. Biotechnol. 64, 806–815. 10.1007/s00253-004-1599-1 [DOI] [PubMed] [Google Scholar]