Abstract
Cigarette smoking is associated with an increased risk of developing mucinous ovarian tumors but whether it is associated with ovarian cancer survival overall or for the different histotypes is unestablished. Furthermore, it is unknown whether the association between cigarette smoking and survival differs according to strata of ovarian cancer stage at diagnosis. In a large pooled analysis, we evaluated the association between various measures of cigarette smoking and survival among women with epithelial ovarian cancer. We obtained data from 19 case-control studies in the Ovarian Cancer Association Consortium (OCAC), including 9,114 women diagnosed with ovarian cancer. Cox regression models were used to estimate adjusted study-specific hazard ratios (HRs), which were combined into pooled hazard ratios (pHR) with corresponding 95% confidence intervals (CIs) under random effects models. Overall, 5,149 (57%) women died during a median follow-up period of 7.0 years. Among women diagnosed with ovarian cancer, both current (pHR = 1.17, 95% CI: 1.08–1.28) and former smokers (pHR = 1.10, 95% CI: 1.02–1.18) had worse survival compared with never smoking women. In histotype-stratified analyses, associations were observed for mucinous (current smoking: pHR = 1.91, 95% CI: 1.01–3.65) and serous histotypes (current smoking: pHR = 1.11, 95% CI: 1.00–1.23; former smoking: pHR = 1.12, 95% CI: 1.04–1.20). Further, our results suggested that current smoking has a greater impact on survival among women with localized than disseminated disease. The identification of cigarette smoking as a modifiable factor associated with survival has potential clinical importance as a focus area to improve ovarian cancer prognosis.
Keywords: cigarette smoking, ovarian cancer, survival, pooled analysis
Ovarian cancer is the most deadly gynaecological disease in the Western World, causing >150,000 deaths worldwide in 2012.1 Currently, no effective technique of routine population screening exists and, because ovarian cancer has nonspecific symptoms,2 up to 80% of all ovarian cancers are diagnosed at advanced stages.3 As a consequence, women with ovarian cancer have a poor prognosis, with an overall 5-year survival of only around 40%.3
Factors known to play a role in ovarian cancer survival include age, stage and grade, but these are unmodifiable.4,5 Thus, identification of modifiable factors that potentially improve prognosis for women diagnosed with ovarian cancer may have clinical and public health importance. However, little is known about modifiable lifestyle factors in ovarian cancer but in a recent paper, Nagle et al. found that higher BMI was associated with adverse survival among women with ovarian cancer.6
Even though the number of female smokers has declined in most parts of the Western world, cigarette smoking is still very common in many countries and it has been estimated that nearly 180 million adult women worldwide smoke cigarettes daily.7 Cigarette smoking is known to affect the risk of developing epithelial ovarian cancer. The association differs by histotype, reflecting their different aetiologies, and the strongest association is observed for mucinous ovarian tumors.8–11 Further, smoking has been found to correlate with survival for several malignancies including lung, breast and laryngeal cancer,12–14 but only a few studies have investigated the association between cigarette smoking and epithelial ovarian cancer survival and the results have been inconclusive.15–21 Four studies found that cigarette smoking was associated with worse survival,15–18 whereas three studies found no association.19–21 However, the results from most previous studies are based on small numbers of participants (n = 61–1,997 women), only one of the studies performed separate analyses by histotype,18 and only two of the studies investigated progression-free survival.17,18
By use of data from 19 case-control studies participating in the Ovarian Cancer Association Consortium (OCAC), the aim of this study was to investigate the prognostic impact of pre-diagnostic cigarette smoking on epithelial ovarian cancer survival, both overall and according to histotype. We furthermore investigated whether the association between smoking status and survival differed according to strata of stage of ovarian cancer at diagnosis (localized vs. advanced stage).
Material and Methods
OCAC, which has been described in detail elsewhere,22 is an international collaboration founded in 2005. For the present analyses, 19 case-control studies provided data on cigarette smoking, potential confounders and clinical follow-up information (Table 1).23–41
Table 1.
Characteristics of the 19 case-control studies included in the pooled analysis of cigarette smoking and survival following a diagnosis of ovarian cancer
| Study1 | Country | Study period | Cases (N) | Age range at diagnosis | Median follow-up time among living (years) | Number of women who died (%) | 5-year survival (%) |
|---|---|---|---|---|---|---|---|
| AUS: Australian Ovarian Cancer Study & Australian Cancer Study28 | Australia | 2002–2006 | 1,007 | 20–80 | 7.1 | 629 (62.5) | 482 (47.9) |
| JPN: Hospital-based Research Programme at Aichi Cancer Center26 | Japan | 2001–2005 | 33 | 32–72 | 5.0 | 12 (36.4) | 10 (30.3) |
| GER: German Ovarian cancer study29 | Germany | 1993–1996 | 188 | 21–75 | 13.6 | 129 (68.6) | 89 (47.3) |
| MAL: The Danish Malignant Ovarian Tumor Study24 | Denmark | 1994–1999 | 516 | 32–80 | 13.5 | 393 (76.2) | 226 (43.8) |
| POL: Polish Ovarian Cancer Study33 | Poland | 2000–2003 | 171 | 32–74 | 5.2 | 90 (52.6) | 55 (32.2) |
| SEA: Study of Epidemiology and Risk Factors in Cancer Heredity35 | United Kingdom | 1998–2010 | 582 | 23–74 | 6.2 | 279 (47.9) | 309 (53.1) |
| UKO: UK Ovarian Cancer Population Study32 | United Kingdom | 2006–2010 | 449 | 19–90 | 3.5 | 150 (33.4) | 196 (43.7) |
| CON: Connecticut Ovary Cancer Study40 | USA | 1998–2003 | 301 | 36–81 | 7.6 | 177 (58.8) | 174 (57.8) |
| DOV: Diseases of the Ovary and their Evaluation Study39 | USA | 2002–2005 | 462 | 35–74 | 6.4 | 256 (55.4) | 251 (54.3) |
| HAW: Hawaii Ovarian Cancer Case-Control Study25 | USA | 1993–2008 | 388 | 24–87 | 6.6 | 200 (51.5) | 190 (49.0) |
| HOP: Hormones and Ovarian Cancer Prediction Study38 | USA | 2003–2009 | 587 | 25–91 | 4.8 | 308 (52.5) | 191 (32.5) |
| MAY: Mayo Clinic Ovarian Cancer Case-Control Study27 | USA | 2000–2009 | 481 | 21–91 | 4.7 | 277 (57.6) | 144 (29.9) |
| NCO: North Carolina Ovarian Cancer study30 | USA | 1999–2008 | 833 | 22–74 | 6.9 | 496 (59.5) | 354 (42.5) |
| NEC: New England Case-Control Study of Ovarian Cancer31 | USA | 1992–2003 | 826 | 21–77 | 12.3 | 476 (57.6) | 486 (58.8) |
| NJO: New Jersey Ovarian Cancer Study23 | USA | 2002–2008 | 189 | 32–81 | 2.2 | 42 (22.2) | 0 (0.0)2 |
| STA: Family Registry for Ovarian Cancer and Genetic Epidemiology of Ovarian Cancer34 | USA | 1997–2001 | 427 | 21–64 | 10.1 | 248 (58.1) | 224 (52.5) |
| TBO: Tampa Bay Ovarian Cancer Study41 | USA | 2000–2012 | 189 | 26–93 | 5.6 | 104 (55.0) | 61 (32.3) |
| USC: Los Angeles County Case-Control Studies of Ovarian Cancer38 | USA | 1993–2005 | 1,138 | 20–84 | 11.4 | 721 (63.4) | 646 (56.8) |
| UCI: University California, Irvine Ovarian Cancer Study37 | USA | 1993–2005 | 347 | 21–86 | 6.2 | 162 (46.7) | 250 (72.0) |
| TOTAL | 1992–2012 | 9,114 | 19–93 | 7.0 | 5,149 (56.5) | 4,338 (47.6) |
All studies were population-based except for UKO, MAY and JPN that were all hospital-based.
In this study, no women were followed for 5 years or more. Therefore, no women in NJO survived 5 years.
Using standardised formats, data from each OCAC study were centrally harmonised. All data were checked for internal consistency and, where necessary, clarification was provided by the original investigators. In this study, women diagnosed with fallopian tube or peritoneal cancer as well as women diagnosed with borderline ovarian tumors were no considered for analyses. Consequently, the initial study population consisted of women diagnosed with epithelial ovarian cancers only (n = 14,150). From these, we excluded women with missing data on vital status or survival time (n = 1,364), smoking status (n = 1,973), age (n = 3), race/ethnicity (n = 20), tumor stage (n = 478) and grade (n = 1,198), leaving 9,114 women diagnosed with epithelial ovarian cancer eligible for analyses. Of these, there were 5,455 serous ovarian tumors (5,014 high-grade and 441 low-grade serous ovarian tumors), 611 mucinous, 1,473 endometrioid, 600 clear cell ovarian tumors and 975 with other types of epithelial ovarian tumors. All individual studies included in OCAC had institutional review board and/or ethics committee approval and all study participants provided informed consent.
Assessment of cigarette smoking
Information on use of tobacco products other than cigarettes was limited to a few studies. Therefore, this study only addressed the prognostic impact of pre-diagnostic cigarette smoking on epithelial ovarian cancer survival. Information on cigarette smoking was obtained through self-administered questionnaires or in-person interviews and assessment of current and former smoking related either to date of diagnosis or interview, or one year prior to this depending on the study. We obtained information on smoking status prior to diagnosis (never, former or current), cigarette consumption (average number of cigarettes per day), total duration of smoking (years) and time since smoking cessation (years). Among the case-control studies included, various definitions were used to classify women who had smoked. Some studies used a definition of at least 100 cigarettes smoked during the lifetime (AUS,28 CON,40 DOV,39 JPN,26 MAY,27 NCO,30 NEC,31 POL,33 TBO41 and UCI37), whereas other studies used daily smoking for a period of 3, 6 or 12 months (GER,29 HAW,25 HOP,38 NJO,23 SEA,35 STA,34 UKO32 and USC36) or self-report of smoking without further specification (MAL24).
Covariate and clinical data
From all 19 studies included, we obtained information about the following covariates associated with smoking and/or survival: age at diagnosis, race/ethnicity (non-Hispanic White, Hispanic White, Black, Asian or others, including unknown race), tumor grade (well, moderately or poorly differentiated, or undifferentiated) and tumor stage at diagnosis. In the OCAC data, tumor stage was classified from a harmonised summary stage variable based on the International Federation of Gynaecology and Obstetrics (FIGO) staging system and the Surveillance, Epidemiology, and End Results (SEER) staging manuals and categorised as: localized, regional, distant or unknown. Information on FIGO and SEER stage was obtained by each OCAC study from a variety of sources including medical records, pathology reports, institutional databases, hospital tumor boards and cancer registries. Furthermore, 15 studies (all studies but SEA, STA, TBO and UKO) had information on recent BMI (1 or 5 years prior to ovarian cancer, depending on the study) and 17 studies (all studies but JPN and TBO) provided information on level of education (≤high school vs. >high school). Information on residual disease remaining after primary surgery was available from seven studies (AUS, HAW, JPN, MAL, MAY, NCO and NEC). In the common OCAC data set, residual disease was defined as the maximum dimension of disease remaining after primary surgery and categorised as: no macroscopic disease, macroscopic disease ≤1 cm, macroscopic disease >1 cm and ≤2 cm, macroscopic disease >2 cm, macroscopic disease (size unknown), tumor not resected or unknown. In the analysis, residual disease was categorised as a dichotomous variable (no macroscopic disease present vs. macroscopic disease present).
Each study reported vital status and survival time and follow-up information was obtained from a variety of data sources including medical record review, patient contact, linkage with state cancer registries, use of the SEER registry and death-record databases. Overall survival time was calculated from date of diagnosis or date of study recruitment whichever came last, until date of death from any cause or, for living patients, date of last follow-up. Cause of death data was only available from seven studies (AUS, HAW, JPN, MAL, MAY, NCO and NEC) corresponding to 968 women of the 5,149 women who had died (19%). In this study, death due to an ovarian cancer diagnosis was defined as death due to progression of the disease. Among the women for whom cause of death data were available, the vast majority (94%) had died from ovarian cancer. Thus, all-cause mortality was used as the primary outcome in these analyses. Further, for the seven studies where data were available (AUS, HAW, JPN, MAL, MAY, NCO and NEC), progression-free survival time was calculated from date of diagnosis to date of documented clinical (e.g., ascites), biochemical (i.e., CA125) or radiological disease progression (CT scan), date of death or date of last follow-up for patients who had not progressed. For all 19 studies included, the time-period from date of diagnosis to date of study recruitment was available and left truncation at recruitment was used in all analyses to account for time elapsed between date of diagnosis and date of study recruitment, in order to reduce the likelihood of survivorship bias arising from the exclusion of eligible women who had died before recruitment.
Statistical analysis
Associations between the various variables of smoking and survival were analyzed using a two-stage approach.42 In stage one, adjusted study-specific hazard ratios (HRs) and corresponding standard errors were obtained from Cox regression models with time since diagnosis as the underlying time scale. Smoking status was included as a categorical variable (never, former or current), whereas “cigarette consumption,” “duration of smoking” and “time since smoking cessation” were parameterised both as categorical and as continuous variables. Each categorical variable was categorised into ordinal groups with never smokers as the reference group. The associations between the continuous variables “cigarette consumption,” “duration of smoking” and ovarian cancer survival were evaluated among ever smokers (former or current smokers), whereas the association between “time since smoking cessation” and survival was evaluated among former smokers only. All study-specific analyses were adjusted for age (continuous, included as a linear variable), tumor stage (localized, regional or distant), tumor grade (well, moderately or poorly differentiated or undifferentiated) and race/ethnicity (non-Hispanic White, Hispanic White, Black, Asian or other, including unknown race). Not all studies had data on BMI (continuous, per 5 kg/m2), level of education (≤high school vs. >high school) or residual disease (no macroscopic disease present vs. macroscopic disease present) and these variables were therefore only included as adjustment factors in a subset of studies in additional statistical models.
In stage two, the study-specific estimates were combined by a random-effects inverse variance-weighted univariate meta-analysis into a pooled hazard ratio (pHR) with corresponding 95% CIs.43 For all analyses, individual studies were included in the meta-analysis only if the following two requirements were met: (i) at least five observations with data on all covariates were available and (ii) there were at least one observation with an event, that is, death (or progression for the analyses on progression free survival). Statistical heterogeneity among studies was evaluated using the Cochran Q and I2 statistics, but as only very little and non-consistent evidence of heterogeneity was observed in the analyses, potential sources of heterogeneity were not investigated further.
Analyses for the associations between smoking and overall survival were also conducted separately for the various standard histotypes of epithelial ovarian cancer (serous, mucinous, endometrioid and clear cell ovarian tumors). Additionally, serous ovarian tumors were categorised as either low- (Grade 1) or high-grade (Grade ≥2) serous tumors. A similar analytic approach was used to assess the association between cigarette smoking and progression-free survival. However, we only investigated the association between smoking status and progression-free survival for ovarian cancer overall and for serous ovarian tumors because a limited number of cases impeded meaningful analyses for the remaining smoking variables and histotypes.
In a stratified analysis, we investigated whether the association between smoking status and overall survival differed according to strata of stage of ovarian cancer at diagnosis (localized vs. advanced stage). For this analysis, pairwise comparisons were made using t-tests based on estimates and standard errors from the stratified analyses and p-values were adjusted for multiple testing by use of the Bonferroni procedure. Finally, sensitivity analyses for the association between cigarette smoking and overall survival were performed where follow-up was restricted at ≤5, >5 – ≤10 and >10 years after the diagnosis of epithelial ovarian cancer. These sensitivity analyses were only conducted for ovarian cancer overall and for serous ovarian tumors, as small numbers of cases prohibited these analyses for the other histotypes. All p-values presented are two-sided. We used the statistical package meta in R (version 3.1.0) for all analyses.
Results
Detailed information on the 19 included case-control studies is shown in Table 1. Twelve studies were conducted in the United States, 5 in Europe and one each in Australia and Japan. The number of women diagnosed with epithelial ovarian cancer included in the studies varied from 33 (JPN) to 1,138 (USC). The age range at diagnosis varied from 19–93 years among women diagnosed between 1992 and 2012. Sixteen studies were population-based and three hospital-based (UKO, MAY and JPN). Eight studies (AUS, GER, JPN, MAY, SEA, TBO, UCI and UKO) involved information obtained from self-completed questionnaires, whereas 11 studies (CON, DOV, HAW, HOP, MAL, NCO, NEC, NJO, POL, STA and USC) collected information by in-person interviews. Approximately 57% of the study women died during the follow-up period and 5-year survival was 48%. The median follow-up time was 7.0 years.
Among the 9,114 women with epithelial ovarian cancer, 54.5% were never smokers at diagnosis, 31.8% were former smokers, whereas current smokers constituted 13.7% of the study population (Table 2). Compared with never and former smokers, current smokers tended to be younger, were more often diagnosed with localized disease and with a mucinous or well-differentiated tumor. They were also more likely to be Black, were less obese, were less likely to have completed more than high school and more likely to have had residual disease compared with never and former smokers (all p-values <0.04).
Table 2.
Age distribution, covariate and clinical characteristics for the 9,114 women included in analysis, according to smoking status at diagnosis
| Smoking status
|
||||||
|---|---|---|---|---|---|---|
| Characteristics | Never N (%) | Former N (%) | Current N (%) | |||
| Number of Women | 4,966 (54.5) | 2,900 (31.8) | 1,248 (13.7) | |||
|
| ||||||
| Age at diagnosis (years)1 | ||||||
|
| ||||||
| <40 | 328 | (6.6) | 114 | (3.9) | 119 | (9.5) |
|
| ||||||
| 40–49 | 953 | (19.2) | 463 | (16.0) | 326 | (26.1) |
|
| ||||||
| 50–59 | 1,528 | (30.8) | 965 | (33.3) | 408 | (32.7) |
|
| ||||||
| 60–69 | 1,356 | (27.3) | 913 | (31.5) | 279 | (22.4) |
|
| ||||||
| ≥70 | 801 | (16.1) | 445 | (15.3) | 116 | (9.3) |
|
| ||||||
| Tumor stage1 | ||||||
|
| ||||||
| Localized | 952 | (19.2) | 529 | (18.2) | 284 | (22.8) |
|
| ||||||
| Regional | 1,158 | (23.3) | 647 | (22.3) | 231 | (18.5) |
|
| ||||||
| Distant | 2,856 | (57.5) | 1,724 | (59,4) | 733 | (58.7) |
|
| ||||||
| Histology1 | ||||||
|
| ||||||
| Serous | 2,899 | (58.3) | 1,829 | (63.1) | 727 | (58.3) |
|
| ||||||
| Serous low-grade | 218 | (4.4) | 142 | (4.9) | 81 | (6.5) |
|
| ||||||
| Serous high-grade | 2,681 | (54.0) | 1,687 | (58.2) | 646 | (51.8) |
|
| ||||||
| Mucinous | 316 | (6.4) | 161 | (5.6) | 134 | (10.7) |
|
| ||||||
| Endometrioid | 839 | (16.9) | 460 | (15.9) | 174 | (13.9) |
|
| ||||||
| Clear cell | 374 | (7.5) | 150 | (5.2) | 76 | (6.1) |
|
| ||||||
| Other | 538 | (10.8) | 300 | (10.3) | 137 | (11.0) |
|
| ||||||
| Grade1 | ||||||
|
| ||||||
| Well differentiated | 634 | (12.8) | 357 | (12.3) | 214 | (17.1) |
|
| ||||||
| Moderately differentiated | 1,286 | (25.9) | 709 | (24.4) | 317 | (25.4) |
|
| ||||||
| Poorly differentiated | 2,739 | (55.2) | 1,657 | (57.1) | 669 | (53.6) |
|
| ||||||
| Undifferentiated | 307 | (6.2) | 177 | (6.1) | 48 | (3.8) |
|
| ||||||
| Race/ethnicity1 | ||||||
|
| ||||||
| Non-Hispanic White | 4,187 | (84.3) | 2,629 | (90.7) | 1,108 | (88.8) |
|
| ||||||
| Hispanic White | 150 | (3.0) | 73 | (2.5) | 25 | (2.0) |
|
| ||||||
| Black | 103 | (2.1) | 71 | (2.4) | 60 | (4.8) |
|
| ||||||
| Asian | 369 | (7.4) | 59 | (2.0) | 18 | (1.4) |
|
| ||||||
| Other | 156 | (3.1) | 67 | (2.3) | 34 | (2.7) |
|
| ||||||
| BMI | ||||||
|
| ||||||
| Median | 24.19 | 24.33 | 23.43 | |||
|
| ||||||
| Interquartile range | 21.48–28. | 34 | 21.64–28.59 | 20.90–27.48 | ||
|
| ||||||
| Missing | 359 | (7.2) | 217 | (7.5) | 52 | (4.2) |
|
| ||||||
| Level of education | ||||||
|
| ||||||
| ≤high school | 2,168 | (43.7) | 1,275 | (44.0) | 771 | (61.8) |
|
| ||||||
| >high school | 2,621 | (52.7) | 1,516 | (52.3) | 438 | (35.1) |
|
| ||||||
| Missing | 177 | (3.6) | 109 | (3.7) | 39 | (3.1) |
|
| ||||||
| Residual disease2 | ||||||
|
| ||||||
| No macroscopic disease present | 539 | (10.9) | 255 | (8.8) | 128 | (10.3) |
|
| ||||||
| Macroscopic disease present | 714 | (14.4) | 420 | (14.5) | 214 | (17.1) |
|
| ||||||
| Missing | 3,713 | (74.8) | 2,225 | (76.7) | 906 | (72.6) |
No missing data.
Only seven studies provided information on residual disease (AUS, HAW, JPN, MAL, MAY, NCO and NEC).
Figure 1 shows the association between cigarette smoking status and overall survival following a diagnosis of epithelial ovarian cancer, by study site (HRs) and overall (pHRs). In Table 3, the adjusted pHRs for the associations between the various smoking variables and overall survival after a diagnosis of epithelial ovarian cancer and according to histotype are presented. For women with epithelial ovarian cancer, both current (pHR = 1.17, 95% CI: 1.08–1.28) (Table 3; Fig. 1a) and former smokers (pHR = 1.10, 95% CI: 1.02–1.18) (Table 3; Fig. 1b) had a worse overall survival compared with women who had never smoked.
Figure 1.
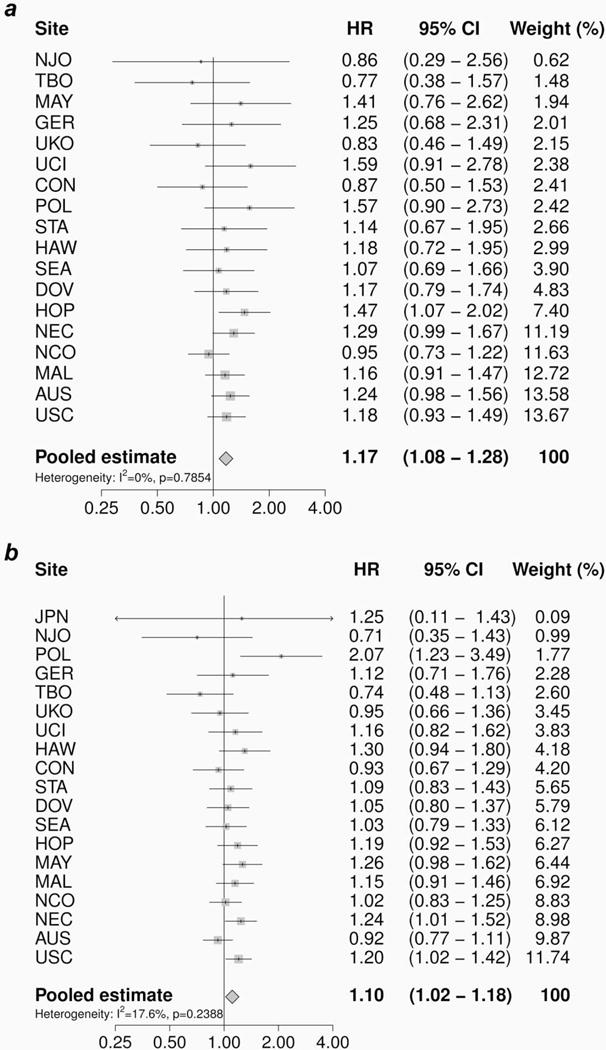
The association between cigarette smoking status at diagnosis and overall survival following a diagnosis of epithelial ovarian cancer, by study site and overall. Study-specific hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox regression models adjusted for age, race/ethnicity, stage and grade. The pooled hazard ratio (pHR) with corresponding 95% CI was estimated using a random effects model. (a) Current versus never smokers; (b) former versus never smokers.
Table 3.
Adjusted pooled hazard ratios (pHRs) and 95% confidence intervals (CIs) for the association between cigarette smoking and overall survival among 9,114 women from 19 studies with epithelial ovarian cancer, overall and by histotype
| Overall
|
Serous
|
Mucinous
|
Endometrioid
|
Clear cell
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | I2 (%) | pHR2 | 95% CI | Cases | I2 (%) | pHR2 | 95% CI | Cases | I2 (%) | pHR2 | 95% CI | Cases | I2 (%) | pH R2 | 95% CI | Cases | I2 (%) | pH R2 | 95% CI | |
| Smoking status3 | ||||||||||||||||||||
|
| ||||||||||||||||||||
| Never smoker4 | 4,966 | – | 1.00 | Ref. | 2,899 | – | 1.00 | Ref. | 316 | – | 1.00 | Ref. | 835 | – | 1.00 | Ref. | 373 | – | 1.00 | Ref. |
|
| ||||||||||||||||||||
| Former smoker | 2,900 | 17.6 | 1.10 | (1.02–1.18) | 1,829 | 0.0 | 1.12 | (1.04–1.20) | 161 | 43.5 | 1.43 | (0.83–2.48)1 | 460 | 33.3 | 0.85 | (0.63–1.15) | 150 | 8.1 | 1.17 | (0.74–1.85) |
|
| ||||||||||||||||||||
| Current smoker | 1,248 | 0.0 | 1.17 | (1.08–1.28) | 727 | 0.0 | 1.11 | (1.00–1.23) | 134 | 0.0 | 1.91 | (1.01–3.65) | 174 | 5.0 | 1.27 | (0.91–1.77) | 76 | 0.0 | 1.08 | (0.67–1.75) |
|
| ||||||||||||||||||||
| Cigarette consumption (per day)5 | ||||||||||||||||||||
|
| ||||||||||||||||||||
| 1–≤10 | 1,630 | 0.0 | 1.12 | (1.04–1.21) | 1,016 | 0.0 | 1.16 | (1.06–1.27) | 103 | 0.0 | 1.46 | (0.74–2.88) | 248 | 21.1 | 0.78 | (0.55–1.10) | 85 | 0.0 | 0.78 | (0.45–1.33) |
|
| ||||||||||||||||||||
| >10–≤20 | 1,535 | 0.0 | 1.12 | (1.03–1.21) | 922 | 0.0 | 1.08 | (0.98–1.19) | 124 | 16.5 | 1.69 | (0.89–3.21) | 227 | 58.9 | 1.17 | (0.72–1.89)1 | 93 | 9.4 | 1.53 | (0.93–2.51) |
|
| ||||||||||||||||||||
| ≤20 | 615 | 10.9 | 1.24 | (1.10–1.40) | 383 | 0.0 | 1.26 | (1.10–1.43) | 45 | 52.4 | 2.59 | (0.59–10.89)1 | 100 | 42.2 | 1.28 | (0.74–2.21) | 22 | 0.0 | 1.23 | (0.53–2.86) |
|
| ||||||||||||||||||||
| Per 5 cigarettes/day6 | 0.0 | 1.01 | (0.99–1.03) | 0.0 | 1.00 | (0.98–1.02) | 0.0 | 1.10 | (0.95–1.26) | 20.2 | 1.06 | (0.98–1.14) | 0.0 | 1.08 | (0.94–1.24) | |||||
|
| ||||||||||||||||||||
| Duration of smoking before diagnosis7 (years) | ||||||||||||||||||||
|
| ||||||||||||||||||||
| 1–≤10 | 923 | 8.5 | 1.07 | (0.97–1.19) | 565 | 0.0 | 1.10 | (0.98–1.23) | 53 | NA10 | 156 | 34.4 | 0.96 | (0.57–1.60) | 48 | 0.0 | 1.80 | (0.85–3.81) | ||
|
| ||||||||||||||||||||
| >10–≤20 | 823 | 14.8 | 1.13 | (1.00–1.26) | 504 | 0.0 | 1.15 | (1.02–1.29) | 64 | 0.0 | 1.52 | (0.60–3.82) | 132 | 0.0 | 0.73 | (0.49–1.08) | 42 | 0.0 | 1.29 | (0.67–2.46) |
|
| ||||||||||||||||||||
| >20 | 2,267 | 24.6 | 1.16 | (1.06–1.25) | 1,411 | 0.0 | 1.14 | (1.06–1.24) | 159 | 45.6 | 1.90 | (0.71–5.05)1 | 322 | 36.7 | 1.05 | (0.75–1.46) | 126 | 6.4 | 1.09 | (0.70–1.69) |
|
| ||||||||||||||||||||
| Per 5-year period6 | 50.1 | 1.02 | (1.00–1.04)1 | 40.9 | 1.01 | (0.99–1.04)1 | 22.4 | 0.95 | (0.79–1.14) | 27.3 | 1.05 | (0.98–1.13) | 9.4 | 1.04 | (0.94–1.16) | |||||
|
| ||||||||||||||||||||
| Time from cessation to diagnosis (years)8 | ||||||||||||||||||||
|
| ||||||||||||||||||||
| 1–≤10 | 739 | 42.1 | 1.21 | (1.04–1.40)1 | 424 | 32.7 | 1.24 | (1.05–1.47) | 55 | 0.0 | 1.93 | (0.84–4.42) | 126 | 21.3 | 1.15 | (0.73–1.81) | 32 | 0.0 | 0.93 | (0.37–2.37) |
|
| ||||||||||||||||||||
| >10–<20 | 599 | 0.0 | 1.22 | (1.09–1.37) | 343 | 0.0 | 1.23 | (1.07–1.41) | 38 | 0.0 | 3.10 | (0.99–9.72) | 98 | 0.0 | 1.32 | (0.86–2.03) | 28 | 0.0 | 2.92 | (1.13–7.54) |
|
| ||||||||||||||||||||
| >20 | 1,086 | 0.0 | 1.08 | (0.98–1.18) | 668 | 0.0 | 1.15 | (1.03–1.28) | 51 | 0.0 | 1.53 | (0.69–3.39) | 172 | 0.0 | 0.79 | (0.55–1.13) | 60 | 9.0 | 1.35 | (0.67–2.74) |
|
| ||||||||||||||||||||
| Per 5-year period9 | 32.7 | 0.97 | (0.95–1.00) | 22.7 | 0.98 | (0.95–1.01) | 0.0 | 0.96 | (0.82–1.12) | 0.0 | 0.98 | (0.90–1.06) | 0.0 | 0.93 | (0.77–1.12) | |||||
Numbers may not sum up to total because of missing data.
p-value for heterogeneity <0.05.
Adjusted for: age (continuous), race/ethnicity (non-Hispanic White, Hispanic White, Black, Asian or other), tumor stage (localized, regional or distant) and grade (well differentiated, moderately differentiated, poorly differentiated or undifferentiated).
Number of studies included for analysis: overall = 19; serous = 19, mucinous = 15; endometrioid = 17; clear cell = 15.
Never smokers was used as the reference group for all categorical analyses.
Number of studies included for analysis: overall = 18; serous = 18, mucinous = 14; endometrioid = 16; clear cell = 14.
Among ever smokers.
Number of studies included for analysis: overall = 19; serous = 19, mucinous = 16; endometrioid = 17; clear cell = 15.
Number of studies included for analysis: overall = 18; serous = 17, mucinous = 12; endometrioid = 15; clear cell = 14.
Among former smokers only.
Not applicable due to unreliable model parameters (small numbers of events).
In addition, an increasing number of cigarettes smoked per day and duration of smoking tended to have a negative impact on overall survival whereas increasing time since smoking cessation tended to have a positive impact on overall survival of epithelial ovarian cancer.
Concerning the histotype-specific analyses, a number of associations are noteworthy. Both former (pHR = 1.12, 95% CI: 1.04–1.20) and current (pHR = 1.11, 95% CI: 1.00–1.23) smokers diagnosed with serous ovarian tumors had a worse overall survival compared with never smokers (Table 3). Additional analyses stratified by grade revealed similar associations for women with high-grade (former smokers: pHR = 1.10, 95% CI: 1.02–1.18; current smokers: pHR = 1.11, 95% CI: 0.99–1.23) and low-grade serous ovarian tumors (former smokers: pHR = 1.43, 95% CI: 1.02–2.02; current smokers: pHR = 1.19, 95% CI: 0.80–1.78). The strongest associations were observed for mucinous ovarian tumors, where current smokers had a statistically significantly 91% worse survival (pHR = 1.91, 95% CI: 1.01–3.65) and former smokers a statistically non-significantly 43% worse survival (pHR = 1.43, 95% CI: 0.83–2.48) than never smokers. Also for this tumor type, an increasing number of cigarettes smoked per day tended to have a negative impact on survival (pHR = 1.10, 95% CI: 0.95–1.26 per each additional 5 cigarettes smoked per day). In addition, current smokers with endometrioid ovarian tumors tended to have a poorer survival (pHR = 1.27, 95% CI: 0.91–1.77), whereas no clear association between smoking status and overall survival was observed for clear cell tumors (Table 3).
Potential confounders in the association between smoking and overall survival of ovarian cancer include BMI and level of education. Additional adjustment for these two variables in a model restricted to studies where this information was available (n = 15, Supporting Information Table 1) made virtually no changes to the estimated associations between smoking and overall survival, both when compared to results from the main statistical model (i.e., without adjustment for BMI and level of education) using data from these 15 studies only (Supporting Information Table 2) and when compared with the results from the main statistical model using data from all 19 studies (Table 3). In addition, a statistical model including information on residual disease was also evaluated for the seven studies in which these data were available. In general, inclusion of this clinical variable did not result in any consistent changes to the pooled estimates (Supporting Information Table 3) when compared with results from the main statistical model including data from these seven studies only (Supporting Information Table 4) and results from the main statistical model using data from all 19 studies (Table 3).
For epithelial ovarian cancer and for serous ovarian tumors, we investigated whether the association between smoking status and overall survival varied by tumor stage (Table 4). Compared with never smokers, current smokers (pHR = 1.63, 95% CI: 1.19–2.22) with all histotypes of ovarian cancer combined had worse overall survival among women with localized stage disease. A significantly weaker association was observed with current smoking among women with advanced stage disease (pHR = 1.16, 95% CI: 1.06–1.28) (p values for pairwise comparison = 0.04). The same pattern was seen for former smoking but the pooled HRs for former smokers were not statistically significantly different across tumor stage strata (p values = 0.21). Comparable, but slightly higher pHRs were observed for serous ovarian tumors as former (pHR = 1.46, 95% CI: 0.87–2.45) and current smokers (pHR = 1.67, 95% CI: 0.84–3.34) with localized disease had a poorer survival compared with never smokers. A less strong association was observed among women with advanced stage disease, but the pooled HRs for current and former smokers were not statistically significantly different across tumor stage strata (both p-values >0.05).
Table 4.
Adjusted pooled hazard ratios (pHRs) and 95% confidence intervals (CIs) for the association between cigarette smoking status at diagnosis and overall survival among 9,114 women from 19 studies diagnosed with epithelial ovarian cancer, overall and for serous ovarian tumors, stratified by stage
| Overall
|
Serous
|
p values | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Localized stage
|
Advanced stage1
|
Localized stage
|
Advanced stage1
|
|||||||||||
| Cases | pHR2 | 95% CI | Cases | pHR2 | 95% CI | p values | Cases | pHR2 | 95% CI | Cases | pHR2 | 95% CI | ||
| Smoking status | ||||||||||||||
|
| ||||||||||||||
| Never | 952 | 1.00 | Ref. | 4,014 | 1.00 | Ref. | 182 | 1.00 | Ref. | 2,705 | 1.00 | Ref. | ||
|
| ||||||||||||||
| Former | 529 | 1.32 | (0.96–1.82) | 2,371 | 1.07 | (1.00–1.15) | 0.21 | 134 | 1.46 | (0.87–2.45) | 1,689 | 1.09 | (1.01–1.17) | 0.27 |
|
| ||||||||||||||
| Current | 284 | 1.63 | (1.19–2.22) | 964 | 1.16 | (1.06–1.28) | 0.04 | 56 | 1.67 | (0.84–3.34) | 665 | 1.09 | (0.98–1.21) | 0.23 |
Advanced stage includes regional and distant stage.
Adjusted for age (continuous), race/ethnicity (non-Hispanic White, Hispanic White, Black, Asian or other) and grade (well differentiated, moderately differentiated, poorly differentiated or undifferentiated).
We also examined the association between smoking and overall survival for epithelial ovarian cancer and for serous ovarian tumors according to follow-up time since ovarian cancer diagnosis (Table 5). Where follow-up was censored at 5 years after diagnosis, both former (pHR = 1.10, 95% CI: 1.02–1.18) and current smokers with ovarian cancer overall (pHR = 1.17, 95% CI: 1.08–1.29) had a worse survival compared with never smokers. For the follow-up period from >5 to ≤10 years after ovarian cancer diagnosis, similar patterns of survival were observed, although the pHRs did not reached statistical significance. Finally, for the follow-up period of >10 years after the ovarian cancer diagnosis, both former (pHR = 1.66, 95% CI: 1.14–2.42) and current smokers (pHR = 2.54, 95% CI: 1.27–5.09) had a poorer survival compared with never smokers. For all follow-up periods, virtually similar survival patterns applied to women with serous ovarian tumors. For both ovarian cancer overall and for serous ovarian tumors, an increasing number of cigarettes smoked per day tended to have a negative impact on survival in the follow-up period of >10 years after the ovarian cancer diagnosis, whereas no association between number of cigarettes smoked per day and survival was found in follow-up ≤10 years since diagnosis. Also, increasing time since smoking cessation tended to have a positive impact on survival only when the length of the follow-up period exceeded 10 years, whereas no consistent pattern between duration of smoking and survival was noted with increasing follow-up time since diagnosis.
Table 5.
Adjusted pooled hazard ratios (pHRs) and 95% confidence intervals (CIs) for the association between cigarette smoking and overall survival among 9,114 women from 19 studies diagnosed with epithelial ovarian cancer, overall and for serous ovarian tumors, according to length of follow-up since ovarian cancer diagnosis
| Length of follow-up
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <5 years
|
>5 – <10 years
|
>10 years
|
|||||||
| Cases | pHR1 | 95% CI | Cases | pHR1 | 95% CI | Cases | pHR1 | 95% CI | |
| Overall epithelial ovarian cancer | |||||||||
|
| |||||||||
| Smoking status | 9,114 | 4,308 | 1,419 | ||||||
|
| |||||||||
| Never | 4,966 | 1.00 | Ref. | 2,425 | 1.00 | Ref. | 775 | 1.00 | Ref. |
|
| |||||||||
| Former | 2,900 | 1.10 | (1.02–1.18) | 1,303 | 1.09 | (0.95–1.25) | 425 | 1.66 | (1.14–2.42) |
|
| |||||||||
| Current | 1,248 | 1.17 | (1.08–1.29) | 580 | 1.13 | (0.90–1.41) | 219 | 2.54 | (1.27–5.09) |
|
| |||||||||
| Cigarette consumption (per day) | |||||||||
|
| |||||||||
| Per 5 cigarettes/day2 | 1.01 | (0.99–1.03) | 1.01 | (0.96–1.05) | 1.09 | (0.95–1.25) | |||
|
| |||||||||
| Duration of smoking before diagnosis (years) | |||||||||
|
| |||||||||
| Per 5-year period2 | 1.02 | (1.00–1.04) | 1.00 | (0.97–1.04) | 1.03 | (0.95–1.12) | |||
|
| |||||||||
| Time from cessation to diagnosis (years) | |||||||||
|
| |||||||||
| Per 5-year period3 | 0.97 | (0.95–1.00) | 0.97 | (0.92–1.02) | 0.90 | (0.75–1.07) | |||
|
| |||||||||
| Serous ovarian tumors | |||||||||
|
| |||||||||
| Smoking status | 5,455 | 2,117 | 583 | ||||||
|
| |||||||||
| Never | 2,899 | 1.00 | Ref. | 1,173 | 1.00 | Ref. | 310 | 1.00 | Ref. |
|
| |||||||||
| Former | 1,829 | 1.12 | (1.04–1.20) | 662 | 1.09 | (0.93–1.29) | 183 | 1.93 | (1.15–3.23) |
|
| |||||||||
| Current | 727 | 1.11 | (1.00–1.23) | 282 | 1.02 | (0.80–1.31) | 90 | 1.88 | (0.90–3.93) |
|
| |||||||||
| Cigarette consumption (per day) | |||||||||
|
| |||||||||
| Per 5 cigarettes/day2 | 1.00 | (0.98–1.02) | 1.00 | (0.94–1.05) | 1.04 | (0.91–1.19) | |||
|
| |||||||||
| Duration of smoking before diagnosis (years) | |||||||||
|
| |||||||||
| Per 5-year period2 | 1.01 | (0.99–1.04) | 1.00 | (0.96–1.04) | 0.99 | (0.88–1.12) | |||
|
| |||||||||
| Time from cessation to diagnosis (years) | |||||||||
|
| |||||||||
| Per 5-year period3 | 0.98 | (0.94–1.01) | 1.00 | (0.94–1.06) | 0.87 | (0.72–1.05) | |||
Adjusted for age (continuous), race/ethnicity (non-Hispanic White, Hispanic White, Black, Asian or other), tumor stage (localized, regional or distant) and grade (well differentiated, moderately differentiated, poorly differentiated or undifferentiated).
Among ever smokers.
Among former smokers only.
Finally, we assessed the prognostic impact of smoking status on progression-free survival for ovarian cancer overall and for serous ovarian tumors in seven studies where this information was available. The pHRs resembled the results obtained for overall survival but the pHRs were not statistically significant, which may be explained by the relatively smaller numbers of women included for these analyses (data not shown).
Discussion
To our knowledge, this is the largest study to date on cigarette smoking and epithelial ovarian cancer survival. We found that smoking status prior to diagnosis was associated with worse overall survival. Our results also showed that the association with smoking seemed to be different across histotypes of epithelial ovarian cancer being most pronounced for mucinous tumors, where current smokers had an almost 2-fold worse survival compared with never smokers. Also, both former and current smoking was associated with worse survival following serous ovarian cancer (both for high-grade and low-grade serous tumors) and among current smokers with endometrioid ovarian tumors, whereas no appreciable relationships were observed for clear cell subtypes, though evaluation of this subtype was limited by small numbers. These associations remained virtually unchanged after additional adjustments for BMI, level of education and residual disease. Stratification by stage showed that smoking had a stronger association with overall survival among women with localized disease. Also, the magnitude of the association between smoking and overall survival appeared to increase with longer follow-up since ovarian cancer diagnosis. Finally, the results for progression-free survival resembled the results obtained for overall survival.
Only seven previous studies, including between 61 and 1,997 study subjects, have investigated the association between smoking and epithelial ovarian cancer survival.15–21 However, one of the studies was based on data from a study site (MAL) that is included in the present analysis and consequently, results from this study will not be discussed further.15 While three previous studies found no marked association,19–21 the survival disadvantage associated with smoking observed in the present study is supported by the results from three other studies.16–18 For example, in a study of 676 women with epithelial ovarian cancer, Nagle et al.16 found that current smokers had 36% worse survival compared with non-smoking women and that worse survival was further increased with increasing number of pack-years and number of cigarettes smoked per day. Most recently, Kelemen et al.18 studied 432 epithelial ovarian cancer patients receiving adjuvant chemotherapy from the Alberta Cancer Registry, Canada and while no association between smoking status and overall or progression-free survival among ovarian cancer overall was observed, histotype-specific analyses showed that smoking women with mucinous ovarian tumors had worse overall and progression-free survival compared with non-smoking women.
The observed associations between smoking and overall as well as histotype-specific ovarian cancer survivals may be explained by a number of mechanisms. It has been suggested that carcinogens in tobacco smoke directly accelerate tumor growth resulting in earlier progression and death. It has also been suggested that smoking is associated with an increased risk of recurrence, postsurgical complications, a poorer response to treatment and an increased treatment-related toxicity.44,45 Finally, smoking is known to be associated with an unhealthy lifestyle,46 which may have a negative effect on survival.
We found that the association between smoking and survival observed for ovarian cancer overall was confined to the serous and especially mucinous histotypes of the disease and perhaps also to endometrioid tumors. Epidemiological studies have consistently found that the strongest association between smoking and risk of epithelial ovarian cancer appears to be with mucinous ovarian tumors,8–11 and the present results add further knowledge about the relationship between smoking and epithelial ovarian cancer. However, biological explanations for histotype-specific survival differences with regard to smoking are not known and should be investigated further.
Our results suggested that current smoking may have a greater impact on survival among women with localized than disseminated disease. Further, we observed a tendency that smoking status was associated with an increasingly poorer survival with increasing follow-up. These results were observed both for ovarian cancer overall and for serous tumors, but evaluation of the other subtypes was hampered by small numbers. The results may reflect differences in stage. Women who are diagnosed in an advanced stage disease are more likely to die shortly after diagnosis, whereas women who survive for a longer time period are more likely to have been diagnosed in a localized stage. Thus, our results suggest that smoking has the most substantial impact on long-term survival, which most often occur among women diagnosed in an early stage. Our findings are not surprising given the poor prognosis among women with advanced stage disease, which leaves little potential for other factors including smoking to have an impact on ovarian cancer survival.
A major strength of our study is the large sample size including >9,000 women with epithelial ovarian cancer, which allowed us to investigate associations between a number of variables of smoking and the various histotypes of epithelial ovarian cancer. For a subset of women, we also investigated progression-free survival and found no marked association, potentially due to insufficient power. We did not include ovarian cancer-specific survival analysis. However, among the limited cause of death data in our dataset, the vast majority died from ovarian cancer (94%) and we are thus confident that all-cause survival is a pertinent proxy for ovarian cancer survival. As the studies included in our pooled analysis were not selected from published studies, our analyses have not been affected by publication bias. Our analyses relied on individual data combined into a single dataset following careful central data harmonisation. By use of a two-stage approach, we were able to consider differences in study design and data collection across studies and to control for a number of potential confounders. Further, by utilising left truncated data, we decreased the likelihood of potential survivorship bias. Most importantly, adverse associations between smoking and survival were still observed after additional adjustment for BMI and level of education as well as the main clinical factors that affect survival: stage, grade and residual disease.
Women who smoke are known to have a higher degree of comorbidity compared with non-smokers47 and comorbid conditions have a negative prognostic impact on survival from ovarian cancer.48 Specifically, women with comorbidities may not tolerate standard treatments and are therefore more often offered less aggressive types of treatment compared with healthier women.49 Unfortunately, we were not able to adjust for degree of comorbidity as this information was not available in our data at the time of analysis and we can therefore not rule out that our results may have been slightly affected by unmeasured confounding from comorbidity. However, as obesity and low socioeconomic status is highly associated with comorbidities,50,51 our adjustment for BMI and level of education may have diminished potential confounding by comorbidity. Further limitations of this study include the fact that information on smoking habits was based on retrospective reports in all studies included in the present paper, which increases the risk of mis-classification, and that these reports of smoking behaviours pertained to time periods prior to diagnosis rather than to during follow-up time. Newly diagnosed women with ovarian cancer could conceivably change their smoking behaviours and such information might not have been captured in the retrospective reporting. However, because the data on smoking were obtained independent of mortality events, any effects of possible misclassification are likely to be non-differential. In general, socially undesirable behaviours such as cigarette smoking may be prone to under-reporting, where current smokers may have categorised themselves as either never or former smokers and this may therefore have underestimated the true association between current smoking status and survival. In support of this idea, one study among others found that approximately one-third of newly diagnosed cancer patients who denied any current smoking had blood cotinine values at levels that supported active smoking.52 Another possible limitation of the present work is that in some studies ovarian tumors may not have undergone systematic histopathological review. Hence, some extent of misclassification of the histotypes cannot be excluded. Finally, our study design did not allow us to investigate how smoking cessation after a diagnosis of epithelial ovarian cancer could affect survival.
In conclusion, the results from this large pooled analysis indicate that cigarette smoking is associated with a worse survival in ovarian cancer patients; primarily among women diagnosed with serous and mucinous ovarian tumors. Furthermore, our results may also suggest that current smoking more strongly impairs survival among women with localized disease and that the effect of smoking on ovarian cancer prognosis increases with longer follow-up since ovarian cancer. Future studies are needed focusing on how smoking patterns after a diagnosis of ovarian cancer affect survival.
Supplementary Material
What’s new?
The number of female smokers is declining worldwide but an estimated 180 million women still smoke daily worldwide. Here the authors examined the association between cigarette smoking and ovarian cancer survival. Current and former smoking shortened survival compared to women who had never smoked, especially in those afflicted with mucinous and serous tumors and with localized disease. The study identifies cigarette smoking as a modifiable factor associated with ovarian cancer survival.
Acknowledgments
We are grateful to the family and friends of Kathryn Sladek Smith for their generous support of the Ovarian Cancer Association Consortium through their donations to the Ovarian Cancer Research Fund. The MALOVA study is grateful to Kirsten Frederiksen for statistical support and to Nick Martinussen for data management assistance. The NJO group thanks the New Jersey State Cancer Registry staff, M. King and L. Rodriguez. The SEARCH group thanks the SEARCH team, Craig Luccarini, Caroline Baynes and Don Conroy. The UKOPS group thanks I. Jacobs, M. Widschwendter, E. Wozniak, A. Ryan, J. Ford and N. Balogun for their contribution to the study (UKO). The German group thanks Ursula Eilber and Tanja Koehler for competent technical assistance (GER). The Australian group thanks all the clinical and scientific collaborators (http://www.aocstudy.org/) and the women for their contribution (AUS). The cooperation of the 32 Connecticut hospitals, including Stamford Hospital, in allowing patient access, is gratefully acknowledged (CON). Certain data in the CON study were obtained from the Connecticut Tumor Registry, Connecticut Department of Public Health. The CON study assumes full responsibility for analyses and interpretation of these data.
Grant sponsor: The Ovarian Cancer Association Consortium (Ovarian Cancer Research Fund); Grant sponsor: European Commission’s Seventh Framework Programme; Grant number: 223175 (HEALTH-F2-2009–223175); Grant sponsor: National Institutes of Health; Grant numbers: R01 CA074850 and R01 CA080742 (CON), R01 CA112523 and R01 CA87538 (DOV), R01 CA58598, N01 CN55424 and N01 PC 67001 (HAW), MO1-RR000056 (HOP), R01 CA61107 (MAL), R01 CA122443, P30 CA15083 and P50 CA136393 (MAY), R01 CA76016 (NCO), R01 CA54419 and P50 CA105009 (NEC), P30 CA072720, K07 CA095666 and K22 CA138563 (NJO), U01 CA71966, R01 CA16056, K07 CA143047 and U01 CA69417 (STA), R01-CA106414 (TBO), R01 CA058860, R01 CA092044 and PSA 042205 (UCI), P30 CA14089, R01 CA61132 and N01 PC67010 (USC); Grant sponsor: Danish Cancer Society; Grant number: 94 222 52 (MAL); Grant sponsors: Mermaid 1 (MAL) and Mermaid 3 (MAL); Grant sponsor: U.S. Army Medical Research and Materiel Command; Grant number: DAMD17-01–1-0729 (AUS); Grant sponsor: National Health & Medical Research Council of Australia; Grant numbers: 199600 and 400281 (AUS); Grant sponsor: Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania (AUS) and Cancer Foundation of Western Australia (AUS); Grant sponsor: German Federal Ministry of Education and Research (Program of Clinical Biomedical Research); Grant number: 01GB9401 (GER); Grant sponsor: German Cancer Research Center (GER); Grant sponsor: US Army Medical Research and Material Command; Grant numbers: DAMD17-02–1-0669 (HOP), DAMD17-02–1-0666 (NCO), W81XWH-10–1-02802 (NEC) and DAMD17-98–1-8659 (TBO); Grant sponsors: Ministry of Education, Science, Sports, Culture and Technology of Japan (Grant-in-Aid for Scientific Research on Priority Areas), Ministry of Health, Labour and Welfare of Japan (Grant-in-Aid for the third Term Comprehensive 10-year Strategy for Cancer Control) and Takeda Science Foundation (JPN); Grant sponsors: Mayo Foundation (MAY), Minnesota Ovarian Cancer Alliance (MAY), Fred C. and Katherine B. Andersen Foundation (MAY), The Cancer Institute of New Jersey (NJO) and National Cancer Institute (POL; Intramural Research Program); Grant sponsor: Cancer Research UK; Grant numbers: C490/ A10119 and C490/A10124 (SEA); Grant sponsor: American Cancer Society; Grant number: CRTG-00–196-01-CCE; Grant sponsor: Celma Mastry Ovarian Cancer Foundation (TBO); Grant sponsor: Lon V Smith Foundation; Grant number: LVS-39420 (UCI); Grant sponsors: Cancer Research UK (UKO, SEA), Eve Appeal (UKO) and OAK Foundation (UKO); Grant sponsor: California Cancer Research Program; Grant numbers: 00-01389V-20170, N01 CN025403, R03 CA113148 and R03 CA115195 (USC); Grant sponsor: California Cancer Research Program; Grant number: 2II0200 (USC); Grant sponsor: US National Cancer Institute; Grant numbers: P01 CA17054 (USC) and K07-CA80668 and P50-CA159981 (HOP); Grant sponsor: Center for Disease Control (The New Jersey State Cancer Registry); Grant number: 5U58DP003931-02; Grant sponsor: The National Cancer Institute’s Surveillance, Epidemiology, and End Results Program; Grant number: HHSN 261201300021I (NCI Control No. N01PC-2013–00021); Grant sponsor: Department of Health (UKO; A portion of this was done at UCLH/UCL within the ’Women’s Health Theme’; of the NIHR UCLH/UCL Comprehensive Biomedical Research Centre); Grant sponsor: Professor Usha Menon has declared a conflict of interest in connection with the paper (Stock ownership and research funding from Abcodia Ltd, a UCL spin-out company with an interest in biomarkers and commercial rights of ROCA used in ovarian cancer screening)
Abbreviations
- BMI
Body Mass Index
- CI
confidence interval
- FIGO
International Federation of Gynaecology and Obstetrics
- HR
hazard ratio
- OCAC
Ovarian Cancer Association Consortium
- pHR
pooled hazard ratio
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Olson SH, Mignone L, Nakraseive C, et al. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212–7. doi: 10.1016/s0029-7844(01)01457-0. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Clark TG, Stewart ME, Altman DG, et al. A prognostic model for ovarian cancer. Br J Cancer. 2001;85:944–52. doi: 10.1054/bjoc.2001.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akeson M, Jakobsen AM, Zetterqvist BM, et al. A population-based 5-year cohort study including all cases of epithelial ovarian cancer in western Sweden: 10-year survival and prognostic factors. Int J Gynecol Cancer. 2009;19:116–23. doi: 10.1111/IGC.0b013e3181991b13. [DOI] [PubMed] [Google Scholar]
- 6.Nagle CM, Dixon SC, Jensen A, et al. Obesity and survival among women with ovarian cancer: results from the Ovarian Cancer Association Consortium. Br J Cancer. 2015;113:817–26. doi: 10.1038/bjc.2015.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksen M, Mackay J, Ross H. The tobacco atlas. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 8.Risch HA, Marrett LD, Jain M, et al. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. Am J Epidemiol. 1996;144:363–72. doi: 10.1093/oxfordjournals.aje.a008937. [DOI] [PubMed] [Google Scholar]
- 9.Faber MT, Kjaer SK, Dehlendorff C, et al. Cigarette smoking and risk of ovarian cancer: a pooled analysis of 21 case-control studies. Cancer Causes Control. 2013;24:989–1004. doi: 10.1007/s10552-013-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beral V, Gaitskell K, Hermon C, et al. Ovarian cancer and smoking: individual participant meta-analysis including 28,114 women with ovarian cancer from 51 epidemiological studies. Lancet Oncol. 2012;13:946–56. doi: 10.1016/S1470-2045(12)70322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick CN, Risch HA. Smoking and Ovarian Cancer. In: Boyle P, Gray N, Henningfield J, Seffrin J, Zatonski W, editors. Tobacco and Public Health: Science and Policy. New York: Oxford University Press; 2004. pp. 511–21. [Google Scholar]
- 12.Braithwaite D, Izano M, Moore DH, et al. Smoking and survival after breast cancer diagnosis: a prospective observational study and systematic review. Breast Cancer Res Treat. 2012:136521–33. doi: 10.1007/s10549-012-2276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebbert JO, Williams BA, Sun Z, et al. Duration of smoking abstinence as a predictor for non-small-cell lung cancer survival in women. Lung Cancer. 2005;47:165–72. doi: 10.1016/j.lungcan.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 14.Dikshit RP, Boffetta P, Bouchardy C, et al. Lifestyle habits as prognostic factors in survival of laryngeal and hypopharyngeal cancer: a multicentric European study. Int J Cancer. 2005;117:992–5. doi: 10.1002/ijc.21244. [DOI] [PubMed] [Google Scholar]
- 15.Kjaerbye-Thygesen A, Frederiksen K, et al. Smoking and overweight: negative prognostic factors in stage III epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:798–803. doi: 10.1158/1055-9965.EPI-05-0897. [DOI] [PubMed] [Google Scholar]
- 16.Nagle CM, Bain CJ, Webb PM. Cigarette smoking and survival after ovarian cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2006;15:2557–60. doi: 10.1158/1055-9965.EPI-06-0592. [DOI] [PubMed] [Google Scholar]
- 17.Ioffe YJ, Elmore RG, Karlan BY, et al. Effect of cigarette smoking on epithelial ovarian cancer survival. J Reprod Med. 2010;55:346–50. [PubMed] [Google Scholar]
- 18.Kelemen LE, Warren GW, Koziak JM, et al. Smoking may modify the association between neoadjuvant chemotherapy and survival from ovarian cancer. Gynecol Oncol. 2016;140:124–30. doi: 10.1016/j.ygyno.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality–beyond established causes. N Engl J Med. 2015;372:631–40. doi: 10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 20.Vessey M, Painter R, Yeates D. Mortality in relation to oral contraceptive use and cigarette smoking. Lancet. 2003;362:185–91. doi: 10.1016/S0140-6736(03)13907-4. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Klint A, Lambe M, et al. Predictors of ovarian cancer survival: a population-based prospective study in Sweden. Int J Cancer. 2008;123:672–9. doi: 10.1002/ijc.23429. [DOI] [PubMed] [Google Scholar]
- 22.Ramus SJ, Vierkant RA, Johnatty SE, et al. Consortium analysis of 7 candidate SNPs for ovarian cancer. Int J Cancer. 2008;123:380–8. doi: 10.1002/ijc.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandera EV, King M, Chandran U, et al. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health. 2011;11:40. doi: 10.1186/1472-6874-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glud E, Kjaer SK, Thomsen BL, Hogdall C, Christensen L, Hogdall E, Bock JE, Blaakaer J. Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med. 2004:1642253–9. doi: 10.1001/archinte.164.20.2253. [DOI] [PubMed] [Google Scholar]
- 25.Goodman MT, Lurie G, Thompson PJ, et al. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer. 2008;15:1055–60. doi: 10.1677/ERC-08-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamajima N, Matsuo K, Saito T, et al. Gene-environment Interactions and Polymorphism Studies of Cancer Risk in the Hospital-based Epidemiologic Research Program at Aichi Cancer Center II (HERPACC-II) Asian Pac J Cancer Prev. 2001;2:99–107. [PubMed] [Google Scholar]
- 27.Kelemen LE, Sellers TA, Schildkraut JM, et al. Genetic variation in the one-carbon transfer pathway and ovarian cancer risk. Cancer Res. 2008;68:2498–506. doi: 10.1158/0008-5472.CAN-07-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merritt MA, Green AC, Nagle CM, et al. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122:170–6. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 29.Royar J, Becher H, Chang-Claude J. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001:95370–4. doi: 10.1002/1097-0215(20011120)95:6<370::aid-ijc1065>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Schildkraut JM, Iversen ES, Wilson MA, et al. Association between DNA damage response and repair genes and risk of invasive serous ovarian cancer. PLoS One. 2010;5:e10061. doi: 10.1371/journal.pone.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terry KL, De VI, Titus-Ernstoff L, et al. Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res. 2005;65:5974–81. doi: 10.1158/0008-5472.CAN-04-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balogun N, Gentry-Maharaj A, Wozniak EL, et al. Recruitment of newly diagnosed ovarian cancer patients proved challenging in a multi-centre biobanking study. J Clin Epidemiol. 2011;64:525–30. doi: 10.1016/j.jclinepi.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Closas M, Brinton LA, Lissowska J, et al. Ovarian cancer risk and common variation in the sex hormone-binding globulin gene: a population-based case-control study. BMC Cancer. 2007;7:60. doi: 10.1186/1471-2407-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGuire V, Felberg A, Mills M, et al. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. Am J Epidemiol. 2004;160:613–8. doi: 10.1093/aje/kwh284. [DOI] [PubMed] [Google Scholar]
- 35.Song H, Ramus SJ, Quaye L, et al. Common variants in mismatch repair genes and risk of invasive ovarian cancer. Carcinogenesis. 2006;27:2235–42. doi: 10.1093/carcin/bgl089. [DOI] [PubMed] [Google Scholar]
- 36.Wu AH, Pearce CL, Tseng CC, et al. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer. 2009;124:1409–15. doi: 10.1002/ijc.24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziogas A, Gildea M, Cohen P, et al. Cancer risk estimates for family members of a population-based family registry for breast and ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:103–11. [PubMed] [Google Scholar]
- 38.Trabert B, Ness RB, Lo-Ciganic WH, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst. 2014;106:djt431. doi: 10.1093/jnci/djt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodelon C, Cushing-Haugen KL, Wicklund KG, et al. Sun exposure and risk of epithelial ovarian cancer. Cancer Causes Control. 2012;23:1985–94. doi: 10.1007/s10552-012-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Risch HA, Bale AE, Beck PA, et al. PGR +331 A/G and increased risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1738–41. doi: 10.1158/1055-9965.EPI-06-0272. [DOI] [PubMed] [Google Scholar]
- 41.Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 42.Stukel TA, Demidenko E, Dykes J, et al. Two-stage methods for the analysis of pooled data. Stat Med. 2001;20:2115–30. doi: 10.1002/sim.852. [DOI] [PubMed] [Google Scholar]
- 43.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 44.Warren GW, Sobus S, Gritz ER. The biological and clinical effects of smoking by patients with cancer and strategies to implement evidence- based tobacco cessation support. Lancet Oncol. 2014;15:568–80. doi: 10.1016/S1470-2045(14)70266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomsen T, Villebro N, Moller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev. 2014(3):CD002294. doi: 10.1002/14651858.CD002294.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lahti-Koski M, Pietinen P, et al. Associations of body mass index and obesity with physical activity, food choices, alcohol intake, and smoking in the 1982–1997 FINRISK Studies. Am J Clin Nutr. 2002;75:809–17. doi: 10.1093/ajcn/75.5.809. [DOI] [PubMed] [Google Scholar]
- 47.Ezzati M, Lopez AD. Regional, disease specific patterns of smoking-attributable mortality in 2000. Tob Control. 2004;13:388–95. doi: 10.1136/tc.2003.005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sperling C, Noer MC, Christensen IJ, et al. Comorbidity is an independent prognostic factor for the survival of ovarian cancer: a Danish register-based cohort study from a clinical database. Gynecol Oncol. 2013;129:97–102. doi: 10.1016/j.ygyno.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 49.Janssen-Heijnen ML, Houterman S, Lemmens VE, et al. Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol. 2005;55:231–40. doi: 10.1016/j.critrevonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Louwman WJ, Aarts MJ, Houterman S, et al. A 50% higher prevalence of life-shortening chronic conditions among cancer patients with low socioeconomic status. Br J Cancer. 2010;103:1742–8. doi: 10.1038/sj.bjc.6605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morales NA, Romano MA, Michael CK, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control. 2013;24:1223–30. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


