Abstract
The memory-enhancing effect of emotion can be powerful and long-lasting. Most studies investigating the neural bases of this phenomenon have focused on encoding and early consolidation processes, and hence little is known regarding the contribution of retrieval processes, particularly after lengthy retention intervals. To address this issue, we used event-related functional MRI to measure neural activity during the retrieval of emotional and neutral pictures after a retention interval of 1 yr. Retrieval activity for emotional and neutral pictures was separately analyzed for successfully (hits) vs. unsuccessfully (misses) retrieved items and for responses based on recollection vs. familiarity. Recognition performance was better for emotional than for neutral pictures, and this effect was found only for recollection-based responses. Successful retrieval of emotional pictures elicited greater activity than successful retrieval of neutral pictures in the amygdala, entorhinal cortex, and hippocampus. Moreover, in the amygdala and hippocampus, the emotion effect was greater for recollection than for familiarity, whereas in the entorhinal cortex, it was similar for both forms of retrieval. These findings clarify the role of the amygdala and the medial temporal lobe memory regions in recollection and familiarity of emotional memory after lengthy retention intervals.
Keywords: affect, arousal, declarative memory, episodic memory, R-K paradigm
Through evolution, the declarative memory system developed the ability to preferentially retain events that are relevant for survival, which are usually those associated with strong emotions and motivational goals (finding food, spotting a predator, etc.). Emotional arousal may enhance one or more of several memory stages, including the creation of new memory traces (encoding), the stabilization and persistence of these traces (consolidation and storage), and/or the final access to stored traces (retrieval). Yet, the vast majority of studies on the neural mechanisms of emotional memory have focused on early memory stages (encoding and consolidation), and very little information is available about retrieval, particularly after long retention intervals. Neurobiological theories of emotional memory will not be complete without an account of retrieval mechanisms. This issue also has implications for understanding dysfunctional accessibility of traumatic memory traces in affective disorders. To fill this void, we used event-related functional MRI (fMRI) to investigate the neural mechanisms underlying the long-term retrieval of emotional memories in healthy adults.
According to the modulation hypothesis, the memory-enhancing effect of emotional arousal reflects the influence of the amygdala (AMY) on the medial temporal lobe (MTL) memory system. Although much animal (1, 2) and functional neuroimaging (3–12) evidence links the memory-enhancing effect of emotional arousal to amygdalar modulation during encoding, it is not clear whether a similar mechanism operates also during retrieval. Evidence from the animal literature suggests that AMY is also involved during emotional memory retrieval (13), but the exact nature of this involvement is a matter of current debate (14, 15).
Similarly, a few functional neuroimaging studies in humans have found amygdalar activity during retrieval (16–20), but these studies have two main limitations: (i) they used short retention intervals (i.e., minutes), which do not allow a clear separation between the involvement of AMY in retrieval and early consolidation processes; and (ii) they did not demonstrate that AMY is differentially more involved in successful than in unsuccessful retrieval of emotional events relative to neutral events.
Thus, the first goal of the present fMRI study was to investigate the effect of emotional arousal on retrieval activity while addressing the two limitations of earlier studies: (i) to distinguish retrieval processes from early consolidation processes, we examined retrieval processes after a retention interval of 1 yr; and (ii) to specifically isolate activity associated with successful retrieval operations, we compared amygdalar and MTL memory system activity for successfully vs. unsuccessfully retrieved items, specifically contrasting old items classified as old (hits) to old items classified as new (misses).
The second goal of the study was to disentangle the effects of emotional arousal on two different forms of episodic memory retrieval: recollection and familiarity (21). Recollection refers to memory for an event (e.g., meeting someone) that is accompanied by the retrieval of contextual information and other associated elements (e.g., time, location, and sensory details), whereas familiarity refers to the feeling that an event happened in the past, but no associated information can be retrieved (e.g., knowing that a face was seen before but without remembering where or when). Distinguishing recollection and familiarity is critical, because there is behavioral evidence that the memory-enhancing effect of emotion specifically modulates recollection rather than familiarity processes (22, 23). However, the neural correlates of this differential effect are unknown. The fMRI studies that investigated recollection vs. familiarity either used neutral stimuli (24–26) or did not distinguish between successful and unsuccessful activity (20). Thus, the present study also investigated the effect of emotional arousal on the neural correlates of successful recollection vs. familiarity, an issue that has not been examined by previous studies.
In the present study, participants encoded high-arousing emotional (pleasant and unpleasant) and low-arousing neutral pictures by rating their valence, and 1 yr later, they were scanned while distinguishing between the pictures they previously saw and equivalent new pictures. Subjects performed a recognition task that distinguishes between recollection-based (R) and familiarity-based (K) responses (27). On the basis of behavioral evidence concerning the memory-enhancing effect of emotion (28, 29), we made a first prediction: (i) recognition would be better for emotional than for neutral pictures, and this effect would be driven mainly by recollection (22, 23). Concerning the neural basis of these effects, we made two additional predictions: (ii) on the basis of our encoding results (7), we predicted that emotion would enhance retrieval success activity (RS) in AMY and the MTL memory regions, specifically the hippocampus (HC) and the entorhinal cortex (EC); and (iii) we also predicted that R responses would be associated with greater activity than K response in AMY and HC but not in cortical MTL regions, such as EC. The AMY and HC were assumed to be the source of differential recollection vs. familiarity effects, because emotional arousal enhances recollective processes behaviorally, which have been more closely associated with hippocampal function than with other cortical MTL regions (21, 30).
Methods
Subjects. Nine young (20–35 yr old; mean age, 26.8; SD, 4.6), healthy, right-handed female adults participated in the study. These subjects also participated in a previous study on short-term retention of the same stimuli (7, 8). Due to high rates of false alarms (FAs), data from two subjects were excluded from analyses. Female participants were chosen because, compared with men, women are physiologically more reactive to emotional stimuli (31) and are more likely to report intense emotional experiences (32). All participants gave written consent to a protocol approved by the Duke Institutional Review Board.
Stimuli. The stimuli were emotional and neutral pictures selected from the International Affective Picture System (33). To equate emotional and neutral pictures for complexity and human presence, neutral pictures were also selected from other sources (34). The procedure for selecting the pictures was previously described (7, 35). In brief, pleasant and unpleasant pictures were high in arousal (mean in the 1–9 arousal scale: pleasant, 6.0; unpleasant, 6.15) and of opposing valence (mean in the 1–9 valence scale: pleasant, 7.1; unpleasant, 2.3), whereas neutral pictures were low in arousal (3.15) and intermediate in valence (5.0).
Procedure. Participants were scanned with fMRI both during encoding and 1 yr later during retrieval. Here we focus on retrieval data; encoding results were reported in a previous paper (7). During encoding, participants were scanned while rating 120 emotional (60 pleasant, 60 unpleasant) and 60 neutral pictures for pleasantness, by using a three-point scale (1, unpleasant; 2, neutral; and 3, pleasant). They were unaware of a subsequent memory test (incidental learning). Forty-five minutes after the scanning session, subjects performed a cued-recall task, whose results were used to analyze the encoding data (7, 8). About 1 yr later (range, 10–16 months; mean, 13 months), participants were scanned with fMRI while recognizing old and new pictures. The test included 180 old pictures and 90 new foils (30 from each category), distributed across nine blocks. Old and new pictures did not reliably differ in normative arousal and valence scores. During retrieval, participants pressed a key to indicate “Remember,” “Know,” or “New.” “Remember” indicated memory for the picture accompanied by specific details about its occurrence during the encoding session, whereas “Know” indicated the belief that the picture was seen during encoding, even though no specific details could be retrieved (27). This single-step procedure of distinguishing R vs. K responses was used to keep the number of response options during the retrieval task consistent with that used during the encoding task (see above). As presented below, this procedure was as effective in identifying R vs. K response differences in the HC as the two-step procedure used by other studies (e.g., ref. 24). During both encoding and retrieval, pictures were randomly mixed and presented every 15 sec (picture, 3 sec; fixation, 12 sec) (7, 8). The pictures were projected to a screen that participants viewed via an angled mirror. Responses were recorded by using a three-button magnetic resonance-compatible response box. During both encoding and retrieval, participants were encouraged to make quick and accurate responses.
fMRI Methods. Anatomical scanning. Scanning was performed on a 1.5-T General Electric scanner. Thirty-four T1-weighted contiguous oblique slices were prescribed parallel to anterior–posterior commissures by using 450-ms repetition time, 9-ms echo time, 24-cm field of view, 2562 matrix, and 3.75-mm slice thickness.
Functional scanning. Thirty-four contiguous gradient-echo echoplanar images (EPIs) sensitive to blood-oxygen level-dependent contrast and coplanar with the high-resolution anatomical images were acquired. The EPIs were acquired with 3-sec repetition time, 40-ms echo time, one radio frequency excitation, 24-cm field of view, 642 image matrix, 90° flip angle, and 3.75-mm slice thickness (resulting in cubic 3.75-mm3 isotropic voxels).
Image preprocessing. Image preprocessing was performed with SPM99 (www.fil.ion.ucl.ac.uk/spm). Functional images were corrected for acquisition order and realigned to correct for motion artifacts. Anatomical images were coregistered with the first functional images for each subject, and both anatomical and functional images were spatially normalized to standard stereotactic space. The functional images were also spatially smoothed by using an 8-mm isotropic Gaussian kernel.
Statistical analyses. Analyses were performed for each individual and then for the group. For individual analyses, the fMRI signal was selectively averaged for each subject as a function of stimulus condition (e.g., remembered-forgotten = RS) and time point (one prestimulus and four poststimulus onset time points were used), by using in-house software. No assumption was made regarding the shape of the hemodynamic response function. These analyses yielded whole-brain activation maps, which were used to calculate the percent signal change relative to stimulus onset for each condition and time point. For group analyses, voxel-wise paired t tests were performed by using the individual percent signal change maps for the conditions of interest and time points. Given behavioral (28) and neuroimaging (7, 8, 36, 37) evidence that the arousal rather than the valence is the main factor determining episodic memory for emotional stimuli, pleasant and unpleasant pictures were collapsed into a single “emotional” category. This procedure was feasible, because pleasant and unpleasant pictures were equated for emotional arousal and yielded similar recognition performance.
All fMRI results reported involve or are based on RS, which is defined as greater activity for hits (H, old pictures correctly classified as old) than for misses (M, old pictures incorrectly classified as new): (RS = H-M). We preferred to compare hits with misses rather than to compare them with correct rejections, because recent evidence suggests that the latter elicit encoding/novelty MTL activity that can subtract out RS (38).
To investigate emotion enhancement on RS activity (EERS), RS was separately calculated for emotional pictures (eRS = eH-eM) and for neutral pictures (nRS = nH-nM), and the two measures were compared with each other (EERS = eRS-nRS). To make sure that this contrast reflected eRS activations rather than nRS deactivations, its results were inclusively masked with eRS activations (P < 0.05). To investigate recollection enhancement on RS activity (RERS), RS was separately calculated for R responses (rRS = rH-M) and for K responses (kRS = kH-M), and the two measures were compared with each other (RERS = rRS-kRS). Again, to make sure that this contrast reflected activations for eRS rather than deactivations for kRS, its results were inclusively masked with rRS activations (P < 0.05).
To investigate the interaction between emotion and recollection enhancement effects, two analyses were performed. In the first analysis, regions showing EERS were interrogated about whether the EERS was greater for R than for K responses (recollection enhancement on EERS: RE-EERS = rEERS-kEERS). To make sure that this contrast reflected activations for rEERS rather than deactivations for kEERS, its results were inclusively masked with rEERS activations (P < 0.05). In the second analysis, regions showing RERS were interrogated about whether the RERS was greater for emotional than for neutral pictures (emotion enhancement on RERS: EE-RERS = eRERS-nRERS). Once more, to make sure that this contrast reflected activations for eRERS rather than deactivations for nRERS, its results were inclusively masked with eRERS activations (P < 0.05).
Because the focus of the study was activity in the MTL regions, the fMRI signal from the active MTL voxels as identified by the group analyses for the conditions of interest was extracted by using a MTL mask, which can localize more precisely activity from various MTL subregions. This procedure involved two steps. First, the active voxels identified for a specific contrast on a time-point-by-time-point basis were clustered together across time points by using the logical function or. Then, the averaged signal from this cluster (extent threshold = four contiguous voxels) was extracted by using the MTL mask. The MTL mask consisted of regions of interest (ROIs) manually traced on a high-resolution anatomical image having the same resolution as those recorded for each subject and normalized to the same Montreal Neurological Institute template. Similar to the procedure used in our encoding study (7), ROI tracing first identified AMY and the main MTL memory regions (i.e., HC and the associated parahippocampal gyrus), which where then further subdivided into their major subregions (i.e., the HC was subdivided into head, body, and tail, and the parahippocampal gyrus was subdivided into the entorhinal, perirhinal, and parahippocampal cortices), based on tracing guidelines for the MTL (39–43).
The MTL mask was used to more precisely localize the activity coming from various MTL subregions and to extract data for confirmatory analyses (i.e., t tests and/or ANOVAs). These confirmatory analyses were performed on averaged signal from clusters of voxels. To reduce the possibility of type I error, an activation within the a priori-defined regions of interest was considered significant if it fulfilled three criteria: (i) it passed a height threshold of P < 0.05 uncorrected; (ii) it involved at least 5% of the voxels in MTL subregions for the contrasts of interest; and (iii) it was confirmed by subsequent statistical analyses. Unless otherwise specified, the statistical results reported are based on confirmatory t tests/ANOVAs performed on percent signal change extracted from clustered voxels. Given the evidence that BOLD activity in the peak voxels is more directly related to electrophysiological recordings of neural activity than the average of voxel clusters (44), statistical results based on t tests performed on activity extracted from peak voxels were also reported (i.e., Table 2).
Table 2. Emotion effect on recollection in the MTL (t scores).
| MTL regions | Emo rRS-kRS | Neu rRS-kRS | (Emo rRS-kRS) > (Neu rRS-kRS) |
|---|---|---|---|
| AMY | 5.29 (R)/2.11 (L) | 4.98 (R) | 3.29 (R) |
| HC (head) | 8.83 (R)/3.21 (L) | 4.36 (R)/3.41 (L) | 5.70 (R) |
| HC (body) | 7.58 (R) | 2.62 (R)/4.13 (L) | 2.26 (L) |
| HC (tail) | 3.95 (R)/3.94 (L) | - | 2.61 (R)/2.04 (L)* |
| APHG (EC) | 3.98 (R) | - | - |
| APHG (PC) | 2.64 (L) | - | - |
| PPHG | 5.52 (R)/6.01 (L) | 3.59 (R) | * |
t statistics were performed on the signal extracted from the peak voxels identified in each MTL subregion and contrast of interest (t = 1.94, P < 0.05) Emo, emotional; Neu, neutral; (RS, Hits - Misses); rRS, recollection-based RS; kRS, familiarity-based RS; R, right hemisphere; L, left hemisphere; PHG, parahippocampal gyrus; APHG, anterior PHG; PPHG, posterior PHG; PC, perirhinal cortex.
For the (Emo rRS-kRS) > (Neu rRS-kRS) contrast, because the peak voxel fell on the border between the HC tail and the posterior parahippocampal gyrus, these two regions were considered together
Finally, data analysis also involved correlation analyses. Based on our results from encoding (7), across-subject pairwise Pearson correlations between RS in AMY and the memory-related MTL regions were performed for both R and K responses and compared for emotional and neutral pictures.
Results
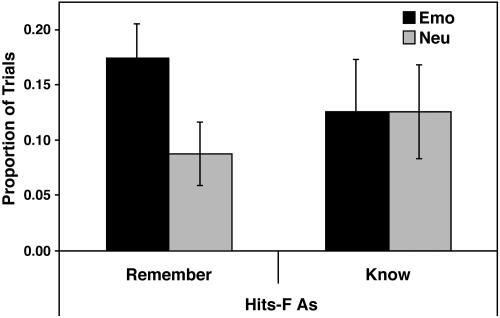
Behavioral Results. Confirming our first prediction, recognition memory was better for emotional than for neutral pictures, and this effect was driven by recollection (see Table 1 and Fig. 1). Recognition memory was similar for pleasant and unpleasant pictures, both when considering hits (pleasant, 0.54; unpleasant, 0.50; P > 0.35) and hits-FAs (pleasant, 0.30; unpleasant, 0.30; P > 0.99), and hence these pictures were collapsed into a single “emotional” category. As Table 1 indicates, overall corrected recognition scores (hits-FAs) were greater for emotional than for neutral pictures, and this difference was driven by R response (see Fig. 1). Actually, the emotional–neutral difference in corrected recognition scores was significant for R response (P < 0.05) but not for K response (P > 0.99), and a 2 (emotional vs. neutral) × 2 (R vs. K responses) ANOVA yielded a significant interaction (P < 0.05). In sum, emotional arousal enhanced memory performance, and this effect was driven by recollective processes.
Table 1. Behavioral results.
| Memory performance | Emotional, % | Neutral, % |
|---|---|---|
| Overall (R + K) | ||
| Hits | 51.9 | 32.8 |
| FAs | 21.9 | 11.4 |
| Hits - FAs | 30 | 21.2 |
| Remember (R) | ||
| Hits | 23.8 | 12.1 |
| FAs | 6.4 | 3.3 |
| Hits - FAs | 17.4 | 8.8 |
| Know (K) | ||
| Hits | 28.1 | 20.7 |
| FAs | 15.5 | 8.1 |
| Hits - FAs | 12.6 | 12.6 |
Hits, old items correctly identified as old; FAs, new items incorrectly identified as old.
Fig. 1.
Corrected recognition scores (hits-FAs) for emotional and neutral pictures are presented. Emo, Emotional; Neu, Neutral; Remember, Recollection-based responses; Know, Familiarity-based responses.
As noted in Methods, participants recalled pictures immediately after scanning 1 yr before the recognition test. To investigate whether the recall test had any effect on recognition 1 yr later, we calculated correlations between these two tasks. These correlations were not significant for either emotional (r = 0.25) or neutral (r = 0.14) pictures (Ps >0.6), suggesting that recall performance did not affect recognition performance for emotional or neutral items. Because the memory advantage for emotional pictures in the present study was driven by Recollection, we further investigated the possibility that memory performance in the recall test selectively affected R responses in the recognition test. However, the correlations between recall and R responses were not statistically significant either (Ps >0.4).
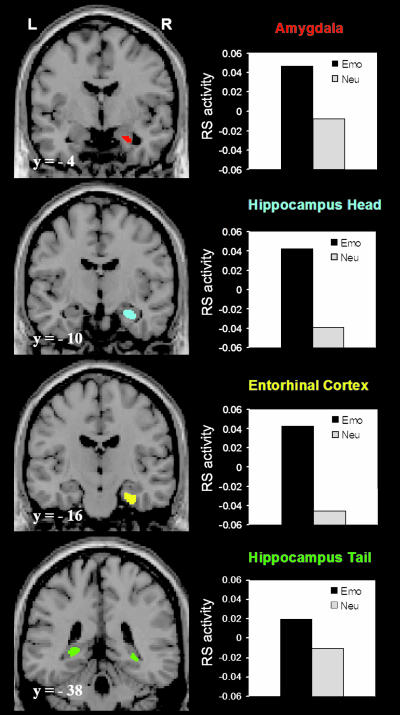
fMRI Results. Confirming our second prediction, retrieval success (RS) activity in the AMY, HC, and EC was greater for emotional than for neutral pictures (Fig. 2). RS activity in other MTL regions was not significantly different for emotional vs. neutral pictures. Paired t tests performed on RS activity at the peak time point yielded significant differences as a function of emotion in the right AMY (t = 2.56, P < 0.05), right EC (t = 2.75, P < 0.04), right HC head (t = 2.98, P < 0.03), and bilaterally in HC tail (left side, t = 2.48, P < 0.05; right side, t = 2.62, P < 0.04).
Fig. 2.
Greater RS in the AMY and MTL memory systems for emotional than for neutral pictures. Compared with the neutral RS, the overall emotional RS was associated with greater activity in the AMY, EC, and HC. In the left column are presented representative brain slices showing the active voxels as identified in the MTL subregions by random-effects group analysis comparing Emo RS with Neu RS. The active voxels (in color) are displayed on a high-resolution anatomical image normalized to the Montreal Neurological Institute (MNI) template. The numbers at the left bottom side of each brain slice (e.g., y =-4) represent the y values in MNI space. On the right column are presented graphs displaying the emotional and neutral RS expressed in percent signal change as extracted from the active voxels identified in the MTL subregions. RS (hits-misses); Emo, emotional; Neu, neutral; L, left; and R, right.
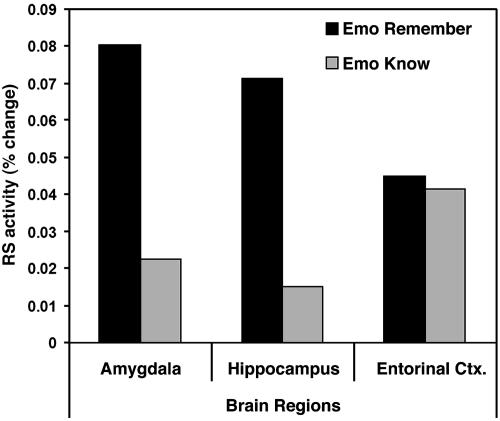
Confirming our third prediction, an effect of recollection (R > K responses) on regions showing an EERS was found in the AMY and HC but not in the EC (see Fig. 3). Paired t tests revealed significant effects of recollection (R > K responses) in the right AMY (t = 2.89, P < 0.03), right HC head (t = 2.57, P < 0.05), and bilaterally in HC tail (left side, t = 2.79, P < 0.04; right side, t = 2.78, P < 0.04), but not in EC (t = 0.68, P > 0.5). Confirming the regional specificity of these recollection effects, 3 (AMY vs. HC vs. EC) × 2 (R vs. K responses) ANOVAs on various HC regions yielded a significant region × recollection interaction. The most significant interaction was obtained when including the HC head [F(2, 12) = 8.16, P < 0.006], as displayed in Fig. 3.
Fig. 3.
Differential effects of recollection on RS activity for emotional pictures on different MTL subregions. In the right AMY and HC head, RS activity for emotional pictures was greater for recollection than for familiarity, whereas in the EC, it was similar for recollection and familiarity. For the AMY and HC head, the bars are based on the percent signal change extracted from the voxels showing the maximum recollection vs. familiarity difference. RS, hits-misses; Remember, recollection-based RS (hits-R > misses); Know, familiarity-based RS (hits-K > misses); Emo, emotional; and Neu, neutral.
The foregoing analyses on recollection effects were limited to regions showing a significant effect of emotion on RS activity. To investigate recollection effects regardless of emotion, we identified regions showing greater RS activity for R than for K responses separately for emotional and neutral pictures. These analyses converge with previous analyses by showing that the recollection effect on several AMY and HC regions was significantly greater for emotional than neutral pictures (see Table 2). Interestingly, in a left HC area, the recollection effect was significant for neutral but not for emotional items (not even at P < 0.05). This result demonstrates that MTL regions were not always more activated for emotional than for neutral pictures, which argues against potential confounds between these conditions, such as in the number of trials. This does not preclude the possibility that differences in the number of trials could have affected other brain regions. Finally, although the present study focused on MTL, we also conducted an exploratory whole-brain analysis investigating the recollection-enhancing effect of emotion outside MTL. Significant effects were found in the lateral cortex, medial prefrontal cortex, temporal cortex, occipital cortex, and cerebellum. These results will be the focus of a separate report, but it is important to note here that they do not alter any of the conclusions made in the present article.
Correlation analyses showed that AMY and the MTL memory regions were more systematically coactivated during recollection of emotional pictures than during recollection of neutral pictures. Correlations were calculated among the MTL subregions showing greater R vs. K effects for emotional pictures compared with neutral pictures (i.e., the AMY, HC head, and posterior hippocampal/parahippocampal regions; see Table 2). As illustrated in Table 3, the greatest differences between the emotional and neutral pictures were in the case of R responses.
Table 3. Correlations between the AMY and the MTL memory regions.
| MTL regions | AMY-MTL correlations: R scores (emotional rRS) | AMY-MTL correlations: R scores (neutral rRS) | AMY-MTL correlations: R scores (emotional kRS) | AMYgdala-MTL correlations: R scores (neutral kRS) |
|---|---|---|---|---|
| AMY (R) | N/A | N/A | N/A | N/A |
| HC (head) (R) | 0.94**** | 0.97**** | 0.98**** | 0.86* |
| HC (body) (L) | 0.82* | - | 0.9*** | - |
| HC (tail)/PPHG (R) | 0.87** | - | - | - |
| HC (tail)/PPHG (L) | 0.77* | - | 0.81* | 0.76* |
Correlations were calculated between the AMY and the MTL memory regions showing greater recollection- vs. familiarity-based retrieval success activity for emotional than for neutral pictures. The greatest differences were in the case of recollection-based responses. Similar to Table 2, the signals from posterior HC/parahippocampal regions were averaged together. RS, (hits - misses); rRS, recollection-based RS; kRS, familiarity-based RS. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.0005.
Taken together, the present fMRI results suggest that the AMY and the MTL memory regions were more engaged and more systematically coactivated during successful retrieval of emotional pictures than during successful retrieval of neutral pictures, and that different MTL subregions have dissociable contributions to recollection- vs. familiarity-based retrieval success.
Discussion
The present study yielded three main results relevant for understanding the psychological and neural mechanisms that mediate emotional memory retrieval. First, 1 yr after their initial encoding, emotionally arousing pictures were remembered better and elicited greater recollection than neutral pictures. Second, emotional content enhanced activity in the AMY and MTL memory systems (HC and EC) related to successful retrieval of individual items from long-term storage (hits vs. misses). Third, the emotion-enhancing effect during retrieval was greater for R than for K responses in AMY and HC but not in EC. These findings are discussed, in turn, below.
Long-Term Retrieval of Emotional Pictures Is Accompanied by a Sense of Recollection. Extending previous behavioral studies using shorter retention intervals (45–48), the present study shows that the memory-enhancing effect of emotion can last over lengthy periods (28). Moreover, we found that such long-lasting memory benefits are equivalent for negatively and positively valent material matched for arousal (4, 7, 28). One-year retention intervals may provide a limiting test case to observe such effects for laboratory-based models of memory, because longer intervals are likely to yield floor effects that mask the modulatory influence of emotion. Nonetheless, empirical studies of real-world events, including flashbulb and autobiographical memories, show emotional retention advantages that extend from years to decades (49). It should be noted that, although 1 yr is “remote” in terms of laboratory-based event memory, it is usually considered “recent” in terms of autobiographical memory. Thus, the present study provides an important bridge between retention intervals that are typically tested across these different episodic memory domains, and additional research is beginning to reveal brain regions common to retrieval of both laboratory-based and real-world events (50, 51).
The results discussed above link emotional arousal to retention advantages as defined by accessibility of the memory trace (hits vs. misses). However, memory retrieval is associated with distinct mechanisms that can be dissociated behaviorally and that may be preferentially targeted by emotional processes. Recollection and familiarity are two types of retrieval that have been found to be differentially affected by emotional arousal in both laboratory (22) and autobiographical (23, 52) memory studies. Here, we extend the laboratory-based findings by showing that memory-enhancing effect of emotional arousal on recollection extends over a period of 1 yr. This issue has not been investigated by earlier laboratory-based studies of emotional memory retrieval after similar retention intervals (28).
Emotion Enhanced RS in AMY and the MTL Memory System. The present report provides strong evidence that successful retrieval of emotional memories involves MTL mechanisms similar to those identified during successful emotional encoding (3–7, 9–11). The few functional neuroimaging studies that investigated emotional memory retrieval (i) did not disentangle retrieval from early consolidation due to short retention intervals (e.g., minutes) and (ii) did not isolate the neural correlates of the difference between successfully vs. unsuccessfully retrieved items. The present study addressed these limitations by (i) investigating retrieval of emotional and neutral pictures after a retention interval of 1 yr and (ii) directly comparing RS activity (hits-misses) for emotional vs. neutral events. In a previous fMRI study (7), we found that emotional arousal enhanced successful encoding activity in the AMY, HC, and EC. In the present study, we show that emotion enhances successful retrieval activity in the same set of regions. The right-lateralization pattern observed in the present study is different from the left-lateralized effects identified in female participants during emotional encoding (53) but is consistent with the pattern observed during emotional retrieval (e.g., ref. 20). It would be interesting to investigate whether the opposite pattern typically observed in males during encoding (53) is also observed during retrieval.
These results clearly show that AMY plays a role in emotional memory not only during successful encoding but also during successful retrieval. It is important to note that AMY activation in the present study cannot be attributed to the emotional nature of the stimuli used as retrieval cues (17, 18, 54). Specifically, AMY activation was found as a difference between retrieval activity for emotional pictures correctly classified as old (emotional hits) and activity for emotional pictures incorrectly classified as new (emotional misses). Moreover, this difference was also identified when RS activity for emotional pictures (emotional hits > emotional misses) was compared to RS activity for neutral pictures (neutral hits > neutral misses). Therefore, activity related to perception of emotion is subtracted out, and the difference reflects the interaction between emotion and memory. In contrast, the fMRI study by Sharot et al. (20) did not compare hits with misses, and hence it could not distinguish between the effects of emotion on memory and perception.
Recollection Enhanced Emotion Effects in AMY and HC but Not in EC. Understanding the differences between recollection and familiarity and the factors that selectively enhance recollection is a fundamental goal of memory research. Emotion is assumed to be a critical factor, but the underlying neural mechanism is only partly understood. Behavioral studies have demonstrated that the ability to recollect past events is enhanced by emotional arousal (22, 23), and functional neuroimaging studies have linked recollection of neutral events to activity in the HC (21). Thus, it was reasonable to predict that the recollection-enhancing effect of emotion was mediated by brain regions associated with arousal (i.e., AMY) and brain regions associated with recollection (i.e., HC). Yet, the connection between these two lines of evidence was lacking. The present study provides this critical missing link: emotion selectively enhanced recollection-based activity indexing retrieval success in both AMY and HC.
Given that AMY is a prototypical emotion region and HC is a prototypical memory region, one way of explaining their coactivation during emotional recollection is that emotion enhances recollection-related activity in the HC, whereas recollection enhances emotion-related activity in AMY. Emotion may enhance recollection, because reinstating the affective context of the original episode is likely to facilitate the recovery of contextual details, such as where, when, and how the original events happened. Conversely, the recollection of the context surrounding an emotional effect is likely to augment the emotional arousal elicited by the event during retrieval. Thus, the AMY and HC could be parts of a synergistic mechanism in which emotion enhances recollection and recollection enhances emotion. Greater correlations for emotional items between successful retrieval activity in the AMY and HC identified in the present study support this idea. The clinical implication of this interpretation is that, in patients suffering from posttraumatic stress disorder, processing of emotional cues related to traumatic events may trigger recollection of traumatic memories, which is accompanied by HC activity. This, in turn, may intensify AMY activity associated with emotional (e.g., fear-related) responses (13).
In EC, in contrast, emotion enhanced familiarity- and recollection-related activity equally (Fig. 3). This may reflect the position of this region within the MTL memory system as a convergence point for information coming from unimodal and multimodal association areas. As a result, this region may be sensitive to the reinstatement of sensory details that give rise to the experience of familiarity. On the other hand, the position of the HC at the top of the MTL hierarchy may be critical for binding content and context information required for the experience of recollection (55). In sum, the AMY, HC, and EC all contribute to the enhancing effect of emotion on retrieval processes, and only the first two regions can additionally differentiate between emotion effects on recollection and familiarity. Different from previous studies, the present results clearly link activity in the AMY and HC not only to enhanced feeling of recollection for emotional stimuli (20) but also to enhanced actual successful recollection of emotional stimuli.
Conclusion
The present study provided behavioral and functional neuroimaging evidence concerning the effect of emotional arousal on memory retrieval processes after a long retention interval. Behavioral results showed that emotionally arousing stimuli were remembered better than neutral ones, and this memory-enhancing effect affected recollection rather than familiarity. Functional neuroimaging results showed that emotion enhances successful retrieval activity in AMY and the MTL memory system, and that in the AMY and HC (but not in the EC), the emotion effect is greater for recollection than for familiarity. Taken together, these results suggest that successful retrieval of remote emotional information involves MTL mechanisms similar to those identified during successful encoding, and that different MTL subregions have dissociable contributions to successful retrieval of emotional memories based on recollection and familiarity.
Acknowledgments
We thank Matthew Budde for assistance with data analysis and Paul Fletcher and Morris Moscovitch for insightful comments on an earlier version of the manuscript. This work was supported by National Institutes of Health Grants R01 AG19731 (to R.C.) and R01 DA14094 (to K.S.L.) and National Science Foundation Career Award 0239614 (to K.S.L.). F.D. was supported by a Chia Ph.D. Scholarship and a Dissertation Fellowship from the University of Alberta, and Research Assistantships from Duke University.
Author contributions: F.D. and R.C. designed research; F.D. performed research; F.D. and R.C. analyzed data; and F.D., K.S.L., and R.C. wrote the paper.
Abbreviations: fMRI, functional MRI; AMY, amygdala; MTL, medial temporal lobe; HC, hippocampus; EC, entorhinal cortex; R, recollection-based response; K, familiarity-based response; RS, retrieval success activity; EERS, emotion enhancement on RS activity; RERS, recollection enhancement on RS activity; FA, false alarm.
References
- 1.McGaugh, J. L. (2000) Science 287, 248-251. [DOI] [PubMed] [Google Scholar]
- 2.McGaugh, J. L. (2004) Annu. Rev. Neurosci. 27, 1-28. [DOI] [PubMed] [Google Scholar]
- 3.Cahill, L., Haier, R. J., Fallon, J., Alkire, M. T., Tang, C., Keator, D., Wu, J. & McGaugh, J. L. (1996) Proc. Natl. Acad. Sci. USA 93, 8016-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamann, S. B., Ely, T. D., Grafton, S. T. & Kilts, C. D. (1999) Nat. Neurosci. 2, 289-293. [DOI] [PubMed] [Google Scholar]
- 5.Canli, T., Zhao, Z., Brewer, J., Gabrieli, J. D. E. & Cahill, L. (2000) J. Neurosci. 20, 1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canli, T., Desmond, J. E., Zhao, Z. & Gabrieli, J. D. E. (2002) Proc. Natl. Acad. Sci. USA 99, 10789-10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolcos, F., LaBar, K. S. & Cabeza, R. (2004) Neuron 42, 855-863. [DOI] [PubMed] [Google Scholar]
- 8.Dolcos, F., LaBar, K. S. & Cabeza, R. (2004) NeuroImage 23, 64-74. [DOI] [PubMed] [Google Scholar]
- 9.Kensinger, E. A. & Corkin, S. (2004) Proc. Natl. Acad. Sci. USA 101, 3310-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson, M. P., Strange, B. A. & Dolan, R. J. (2004) Nat. Neurosci. 7, 278-285. [DOI] [PubMed] [Google Scholar]
- 11.Kilpatrick, L. & Cahill, L. (2003) NeuroImage 20, 2091-2099. [DOI] [PubMed] [Google Scholar]
- 12.Phelps, E. A. (2004) Curr. Opin. Neurobiol. 14, 198-202. [DOI] [PubMed] [Google Scholar]
- 13.LeDoux, J. E. (2000) Annu. Rev. Neurosci. 23, 155-184. [DOI] [PubMed] [Google Scholar]
- 14.Nadel, L. (2000) Nat. Rev. Neurosci. 1, 209-212. [DOI] [PubMed] [Google Scholar]
- 15.Nader, K. (2003) Trends Neurosci. 26, 65-72. [DOI] [PubMed] [Google Scholar]
- 16.Dolan, R. J., Lane, R., Chua, P. & Fletcher, P. (2000) NeuroImage 11, 203-209. [DOI] [PubMed] [Google Scholar]
- 17.Maratos, E. J., Dolan, R. J., Morris, J. S., Henson, R. N. & Rugg, M. D. (2001) Neuropsychologia 39, 910-920. [DOI] [PubMed] [Google Scholar]
- 18.Smith, A. P. R., Henson, R. N. A., Dolan, R. J. & Rugg, M. D. (2004) NeuroImage 22, 868-878. [DOI] [PubMed] [Google Scholar]
- 19.Fossati, P., Hevenor, S. J., Lepage, M., Graham, S. J., Grady, C., Keightley, M. L., Craik, F. I. & Mayberg, H. S. (2004) NeuroImage 22, 1596-1604. [DOI] [PubMed] [Google Scholar]
- 20.Sharot, T., Delgado, M. R. & Phelps, E. A. (2004) Nat. Neurosci. 7, 1376-1380. [DOI] [PubMed] [Google Scholar]
- 21.Yonelinas, A. P. (2002) J. Mem. Lang. 46, 441-517. [Google Scholar]
- 22.Ochsner, K. N. (2000) J. Exp. Psychol. Gen. 129, 242-261. [DOI] [PubMed] [Google Scholar]
- 23.Talarico, J. T., LaBar, K. S. & Rubin, D. C. (2005) Memory Cognit., in press. [DOI] [PubMed]
- 24.Eldridge, L. L., Knowlton, B. J., Furmanski, C. S., Bookheimer, S. Y. & Engel, S. A. (2000) Nat. Neurosci. 3, 1149-1152. [DOI] [PubMed] [Google Scholar]
- 25.Dobbins, I. G., Rice, H. J., Wagner, A. D. & Schacter, D. L. (2003) Neuropsychologia 41, 318-333. [DOI] [PubMed] [Google Scholar]
- 26.Henson, R. N. A., Rugg, M. D., Shallice, T., Josephs, O. & Dolan, R. J. (1999) J. Neurosci. 19, 3962-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tulving, E. (1985) Can. Psychol. 26, 1-12. [Google Scholar]
- 28.Bradley, M. M., Greenwald, M. K., Petry, M. C. & Lang, P. J. (1992) J. Exp. Psychol. Learn. Mem. Cognit. 18, 379-390. [DOI] [PubMed] [Google Scholar]
- 29.Christianson, S.-A. (1992) The Handbook of Emotion and Memory: Research and Theory (Lawrence Erlbaum, Mahwah, NJ).
- 30.Moscovitch, D. A. & McAndrews, M. P. (2002) Neuropsychologia 40, 1335-1342. [DOI] [PubMed] [Google Scholar]
- 31.Lang, P. J., Greenwald, M. K., Bradley, M. M. & Hamm, A. O. (1993) Psychophysiology 30, 261-273. [DOI] [PubMed] [Google Scholar]
- 32.Shields, S. (1991) in International Review of Studies on Emotion, ed. Strongman, K. (Wiley, New York), pp. 227-245.
- 33.Lang, P. J., Bradley, M. M. & Cuthberg, B. N. (1997) International Affective Picture System (IAPS) (National Institute of Mental Health Center for the Study of Emotion and Attention, Gainesville, FL).
- 34.Yamasaki, H., LaBar, K. S. & McCarthy, G. (2002) Proc. Natl. Acad. Sci. USA 99, 11447-11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dolcos, F. & Cabeza, R. (2002) Cognit. Affect. Behav. Neurosci. 2, 252-263. [DOI] [PubMed] [Google Scholar]
- 36.Anderson, A. K., Christoff, K., Stappen, I., Panitz, D., Ghahremani, D. G., Glover, G., Gabrieli, J. D. & Sobel, N. (2003) Nat. Neurosci. 6, 196-202. [DOI] [PubMed] [Google Scholar]
- 37.Hamann, S. B., Ely, T. D., Hoffman, J. M. & Kilts, C. D. (2002) Psychol. Sci. 13, 135-141. [DOI] [PubMed] [Google Scholar]
- 38.Stark, E. L. & Okado, Y. (2003) J. Neurosci. 23, 6748-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brierley, B., Shaw, P. & David, A. S. (2002) Brain Res. Rev. 39, 84-105. [DOI] [PubMed] [Google Scholar]
- 40.Duvernoy, H. M., Bourgouin, P., Cabanis, E. A., Cattin, F., Guyot, J., Iba-Zizen, M. T., Maeder, P., Parratte, B., Tatu, L. & Fuillier, F. (1999) The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI and Blood Supply (Springer, New York).
- 41.Insausti, R., Juottonen, K., Soininen, H., Insausti, A. M., Partanen, K., Vainio, P., Laakso, M. P. & Pitkanen, A. (1998) Am. J. Neuroradiol. 19, 659-671. [PMC free article] [PubMed] [Google Scholar]
- 42.Pruessner, J. C., Li, L. M., Serles, W., Pruessner, M., Collins, D. L., Kabani, N., Lupien, S. & Evans, A. C. (2000) Cereb. Cortex 10, 433-442. [DOI] [PubMed] [Google Scholar]
- 43.Pruessner, J. C., Kohler, S., Crane, J., Pruessner, M., Lord, C., Byrne, A., Kabani, N., Collins, D. L. & Evans, A. C. (2002) Cereb. Cortex 12, 1342-1353. [DOI] [PubMed] [Google Scholar]
- 44.Arthurs, O. J. & Boniface, S. J. (2003) Clin. Neurophysiol. 114, 1203-1209. [DOI] [PubMed] [Google Scholar]
- 45.Kleinsmith, L. J. & Kaplan, S. (1963) J. Exp. Psychol. 65, 190-193. [DOI] [PubMed] [Google Scholar]
- 46.LaBar, K. S. & Phelps, E. A. (1998) Psychol. Sci. 9, 490-493. [Google Scholar]
- 47.Cahill, L., Babinsky, R., Markowitsch, H. & McGaugh, J. L. (1995) Nature 377, 295-296. [DOI] [PubMed] [Google Scholar]
- 48.Heuer, F. & Reisberg, D. (1990) Mem. Cognit. 18, 496-506. [DOI] [PubMed] [Google Scholar]
- 49.Maguire, E. A. (2001) Philos. Trans. R. Soc. London B 356, 1441-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cabeza, R., Prince, S. E., Daselaar, S. M., Greenberg, D. L., Budde, M., Dolcos, F., LaBar, K. S. & Rubin, D. C. (2004) J. Cognit. Neurosci. 16, 1583-1594. [DOI] [PubMed] [Google Scholar]
- 51.Gilboa, A. (2004) Neuropsychologia 42, 1336-1349. [DOI] [PubMed] [Google Scholar]
- 52.Reisberg, D., Heuer, F., McLean, J. & O'Shaughnessy, M. (1988) Bull. Psychonom. Soc. 26, 100-103. [Google Scholar]
- 53.Cahill, L., Uncapher, M., Kilpatrick, L., Alkire, M. T. & Turner, J. (2004) Learn. Mem. 11, 261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaBar, K. S. (2003) Curr. Neurol. Neurosci. Rep. 3, 363-364. [DOI] [PubMed] [Google Scholar]
- 55.Gilboa, A., Winocur, G., Grady, C., Hevenor, S. J. & Moscovitch, M. (2004) Cereb. Cortex 14, 1214-1225. [DOI] [PubMed] [Google Scholar]