Abstract
Introduction
Primary anorectal malignant melanoma is a rare and aggressive tumor that carries a poor prognosis. Anorectal melanoma (ARM) is often misdiagnosed as hemorrhoids adenocarcinoma polips and rectal cancer. ARM spreads along sub-mucosal planes and is often to wide-spread for complete resection at time of diagnosis and almost all patients die because of metastases.
Presentation of the case
A 77-year-old male patient presented a history of recurrent rectal bleeding and whose histopathological diagnosis was melanoma.
Discussion
The treatment of choice remains controversial. Surgery with complete resection represents the typical treatment. However standard operative procedures related to the area of resection and lymph dissection have yet to be established. Abdominal perineal resection (APR) with or without bilateral inguinal lymphadenectomy or wide local excision (WLE) have been used to manage patients with ARM.
Conclusion
The higher serum levels of LDH and YKL-40 are suggestive for Anorectal Melanoma diagnosis. The decrease of these findings may be associated with good prognosis. The review of both APR and WLE options suggests no significant difference in survival among patients.
Keywords: Melanoma treatment, Primary anorectal melanoma, Abdominoperineal resection, Wilde local excision, Case report
Highlights
-
•
Anorectal melanoma (ARM) is a systemic disease.
-
•
Regardless how aggressive is ARM, no surgical treatment will truly change the outcome.
-
•
If surgical teqchniques are available, patients should undergo to wide local excision.
-
•
In case of recurrence, the abdominal resection should be considered as the best surgical treatment.
1. Introduction
Primary anorectal malignant melanoma (AMM) is a rare and aggressive tumor with poor prognosis. It represents 1–2% of all melanomas and it is the third most common form of melanoma after the skin and retina. Nonetheless this condition is known, any description of single case may give more useful information on this pathology.
The disease typically affects Caucasians, females, and patients between the fifth and eighth decade of life with a mean incidence of 64.3 yrs (58.1 yrs and 70.2 yrs) [1].
The symptoms such as elimination of mucus and blood through the anal canal, presence of anal pain, feeling of rectal fullness or incomplete evacuation, externalization of tumor, and changes in bowel habits, are common to other tumors in anorectal region.
Many treatments including surgery, chemotherapy and radiotherapy have been used, but AMM is frequently radiotherapy-resistant and shows a poor response to chemotherapy [2]. Whether surgical procedure is better than other treatments is still an issue, and also it is controversial whether abdominoperineal resection of the anorectum (APR) or Wilde local excision (WLE) of the tumor has better outcomes.
In this report, we present the case of a patient with anorectal melanoma, without lymph node or distance metastases who underwent laparoscopic abdominoperineal resection (APR) [3], [4].
2. Case presentation
A 77-year-old man was admitted to the hospital complaining of 8-months history of painless rectal bleeding. A digital rectal examination revealed a hemorrhagic, soft mass of rectum, 5 cm from the anal verge. The findings of the remainder of the physical examination were within normal limits. At the time of admission and during the follow up the serum was analyzed for evaluation for biochemical and tumor markers [5]. After transanal polypectomy, with sufficient macroscopically negative margins, the histologic result was consistent with ulcerated malignant epithelioid melanoma, without BRAF V600 mutation, involving rectal mucosa and submucosa, with positive lateral surgical resection margin and unclear deep margin. At admission LDH and YKL-40 are respectively 987 IU/L and 852 μg/L (Table 1).
Table 1.
Serum markers of the patient.
| CEA | Ca 19-9 | LDH | YKL-40 | |
|---|---|---|---|---|
| Normal value | 0–5 IU/L | 0.39 IU/ml | 120–250 IU/L | 45–500 μg/L |
| At admission | 4.88 | 44 | 987 | 852 |
| 7 days after surgery | 3.25 | 38 | 654 | 528 |
| 1 month after surgery | 3.40 | 35 | 525 | 460 |
| 1 year after surgery | 2.88 | 27 | 256 | 396 |
Clinical history for primary melanoma in the skin and in other noncutaneous sites, including the eye, was negative. A computed tomographic scan of the abdomen, chest, brain, and PET/CT (Positron Emission Tomography - Computed Tomography) showed a non homogeneous and partially calcified mass occupying the prostatic bed, and excluded lymph node and distant metastases.
A laparoscopic abdominal perineal resection with anorectal amputation was carried out, along with total mesorectal excision using curved harmoning shears Ultracision®.
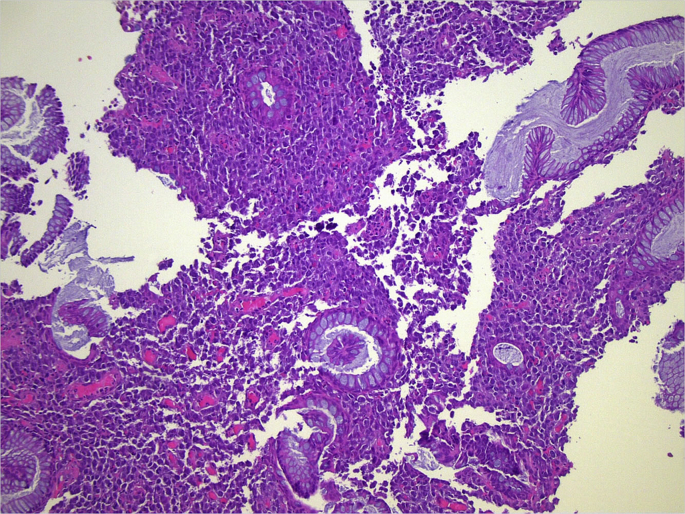
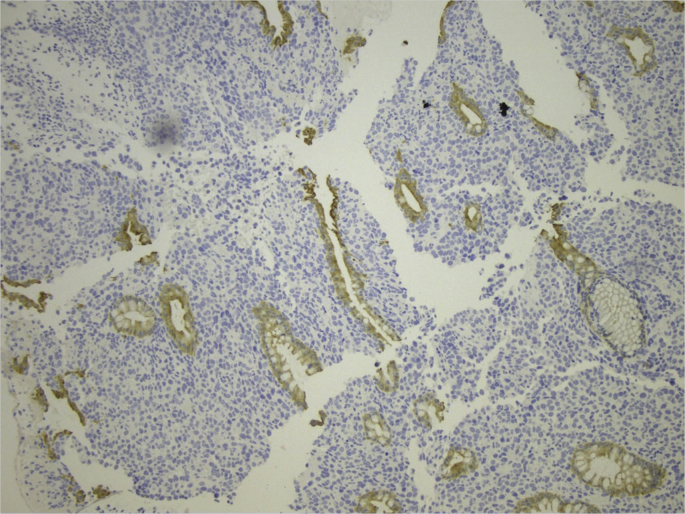
The histologic examination of the operative specimen showed only a tumor to invade through the lamina propria of mucosa at anorectal junction. At histology, the case was diagnosed as stage I and T1b Breslow's thickness (Fig. 1, Fig. 2).
Fig. 1.
Presence of melanoma in intestinal cells (hematoxylin and eosin staining).
Fig. 2.
Presence of cytokeratin (CKAE1/AE3) in intestinal cells.
One year after surgical intervention a full-body computed-tomography (CT) scans, LDH and YKL-40 excluded lymph node and distant metastases.
3. Discussion
Anorectal melanomas are rare but aggressive tumors. Compared with cutaneous melanomas, anorectal melanomas have the lowest percent of five years survivals, only 25%. Best hope for survival is offered by early detection and complete surgical removal. However is usually delayed because of occult site of recurrence and unspecific symptoms diagnosis. The most common presenting symptom of anorectal melanoma is bleeding with 53–89% of patients reporting this as the predominant complaint. Other symptoms are suspicion of hemorrhoids, discomfort or pain, an anal mass, change in bowel habit, tenesmus or pruritus. The higher serum levels of LDH and YKL-40 are suggestive for suspicion of anorectal melanoma diagnosis. A smaller proportion of patients present with nodal disease and inguinal lymphadenopathy. Due to absence of specific symptoms and comparing with other anorectal disease, anorectal melanoma is often diagnosed accidentally. Although rare, several cases have been described, each case gives information on both treatment and prognosis of AMM [6], [7].
There are two methods of staging in anorectal melanoma: 1) developed by The American Joint Commission on Cancer, which is a staging method based on depth of primary tumor, presence of tumor in lymph nodes and presence of distant metastasis. 2) Another staging system based only on disease spread: it describes local disease only as stage 1, regional lymph node disease as stage 2, and metastatic disease as stage 3 [8].
Tumors are most commonly located in the anal canal, followed by at the dentate line with fewer melanomas located in the rectum itself [9].
There is no consensus on which surgical approach is preferred. A number of studies claim that abdomino-perineal resection (APR) is the treatment of choice [10] because it can control better lymphatic spread and it allow to obtain larger negative margins for local control.
Other studies instead have recommended only a sphincter-saving excision (wide local excision) because treatment is often palliative and wide radical surgery is unnecessary mutilating [11]. Wide local excision is defined as a sphincter-saving operation with a defined margin around the tumor in two dimensions. The benefits of a WLE are quicker recovery, no need for a stoma, and minimal impact on bowel function [12].
There is no value in a prophylactic lymph node dissection during a WLE, even when there are clinically positive nodes, also because locoregional recurrence of AMM occurs more at the inguinal lymph nodes than at the pelvic lymph nodes [13].
Neither APR nor WLE affect any of the inguinal lymph nodes, so they do not offer an advantage in controlling locoregional recurrence [14].
WLE may not always be possible, for example, when tumor is invading the sphincter complex or when tumors are causing chronic bleeding or obstruction [15]. The excision should be performed up to the internal sphincter muscle and side margin of 2 cm to the tumor [16].
Abdominal perineal resection seems to better control local disease, but does not change the incidence of distant metastasis or survival. Ramalingam et al. [17] documented that laparoscopic APR could control disease and reduce morbidity at the same time.
In our case, APR was performed because the histological result of transanal polypectomy revealed positive surgical resection margin, and, according to the literature [17], [18], APR should be performed when the margins of the local excision are positive, or in the event of recurrence.
Many studies have compared 5-year survival rates between patients underwent APR and WLE:
-
•
A systematic review was perfomed by Anna Heeney et al. over 368 patients with anorectal melanoma, 161 underwent APR, 132 WLE; they identified no statistical difference in survival between these 2 groups [9].
-
•
Nilsson et al. have performed a study over 251 patients with anorectal melanoma identified from 1960 to 1999 from the Swedish National Cancer Registry [13]. 66 and 86 patients underwent APR and local excision respectively. Median survival among patients treated with APR or local excision was 14 months and the overall 5-year survival rate 11.2%. There was no statistically significant difference with regard to median survival between patients treated with APR or local excision (11 versus 14 months, 5-year survival rate 7 versus 15%; P = 0.084).
-
•
In a review that included 17 large case series, Yap et al. compared the survival of patients who underwent APR or LE. The analysis revealed no statistically significant difference in 5-year survival between two treatment modalities, even at all stages of the disease [14].
-
•
Akihisa Matsuda et al. perfomed a systematic review of the literature. They identified Thirty-one studies, with a total of 1006 patients [544 (54.1%) APR and 462 (45.9%) LE]. Meta-analyses showed that overall survival (OR, 1.14; 95% CI, 0.74–1.76; P = 0.54) and relapse-free survival (OR, 0.95; 95% CI, 0.43–2.09; P = 0.89) did not differ significantly between the APR and LE groups. APR significantly reduced local recurrence compared with LE (OR, 0.18; 95% CI, 0.09–0.36; P < 0.00001) [18].
Recent studies show that sphincter-saving local excision combined with adjuvant loco-regional radiotherapy at the primary site of the tumor and the regional pericolic and inguinal lymphatics (5 X 6 GY) results in the same loco-regional control with less loss of function compared to APR (70% vs 74%) [15], and in better loco-regional control compared to WLE alone [19], [20], [21], but there is no consensus on this type of treatment strategy.
Sentinel lymph node detection, excision and histology are important in sparing the patient a futile inguinal lymph node dissection, but it no predictive of prognosis. In fact in a case report of Mariolis-Spasakos et al. a patient underwent Lymphoscintigraphy and intraoperative gamma-probe guided detection of the Sentinel Lymph Node (SLN). SLNs were localized in the inguinal basins bilaterally and were negative on histology. Thirty months later the patient developed distant metastases and died six months later [20].
4. Conclusion
The abdominoperineal resection was considered as the initial therapeutic choice for anorectal malignant melanoma, concluded that abdominoperineal resection had no prominent long-term advantage on survival time and the surgical modality was more risky besides patients were required to undergo permanent colostomy [22], [23]. This suggests that anorectal melanoma is a systemic disease at the time of diagnosis and no surgical treatment, regardless how aggressive, change the outcome. Therefore, it was only when patient could not receive wide local excision technically that he or she would undergo the abdominoperineal resection. For curative intent, patients with stage 0 and 1 should have a WLE, but higher stage patients should have an APR, because an APR may improve quality of life from chronic bleeding or obstruction from tumor for stage IV patients [24].
Therefore, if surgical techniques are available, patients should undergo wide local excision. Only when local excision of tumor mass is not possible technically, or in case of recurrence, the abdominoperineal resection should be considered as the best surgical treatment.
Finally, when technically possible laparoscopic abdominoperineal resection could be done, it will reduce morbidity and quicker recovery will be obtained. The higher serum levels of LDH and YKL-40 are suggestive for Anorectal Melanoma diagnosis. The decrease of these findings may be associated with good prognosis.
Adjuvant therapies such as radiotherapy, chemiotherapy and targeted therapies are administered in a number of cases, but due to the rarity of the disease, randomized controlled trials have not been conducted to evalute the additional benefit of these treatment mobilities.
Ethical approval
The study had been approved by Ethic Committee of Cannizzaro Hospital, Catania, Italy. The patient subscribed written informed consensus.
Sources of funding
The research was supported by MIUR.
Author contribution
All authors contributed equally to the manuscript.
Conflicts of interest
No authors have conflict of interest.
Guarantor
Saverio Latteri.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Registration of research studies
As case reports we did not registered this study.
Acknowledgements
This research had been supported by MIUR.
References
- 1.Quan S.H.Q. Anal cancers. Squamous and melanoma. Cancer. 1992;70:1384–1389. doi: 10.1002/1097-0142(19920901)70:3+<1384::aid-cncr2820701528>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Trzcinski R., Kujawski R., Mik M., Sygut A., Dziki L., Dziki A. Malignant melanoma of the anorectum—a rare entity. Langenbecks Arch. Surg. 2010;395(6):757–760. doi: 10.1007/s00423-009-0586-5. [DOI] [PubMed] [Google Scholar]
- 3.Malik A., Hull T.L., Milsom J. Long-term survival of anorectal melanoma: report of a case. Dis. Colon Rectum. 2002;45:1412–1415. doi: 10.1007/s10350-004-6435-2. [DOI] [PubMed] [Google Scholar]
- 4.Singer M., Mutch M.G. Anal melanoma. Clin. Colon Rectal Surg. 2006;19(2):78–87. doi: 10.1055/s-2006-942348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrotta R., Bevelacqua Y., Malaguarnera G., Paladina I., Giordano M., Malaguarnera M. Serum markers of cutaneous melanoma. Front. Biosci. Elite Ed. 2010 Jun 1;2:1115–1122. doi: 10.2741/e170. [DOI] [PubMed] [Google Scholar]
- 6.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., SCARE Steering Group A protocol for the development of reporting criteria for surgical case reports: the SCARE statement. Int. J. Surg. 2016 Mar;27:187–189. doi: 10.1016/j.ijsu.2016.01.094. [DOI] [PubMed] [Google Scholar]
- 7.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P. The SCARE Statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016 doi: 10.1016/j.ijsu.2016.08.014. (article in press) [DOI] [PubMed] [Google Scholar]
- 8.Falch C., Stojadinovic A., Hann-von-Weyhern C., Protic M., Nissan A., Faries M.B., Daumer M., Bilchik A.J., Itzhak A., Brücher B.L. Anorectal malignant melanoma: extensive 45-year review and proposal for a novel staging classification. J. Am. Coll. Surg. 2013 Aug;217(2):324–335. doi: 10.1016/j.jamcollsurg.2013.02.031. Epub 2013 May 19. [DOI] [PubMed] [Google Scholar]
- 9.Heeney Anna, Mulsow Jurgen, Hyland John M.P. Treatment and outcomes of anorectal melanoma. Surgery. 2011;9:27–32. doi: 10.1016/j.surge.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Brady M.S., Kavolius J.P., Quan S.H. Anorectal melanoma. A 64-year experience at Memorial sloan-kettering cancer center. Dis. Colon Rectum. 1995;38:146–151. doi: 10.1007/BF02052442. [DOI] [PubMed] [Google Scholar]
- 11.Stoidis C.N., Spyropoulos B.G., Misiakos E.P., Fountzilas C.K., Paraskeva P.P., Fotiadis C.I. Diffuse anorectal melanoma; review of the current diagnostic and treatment aspects based on a case report. World J. Surg. Oncol. 2009;7:64. doi: 10.1186/1477-7819-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross M., Pezzi C., Pezzi T., Meurer D., Hickey R., Balch C. Patterns of failure in anorectal melanoma. A guide to surgical therapy. Arch. Surg. 1990;125:313–316. doi: 10.1001/archsurg.1990.01410150035007. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson P.J., Ragnarsson-Olding B.K. Importance of clear resection margins in anorectal malignant melanoma. Br. J. Surg. 2010;97:98–103. doi: 10.1002/bjs.6784. [DOI] [PubMed] [Google Scholar]
- 14.Yap L.B., Neary P. A comparison of wide local excision with abdominoperineal resection in anorectal melanoma. Melanoma Res. 2004;14:147–150. doi: 10.1097/00008390-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Weyandt G.H., Eggert A.O., Houf M., Raulf F., Bröcker E.B., Becker J.C. Anorectal melanoma: surgical management guidelines according to tumour thickness. Br. J. Cancer. 2003;89(11):2019–2022. doi: 10.1038/sj.bjc.6601409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballo M.T., Gershenwald J.E., Zagars G.K. Sphinctersparing local excision and adjuvant radiation for anal-rectal melanoma. J. Clin. Oncol. 2002;20(23):4555–4558. doi: 10.1200/JCO.2002.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Ramalingam G., Gan E.Y., Kutt-Sing W. Laparoscopic abdominoperineal resection for anorectal melanoma: a case report and review of the literature. Surg. Laparosc. Endosc. Percutan Tech. 2009;19 doi: 10.1097/SLE.0b013e3181b13592. e149–51. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda Akihisa, Miyashita Masao, Matsumoto Satoshi, Takahashi Goro, Matsutani Takeshi, Yamada Takeshi, Kishi Taro, Uchida Eiji. Abdominoperineal resection provides better local control but equivalent overall survival to local excision of anorectal malignant melanoma. Ann. Surg. 2015 Apr;261(4):670–677. doi: 10.1097/SLA.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 19.Ishizone S., Koide N., Karasawa F. Surgical treatment for anorectal malignant melanoma: report of five cases and review of 79 Japanese cases. Int. J. Colorec. Dis. 2008;23(12):1257–1262. doi: 10.1007/s00384-008-0529-6. [DOI] [PubMed] [Google Scholar]
- 20.Mariolis-Sapsakos T., Malamitsi J., Yakoumakis E., Orfanos F. Is sentinel node mapping useful in anorectal melanoma? Hell. J. Nucl. Med. 2008 Jan-Apr;11(1):39–42. [PubMed] [Google Scholar]
- 21.Kelly P., Zagars G.K., Cormier J.N., Ross M.I., Guadagnolo B.A. Sphincter-sparing local excision and hypofractionated radiation therapy for anorectal melanoma: a 20- year experience. Cancer. 2011;117:4747–4755. doi: 10.1002/cncr.26088. [DOI] [PubMed] [Google Scholar]
- 22.Belli F., Gallino G.F., Lo Vullo S., Mariani L., Poiasina E., Leo E. Melanoma of the anorectal region: the experience of the national cancer institute of Milano. Eur. J. Surg. Oncol. 2009;35:757–762. doi: 10.1016/j.ejso.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Yeh J.J., Shia J., Hwu W.J. The role of abdominoperineal resection as surgical therapy for anorectal melanoma. Ann. Surg. 2006;244(6):1012–1017. doi: 10.1097/01.sla.0000225114.56565.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Che Xu, Zhao Dong-Bing, Wu Yong-Kai, Wang Cheng-Feng, Cai Jian-Qiang, Shao Yong-Fu, Zhao Ping. Anorectal malignant melanomas: retrospective experience with surgical management. World J. Gastroenterol. 2011 January 28;17(4):534–539. doi: 10.3748/wjg.v17.i4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]