Abstract
Predation is a fundamental interaction between species, yet it is unclear what escape strategies are effective for prey survival. Classical theory proposes that prey should either escape in a direction that conforms to a performance optimum or that is random and therefore unpredictable. Here, we show that larval zebrafish (Danio rerio) instead use a mixed strategy that may be either random or directed. This was determined by testing classic theory with measurements of the escape direction in response to a predator robot. We found that prey consistently escaped in a direction contralateral to the robot when approached from the side of the prey's body. At such an orientation, the predator appeared in the prey's central visual field and the contralateral response was consistent with a model of strategy that maximizes the distance from the predator. By contrast, when the robot approached the rostral or caudal ends of the body, and appeared in the prey's peripheral vision, the escape showed an equal probability of a contralateral or ipsilateral direction. At this orientation, a contralateral response offered little strategic advantage. Therefore, zebrafish larvae adopt an escape strategy that maximizes distance from the threat when strategically beneficial and that is otherwise random. This sensory-mediated mixed strategy may be employed by a diversity of animals and offers a new paradigm for understanding the factors that govern prey survival.
Keywords: pursuit–evasion model, locomotion, predation, sensing, strategy
1. Introduction
Predation plays a crucial role in the population dynamics, trophic interactions and individual fitness of a diversity of species. Although the ability of prey to evade predators may have broad biological implications, the strategies used for predator evasion are largely unclear. Classic theory suggests that prey may direct their escape with two major strategies to enhance survival. The protean strategy [1] favours high variability in escape direction to challenge a predator's ability to anticipate the prey's heading. Prey using an optimal strategy, by contrast, will conform to the direction that maximizes the distance from the predator [2]. Although the escape direction has been measured in a broad array of animals [3,4], the predictions of strategic theory are largely untested (with some exceptions [5,6]). It is consequently unclear which animals use protean or optimal strategies and what conditions favour one strategy over another.
Predator evasion is facilitated by an animal's escape response, an explosively fast maneouver performed when a prey senses a threat. Although its role in survival is not well studied [7], the neuromuscular control of the escape response has been extensively explored in animals as diverse as rodents, cephalopods, flies and fishes [8]. This research has revealed specialized circuits of neurons that activate muscles at high speed with some directional control [9]. One of the most extensively studied escape responses is the ‘fast start’ of fishes, which is controlled by the Mauthner neuron and its serial homologues [10,11]. The fast start is characterized by the body bending into a preparatory ‘C’ shape, followed by a rapid acceleration as the body unfurls [12]. Recent advances in the study of this behaviour have been aided by techniques in optogenetics and functional imaging developed in zebrafish (Danio rerio, Hamilton 1922) larvae [10,13,14]. Zebrafish larvae are also amenable to laboratory study of predator–prey interactions where they use the fast start to escape predation by adults of the same species [15]. For these reasons, the present study used the zebrafish system to test models of strategy with experiments that simulated the approach of an adult predator using a predator robot.
2. Material and methods
(a). Escape-response kinematics
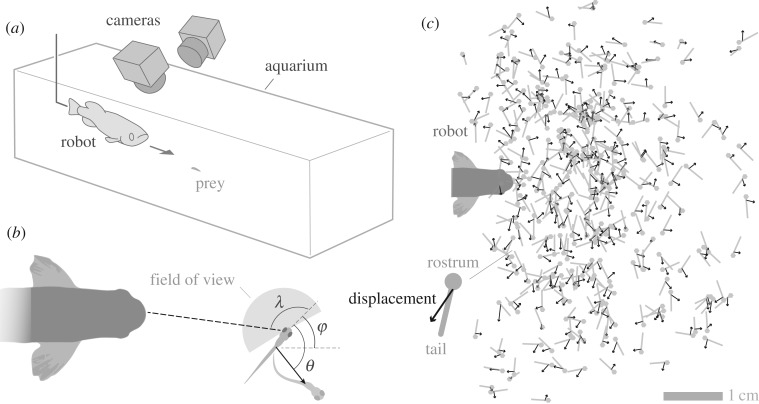
All zebrafish larvae were bred from wild-type (AB line) colonies in a flow-through tank system (Aquatic Habitats, Apopka, FL, USA) that was maintained at 28.5°C on a 14 L : 10 D cycle. These larvae were exposed to a predator robot to present a controlled and repeatable visual stimulus that elicited a fast start (figure 1a) [16]. The robot consisted of a dead adult zebrafish that was suspended in the centre of an aquarium populated with larvae, as described previously [16]. Through the action of a linear servomotor (figure 1a), the fish body was translated through the aquarium at a constant speed (11 cm s−1), like a foraging predator [15]. This motor also propelled two high-speed (250 frames s−1) cameras that were mounted above the predator to record the responses of larval zebrafish. The prey were generally motionless until exhibiting a fast start in response to the robot.
Figure 1.
Measurements of the escape response stimulated by a predator robot. (a) The robot consisted of a dead adult zebrafish that was suspended in the centre of an aquarium populated with larvae and translated through the water at constant speed (11 cm s−1), like that of a live predator while foraging. Escape responses were recorded with two high-speed video cameras (250 frames s−1 at 640 × 480 pixels) that moved with the robot as it translated through a rectangular aquarium. The prey were generally motionless until exhibiting a fast start in response to the robot. (b) We calculated the stimulus angle (λ) presented by the robot in the prey's field of view (in grey), the direction of the escape response (θ) and the initial orientation of the body (ϕ). (c) The position (in grey) and displacement (black arrow) of the bodies of larvae achieved during the escape response were measured from the video recordings.
We performed a kinematic analysis of the fast start to determine how visual cues affect the direction of the escape response in prey by recording the three-dimensional location of each larva before and after an escape. This was achieved with custom software developed in Matlab (v.2014b, MathWorks, Natick, MA, USA) to digitize three landmarks along the prey body (rostrum, swim bladder and tail) from the video recordings of both cameras. Coordinates were transformed into three-dimensional space using ‘Digitizing Tools’ software in Matlab [17] and expressed with respect to the rostrum and heading of the robot. We consequently calculated the angular position (ψ) and orientation (ϕ) of each larva in the video frame prior to escape (figure 1b). The prey's field of view was defined as extending from −16° to 167° with respect to the central axis of the body, where 0° is directed anteriorly. This was previously determined from the retinal anatomy and eye rotation during saccades [18,19]. Within the field of view, we found the stimulus angle (λ), the position of the predator's centre, and examined the responses elicited by stimuli in 10 equal intervals of this angle, which yielded n > 5 in each bin.
(b). Mathematical modelling
We used differential game theory to model optimal strategy. Consistent with the theoretical literature [2,20], our model considered how the minimum distance (d, normalized by escape distance) is affected by the kinematics of predator and prey. Assuming a fixed velocity for both animals, the minimum distance depends on the escape heading (α) and angular position (ψ) relative to the predator's heading, as follows [21]:
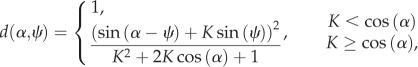 |
2.1 |
where K is the ratio of predator to prey speed (K = 0.5) [15,22]. Based on previous results [10,16] and our preliminary findings, we modelled the escape heading to be perpendicular to the body orientation prior to the escape. We calculated the minimum distance as a function of body orientation for contralateral (αC = ϕ − 90°) and ipsilateral (αI = ϕ + 90°) responses. From these results, we found the contralateral advantage as the difference between the minimum distance for contralateral (dC) or ipsilateral (dI) responses. The contralateral advantage expresses the strategic benefit of a contralateral escape relative to the alternative.
A purely protean strategy predicts that an escape has an equal probability of occurring among all possible directions. Because larval zebrafish escape in a relatively narrow range of directions with respect to the initial orientation of the body [10,16], the escape direction was defined as towards the side of the body that is either contralateral or ipsilateral to the stimulus. Therefore, the protean strategy predicts an equal probability between these directions (pC = 0.5, where pC is the probability of a contralateral response). We tested whether the escape responses conformed to this prediction for larvae with variable orientation with respect to the predator.
3. Results
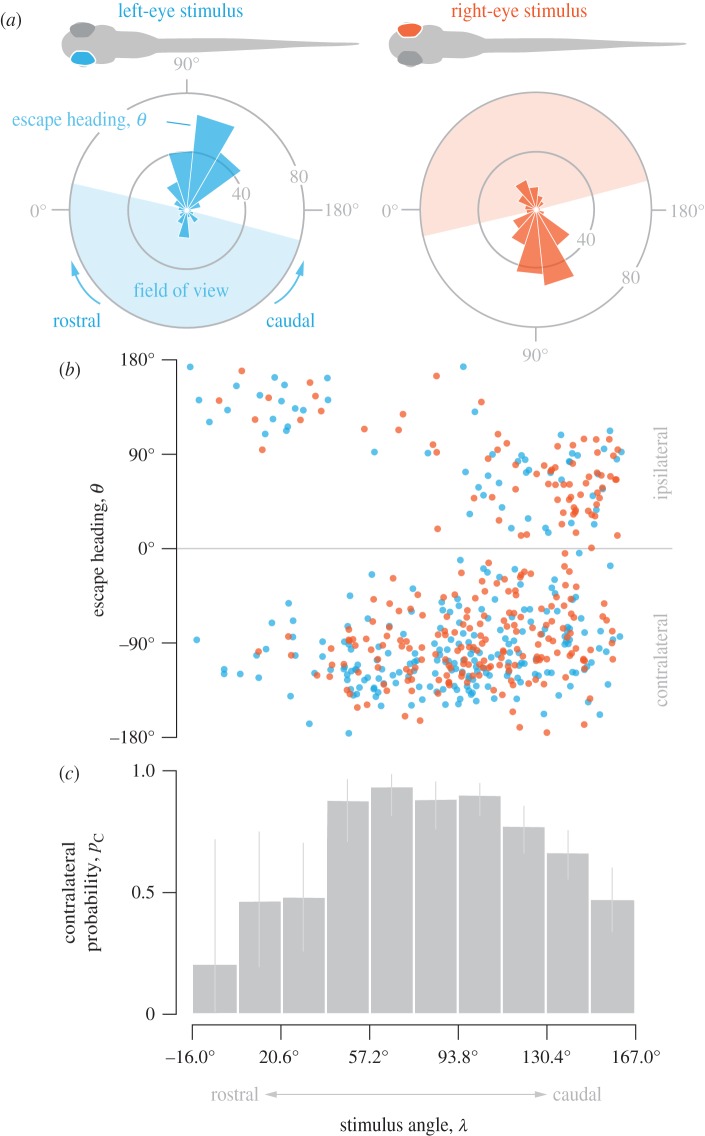
Most escape responses were directed away from the predator robot (figure 1c). This occurred because most larvae escaped towards the side of the body facing away from the predator. This was discovered by transforming the escape heading with respect to the prey's frame of reference prior to its escape (figure 2). Whether the predator appeared in the left eye (pC = 0.80 ± 0.05, ±95% confidence intervals for a binomial distribution, n = 224) or right eye (pC = 0.73 ± 0.06, n = 239), about three-quarters of escapes were directed contralateral to the predator (figure 2a). The escape heading was not correlated with the stimulus angle of the predator (linear regression, p = 0.75; figure 2b), but rather was approximately perpendicular to the body's initial orientation (θ = 106° ± 10°, n = 502). The stimulus angle, the position of the predator in the prey's visual field, did influence the probability of a contralateral escape. In particular, contralateral responses occurred in the vast majority of instances when the predator approached from the side of the prey's body and consequently appeared in the central visual field (pC = 0.87 ± 0.04, for 30° < λ < 30°, where λ is the stimulus angle n = 265; figure 2c). By contrast, prey were as likely to respond with an ipsilateral response as a contralateral response when the predator approached the rostrum (pC = 0.39 ± 0.23, for −16.0° < λ < 20.6°, n = 18) or tail (pC = 0.58 ± 0.08, for 130.4° < λ < 167°, n = 154; figure 2c) of the prey. Therefore, peripheral visual stimuli generated responses that were consistent with a protean strategy.
Figure 2.
The direction of the escape response relative to the visual stimulus. (a) The frequency of the escape direction when the predator appeared on the left eye (in blue) and right eye (in red) of the prey. (b) The escape heading was not correlated with the stimulus angle (λ, linear regression, p = 0.75). (c) The probability of a contralateral response in equal intervals (18.3°) of the stimulus angle (±95% confidence intervals, assuming binomial distribution, n = 502).
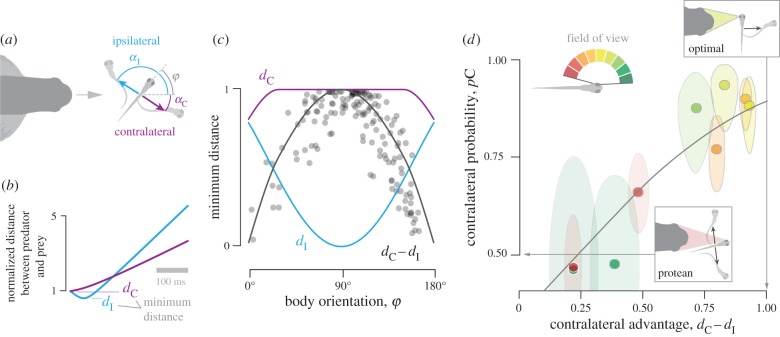
We examined how our kinematics compared to the predictions of optimal strategy. Assuming an escape that is perpendicular to the initial orientation of the body, predictions of minimum distance differed substantially between ipsilateral and contralateral responses (figure 3a,b). The minimum distance is maximal for contralateral responses over a broad range of body orientations (figure 3c), but varies greatly with body orientation among ipsilateral responses. This relationship resembles an inverse parabola, with an advantage to a contralateral response that is greatest when the body is perpendicular to the predator's heading. The contralateral advantage, the difference in minimum distance between contralateral and ipsilateral responses (dC − dI), indicates that a contralateral response is optimal when the prey is oriented perpendicular to a predator's heading (figure 3c).
Figure 3.
The strategic implications of escape heading. (a) We modelled the escape response as occurring at a right angle from the initial orientation of the body (ϕ) for contralateral (purple) and ipsilateral (blue) escape responses. (b) For each response, the minimum distance between predator and prey were predicted for contralateral (dC) and ipsilateral (dI) escapes, and (c) this was determined for all body orientations. The difference in minimum distance between ipsilateral and contralateral responses (black curve, dC − dI) represents the contralateral advantage. We calculated the contralateral advantage (grey circles) for all positions and orientations recorded in our experiments (figure 1c). (d) The contralateral probability (values from figure 2c) was correlated with the contralateral advantage (logistic regression, p ≪ 0.001, n = 9), when binned with respect to the visual field. These regions of the visual field are colour-coded, as shown in the legend.
We found that the observed probability of a contralateral response may be predicted by the contralateral advantage (figure 3d). Using the results of our model, we determined the contralateral advantage (figure 3c) for equal intervals of the prey's visual field. These predictions were compared with the probability of a contralateral response for the same intervals (figure 2c). We found that a logistic regression (pC = (1 + exp(−2.3(dC − dI) + 0.126))−1, p ≪ 0.001, n = 9) significantly characterizes the positive relationship between these quantities. This relationship demonstrates that contralateral responses occur with greater frequency when they are strategically advantageous. These conditions may be detected by the position of the predator in the prey's field of view.
4. Discussion
Through a combination of experimental measurements and mathematical modelling, our results suggest that zebrafish larvae employ a mixed strategy that combines protean and optimal responses. Prey escaped contralateral to the predator when approached from the side (figure 2c), which is consistent with a strategy to maximize distance. However, the strategic advantage of a contralateral response diminishes at orientations deviating from the perpendicular, and prey under these conditions were increasingly likely to exhibit an ipsilateral response. At the extremes, prey aligned with the predator's heading responded with a protean response and were therefore unpredictable in direction (figure 3d). Therefore, the escape strategy depended on the direction of the predator approach, as detected by the prey's visual system.
Our findings are compatible with current understanding of the neurophysiology of zebrafish larvae. A looming stimulus is detected in a brain region known as the optic tectum [10], which is structured with a topographic map of the retinal cells that span the visual field [23,24]. Therefore, a predator approaching the side of a prey's body that stimulates a central portion of the retina activates a region of the brain that is distinct from that activated by a peripheral stimulus. Each region of the optic tectum is capable of activating the motor programme for an escape [10,25]. Escapes stimulated by the optic tectum are controlled by left and right sets of the Mauthner neuron and its serial homologues [10]. The premotor interneurons that activate these regions can inhibit one side of the body while activating the other and thereby creating a competition that determines the side of the body that activates an escape [14]. Our results suggest that the region of the optic tectum activated by a central stimulus strongly biases the outcome of this competition in favour of the contralateral side. The brain regions activated by a peripheral stimulus offer no such favouritism and consequently exhibit an equal probability of contralateral and ipsilateral responses. In this manner, a mixed strategy may be facilitated through the activation of distinct premotor pathways by different regions of a larva's retina. One great advantage to this arrangement is that directed responses may be triggered with minimal neuronal processing and may consequently be executed at high speed.
A broad diversity of animals may employ a mixed strategy like that of zebrafish larvae. Similar kinematics have been measured in shrimp [26] and crabs [27], which also escape with a limited range in heading. These animals likewise exhibit an equal probability of contralateral and ipsilateral escapes when approached from behind. They also show a high frequency of contralateral escapes when a predator approaches from the side, which is consistent with an optimal strategy. Unlike larval zebrafish, adult fish have the capacity to direct the fast start with a heading that is directed away from a threat, irrespective of their initial orientation [9]. Nonetheless, some adult fishes, such as herring [28], exhibit a protean strategy when aligned with a predator's heading and show consistent contralateral responses to a lateral approach. A mixed strategy may therefore offer a common means for prey to combine the benefits of optimal and protean responses based on the direction of a threat. The predictions of optimal [2] and protean [1] theories have rarely been tested against measurements of the escape direction [3,4], and it consequently remains unclear what strategies are supported by the empirical literature. By contrast, the integration of directional measurements with a mathematical model of strategy [5,6] provides the means to distinguish between hypothetical strategies, as done presently. It remains an exciting prospect to consider how other prey species similarly combine strategies to survive encounters with predators.
(a). Summary
Our measurements of the escape responses of larval zebrafish to a predator robot (figure 1c) were compared with the predictions of mathematical models of prey strategy. These responses were directed approximately perpendicular to the initial orientation of a prey's body prior to an escape (figure 2a). Therefore, the body orientation largely determined both the direction of an escape and where the predator appeared in the prey's visual field (figure 3a). When predators stimulated the peripheral visual field, either by approaching the tail or rostrum, then prey responded with a protean response (pC ∼ 0.5, figure 2c). However, when approaching at angles closer to the perpendicular, prey showed a greater strategic advantage to a contralateral response (figure 3c) and exhibited a higher probability of such a response (figure 3d). Therefore, larval zebrafish employ a mixed strategy for surviving encounters with predators that depends on the direction of the predator's approach, as detected by the visual system. These responses are compatible with our understanding of the neurophysiology of zebrafish larvae and may be achieved with rapid neuronal processing. A diversity of animals exhibit similar responses to visual threats [26–28], which suggests that a mixed strategy may offer a common solution to predator evasion.
Ethics
All experimental procedures were approved by the Institutional Animal Care and Use Committee at UC Irvine (protocol no. AUP-17-12).
Data accessibility
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.47mq9 [29].
Author's contributions
The study was designed, analysed and written in collaboration between M.J.M. and A.N. W.J.S. provided critical technical assistance on the set-up and K.C. executed the experiments.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by grants to M.J.M. from the National Science Foundation (IOS-1354842) and the Office of Naval Research (N00014-15-1-2249).
References
- 1.Humphries DA, Driver PM. 1970. Protean defence by prey animals. Oecologia 5, 285–302. ( 10.1007/BF00815496) [DOI] [PubMed] [Google Scholar]
- 2.Weihs D, Webb PW. 1984. Optimal avoidance and evasion tactics in predator-prey interactions. J. Theor. Biol. 106, 189–206. ( 10.1016/0022-5193(84)90019-5) [DOI] [Google Scholar]
- 3.Domenici P, Blagburn JM, Bacon JP. 2011. Animal escapology II: escape trajectory case studies. J. Exp. Biol. 214, 2474–2494. ( 10.1242/jeb.053801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domenici P, Blagburn J, Bacon JP. 2011. Animal escapology I: theoretical issues and emerging trends in escape trajectories. J. Exp. Biol. 214, 2463–2473. ( 10.1242/jeb.029652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcoran AJ, Conner WE. 2016. How moths escape bats: predicting outcomes of predator–prey interactions. J. Exp. Biol. 219, 2704–2715. ( 10.1242/jeb.137638) [DOI] [PubMed] [Google Scholar]
- 6.Gal S, Alpern S, Casas J. 2015. Prey should hide more randomly when a predator attacks more persistently. J. R. Soc. Interface 12, 20150861 ( 10.1098/rsif.2015.0861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker JA, Ghalambor CK, Griset OL, McKenney D, Reznick DN. 2005. Do faster starts increase the probability of evading predators? Funct. Ecol. 19, 808–815. ( 10.1111/j.1365-2435.2005.01033.x) [DOI] [Google Scholar]
- 8.Eaton RC (ed.) 1984. Neural mechanisms of startle behavior. Boston, MA: Springer US. [Google Scholar]
- 9.Foreman MB, Eaton RC. 1993. The direction change concept for reticulospinal control of goldfish escape. J. Neurosci. 13, 4101–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn TW, Gebhardt C, Naumann EA, Riegler C, Ahrens MB, Engert F, Del Bene F. 2016. Neural circuits underlying visually evoked escapes in larval zebrafish. Neuron 89, 613–628. ( 10.1016/j.neuron.2015.12.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimmel C, Patterson J, Kimmel R. 1972. The development and behavioral characteristics of the startle response in the zebra fish. Dev. Psychobiol. 24, 47–60. [DOI] [PubMed] [Google Scholar]
- 12.Weihs D. 1973. The mechanism of rapid starting of slender fish. Biorheology 10, 343–350. [DOI] [PubMed] [Google Scholar]
- 13.Liu K, Fetcho JR. 1999. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron 23, 325–335. ( 10.1016/S0896-6273(00)80783-7) [DOI] [PubMed] [Google Scholar]
- 14.Koyama M, Minale F, Shum J, Nishimura N, Schaffer CB, Fetcho JR, Calabrese RL. 2016. A circuit motif in the zebrafish hindbrain for a two alternative behavioral choice to turn left or right. eLife 5, e16808 ( 10.7554/eLife.16808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart WJ, Cardenas GS, McHenry MJ. 2013. Zebrafish larvae evade predators by sensing water flow. J. Exp. Biol. 216, 388–398. ( 10.1242/jeb.072751) [DOI] [PubMed] [Google Scholar]
- 16.Stewart WJ, Nair A, Jiang H, McHenry MJ. 2014. Prey fish escape by sensing the bow wave of a predator. J. Exp. Biol. 217, 4328–4336. ( 10.1242/jeb.111773) [DOI] [PubMed] [Google Scholar]
- 17.Hedrick TL. 2008. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 3, 034001 ( 10.1088/1748-3182/3/3/034001) [DOI] [PubMed] [Google Scholar]
- 18.Easter S Jr, Nicola GN. 1996. The development of vision in the zebrafish (Danio rerio). Dev. Biol. 180, 646–663. ( 10.1006/dbio.1996.0335) [DOI] [PubMed] [Google Scholar]
- 19.Patterson BW, Abraham AO, MacIver MA, McLean DL. 2013. Visually guided gradation of prey capture movements in larval zebrafish. J. Exp. Biol. 216, 3071–3083. ( 10.1242/jeb.087742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaacs R. 1965. Differential games. A mathematical theory with applications to warfare and pursuit, control and optimization. New York, NY: John Wiley & Sons, Inc. [Google Scholar]
- 21.Soto A, Stewart WJ, McHenry MJ. 2015. When optimal strategy matters to prey fish. Int. Comp. Biol. 55, 110–120. ( 10.1093/icb/icv027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller UK, van Leeuwen JL. 2004. Swimming of larval zebrafish: ontogeny of body waves and implications for locomotory development. J. Exp. Biol. 207, 853–868. ( 10.1242/jeb.00821) [DOI] [PubMed] [Google Scholar]
- 23.Stuermer CA. 1988. Retinotopic organization of the developing retinotectal projection in the zebrafish embryo. J. Neurosci. 8, 4513–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolaou N, Lowe AS, Walker AS, Abbas F, Hunter PR, Thompson ID, Meyer MP. 2012. Parametric functional maps of visual inputs to the tectum. Neuron 76, 317–324. ( 10.1016/j.neuron.2012.08.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zottoli SJ, Hordes AR, Faber DS. 1987. Localization of optic tectal input to the ventral dendrite of the goldfish Mauthner cell. Brain Res. 401, 113–121. ( 10.1016/0006-8993(87)91170-X) [DOI] [PubMed] [Google Scholar]
- 26.Arnott SA, Neil DM, Ansell AD. 1999. Escape trajectories of the brown shrimp Crangon crangon and a theoretical consideration of initial escape angles from predators. J. Exp. Biol. 202, 193–209. [DOI] [PubMed] [Google Scholar]
- 27.Woodbury PB. 1986. The geometry of predator avoidance by the blue crab Callinectes sapidus Rathbun. Anim. Behav. 34, 28–37. [Google Scholar]
- 28.Domenici P, Batty RS. 1994. Escape manoeuvres of schooling Clupea harengus. J. Fish. Biol. 45, 97–110. ( 10.1111/j.1095-8649.1994.tb01086.x) [DOI] [Google Scholar]
- 29.Nair A, Changsing K, Stewart WJ, McHenry MJ. 2017. Data from: Fish prey change strategy with the direction of a threat. Dryad Digital Repository. ( 10.5061/dryad.47mq9) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Nair A, Changsing K, Stewart WJ, McHenry MJ. 2017. Data from: Fish prey change strategy with the direction of a threat. Dryad Digital Repository. ( 10.5061/dryad.47mq9) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.47mq9 [29].