Abstract
Increased predation risk is considered a cost of having conspicuous colours, affecting the anti-predator behaviour of colourful animals. However, this is difficult to test, as individual factors often covary with colour and behaviour. We used alarm call playback and behavioural observations to assess whether individual birds adjust their response to risk according to their plumage colour. Male superb fairy-wrens (Malurus cyaneus) change from a dull brown to conspicuous blue plumage each year, allowing the behaviour of different coloured birds to be compared while controlling for within-individual effects. Because the timing of colour change varies among males, blue and brown birds can also be compared at the same time of year, controlling for seasonal effects on behaviour. While blue, fairy-wrens fled more often in response to alarm calls, and took longer to emerge from cover. Blue fairy-wrens also spent more time foraging in cover and being vigilant. Group members appeared to benefit from the presence of blue males, as they reduced their response to alarms, and allocated less time to sentinel behaviour when a blue male was close by. We suggest that fairy-wrens perceive themselves to be at a higher risk of predation while in conspicuous plumage and adjust their behaviour accordingly.
Keywords: alarm call, anti-predator, colour, conspicuous, playback, predation
1. Background
Higher predation risk is considered a key cost borne by conspicuously coloured animals, as this is a logical outcome of being readily detected in the natural environment ([1], but see aposematic colours; [2]). Evidence of predator bias towards conspicuous prey has been found in field experiments, which show that conspicuous models are attacked more often compared with dull models of the same size and shape ([3–6] but see [7,8]). However, unlike static models, conspicuous animals counteract predation risk by spending more time hiding [9], scanning for predators [10,11] and being more responsive to perceived threats ([12,13] but see [14,15]). So, while there is evidence for direct (lethal) costs due to bright colours [16], costly behaviours to mitigate risk may be more pervasive [17]. Such behaviours can reduce the time available for foraging [18,19] or other important activities and may have profound impacts on population dynamics [20] and colour signal honesty [21].
The relationship between conspicuous colours and predation risk is intuitive, but demonstrating that conspicuousness itself has an effect on the anti-predator behaviour of colourful animals has proven difficult. This is because individuals may differ in factors that covary with colour, predation risk and the propensity for risk-taking behaviour [22]. When comparing different species, ornamentation may be related to differences in body size, escape strategies and trade-offs between reproduction and survival [23–25]. For example, comparative analyses found that conspicuous bird species spent less time feeding in an exposed location, but they were also consistently smaller than the cryptic species, perhaps making them easier prey [26]. Studies of sexually dichromatic species have shown that colourful males scan more often than dull females [10,11]; however, the sexes also differ in other factors that influence vigilance behaviour, such as their need to forage or keep a look out for competitors or mates [27]. Within sexes, anti-predator behaviours of conspecific males have been shown to increase with the intensity of their colour signals [9,11,28]. However, these males may also differ in factors such as body condition, age, personality and likelihood of future reproductive success [21,28,29], which have been shown to affect the propensity to take risks [30–32]. To address these issues, it is helpful to test for changes in conspicuousness and anti-predator behaviour within individuals.
We are aware of one experimental test of the effect of conspicuousness on an individual's perception of predation risk [33]. Individual hermit crabs (Pagurus bernhardus) were assigned different shell and background colours, and spent more time hiding in their shell when given a contrasting shell–background combination. This suggests that hermit crabs were aware of their conspicuousness to potential predators and adjusted their behaviour accordingly [33]. Hermit crabs are naturally cryptic however, and adapted to use matching habitat backgrounds in order to avoid detection [33]. Whether individuals with conspicuous colours used for signalling adjust their anti-predator behaviour in a similar way is yet to be shown.
We performed within-individual comparisons to assess whether conspicuous colours affect anti-predator behaviours consistent with increased predation risk in superb fairy-wrens, Malurus cyaneus. This was achieved using alarm call playback to determine responses to a perceived threat. In addition, we conducted behavioural observations of undisturbed birds to determine the general cautiousness of individuals. Male fairy-wrens moult twice per year, alternating between cryptic brown, and conspicuous blue-and-black plumage (‘blue plumage’), while females have brown plumage year-round [34,35]. This allows the behaviour of blue and brown fairy-wrens to be compared while controlling for individual differences in behaviour due to dominance, personality and other factors. Because individual males differ in the time they undergo colour change [36,37], we were also able to compare different-coloured fairy-wrens at the same time of year, allowing us to control for possible seasonal effects on behaviour.
2. Methods
(a). Study site and species
Superb fairy-wrens are small passerines native to southeastern Australia. They live in groups comprised of a dominant pair and, often, several male helpers [35]. Although the dominant male and female form a stable social pair, extra-pair paternity is high, accounting for up to 70% of the offspring in the population [38]. Females appear to be unselective of their social partner, but choose males that moult into blue plumage earliest in the year as extra-pair mates [36]. These preferred males are blue for the longest period of time (11–12 months of the year; [35]).
Fieldwork was undertaken before the onset of the superb fairy-wren breeding season, from June to September in 2015, at Lysterfield Park (Victoria, Australia, 37.95°S, 145.30°E) using a population of individually colour-banded birds. The reserve is comprised of open woodland, including areas with dense shrubs and open grassland, and avian predators are common (for details, see the electronic supplementary material).
(b). Alarm playback
(i). Design
Superb fairy-wrens produce high-frequency alarm calls in response to aerial predators. A greater number of elements in the call signals more urgent danger [39]. During the playback study, we broadcast single-element ‘low-danger’ and four-element ‘high-danger’ alarm calls. Our rationale for the two alarm types was that we could analyse perceived risk in two ways; past research has shown that fairy-wrens demonstrate a range of immediate responses to low-danger alarms [39], so we used the strength of their immediate response as an estimate of perceived risk. By contrast, fairy-wrens flee in response to high-danger alarms in almost all cases (e.g. [39–47]) but vary in their time to emerge from cover [39], so we used the time in cover as our estimate of perceived risk. Throughout our study, ‘cover’ is defined as a location where fairy-wrens are surrounded by vegetation, so that they are concealed from above.
We performed playback tests on male and female fairy-wrens, where each test included the playback of a high- and low-danger alarm call as well as a control sound. The control sound was the contact call of the crimson rosella (Platycercus elegans), a harmless parrot common to the field site. All sounds were broadcast at a natural alarm call amplitude of 60 dB SPL at 5 m and in random order [47]. There was a minimum 5-min interval between the bird returning to its previous behaviour after the first alarm and the playback of the next alarm. To limit habituation, we used 25 different aerial alarm and rosella call recordings at random throughout the playback study ([39,47]; for full details of sound recordings, equipment and sound calibration, see the electronic supplementary material). We sampled up to four individual fairy-wrens per territory, either simultaneously, if more than one bird satisfied playback criteria (see below), or at least 3 days apart.
The study was designed to assess responses to perceived risk by the same individual multiple times, in order to record their behaviour over time and according to changes in plumage colour (mean samples per individual = 3, range = 1–6). We sampled (i) individual males in brown as well as blue plumage, (ii) males that were brown or (iii) blue for all tests, and (iv) females, which remain brown year-round. While we attempted to record the response to all sounds within a test, this was not always possible due to field logistics; out of 182 playback tests, immediate responses to both alarms types were recorded for 142, while only low-danger alarm responses were obtained for 16 (total immediate responses to low-danger alarms, N = 158) and only high-danger alarms for 24 tests; out of 166 immediate responses to high-danger alarms, fairy-wrens fled for 148 and the time to emerge from cover was recorded for N = 92 (see criteria below). Rosella calls were played in 170 tests and were ignored in all cases. For a full breakdown of sample sizes, see electronic supplementary material, figure S1.
(ii). Playback procedure
Fairy-wrens were observed for at least 5 min before the broadcast of the first sound. The playback test proceeded if all fairy-wrens appeared to be undisturbed by the observer, there were no signs of predators, and at least one individual was clearly visible to the observer while at least 30 cm from cover. The responses of between one and four individuals were recorded, depending on how many birds met the playback criteria. Of 136 low-danger playbacks, 117 gathered data for a single individual and 19 gathered data for more than one (mean 2.2). Similarly, of 83 high-danger playbacks where the time in cover was recorded, 75 gathered data for a single individual and eight gathered data on more than one (mean 2.1). To test whether multiple individual samples per playback could have biased our results, we ran models with a restricted dataset, sampling one individual per playback according to pre-determined criteria consistent with the aims of the study (i.e. maximizing sample sizes per individual, sampling individuals with repeated measures in different plumage colours). Results from the restricted dataset did not qualitatively differ from the full dataset (see Results and electronic supplementary material, tables S1–S4).
We recorded the sex, plumage colour (brown or blue) and pre-playback activity of sampled individuals. Fairy-wrens were classed as ‘brown’ if they were female or males with less than two or three blue or black feathers. Males with complete or near-complete (less than two or three brown feathers) breeding plumage were classed as ‘blue’. Activities prior to playback included foraging, acting as a sentinel, preening, resting or singing. We recorded the number of bystander fairy-wrens (mean = 1.4, range = 0–10), classified as individuals less than 5 m from the sampled wren immediately prior to sound playback. Because the colour of bystanders could affect an individual's predation risk [48], we also recorded whether there were any bystanders in blue plumage (yes/no, as there was only one occasion with two blue bystanders). Finally, we estimated the distance between the sampled individuals and the observer (mean = 10.8, range = 6–15 m), the distance between the sampled individuals and the nearest cover (mean = 1.7, range = 0.3–10 m) and the per cent cover within a 5 m radius of the sampled individuals (mean = 36, range = 5–95%).
We recorded the immediate response to the playback of each sound as no response (0), intermediate response (1) and flee to cover (2). Intermediate responses included pausing, looking up, ducking, flying to an open area and pausing before flying to cover. The ‘flee to cover’ response occurred when the individual flew directly to cover without delay. If this occurred, we also attempted to record the time taken to re-emerge into the open (time spent in cover). This was not recorded if the individual was resighted in the open without being seen to re-emerge, if it disappeared from view for more than 2 min, or if an additional (natural) aerial alarm was made by any fairy-wren prior to the sampled individual re-emerging.
(c). Time-budget observations
We used time-budget observations to estimate the proportion of time fairy-wrens devoted to foraging relative to vigilance. In addition, we assessed the time spent foraging in cover relative to open areas.
Observations were conducted for 24 individual fairy-wrens. Of these, 20 individuals were observed twice, with repeated observations at least 15 days apart. We recorded the sex and plumage colour of the focal individual, group size and whether blue males were present in addition to the focal individual. Fairy-wrens were counted as being present in the group if they were seen in the same area (within 10 m of the focal individual) during the observation period.
The behaviour of a single, focal individual was recorded every 30 s until approximately 60 samples had been taken (range = 46–60 samples; 80% of observations obtained 60 samples), excluding instances where the focal individual was ‘out of sight’ (0–57% of the 30 s scans within a time-budget observation). This instantaneous scan sampling method is commonly used to approximate the time spent on different behaviours in field studies [49], although we acknowledge this is an estimate rather than a direct record of lengths of time. We found no difference in the proportion of out-of-sight scans according to plumage colour, sex, group size or whether or not an additional blue male was present in the group (in all cases p > 0.1).
Behaviours recorded included foraging, acting as a sentinel, scanning, flying, preening, singing, alarm calling and resting. Sentinel behaviour is performed in an exposed location well above the ground (greater than 1 m); the fairy-wren stands tall, with its tail erect and swiftly turns from side to side, as if surveying the area [50,51]. Scanning occurred when a fairy-wren paused (greater than 2 s) and looked around or upwards. Although the behaviour was scored instantaneously (i.e. exactly at the 30 s mark), we considered the context immediately before the sample to distinguish ‘scanning’ from searching for insects during foraging. While scanning and acting as a sentinel are both aspects of vigilance, sentinel behaviour might be risky as the fairy-wren is perched in a conspicuous location, where it may be exposed to predators [50]. We therefore expected blue males to spend more time scanning, but not necessarily more time acting as a sentinel.
(d). Statistical analyses
(i). Response to alarm call playback
Fairy-wrens fled in response to about half of the low-danger alarm playbacks (see Results), so we used the bird's ranked immediate response (none, intermediate, flee to cover) to estimate perceived risk [39]. This was analysed using a cumulative link mixed model (CLMM) from the package ‘ordinal’ [52]. The model included the following fixed factors: plumage colour (blue/brown), sex, date, time (hour), number of bystanders, blue bystanders present (y/n), activity prior to playback (five categories), playback sequence (first or second alarm played per playback test), the distance between the sampled individual and nearest cover (m), the percentage of available cover, observer ID (two observers) and the distance between the observer and the sampled individual (m). These factors were included as they have been shown to impact anti-predator behaviour (e.g. [27,31,48,53,54]) or were required to control for variation in the study design. Random factors were individual and territory ID, as well as the interaction between plumage colour and individual ID (random slopes model; [55]). The use of the random slopes allows us to control for multiple within-individual comparisons and repeated observations for plumage colour within each individual.
Fairy-wrens almost always fled in response to high-danger alarms, so we analysed the time spent in cover after fleeing to estimate perceived risk [39–47] using a linear mixed model (LMM) run with the package ‘lme4’ [56]. We transformed time in cover (s) by x−0.2 to improve normality of residuals. The transformation exponent was selected using the ‘box-cox’ function in the package ‘MASS’ [57]. We used the same fixed and random factors used in the low-danger alarm model (see above) except that ‘activity’ was excluded as the individuals had already fled to cover.
Older male fairy-wrens moult into blue plumage earlier in the year [36,37]. As a result, we expect to have more observations for older males in blue plumage. To test if this could bias our results, we re-ran the models using male observations only and included moult date as a fixed effect. We found no effect of moult date on the fairy-wren's response to playback (immediate response to low-danger alarm: β = 0.0, s.e. = 0.1, p = 0.97; time in cover after fleeing high-danger alarm: β = −2.7 s, s.e. = 4.6, p = 0.33).
In order to check for inaccuracies due to potential over-parameterization, we compared our results with those from simplified models. Simplified models were selected according to their AICc criteria using the function ‘dredge’ in the package ‘MuMIn’ [58], with the predictor ‘plumage colour’ included in all models. Results from simplified models did not qualitatively differ from those of the full models (electronic supplementary material, tables S5 and S6) so we refer to estimates from the full models throughout [59].
Additional analyses showing the immediate response to high-danger alarms and time in cover after fleeing the low-danger alarms are available in the electronic supplementary material; these are not presented here as, due to sample limitations, they had limited power to test our hypothesis and did not alter our conclusions.
(ii). Time-budget observations
Behavioural data from the time-budget observations were analysed using LMMs. We ran separate models for the most common behaviours: foraging, acting as a sentinel, scanning, flying and preening. In addition, we analysed the proportion of scan samples where birds foraged in cover relative to open areas. In each model, we included the following fixed effects: plumage colour, sex, date, time, group size and blue bystander. We included the random effects of individual and territory ID.
As for the playback analyses, we tested whether overrepresentation of old, blue males could bias our results by re-running time-budget models using male observations only and including moult date as a fixed effect. We found no effect of moult date on the estimated time spent on activities recorded during the behavioural observations (foraging: β = 3%, s.e. = 4, p = 0.46; acting as a sentinel: β = −3%, s.e. = 2, p = 0.16; scanning: β = 0%, s.e. = 2, p = 0.96; flying: β = −1%, s.e. = 1, p = 0.12; preening: β = 1%, s.e. = 1, p = 0.70; foraging in cover: β = 8%, s.e. = 5, p = 0.15). Including moult date also did not alter the direction of the results.
(iii). Within-study meta-analysis
To assess the general ‘cautiousness’ of fairy-wrens in blue plumage, we performed a within-study multivariate meta-analysis of our full dataset, using the package ‘metafor’ [60] and equations described in Nakagawa & Cuthill [61]. We predicted cautious fairy-wrens should flee more often in response to low-danger alarms, spend longer hiding in cover after fleeing high-danger alarms and spend more time foraging in cover, more time scanning and less time foraging overall. We used a weighted model with restricted maximum-likelihood to account for variation in sample sizes between tests. The model accounted for non-independence between tests using a variance–covariance matrix where the diagonal elements corresponded to the variance associated with each effect and the off-diagonal elements to the covariances between dependent variables. These were computed based on the correlation coefficients obtained for each combination of response variables, where the same individual was sampled for both variables. All statistical tests were performed in R (version 3.2.3, R Core Team, 2015).
3. Results
(a). Alarm playback
Fairy-wrens were less responsive to low-danger (single-element) alarms compared with high-danger (four-element) alarms. For the 158 immediate responses to low-danger alarms, 47% resulted in the fairy-wren fleeing to cover, 23% provoked an intermediate response and 31% resulted in no response. By contrast, fairy-wrens fled in response to almost all high-danger alarms; for the 166 immediate responses to high-danger alarms, 89% resulted in the fairy-wren fleeing to cover and 10% led to an intermediate response; only one individual on one occasion did not respond (comparison of immediate response to low- versus high-danger alarm: β = −0.7, s.e. = 0.1, p < 0.01). Fairy-wrens also spent less time in cover after fleeing in response to low- compared with high-danger alarms (β = −7 s, s.e. = 4.4, p = 0.01).
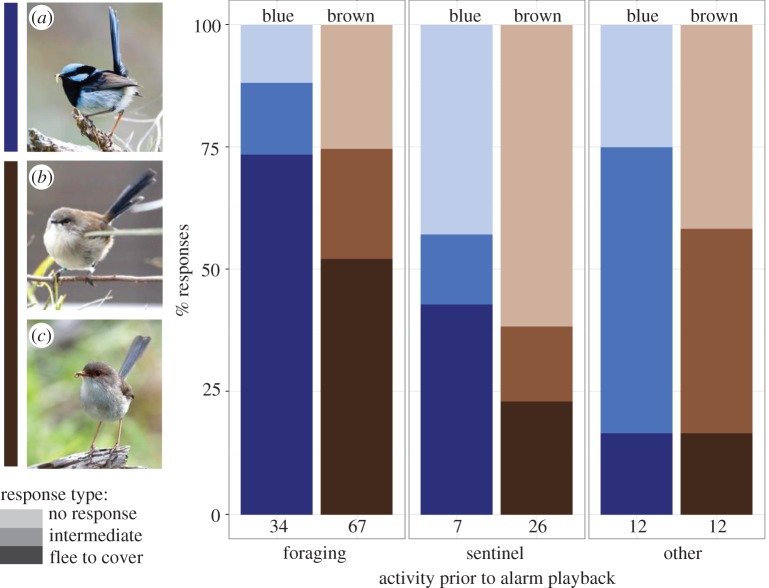
Males in blue plumage showed a stronger immediate response to the playback of low-danger alarms compared with brown males or females (figure 1; β = 1.4, s.e. = 0.6, p = 0.01, N= 158, electronic supplementary material, table S1). Their activity prior to playback also affected the response (electronic supplementary material, table S1). Fairy-wrens were more likely to flee in response to the low-danger alarm when they had been foraging at the time of playback compared with acting as a sentinel, preening or resting. Individuals acting as a sentinel often failed to respond to the low-danger alarm, while preening and resting fairy-wrens were more likely to show an intermediate response. Within each behavioural category, males in blue plumage remained the most responsive (figure 1).
Figure 1.
Blue males are more likely to flee in response to the playback of a conspecific alarm call, independent of their prior activity. Responses to low-danger alarm playbacks are shown for fairy-wrens in blue plumage (blue males, image a; blue bars) and brown plumage (brown bars), including males (image b) and females (image c). Response types are flee to cover (dark), intermediate (medium) and no response (light). Individuals were foraging, acting as a sentinel or performing other behaviours (preening, resting and singing) immediately prior to the alarm playback. Values are based on raw data. Numbers indicate sample sizes for responses to the low-danger alarm. (Online version in colour.)
Compared with brown fairy-wrens, males in blue plumage tended to spend more time in cover after fleeing in response to a high-danger alarm (figure 2a; β = 12.5 s, s.e. = 6.2, p = 0.07; electronic supplementary material, table S2). In addition, fairy-wrens spent less time hiding in cover when there was a blue male bystander (figure 2b; β = −7.6 s, s.e. = 4.2, p < 0.01; electronic supplementary material, table S2). The birds took longer to emerge into the open if they were far from cover at the time of hearing the alarm (electronic supplementary material, table S2). We found no difference in responses to alarm playbacks according to sex or date (electronic supplementary material, tables S1 and S2).
Figure 2.
Time taken for superb fairy-wrens to re-emerge from cover after fleeing in response to high-danger alarm playback. Model predicted means are shown for (a) fairy-wrens in blue and brown plumage and (b) according to whether or not a blue individual was close by at the time of alarm playback. Error bars show standard error. For full statistics, see electronic supplementary material, table S2.
Results using a restricted dataset with the response of one bird per playback did not qualitatively differ from those described above (effect of blue plumage on response to low-danger alarm: β = 1.1, s.e. = 0.6, p = 0.06, time in cover after fleeing high-danger alarm: β = 14.3 s, s.e. = 6.5, p = 0.05; electronic supplementary material, tables S3 and S4).
(b). Time-budget observations
Based on our instantaneous scan samples, fairy-wrens spent most of their time foraging (61%) and performing sentinel behaviour (18%). The remaining 20% of the time was spent scanning (8%), flying (5%), preening (4%) and other behaviours (less than 2% for singing, alarm calling and resting). When foraging, fairy-wrens spent 53% of their time in cover and 47% in the open.
Blue males appear to be more cautious compared with brown conspecifics. They spent more time scanning their surroundings, more time foraging in cover and tended to spend less time foraging overall. They also spent more time flying relative to brown conspecifics (figure 3; electronic supplementary material, tables S7–S10). We did not detect a difference in the proportion of time blue and brown fairy-wrens spent acting as a sentinel or preening (figure 3; electronic supplementary material, tables S11 and S12).
Figure 3.
Fairy-wrens differ in the proportion of time (estimated by proportion of instantaneous scan samples) they allocate to activities according to whether they are in bright blue (dark bars) or dull brown (light bars) plumage. Foraging is shown separately for foraging in cover and foraging in the open. Means are derived from model predicted values, error bars show standard error. For full statistics, see electronic supplementary material, tables S7–S12. **p < 0.01, ***p < 0.001.
The presence of nearby conspecifics influenced the behaviour of focal individuals. Fairy-wrens spent less time acting as a sentinel when there was a blue male close by (β = −6%, s.e. = 3, p = 0.02; electronic supplementary material, table S11) and when in larger groups (β = −4%, s.e. = 1, p < 0.01; electronic supplementary material, table S11). Individuals also spent more time foraging when in larger groups (β = 5%, s.e. = 2, p = 0.03; electronic supplementary material, table S9). We found no significant change in the time spent on other behaviours according to whether or not a blue male was present in the group (electronic supplementary material, tables S7, S9, S10 and S12).
(c). Within-study meta-analysis
The within-study meta-analysis confirms that fairy-wrens are overall more cautious when in blue plumage (figure 4; β = 0.35, s.e. = 0.12, p < 0.01). This analysis combines the effect of each anti-predator variable (responses to low- and high-danger alarm playbacks, and foraging and vigilance behaviours during time-budget observations).
Figure 4.
Results from the within-study meta-analysis, showing increased cautiousness in fairy-wrens with blue plumage. Standardized effect sizes (Zr) that support the hypothesis that blue males are more cautious have been given a positive sign. Error bars show 95% confidence intervals.
4. Discussion
Superb fairy-wrens behave as though they perceive themselves to be at increased risk of predation while in conspicuous blue plumage (figure 4). Blue males responded more strongly to alarm playbacks than brown birds; they were more likely to flee in response to low-danger alarms (figure 1), and tended to take longer to emerge from cover after fleeing in response to high-danger alarms (figure 2a). Blue individuals also spent more time foraging in cover, more time scanning their surroundings and less time foraging overall compared with brown conspecifics (figure 3). Group members emerged from cover sooner after fleeing in response to a high-danger alarm (figure 2b) and devoted less time to sentinel behaviour when a blue male was close by, consistent with the blue males' greater vigilance or perhaps viewing the blue male as a ‘decoy’ in the event of an attack.
(a). Conspicuous colours and cautious behaviour
By controlling for within-individual effects, we show that being conspicuous increases perceived predation risk independent of individual differences in factors such as age, dominance, personality and foraging capability. In addition, the effect was independent of differences between the sexes, because males in blue and brown plumage differed in their response to playback while brown males and females did not. The effect of colour on perceived predation risk was also independent of differences in sexual attractiveness [30], as males did not differ in their responses to alarm playback according to moult date, which is the criterion used by females to select extra-pair mates [37]. Finally, responses to perceived risk were independent of seasonal changes in behaviour, as we compared brown and blue fairy-wrens at the same time of year and completed the study prior to the onset of breeding. Our results therefore provide strong evidence that conspicuous colours increase predation risk independent of other factors.
Increased anti-predator behaviour in conspicuous birds is likely to be costly, as blue males spent more time scanning at the expense of foraging (figure 3). Foraging in cover may also be less efficient, as fairy-wrens regularly forage in the open despite increased exposure to predators [62]. Blue males spent more time flying compared with those in brown plumage, perhaps due to their tendency to leave their social group and display to off-territory females [35] or because they must fly between patches of cover to avoid open areas during foraging. As alarm calls are common among superb fairy-wrens [45], increased flight to cover and latency to re-emerge may come at an additional cost, requiring increased energy expenditure and further reducing the time available for foraging [18]. Taken together, we suggest the behaviours used to mitigate predation risk in blue fairy-wrens are likely to be energetically costly or else require that males who are blue for long periods of time be highly efficient foragers. This is because blue fairy-wrens: (i) scan more and forage less, (ii) forage more often in potentially less productive areas, and (iii) flee more often and spend more time hiding in response to alarm calls.
The costs of mitigating predation risk are likely to be highest for the most attractive males. In this species, females show a strong and unanimous preference for extra-pair males that moult earliest in the year and remain in blue plumage for longest, with the time spent blue ranging from four to 12 months per year [36]. The display of blue plumage by male superb fairy-wrens is therefore thought to be a signal of endurance [38]; early moulting males pay the cost of maintaining blue plumage for a long time, which is associated with high testosterone and related immune costs [63]. Here, we show that these physiological costs are likely to be compounded by increased anti-predator behaviours, as highly attractive, early-moulting males may be required to reduce foraging and maintain high vigilance for longer compared with less attractive males. In addition, the higher cumulative predation risk faced by early-moulting males may be exacerbated by being the only blue male in the group, while late-moulting fairy-wrens should benefit from a dilution effect [10]. Past research on fish has shown that predators are crucial for maintaining an honest correlation between body condition and male colours, where the absence of predators led to the evolution of more vivid colours that were unrelated to condition [21]. Perhaps predation risk could similarly enforce signal honesty in fairy-wrens, by limiting early moult to males with sufficient energetic resources or foraging capabilities required to meet the costs of mitigating high risk. Further research could investigate the relationship between colour signals and predation risk in fairy-wrens by assessing whether: (i) high testosterone levels associated with blue plumage [63] provide a proximate mechanism for changes in risk-taking behaviour and (ii) whether experimentally manipulated perceived predation risk influences the timing of male colour change (see [64]).
(b). Benefits to group members
Fairy-wrens within the group appear to benefit from having a blue male in the vicinity, as they return from cover sooner after fleeing in response to high-danger alarms and reduce their time devoted to sentinel behaviour when a blue male is nearby (figure 2b). We consider three possible explanations. First, fairy-wrens could perceive themselves to face a lower risk of predation when in the presence of a blue ‘decoy’ individual. The decoy effect has been predicted to occur using models of a predator's sensory system [48]. A laboratory experiment using Daphnia dyed with cryptic and conspicuous colours supports this prediction [65], although to date there has been no evidence from natural populations. Second, fairy-wrens might take advantage of the heightened vigilance of blue males. Past research has shown that birds reduce sentinel behaviour when in the company of highly alert individuals [66–70]. Fairy-wrens could similarly take advantage of the heightened vigilance of conspicuous males, allowing them to reduce their sentinel behaviour and return from cover sooner after fleeing in response to a perceived threat. Thirdly, blue males might have a tendency to give more false alarms, so that other group members reduce their time hiding in cover due to an increase in unreliable warning signals [45]. A higher false alarm rate is possible because animals perceiving greater risk should have a lower threshold for anti-predator behaviour [71]. Overall, our study contrasts with previous work on the disadvantages of having conspicuous animals within a group [72,73]. Instead, it shows that animals may perceive themselves to have reduced predation risk when accompanied by conspicuous individuals in a naturally occurring, multi-coloured group.
(c). Flexible responses to risk
Throughout this study, birds have shown flexible responses to perceived predation risk, adjusting their behaviour according to the degree of danger encoded in the alarm call, distance to cover and group size. In addition, responses to the low-danger alarm calls varied according to the activity individuals were engaged in at the time of playback. Foraging individuals fled most often, while preening and resting birds had more intermediate responses. Individuals acting as a sentinel were more likely to ignore the alarm (figure 1). These different responses could be explained by differences in prior awareness. Individuals foraging close to the ground with their attention occupied should flee even in response to low-danger alarms, because they are less able to rapidly assess their situation [74,75]. By contrast, sentinels have a good vantage point and are already alert to surroundings, making them less likely to respond to a false alarm. Intermediate responses in preening and resting fairy-wrens may be adaptive as they are already relatively still and may benefit from pausing to avoid detection [12]. Despite flexible changes in anti-predator behaviours according to the activity and context of the individual, there is a large and consistent effect of colour (figures 1 and 4). Taken together, our study demonstrates a link between risk-related behaviour and conspicuous colours, independent of the context, individual factors and temporal changes in behaviour.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank M. Roast for help with population surveys and for providing feedback on the manuscript as well as J. Hadfield and S. Nakagawa for statistical advice. We thank R. Montgomerie, D. Blumstein and an anonymous reviewer for their constructive feedback which improved the quality of the manuscript. We are grateful to Parks Victoria staff from Lysterfield Park for providing access to the park.
Ethics
Research was approved by the Monash University Animal Ethics Committee, permit nos. BSCI/2013/10 and BSCI/2015/05 and the Department of Environment and Primary Industries, permit no. 10007370.
Data accessibility
Data supporting this article are available in the electronic supplementary material.
Authors' contributions
Conceived study: A.M., A.P., K.D. and R.D.M.; collected data: A.C.N., A.M. and N.T.; analysed data: A.M., A.P. and K.D.; wrote manuscript: A.M., A.P., K.D. and R.D.M., with contributions from A.C.N. and N.T.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the Australian Research Council (FT 110100505 to A.P. and DE 120102323 to K.D.) and Monash University.
References
- 1.Endler JA. 1992. Signals, signal conditions, and the direction of evolution. Am. Nat. 139, 125–153. ( 10.1086/285308) [DOI] [Google Scholar]
- 2.Stevens M, Ruxton GD. 2012. Linking the evolution and form of warning coloration in nature. Proc. R. Soc. B 279, 417–426. ( 10.1098/rspb.2011.1932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stuart-Fox DM, Moussalli A, Marshall NJ, Owens IPF. 2003. Conspicuous males suffer higher predation risk: visual modelling and experimental evidence from lizards. Anim. Behav. 66, 541–550. ( 10.1006/anbe.2003.2235) [DOI] [Google Scholar]
- 4.Husak JF, Macedonia JM, Fox SF, Sauceda RC. 2006. Predation cost of conspicuous male coloration in collared lizards (Crotaphytus collaris): an experimental test using clay-covered model lizards. Ethology 112, 572–580. ( 10.1111/j.1439-0310.2005.01189.x) [DOI] [Google Scholar]
- 5.Ruiz-Rodríguez M. et al 2013. Does avian conspicuous colouration increase or reduce predation risk? Oecologia 173, 83–93. ( 10.1007/s00442-013-2599-6) [DOI] [PubMed] [Google Scholar]
- 6.Marshall KLA, Philpot KE, Stevens M. 2015. Conspicuous male coloration impairs survival against avian predators in Aegean wall lizards, Podarcis erhardii. Ecol. Evol. 5, 4115–4131. ( 10.1002/ece3.1650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres-Dowdall J, Machado-Schiaffino G, Kautt AF, Kusche H, Meyer A. 2014. Differential predation on the two colour morphs of Nicaraguan Crater lake Midas cichlid fish: implications for the maintenance of its gold–dark polymorphism. Biol. J. Linn. Soc. 112, 123–131. ( 10.1111/bij.12271) [DOI] [Google Scholar]
- 8.Götmark F. 1994. Does a novel bright colour patch increase or decrease predation? Red wings reduce predation risk in European blackbirds. Proc. R. Soc. Lond. B 256, 83–87. ( 10.1098/rspb.1994.0053) [DOI] [Google Scholar]
- 9.Ibáñez A, López P, Martín J. 2014. Inter-individual variation in antipredator hiding behavior of Spanish terrapins depends on sex, size, and coloration. Ethology 120, 742–752. ( 10.1111/eth.12245) [DOI] [Google Scholar]
- 10.Hart PJ, Freed LA. 2005. Predator avoidance as a function of flocking in the sexually dichromatic Hawaii akepa. J. Ethol. 23, 29–33. ( 10.1007/s10164-004-0124-4) [DOI] [Google Scholar]
- 11.Pascual J, Senar JC, Domènech J. 2014. Plumage brightness, vigilance, escape potential, and predation risk in male and female Eurasian siskins (Spinus spinus). Auk 131, 61–72. ( 10.1642/AUK-13-220.1) [DOI] [Google Scholar]
- 12.Ortega J, López P, Martín J. 2014. Conspicuous blue tails, dorsal pattern morphs and escape behaviour in hatchling Iberian wall lizards (Podarcis hispanicus). Biol. J. Linn. Soc. 113, 1094–1106. ( 10.1111/bij.12379) [DOI] [Google Scholar]
- 13.Journey L, Drury JP, Haymer M, Rose K, Blumstein DT. 2013. Vivid birds respond more to acoustic signals of predators. Behav. Ecol. Sociobiol. 67, 1285–1293. ( 10.1007/s00265-013-1556-z) [DOI] [Google Scholar]
- 14.Hensley NM, Drury JP, Garland T, Blumstein DT. 2015. Vivid birds do not initiate flight sooner despite their potential conspicuousness. Curr. Zool. 61, 773–780. ( 10.1093/czoolo/61.4.773) [DOI] [Google Scholar]
- 15.Cooper WE. 2003. Sexual dimorphism in distance from cover but not escape behavior by the keeled earless lizard Holbrookia propinqua. J. Herpetol. 37, 374–378. ( 10.1670/0022-1511(2003)037%5B0374:SDIDFC%5D2.0.CO;2) [DOI] [Google Scholar]
- 16.Huhta E. 2003. Plumage brightness of prey increases predation risk: an among-species comparison. Ecology 84, 1793–1799. ( 10.1890/0012-9658(2003)084%5B1793:PBOPIP%5D2.0.CO;2) [DOI] [Google Scholar]
- 17.Lima SL. 1998. Nonlethal effects in the ecology of predator–prey interactions. Bioscience 48, 25–34. ( 10.2307/1313225) [DOI] [Google Scholar]
- 18.Martín J, López P. 1999. An experimental test of the costs of antipredatory refuge use in the wall lizard, Podarcis muralis. Oikos 84, 499–505. ( 10.2307/3546428) [DOI] [Google Scholar]
- 19.Baker DJ, Stillman RA, Smart SL, Bullock JM, Norris KJ. 2011. Are the costs of routine vigilance avoided by granivorous foragers? Funct. Ecol. 25, 617–627. [Google Scholar]
- 20.Zanette LY, White AF, Allen MC, Clinchy M. 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 80, 1398–1401. ( 10.1126/science.1210908) [DOI] [PubMed] [Google Scholar]
- 21.Giery ST, Layman CA. 2015. Interpopulation variation in a condition-dependent signal: predation regime affects signal intensity and reliability. Am. Nat. 186, 187–195. ( 10.1086/682068) [DOI] [PubMed] [Google Scholar]
- 22.Katz MW, Abramsky Z, Kotler BP, Rosenzweig ML, Alteshtein O. 2014. All that glitters is not gold: different anti-predatory behavior of two color morphs of goldfish (Carassius auratus). Environ. Biol. Fishes 98, 377–383. ( 10.1007/s10641-014-0268-1) [DOI] [Google Scholar]
- 23.Fowler-Finn KD, Hebets EA. 2011. More ornamented males exhibit increased predation risk and antipredatory escapes, but not greater mortality. Ethology 117, 102–114. ( 10.1111/j.1439-0310.2010.01852.x) [DOI] [Google Scholar]
- 24.Dale J, Dey C, Delhey K, Kempenaers B, Valcu M. 2015. The effects of life-history and social selection on male and female plumage coloration. Nature 527, 367–370. ( 10.1038/nature15509) [DOI] [PubMed] [Google Scholar]
- 25.Møller AP, Samia DSM, Weston MA, Guay PJ, Blumstein DT. 2015. Flight initiation distances in relation to sexual dichromatism and body size in birds from three continents. Biol. J. Linn. Soc. 117, 823–831. ( 10.1111/bij.12706) [DOI] [Google Scholar]
- 26.Silva IA, de Figueiredo RA, Matos DM da S. 2008. Feeding visit time of fruit-eating birds in Cerrado plants: revisiting the predation risk model. Rev. Bras. Zool. 25, 682–688. ( 10.1590/S0101-81752008000400013) [DOI] [Google Scholar]
- 27.Barnier F, Duncan P, Fritz H, Blanchard P, Rubenstein DI, Pays O. 2016. Between-gender differences in vigilance do not necessarily lead to differences in foraging-vigilance tradeoffs. Oecologia 181, 757–768. ( 10.1007/s00442-016-3614-5) [DOI] [PubMed] [Google Scholar]
- 28.Martin J, López P. 1999. Nuptial coloration and mate guarding affect escape decisions of male lizards Psammodromus algirus. Ethology 105, 439–447. ( 10.1046/j.1439-0310.1999.00418.x) [DOI] [Google Scholar]
- 29.Mateos-Gonzalez F, Senar JC. 2012. Melanin-based trait predicts individual exploratory behaviour in siskins, Carduelis spinus. Anim. Behav. 83, 229–232. ( 10.1016/j.anbehav.2011.10.030) [DOI] [Google Scholar]
- 30.Engqvist L, Cordes N, Reinhold K. 2014. Evolution of risk-taking during conspicuous mating displays. Evolution 62, 395–406. ( 10.1111/evo.12591) [DOI] [PubMed] [Google Scholar]
- 31.Chmura HE, Wey TW, Blumstein DT. 2016. Assessing the sensitivity of foraging and vigilance to internal state and environmental variables in yellow-bellied marmots (Marmota flaviventris). Behav. Ecol. Sociobiol. 70, 1901–1910. ( 10.1007/s00265-016-2195-y) [DOI] [Google Scholar]
- 32.van Oers K, Drent PJ, de Goede P, van Noordwijk AJ. 2004. Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proc. R. Soc. Lond. B 271, 65–73. ( 10.1098/rspb.2003.2518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briffa M, Twyman C. 2011. Do I stand out or blend in? Conspicuousness awareness and consistent behavioural differences in hermit crabs. Biol. Lett. 7, 330–332. ( 10.1098/rsbl.2010.0761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina I, Delhey K, Peters A, Cain KE, Hall ML, Mulder RA, Langmore NE. 2017. Habitat structure is linked to the evolution of plumage colour in female, but not male, fairy-wrens. BMC Evol. Biol. 17, 1–9. ( 10.1186/s12862-016-0861-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins PJ, Peter JM, Steele WK (eds) 2001. Handbook of Australian, New Zealand and Antarctic birds. Volume 5: tyrant-flycatchers to chats. Melbourne, Australia: Oxford University Press. [Google Scholar]
- 36.Mulder RA, Magrath MJL. 1994. Timing of prenuptial molt as a sexually selected indicator of male quality in superb fairy-wrens (Malurus cyaneus). Behav. Ecol. 5, 393–400. ( 10.1093/beheco/5.4.393) [DOI] [Google Scholar]
- 37.Double M, Cockburn A. 2000. Pre-dawn infidelity: females control extra-pair mating in superb fairy-wrens. Proc. R. Soc. Lond. B 267, 465–470. ( 10.1098/rspb.2000.1023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green DJ, Osmond HL, Double MC, Cockburn A. 2000. Display rate by male fairy-wrens (Malurus cyaneus) during the fertile period of females has little influence on extra-pair mate choice. Behav. Ecol. Sociobiol. 48, 438–446. ( 10.1007/s002650000258) [DOI] [Google Scholar]
- 39.Fallow PM, Magrath RD. 2010. Eavesdropping on other species: mutual interspecific understanding of urgency information in avian alarm calls. Anim. Behav. 79, 411–417. ( 10.1016/j.anbehav.2009.11.018) [DOI] [Google Scholar]
- 40.Murray TG, Magrath RD. 2015. Does signal deterioration compromise eavesdropping on other species’ alarm calls? Anim. Behav. 108, 33–41. ( 10.1016/j.anbehav.2015.07.015) [DOI] [Google Scholar]
- 41.Fallow PM, Pitcher BJ, Magrath RD. 2013. Alarming features: birds use specific acoustic properties to identify heterospecific alarm calls. Proc. R. Soc. B 280, 20122539 ( 10.1098/rspb.2012.2539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haff TM, Magrath RD. 2013. Eavesdropping on the neighbours: fledglings learn to respond to heterospecific alarm calls. Anim. Behav. 85, 411–418. ( 10.1016/j.anbehav.2012.11.016) [DOI] [Google Scholar]
- 43.Magrath RD, Bennett TH. 2012. A micro-geography of fear: learning to eavesdrop on alarm calls of neighbouring heterospecifics. Proc. R. Soc. B 279, 902–909. ( 10.1098/rspb.2011.1362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fallow PM, Gardner JL, Magrath RD. 2011. Sound familiar? Acoustic similarity provokes responses to unfamiliar heterospecific alarm calls. Behav. Ecol. 22, 401–410. ( 10.1093/beheco/arq221) [DOI] [Google Scholar]
- 45.Magrath RD, Pitcher BJ, Gardner JL. 2009. An avian eavesdropping network: alarm signal reliability and heterospecific response. Behav. Ecol. 20, 745–752. ( 10.1093/beheco/arp055) [DOI] [Google Scholar]
- 46.Magrath RD, Pitcher BJ, Gardner JL. 2009. Recognition of other species' aerial alarm calls: speaking the same language or learning another? Proc. R. Soc. B 276, 769–774. ( 10.1098/rspb.2008.1368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magrath RD, Pitcher BJ, Gardner JL. 2007. A mutual understanding? Interspecific responses by birds to each other's aerial alarm calls. Behav. Ecol. 18, 944–951. ( 10.1093/beheco/arm063) [DOI] [Google Scholar]
- 48.Tosh CR, Jackson AL, Ruxton GD, Ruxton D. 2007. Individuals from different-looking animal species may group together to confuse shared predators: simulations with artificial neural networks. Proc. R. Soc. B 274, 827–832. ( 10.1098/rspb.2006.3760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–267. ( 10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 50.Yasukawa K, Cockburn A. 2009. Antipredator vigilance in cooperatively breeding superb fairy-wrens (Malurus cyaneus). Auk 126, 147–154. ( 10.1525/auk.2009.08074) [DOI] [Google Scholar]
- 51.Colombelli-Négrel D, Robertson J, Sulloway FJ, Kleindorfer S. 2010. Extended parental care of fledglings: parent birds adjust anti-predator response according to predator type and distance. Behaviour 147, 853–870. ( 10.1163/000579510X495771) [DOI] [Google Scholar]
- 52.Christensen RHB. 2015. A regression models for ordinal data. R package version 2015.6-28. See http://www.cran.r-project.org/package=ordinal/ [Google Scholar]
- 53.Abbey-lee RN, Kaiser A, Mouchet A, Dingemanse NJ. 2016. Immediate and carry-over effects of perceived predation risk on communication behavior in wild birds. Behav. Ecol. 27, 708–716. ( 10.1093/beheco/arv210) [DOI] [Google Scholar]
- 54.Monclús R, Anderson A, Blumstein DT. 2015. Do yellow-bellied marmots perceive enhanced predation risk when they are farther from safety? An experimental study. Ethology 121, 831–839. ( 10.1111/eth.12397) [DOI] [Google Scholar]
- 55.Schielzeth H, Forstmeier W. 2009. Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 20, 416–420. ( 10.1093/beheco/arn145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bates D, Maechler M, Bolker B. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 57.Venables WD, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer. [Google Scholar]
- 58.Barton K. 2013. MuMIn: Multi-model inference. R package version 1.9.0 ed. [Google Scholar]
- 59.Forstmeier W, Schielzeth H. 2010. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner's curse. Behav. Ecol. Sociobiol. 65, 47–55. ( 10.1007/s00265-010-1038-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 61.Nakagawa S, Cuthill IC. 2007. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605. ( 10.1111/j.1469-185X.2007.00027.x) [DOI] [PubMed] [Google Scholar]
- 62.Watts BD. 1990. Cover use and predator-related mortality in song and savannah sparrows. Auk 107, 775–778. ( 10.2307/4088011) [DOI] [Google Scholar]
- 63.Peters A. 2000. Testosterone treatment is immunosuppressive in superb fairy-wrens, yet free-living males with high testosterone are more immunocompetent. Proc. R. Soc. Lond. B 267, 883–889. ( 10.1098/rspb.2000.1085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hemmi JM, Marshall J, Pix W, Vorobyev M, Zeil J. 2006. The variable colours of the fiddler crab Uca vomeris and their relation to background and predation. J. Exp. Biol. 209, 4140–4153. ( 10.1242/jeb.02483) [DOI] [PubMed] [Google Scholar]
- 65.Rodgers GM, Kimbell H, Morrell LJ. 2013. Mixed-phenotype grouping: the interaction between oddity and crypsis. Oecologia 172, 59–68. ( 10.1007/s00442-012-2473-y) [DOI] [PubMed] [Google Scholar]
- 66.Ridley AR, Wiley E, Thompson A, Ridley AR, Wiley EM, Thompson AM. 2013. The ecological benefits of interceptive eavesdropping. Funct. Ecol. 28, 197–205. ( 10.1111/1365-2435.12153) [DOI] [Google Scholar]
- 67.Ridley AR, Raihani NJ. 2007. Facultative response to a kleptoparasite by the cooperatively breeding pied babbler. Behav. Ecol. 18, 324–330. ( 10.1093/beheco/arl092) [DOI] [Google Scholar]
- 68.Radford AN, Hollén LI, Bell MBV. 2009. The higher the better: sentinel height influences foraging success in a social bird. Proc. R. Soc. B 276, 2437–2442. ( 10.1098/rspb.2009.0187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bell MBV, Radford AN, Rose R, Wade HM, Ridley AR. 2009. The value of constant surveillance in a risky environment. Proc. R. Soc. B 276, 2997–3005. ( 10.1098/rspb.2009.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hollén LI, Bell MBV, Radford AN. 2008. Cooperative sentinel calling? Foragers gain increased biomass intake. Curr. Biol. 18, 576–579. ( 10.1016/j.cub.2008.02.078) [DOI] [PubMed] [Google Scholar]
- 71.Wiley RH. 2013. Signal detection, noise, and the evolution of communication. In Animal communication and noise (ed. Brumm H.), pp. 7–30. Heidelberg: Springer; ( 10.1007/978-3-642-41494-7) [DOI] [Google Scholar]
- 72.Pocklington R, Dill LM. 1995. Predation on females or males: who pays for bright male traits? Anim. Behav. 49, 1122–1124. ( 10.1006/anbe.1995.0141) [DOI] [Google Scholar]
- 73.Colombelli-Negrel D, Kleindorfer S. 2010. Video nest monitoring reveals male coloration-dependant nest predation and sex differences in prey size delivery in a bird under high sexual selection. J. Ornithol. 151, 507–512. ( 10.1007/s10336-009-0480-5) [DOI] [Google Scholar]
- 74.Goodale E, Kotagama SW. 2008. Response to conspecific and heterospecific alarm calls in mixed-species bird flocks of a Sri Lankan rainforest. Behav. Ecol. 19, 887–894. ( 10.1093/beheco/arn045) [DOI] [Google Scholar]
- 75.Martınez AE, Zenil RT. 2012. Foraging guild influences dependence on heterospecific alarm calls in Amazonian bird flocks. Behav. Ecol. 23, 544–550. ( 10.1093/beheco/arr222) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting this article are available in the electronic supplementary material.