Abstract
A long-standing tenet of evolutionary endocrinology states that testosterone mediates the life-history trade-off between mating and paternal care. However, the support for a role of testosterone in suppressing paternal care is mixed: implantation studies in birds suggest that high-level testosterone implants suppress paternal care, but circulating levels of testosterone and paternal care are typically not correlated. Because any trade-off in real life must be realized with hormone levels that are within an individual's reaction norm, it is crucial to show that natural changes in the hormone can modulate behaviour. Here, we used GnRH-injections to alter testosterone levels of free-living male black redstarts within each individual's hormonal reaction norm: individuals experiencing a short-term peak in testosterone resumed feeding their offspring later and showed a stronger suppression of offspring-feeding behaviour than control males. For the first time, this study demonstrated that short-term peaks in testosterone within the hormonal reaction norm of individuals can suppress paternal behaviour. Our findings reconcile previous seemingly contradictive effects that testosterone implants had on paternal care and the absence of correlations between circulating testosterone levels and paternal care, and demonstrate that the differential production of testosterone within the hormonal reaction norm of single individuals can indeed function as a mechanism to mediate a potential trade-off between mating and parenting. On a broader note, our results suggest that natural and short peaks in testosterone can elicit adaptive behavioural changes.
Keywords: hormonal reactive scope, parental care, trade-off, gonadotropin-releasing hormone, reaction norm
1. Introduction
Resources such as space, energy and time are typically limited. Given such constraints, life-history theory predicts trade-offs, i.e. resources invested into one trait contributing to fitness limit the possibility to invest into other fitness-relevant traits [1]. For example, searching for mating partners and providing parental care represent fundamental life-history traits with often conflicting needs. In particular, when periods of mating and offspring care overlap, individuals have to trade off mating effort with parental effort, because in most cases time and energy invested into searching for additional mating partners cannot be allocated to offspring care [2,3]. Various studies have suggested that in many vertebrates, this trade-off is regulated by the sex steroid testosterone (e.g. [4–8]). In particular, it is thought that in male birds, high levels of testosterone promote male mating effort and suppress paternal care behaviour (e.g. [4,8–10]). For males of species with biparental care, the challenge hypothesis [4] predicts rapid adjustments of circulating testosterone concentrations (i.e. high androgen responsiveness). Testosterone concentrations should rise during periods of mate guarding and when fertile females become available, but they should remain at baseline during other times, in particular during parenting [4,11,12]. When periods with mating opportunities and paternal care overlap there is conflict, and testosterone may mediate the immediate decision to either invest into mating or into paternal behaviour. The notion that high levels of testosterone promote mating effort and suppress paternal care in birds is a central tenet in evolutionary endocrinology, but it is still subject of debate because of conflicting empirical evidence.
A large number of studies suggest that testosterone increases mating behaviour in male vertebrates (reviewed in [6,13,14]), and implants of this hormone have been demonstrated to also decrease paternal care in many species of birds. Testosterone implants suppressed incubation behaviour of male blue-headed vireos (Vireo solitaries; [15]), rufous whistlers (Pachycephala rufiventris; [16]), spotted sandpipers (Actitis macularia; [17]) and yellow-legged gulls (Larus cachinnans; [18]), and paternal feeding rates in barn swallows (Hirundo rustica [19]), blue-headed vireos [15], dark-eyed juncos (Junco hyemalis; [20,21]), house finches (Carpodacus mexicanus; [22]), house sparrows (Passer domesticus; [23]), Lapland longspurs (Calcarius lapponicus; [24]), pied flycatchers (Ficedula hypoleuca; [25]), reed warblers (Acrocephalus ssp.; [26]), rufous-collared sparrows (Zonotrichia capensis; [27]), spotless starlings (Sturnus unicolor; [28]), starlings (Sturnus vulgaris; [29]), snow buntings (Plectrophenax nivalis; [30]), superb fairy wrens (Malurus cyaneus [31]), and yellow-headed blackbirds (Xanthocephalus xanthocephalus; [32]). Only in chestnut-collared longspurs (Calcarius ornatus; [33]) and great tits (Parus major; [34,35]) testosterone implants did not affect paternal care. Thus, the majority of testosterone manipulation studies support a role of this hormone in suppressing paternal care.
However, results of testosterone manipulations are in contrast to published studies relating the naturally occurring variance in testosterone levels with paternal care: post-capture testosterone concentrations (termed ‘baseline testosterone’ for the remainder of this article) were not related to paternal care in barn swallows [36], black redstarts (Phoenicurus ochruros; [37]), dark-eyed juncos [38], eastern bluebirds (Sialia sialia [39]), European starlings [40], and northern cardinals (Cardinalis cardinalis; [41]; note, however, that in dark-eyed juncos maximum testosterone was related to pre-capture levels of paternal care [38]). Male tawny owls (Strix aluco) with higher testosterone levels showed even higher provisioning rates than males with lower circulating levels of the hormone [42]. Thus, there is little indication that males with higher baseline levels of testosterone are less paternal than males with lower baselines.
Where does this discrepancy between implantation studies and the natural variance in testosterone in relation to paternal care come from? Implantation studies have some drawbacks limiting their relevance in ecological and evolutionary contexts. First, testosterone implants lead to permanent long-term increases in testosterone disrupting the circadian pattern (e.g. [43–47]) and other natural short-term fluctuations in testosterone production. The permanent testosterone elevation by implants renders it difficult to differentiate between acute effects of short-term changes in testosterone (likely to be ecologically relevant), from those of chronic effects of the hormone (likely to represent ecologically less meaningful artefacts). Second—and more importantly—testosterone implants do not take into account the huge amount of variation in testosterone concentrations among individuals even within one life-history stage. For example, baseline testosterone concentrations differ by 60-fold (from 0.2 to 12 ng ml−1) between individual male black redstarts sampled during the mating life-history stage [48]. These substantial differences in individual baselines were strongly correlated with the respective individual hormonal reaction norms (or hormonal reactive scope [49]; defined as the range of concentrations of a hormone an individual can express in response to external or internal cues in a particular life-history stage): individuals with a low baseline also showed a low maximum testosterone release, whereas individuals with a high baseline also expressed high maximum levels. Baseline testosterone concentrations of high-baseline individuals were often even higher than the maxima that low-baseline individuals could reach [49].
Selection acts on the level of the individual, and in real life a hormone-dependent trait can only be affected by a change in hormone concentrations that is within the hormonal reactive scope of an individual. Thus, it is crucial to study a potential trade-off between mating and parenting at the level of the individual and at the level of the hormonal changes an individual can actually realize [36,50]. Testosterone implantation studies fail to achieve such an individual-level resolution, because testosterone implants typically elevate hormone concentrations to the population maximum during the period of territory establishment or even higher. Hence, the majority of males who receive testosterone implants during the parental phase experience testosterone concentrations that are many times higher than their own peak endogenous concentrations of testosterone during this phase. In most cases, the implants are likely to initially induce even supra-physiological concentrations [51,52], rendering it even more difficult to interpret behaviour associated with such chronic high hormone concentrations [13].
However, studies relating natural variation in circulating levels of testosterone to paternal care also have limitations. First, a point-measure of testosterone is correlated with an interval measure of paternal behaviour. Thus, one has to assume that the measured testosterone level is representative for the whole interval during which paternal behaviour was observed [13,50]. Second, hormones affect behaviour probabilistically, i.e. they modulate the likelihood of a behaviour to occur should the appropriate context arise (e.g. [13,53]). Such effects can be mediated by a hormonal threshold function [13,53,54]. If so, the level of the hormone is unlikely to correlate with the occurrence of the behavioural trait. When individuals differ in their respective hormonal thresholds, a correlation between hormone level and the frequency of the behavioural trait does not exist (for a detailed discussion of this topic, see [13,53,54]).
Testosterone implantation studies and correlations with behaviour have been pivotal to advance our knowledge regarding how hormones affect behaviour, but, as demonstrated above, both approaches have limits. To find out whether testosterone reduces the amount of care males provide and thus may regulate the trade-off between mating and paternal care, it is necessary to study changes in testosterone at the individual level and ask the following questions: first, do increases in testosterone within an individual's hormonal reactive scope reduce paternal care? And second—considering the fact that natural increases in testosterone above the breeding-baseline are likely to be short in duration [4]—do such short-term surges in testosterone affect paternal behaviour? In an ecologically relevant context, testosterone can only act as a mediator of the trade-off between mating and parenting if the answer to these questions is ‘yes’.
Here, we manipulated testosterone concentrations within the hormonal reactive scope of each individual, by injecting male black redstarts with gonadotropin-releasing hormone (GnRH). GnRH is the hypothalamic hormone that induces a hormonal cascade leading to a short-term peak-release of testosterone from the testes (reviewed by [13]). GnRH-injections can maximize testosterone release. By definition, the resulting increase in testosterone remains within the hormonal reactive scope of each individual, because the testes cannot produce more testosterone than a maximal stimulation with GnRH can induce them to do. We assumed that any effect of the GnRH-injection was due to an increase in circulating testosterone (or its oestrogenic metabolites after local conversion in the brain), because there is no evidence that GnRH or the pituitary hormone release triggered by GnRH would affect sexual, aggressive or parental behaviour of male birds [13,55–57]. We compared offspring-feeding rates of males immediately before and right after the induction of such a short peak-release of testosterone and compared their change in paternal behaviour with that of control males injected with saline. A similar approach has been used previously to investigate the effect of short-term surges in testosterone on aggression in European ground squirrels (Spermophilus citellus; [58]) and on territorial behaviour of black redstarts [49]. But to the best of our knowledge, this is the first study to test whether changes in testosterone within the physiological reactive scope of an individual suppresses paternal care.
Male black redstarts are ideal to study the behavioural effects of short-term increases in testosterone, because in this species baseline testosterone strongly varies between individuals (see above and [48]), and GnRH-injections lead to marked short-term increases in testosterone that vary in magnitude depending on the hormonal reactive scope of an individual [49]. Furthermore, the large natural variation in baseline testosterone between individual male black redstarts is unrelated to paternal behaviour [37]. At the same time, testosterone controls aspects of behaviour that may influence mating success in this species, i.e. it affects song parameters likely to influence female choice [59], and males that maintain higher levels of testosterone until the end of the breeding season were less likely to lose paternity [37]. Furthermore, black redstarts of our study population experience a long breeding season with little synchrony among pairs. Some pairs raise up to three broods [60], and there is a high incidence of extra-pair paternity, with 28.8% of nestlings not being fathered by the social mate [37]. Hence, if short-term peaks in testosterone that lie within an individuals' potential to produce this hormone would indeed suppress paternal care, this would be strong evidence for a role of testosterone in modulating the amount of paternal care and its potential to mediate the trade-off between mating and paternal care.
2. Material and methods
To validate the experimental protocol for this study, we first explored for how long a single injection of GnRH elicits a rise in circulating testosterone above baseline levels (see the electronic supplementary material). From these results regarding the effectiveness of GnRH-injections (testosterone remained elevated for a period of 60–90 min after injection of GnRH, see the electronic supplementary material), we derived the experimental protocol for studying changes in parental behaviour of male black redstarts in response to short-term increases in testosterone. This experiment was performed between 18 May–16 July 2015 and 13 May–6 July 2016, in the vicinity of the Max Planck Institute for Ornithology, Germany (N 47°, E 11°, 500–600 m.a.s.l.). We searched for nests of pairs of black redstarts containing nestlings that were at least one week old. For 60 min, baseline nestling feeding rates were established by observing nests from a distance, using binoculars. We established how often the female and the male entered the nest with food. After this initial observation period, males were caught with mealworm-baited ground traps or spring traps in the vicinity of the nest (approx. 5–20 m distance). Traps were observed at all times and upon capture, males were immediately removed from the traps. After measurement (body mass in g, lengths of the right tarsus and the wing in mm) and banding with a numbered aluminium ring (Vogelwarte Radolfzell) and a combination of three colour rings males were either injected with 50 µl saline (control group, N = 13), or with 1.25 µg chicken GnRH-I (Bachem H 3106) in 50 µl isotonic saline (GnRH group, N = 16) into the pectoralis major muscle. The experimenters were blind to the treatment. Within 5–10 min after capture, the birds were released back onto their respective territories. Based on the above observation that testosterone concentrations remained elevated after injection of GnRH for at least 30–60 min and had returned to or below baseline after 90–120 min (electronic supplementary material), we decided to observe nests for a total of 120 min after releasing the freshly injected bird to establish the latency of males to return to their nest with food, and to measure the feeding rates of males (and their female partners) after the respective injections. Even if testosterone was elevated only for a period of 30–60 min, we expected a longer lasting effect, because testosterone does not act as an on/off switch of behaviour, but influences behaviour by binding to genomic androgen receptors in the brain, or by being locally converted to oestradiol and binding to genomic oestrogen receptors (reviewed by [8,13]). In both of these cases, these receptors then affect gene expression, which will ultimately modulate behaviour. In addition, oestradiol can bind to a membrane receptor that directly affects protein synthesis resulting in faster effects [56]. Because of this cascade of hormone expression, conversion, receptor binding, and resulting changes in gene expression and protein synthesis, we expected a longer lasting effect on behaviour, even if the peak in testosterone occurred only for 30–60 min. Furthermore, we are not aware of any published evidence that releasing hormones (such as GnRH) or gonadotropins (such as luteinizing hormone (LH), i.e. the pituitary hormone that is released upon GnRH stimulation and induces the production of testosterone) would directly affect paternal care. Also, earlier reports from the 1950s and 1960s suggesting GnRH or LH could directly affect sexual or aggressive behaviour in male birds independent of gonadal steroids have been refuted [13,55–57]. We thus assumed that any effect of the GnRH-injection was due to the increase in circulating testosterone concentrations (or its local conversion to oestradiol or dihydrotestosterone) and not due to direct effects of the peptides involved in the signalling cascade of the hypothalamic pituitary gonadal axis. Finally, we are also not aware of any report suggesting that adrenaline or corticosterone would be differentially affected by capture and injection with either saline or GnRH, and we thus assumed that capture and saline injection represent an appropriate control treatment for capture and GnRH-injection.
Saline and GnRH-injected males were similar in body mass (reported as means and their 95% credible intervals in squared brackets: saline: 17.2 [16.7–17.8] g; GnRH: 17.2 [16.7–17.7] g), tarsus length (saline: 23.4 [23.0–23.8] mm; GnRH: 23.4 [23.1–23.8] mm), and wing length (saline: 85.3 [84.3–86.3] mm; GnRH: 85.9 [84.9–86.8] mm). Also, paternal feeding rates before capture did not differ between the two groups (for details on this, see Results).
(a). Statistical analysis
Statistical analyses were conducted using R v. 3.03 [61] and a Bayesian statistical approach using the packages ‘BayesianFirstAid’ [62], ‘arm’ [63] and ‘lme4’ [64]. We looked at two different components of the feeding behaviour of male black redstarts that are not independent, but reveal different aspects of the behaviour: (i) the latency period between releasing males until they resumed feeding their offspring as a measure of whether or not they are feeding at all and (ii) the frequency of feeding behaviour as a measure of the intensity of the behaviour.
The latency period between releasing males until they resumed feeding their offspring was compared using the Bayesian equivalent of a t-test (bayes.t.test in ‘BayesianFirstAid’). A generalized linear mixed effect model (glmer in package ‘lme4’) with Poisson distribution was used to test whether feeding rates of males differed before and after injection of either GnRH or saline. Individual ID was included as a random effect and to control for overdispersion we added an observation level random effect (following the recommendations by Korner-Nievergelt et al. [65], resulting in an overdispersion value of 1.05, which seemed acceptable). We also compared the subset of control and GnRH-injected males that were feeding during the second hour to see whether feeding rates differed between males of both groups that were actually feeding their offspring, using a general linear model with a Poisson distribution. The feeding rates of females were analysed using a similar glmer approach as described for males (overdispersion: 1.02). Model residuals were analysed using graphical methods (i.e. qq plots of residuals, fitted values versus residuals) for homogeneity of variance, violation of normality assumptions or other departures from model assumptions and model fit. For inferences from the models, we obtained Bayesian parameter estimates and their 95% credible intervals (running 10 000 simulations of the function bsim of the R package ‘arm’; [63]), using an uninformed prior distribution [65]. In frequentist statistics, the statistical test provides a p-value describing the probability that the null hypothesis is true given the data. Bayesian statistics does not provide such p-values. Instead, meaningful differences between groups can be assessed by comparing the ranges of the 95% credible intervals between groups. The 95% credible interval provides an estimate for the group mean with a probability of 0.95. If the credible interval of one group does not overlap with the mean estimate of another group, the groups can be assumed to differ from each other. In our view, Bayesian estimates and credible intervals enable a more intuitive and meaningful comparison of groups than the frequentist statistics and its reliance on an arbitrary α-value referring to the likelihood of the null hypothesis being true. If not indicated otherwise, data are presented as individual data points in combination with Bayesian posterior means and their respective 95% credible intervals (reported in the text within squared brackets). We also provide the posterior probability P(β) of the likelihood that the parameter estimates are larger than zero, with values of P(β) close to either zero or one indicating statistically meaningful effects. A P(β) of zero indicates a negative effect with a mean effect size and its 95% credible interval being negative (smaller than zero), and a P(β) of one or close to one indicates a positive effect with a mean effect size and its 95% credible interval being positive (larger than zero). Finally, we provide measures of the goodness of fit of the models (i.e. how much of the variance they explain) by reporting R2-values for linear models, or the respective marginal and conditional R2-values for mixed models following Nakagawa & Schielzeth [66]. The marginal R2-value represents the variation explained by the fixed effects of a mixed model, whereas the conditional R2-value reflects the combined variation explained by fixed and random effects.
3. Results
(a). Latency to start refeeding after capture
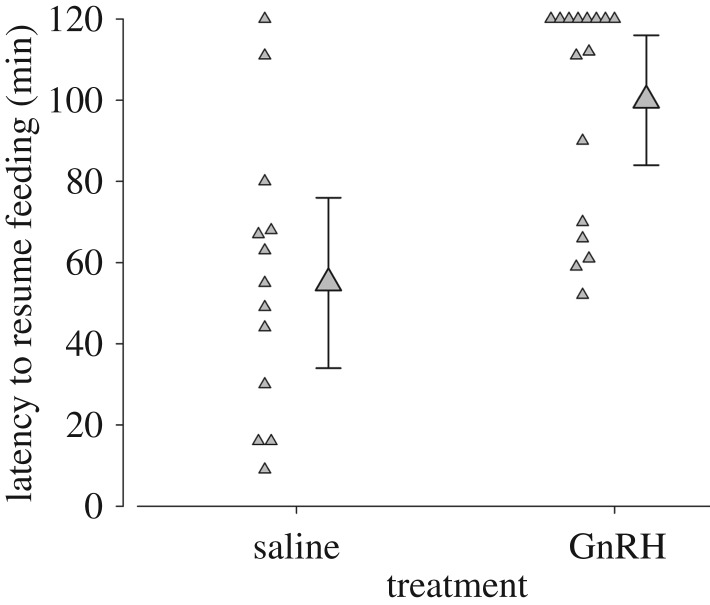
Saline-injected males had a much shorter latency to resume feeding their offspring after having been released than males injected with GnRH (figure 1). The data suggest with a 99.8% likelihood that the latency of saline-injected males was shorter than that of GnRH-injected males, rendering strong support for a true difference between the two groups.
Figure 1.
Saline- and GnRH-treated males differed in the latency until they resumed feeding their offspring. If males did not resume feeding within 120 min after they had been released, then latency was set to 120 min. Large, grey triangles and error bars represent posterior means and their 95% credible intervals. If the 95% credible intervals of one group do not overlap with the mean of the other group a statistically meaningful difference between the groups can be assumed. Smaller triangles represent data points of each single individual (N = 13 saline-treated and 16 GnRH-treated males).
(b). Reduction in feeding rates
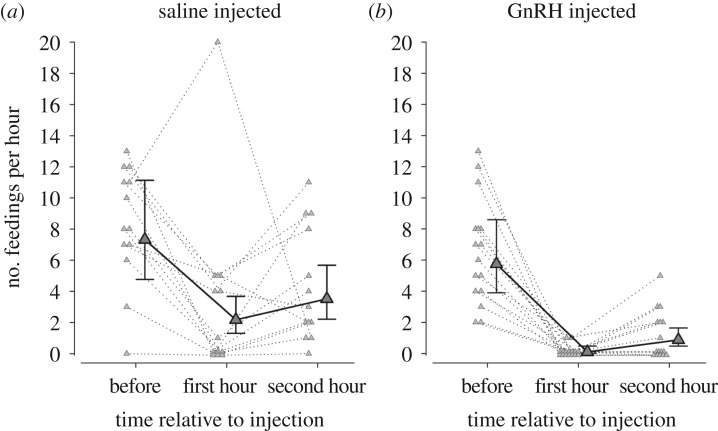
As an alternative—albeit not independent—measure to latency, we also examined the change in feeding frequency. Feeding rates of male black redstarts in the saline- and GnRH-injected groups were similar before the treatment (i.e. the credible intervals of the feeding rates of saline and GnRH-injected males before the treatment overlap with the respective other mean estimate in figure 2), but strongly differed after the treatment. Both groups reduced feeding during the first and second hour after having been released, but GnRH-injected males did so to a much stronger degree than saline-injected males (figure 2; treatment (P(β) = 0.21); time (before first hour: P(β) = 0; before second hour: P(β) = 0.0078); interaction between treatment and time (before first hour: P(β) = 0.0007; before second hour: P(β) = 0.006). The fixed effects explained 67.7% of the variance in the data (conditional R2 = 0.677), and the random effect (male identity) explained an additional 2.3% of the variance (marginal R2 = 0.700).
Figure 2.
(a,b) The effect of injecting male black redstarts with either 50 µl saline or 1.25 µg GnRH in 50 µl saline on offspring-feeding rates during the hour before, and 1 and 2 h after the respective treatment. Before treatment there was no difference, but during the first and the second hour GnRH-injected males showed a stronger suppression of feeding rates than control males. Large, grey triangles and error bars represent posterior means and their 95% credible intervals. Small triangles and dotted lines represent data points of each single individual (males that showed a feeding rate of zero are depicted in two parallel rows). The lack of overlap of the 95% credible intervals with the respective means of the saline or GnRH-injected group during the first and second hour after treatment illustrate the large treatment effect (N = 13 saline-treated and 16 GnRH-treated males).
Comparing the feeding rates of only those males that fed their offspring in the second hour after the treatment, we found that control males fed their offspring more often (4.75 [3.65–6.15] feedings per hour) than GnRH-injected males (2.58 [1.61–4.09] feedings per hour; P(β) = 0.011).
Females did not compensate for the reduction in feeding rates of their male partners (treatment: P(β) = 0.825, time: P(β) = 0.66; interaction between treatment and time: P(β) = 0.25). The fixed effects explained only 0.5% of the variance in the female feeding rate data (conditional R2 = 0.005). Most of the explained variance (8.5%) stems from the random effect of nest identity (marginal R2 = 0.09).
4. Discussion
A fundamental tenet of evolutionary endocrinology of tetrapods with paternal contributions to offspring care has been that testosterone mediates the trade-off between mating and paternal care (e.g. [4,8–10]). Despite this, no study has as of yet demonstrated that testosterone concentrations within the hormonal reaction norm of an individual can suppress paternal behaviour. This study showed that short-term peaks in testosterone within the hormonal reaction norm of individual black redstarts suppressed paternal care: males that were caught and injected with GnRH to induce an individual short-term peak in endogenous testosterone production refrained from feeding their offspring for a longer period of time and showed a stronger reduction in feeding rates than males that were treated with saline only. Because females of saline and GnRH-injected males maintained similar feeding rates throughout the 3-h period we can exclude that variables or disturbances other than capture and treatment influenced feeding rates of males. To the best of our knowledge, this is the first study to investigate the role of testosterone in paternal care at the level of individually achievable testosterone concentrations. We demonstrated that short testosterone surges within the individual hormonal reaction norm affected paternal care.
Male black redstarts are highly territorial throughout most of the year and testosterone does not seem to play a large role in regulating their territorial behaviour [49,59,60,67]. However, testosterone does modulate song characteristics of male black redstarts likely to be important in male–male competition and female choice [59,68]. Furthermore, males that maintain higher levels of testosterone until the end of the breeding season are less likely to lose paternity [37]. Thus, testosterone is likely to be involved in mediating mating effort and success in this species. This study now demonstrated that changes in testosterone can adjust paternal effort, demonstrating that changes in circulating levels of testosterone within an individual's reaction norm could mediate a trade-off between mating and paternal care. In addition to the relevance of our data for understanding the hormonal regulation of behaviour in our particular study species, we believe that the results of this study represent a crucial advance in the field of ecological and evolutionary endocrinology for several reasons.
First, our study accounted for the large variation in testosterone concentrations that exists among male birds even within the same breeding stage (e.g. [48,69]) and the variation of maximal testosterone concentrations that different individuals can realize [49,70]. By default, the GnRH-injections increased testosterone only within the hormonal reactive scope of each individual, showing that testosterone increases within an individual's reaction norm can suppress paternal care. This represents a crucial demonstration of the ecological and evolutionary relevance of testosterone as a potential mediator of trade-offs. Natural and sexual selection act on the level of the individual phenotype, and for this reason testosterone can only mediate the trade-off between mating and paternal care if changes in testosterone concentrations within the reactive scope of an individual are effective in modulating behaviour [50]. By default, testosterone implantation studies cannot demonstrate such an ecologically relevant effect, because they typically increase the hormone concentrations far beyond the reactive scope of most, if not all individuals.
Second, our findings reconcile the absence of a correlation between post-capture levels of testosterone and the degree of paternal care in black redstarts [37] and other bird species [36,38,40,41] with the function of testosterone as a potential mediator of the trade-off between mating and paternal care. From previous studies in redstarts, we know that individuals differ in baseline- and peak-testosterone concentrations, indicating individual hormonal reaction norms (e.g. [37,48,49,67]). This variation in hormonal reaction norms between individuals renders it difficult to correlate the natural variance in testosterone and paternal care. An example may illustrate this: the scheme in figure 3a illustrates that baseline testosterone concentrations are unrelated to paternal care. The blue individual has a naturally high-baseline level of testosterone (level X) and a reaction norm starting at level X and ending at level Y. At level X paternal behaviour of the blue individual is not suppressed, but when testosterone rises to level Y, feeding rates decrease (figure 3). By contrast, the red individual has a naturally lower baseline level W and its reaction norm spans from level W to level X. Baseline concentration W does not affect paternal behaviour of the red individual, but at level X its paternal behaviour is reduced (figure 3). Thus, the same absolute concentration of testosterone (level X) may or may not suppress paternal behaviour depending on individual hormonal reaction norms or threshold levels upon which a suppression of paternal care is realized (for a detailed discussion of this effect, see [53]). This may explain why a correlation between baseline or maximum testosterone and paternal care among individuals does not exist (scheme in figure 3a and actual data in [37]), but why injections of GnRH can lead to a reduction in paternal behaviour (this study). In line with this notion, the degree of paternal care in dark-eyed juncos (measured only before capture) did not correlate with post-capture concentrations of baseline and GnRH-induced testosterone, but with the increase in testosterone between baseline and GnRH-induced maximum ([38]; but see the studies on black redstarts [37], northern cardinals [41] and eastern bluebirds [71] for contrasting results). In summary, previous studies have shown that individual black redstarts differ largely in baseline testosterone and in the magnitude of their testosterone response to GnRH-injections. Furthermore, baseline testosterone and the degree of paternal care are unrelated in this (and other) species, but injections of GnRH led to (individually distinct) testosterone concentrations that suppressed paternal behaviour in all individuals. While we cannot rule out the possibility that baseline testosterone and paternal care are unrelated because a hormonal point-measure is correlated with an interval measure of behaviour (see Introduction and [13,50]), our results are consistent with the existence of individual reaction norms and individual thresholds of testosterone necessary to suppress paternal care.
Figure 3.
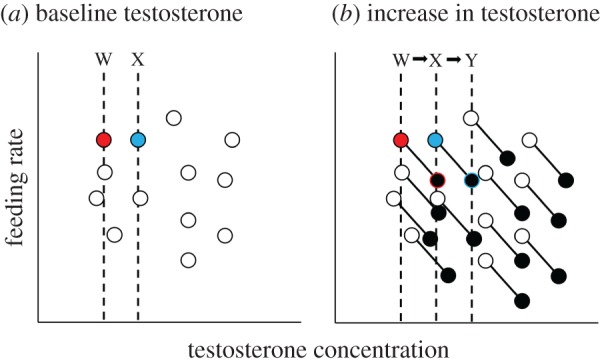
Schematic drawing of the relationship between circulating testosterone and paternal care in black redstarts. Baseline testosterone concentrations differ between individuals and are statistically unrelated to feeding rates (a; open circles; see also [37]). Also maximum testosterone concentrations differ between individuals and are statistically unrelated to feeding rates (b; closed circles; see also [37]). However, as demonstrated by the current study, a short-term increase in testosterone decreases feeding rates of individual males. (b) The hormonal reaction norm of each individual and its effect on feeding rates represented by the connected open and closed circles (for simplicity, we are assuming similar slopes and magnitudes in testosterone increase). For example, the red and the blue individual exert similar baseline feeding rates, but differ in baseline testosterone (a) and their hormonal reaction norms (b). The red male has a lower hormonal reaction norm (level W to X) than the blue male (level X to Y). At level X, paternal care of the blue male is not suppressed, but at the same concentration paternal care of the red individual is suppressed. Because hormonal reaction norms differ, different levels of testosterone are required to suppress paternal care, i.e. the blue individual ‘needs’ much higher levels of testosterone than the red individual to suppress paternal care (for further details, see main text). Note that this scheme represents a simplification of real life, where the magnitude of the testosterone increase and an individuals' paternal responsiveness to the increase in testosterone (i.e. the slope of the relationship between testosterone and paternal care) may differ between individuals.
Third, the results of our study demonstrate that even short-term peaks of testosterone can affect paternal care. Because our manipulation of testosterone involved capture, we cannot distinguish exactly when the additional effect of an increase in testosterone surmounted the suppressive effect of capture-stress. However, most saline-injected individuals resumed feeding their offspring within less than 60 min after release, whereas GnRH-injected males typically took 100 min or more to resume feeding. Hence, the additional suppressive effect of a short peak of testosterone on paternal behaviour must have been effective at least within 60 min after the injection and lasted for at least another hour in the majority of cases. Because the effects occur on such a short time-scale, it is possible that testosterone did not only act via genomic receptors, but also via local conversion to oestradiol and binding to an oestrogenic membrane receptor [56].
Fourth, our findings reconcile results of testosterone implantation studies with the absence of a correlation of baseline testosterone and paternal behaviour in birds. Testosterone implants lead to chronic elevations of testosterone to a level that is either close to the maximum of a population or may be even supra-physiological. Hence, results from implantation studies may theoretically be of limited validity in ecologically relevant contexts. However, we have shown that even short-term elevation of testosterone within an individual's hormonal reaction norm suppress paternal care in a similar way as implantation studies. This result may be thus considered as a validation of implantation studies: even though testosterone implants raise endogenous testosterone concentrations to the population maximum or even higher, the effect of such implants is similar to that of endogenously elevated levels of testosterone within an individual's reactive scope. This may hint at a threshold mechanism of testosterone function in mediating the trade-off between mating and paternal care: below a certain level of testosterone, paternal care is not suppressed, but once an individual's threshold is reached, paternal care is reduced, regardless of how high testosterone rises even above this particular threshold [13,53,54].
In conclusion, to the best of our knowledge, our study is the first unambiguous demonstration that testosterone levels realized within the reactive scope of single individuals are capable of suppressing paternal care, showing that real-life changes in testosterone can indeed mediate a life-history trade-off between mating and paternal care behaviours. Thus, natural individual short-term changes in testosterone may elicit behavioural changes that are relevant in the contexts of ecology and Darwinian fitness.
Supplementary Material
Acknowledgements
We thank Manfred Gahr for support, Martin Küblbeck for assistance in the field, Monika Trappschuh for assistance with the hormone analysis, and Michaela Hau, Henrik Brumm, and two anonymous referees for constructive feedback on earlier versions of the manuscript.
Ethics
All experimental procedures were conducted according to the legal requirements of Germany and were approved by the governmental authorities of Oberbayern, Germany.
Data accessibility
The data for this contribution are available at: http://dx.doi.org/10.5061/dryad.40kp5 [72].
Authors' contributions
W.G. conceived of the study, conducted the analysis and drafted the manuscript; W.G. and P.F. designed the study, acquired the data and revised the manuscript, and both W.G. and P.F. approved of the final version.
Competing interests
We declare we have no competing interests.
Funding
We acknowledge funding and support from the Max-Planck-Gesellschaft.
References
- 1.Stearns SC. 1992. The evolution of life histories. Oxford, NY: Oxford University Press. [Google Scholar]
- 2.Trivers R. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man 1871–1971 (ed. Campbell BG.), pp. 136–179. Chicago, IL: Aldine Publishing Company. [Google Scholar]
- 3.Magrath MJL, Komdeur J. 2003. Is male care compromised by additional mating opportunity? Trends Ecol. Evol. 18, 424–430. ( 10.1016/S0169-5347(03)00124-1) [DOI] [Google Scholar]
- 4.Wingfield JC, Hegner RE, Dufty AM, Ball GF. 1990. The ‘challenge hypothesis': theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846. ( 10.1086/285134) [DOI] [Google Scholar]
- 5.Hirschenhauser K, Oliveira RF. 2006. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim. Behav. 71, 265–277. ( 10.1016/j.anbehav.2005.04.014) [DOI] [Google Scholar]
- 6.Lynn SE. 2008. Behavioral insensitivity to testosterone: Why and how does testosterone alter paternal and aggressive behavior in some avian species but not others? Gen. Comp. Endocrinol. 157, 233–240. ( 10.1016/j.ygcen.2008.05.009) [DOI] [PubMed] [Google Scholar]
- 7.Ketterson ED, Nolan V. 1992. Hormones and life histories: an integrative approach. Am. Nat. 140(Suppl.), S33–S62. ( 10.1086/285396) [DOI] [PubMed] [Google Scholar]
- 8.Hau M. 2007. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. Bioessays 29, 133–144. ( 10.1002/bies.20524) [DOI] [PubMed] [Google Scholar]
- 9.Ketterson ED, Nolan V. 1999. Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 154, S4–S25. ( 10.1086/303280) [DOI] [PubMed] [Google Scholar]
- 10.Goymann W. 2010. Pair-bonding, mating systems and hormones. In Encyclopedia of animal behavior (eds Breed MD, Moore J), pp. 611–617. Oxford, UK: Academic Press. [Google Scholar]
- 11.Goymann W, Landys MM, Wingfield JC. 2007. Distinguishing seasonal androgen responses from male-male androgen responsiveness - revisiting the challenge hypothesis. Horm. Behav. 51, 463–476. ( 10.1016/j.yhbeh.2007.01.007) [DOI] [PubMed] [Google Scholar]
- 12.Goymann W. 2009. Social modulation of androgens in male birds. Gen. Comp. Endocrinol. 163, 149–157. ( 10.1016/j.ygcen.2008.11.027) [DOI] [PubMed] [Google Scholar]
- 13.Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton, NJ: Princeton University Press. [Google Scholar]
- 14.Wingfield JC, Moore IT, Goymann W, Wacker D, Sperry T. 2006. Contexts and ethology of vertebrate aggression: implications for the evolution of hormone-behavior interactions. In Biology of aggression (ed. Nelson R.), pp. 179–210. New York, NY: Oxford University Press. [Google Scholar]
- 15.Van Roo BL. 2004. Exogenous testosterone inhibits several forms of male parental behavior and stimulates song in a monogamous songbird: the blue-headed vireo (Vireo solitarius). Horm. Behav. 46, 678–683. ( 10.1016/j.yhbeh.2004.06.011) [DOI] [PubMed] [Google Scholar]
- 16.McDonald PG, Buttemer WA, Astheimer LB. 2001. The influence of testosterone on territorial defence and parental behavior in male free-living rufous whistlers, Pachycephala rufiventris. Horm. Behav. 39, 185–194. ( 10.1006/hbeh.2001.1644) [DOI] [PubMed] [Google Scholar]
- 17.Oring LW, Fivizzani AJ, El Halawani ME. 1989. Testosterone-induced inhibition of incubation in the spotted sandpiper (Actitis macularia). Horm. Behav. 23, 412–423. ( 10.1016/0018-506X(89)90053-6) [DOI] [PubMed] [Google Scholar]
- 18.Alonso-Alvarez C. 2001. Effects of testosterone implants on pair behaviour during incubation in the yellow-legged gull Larus cachinnans. J. Avian Biol. 32, 326–332. ( 10.1111/j.0908-8857.2001.320406.x) [DOI] [Google Scholar]
- 19.Saino N, Møller AP. 1995. Testosterone-induced depression of male parental behavior in the barn swallow: female compensation and effects on seasonal fitness. Behav. Ecol. Sociobiol. 36, 151–157. ( 10.1007/BF00177791) [DOI] [Google Scholar]
- 20.Ketterson ED, Nolan V, Wolf L, Ziegenfus C. 1992. Testosterone and avian life histories: effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis). Am. Nat. 140, 980–999. ( 10.1086/285451) [DOI] [Google Scholar]
- 21.Schoech SJ, Ketterson ED, Nolan V, Sharp PJ, Buntin JD. 1998. The effect of exogenous testosterone on parental behavior, plasma prolactin, and prolactin binding-sites in dark-eyed juncos. Horm. Behav. 34, 1–10. ( 10.1006/hbeh.1998.1455) [DOI] [PubMed] [Google Scholar]
- 22.Stoehr AM, Hill GE. 2000. Testosterone and the allocation of reproductive effort in male house finches (Carpodacus mexicanus). Behav. Ecol. Sociobiol. 48, 407–411. ( 10.1007/s002650000247) [DOI] [Google Scholar]
- 23.Hegner RE, Wingfield JC. 1987. Effects of experimental manipulations of testosterone levels on parental investment and breeding success in male house sparrows. Auk 104, 462–469. ( 10.2307/4087545) [DOI] [Google Scholar]
- 24.Hunt KE, Hahn TP, Wingfield JC. 1999. Endocrine influences on parental care during a short breeding season: testosterone and male parental care in Lapland longspurs (Calcarius lapponicus). Behav. Ecol. Sociobiol. 45, 360–369. ( 10.1007/s002650050572) [DOI] [Google Scholar]
- 25.Silverin B. 1980. Effects of long-acting testosterone treatment on free-living pied flycatchers, Ficedula hypoleuca, during the breeding period. Anim. Behav. 28, 906–912. ( 10.1016/S0003-3472(80)80152-7) [DOI] [Google Scholar]
- 26.Dittami J, Hoi H, Sageder G. 1991. Parental investment and territorial/sexual behavior in male and female reed warblers: are they mutually exclusive? Ethology 88, 249–255. ( 10.1111/j.1439-0310.1991.tb00279.x) [DOI] [Google Scholar]
- 27.Lynn SE, Prince LE, Schook DM, Moore IT. 2009. Supplementary testosterone inhibits paternal care in a tropically breeding sparrow, Zonotrichia capensis. Physiol. Biochem. Zool. 82, 699–708. ( 10.1086/605915) [DOI] [PubMed] [Google Scholar]
- 28.Moreno J, Veiga JP, Cordero PJ, Minguez E. 1999. Effects of paternal care on reproductive success in the polygynous spotless starling Sturnus unicolor. Behav. Ecol. Sociobiol. 47, 47–53. ( 10.1007/s002650050648) [DOI] [Google Scholar]
- 29.De Ridder E, Pinxten R, Eens M. 2000. Experimental evidence of a testosterone-induced shift from paternal to mating behaviour in a facultatively polygynous songbird. Behav. Ecol. Sociobiol. 49, 24–30. ( 10.1007/s002650000266) [DOI] [Google Scholar]
- 30.Lynn SE, Walker BG, Wingfield JC. 2005. A phylogenetically controlled test of hypotheses for behavioral insensitivity to testosterone in birds. Horm. Behav. 47, 170–177. ( 10.1016/j.yhbeh.2004.10.004) [DOI] [PubMed] [Google Scholar]
- 31.Peters A. 2002. Testosterone and the trade-off between mating and paternal effort in extrapair-mating superb fairy-wrens. Anim. Behav. 64, 103–112. ( 10.1006/anbe.2002.3037) [DOI] [Google Scholar]
- 32.Beletsky LD, Gori DF, Freeman S, Wingfield JC. 1995. Testosterone and polygyny in birds. Curr. Ornithol. 12, 1–41. ( 10.1007/978-1-4615-1835-8_1) [DOI] [Google Scholar]
- 33.Lynn SE, Hayward LS, Benowitz-Fredericks ZM, Wingfield JC. 2002. Behavioural insensitivity to supplementary testosterone during the parental phase in the chestnut-collared longspur, Calcarius ornatus. Anim. Behav. 63, 795–803. ( 10.1006/anbe.2001.1980) [DOI] [Google Scholar]
- 34.Van Duyse E, Pinxten R, Eens M. 2002. Effects of testosterone on song, aggression, and nestling feeding behavior in male great tits, Parus major. Horm. Behav. 41, 178–186. ( 10.1006/hbeh.2001.1747) [DOI] [PubMed] [Google Scholar]
- 35.Van Duyse E, Pinxten R, Eens M. 2000. Does testosterone affect the trade-off between investment in sexual/territorial behaviour and parental care in male great tits? Behaviour 137, 1503–1515. ( 10.1163/156853900502691) [DOI] [Google Scholar]
- 36.Eikenaar C, Whitham M, Komdeur J, van der Velde M, Moore IT. 2011. Endogenous testosterone is not associated with the trade-off between paternal and mating effort. Behav. Ecol. 22, 601–608. ( 10.1093/beheco/arr030) [DOI] [Google Scholar]
- 37.Villavicencio CP, Apfelbeck B, Goymann W.. 2014. Parental care, loss of paternity and circulating levels of testosterone and corticosterone in a socially monogamous song bird. Front. Zool. 11, 11 ( 10.1186/1742-9994-11-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGlothlin JW, Jawor JM, Ketterson ED. 2007. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. Am. Nat. 170, 864–875. [DOI] [PubMed] [Google Scholar]
- 39.Burtka JL, Lovern MB, Grindstaff JL. 2016. Baseline hormone levels are linked to reproductive success but not parental care behaviors. Gen. Comp. Endocrinol. 229, 92–99. ( 10.1016/j.ygcen.2016.03.010) [DOI] [PubMed] [Google Scholar]
- 40.Pinxten R, de Ridder E, Arckens L, Darras V, Eens M. 2007. Plasma testosterone levels of male European starlings (Sturnus vulgaris) during the breeding cycle and in relation to song and paternal care. Behaviour 144, 393–410. ( 10.1163/156853907780756003) [DOI] [Google Scholar]
- 41.DeVries MS, Jawor JM. 2013. Natural variation in circulating testosterone does not predict nestling provisioning rates in the northern cardinal, Cardinalis cardinalis. Anim. Behav. 85, 957–965. ( 10.1016/j.anbehav.2013.02.019) [DOI] [Google Scholar]
- 42.Sasvári L, Péczely P, Hegyi Z. 2009. Plasma testosterone profile of male tawny owls Strix aluco in relation to breeding density, breeding experience, and offspring provisioning. Acta Ornithol. 44, 59–68. ( 10.3161/000164509X464894) [DOI] [Google Scholar]
- 43.Aschoff J. 1979. Circadian rhythms: general features and endocrinological aspects. In Endocrine rhythms (ed. Krieger DT.), pp. 1–61. New York, NY: Raven Press. [Google Scholar]
- 44.Guchhait P, Haldar C. 1999. Circadian rhythms of melatonin and sex steroids in a nocturnal bird, Indian spotted owlet Athene brama during reproductively active and inactive phases. Biol. Rhythm Res. 30, 508–516. ( 10.1076/brhm.30.5.508.1400) [DOI] [Google Scholar]
- 45.Hau M, Romero LM, Brawn JD, Van't Hof TJ. 2002. Effect of polar day on plasma profiles of melatonin, testosterone, and estradiol in high-arctic lapland longspurs. Gen. Comp. Endocrinol. 126, 101–112. ( 10.1006/gcen.2002.7776) [DOI] [PubMed] [Google Scholar]
- 46.Laucht S, Dale J, Mutzel A, Kempenaers B. 2011. Individual variation in plasma testosterone levels and its relation to badge size in house sparrows Passer domesticus: It's a night-and-day difference. Gen. Comp. Endocrinol. 170, 501–508. ( 10.1016/j.ygcen.2010.11.007) [DOI] [PubMed] [Google Scholar]
- 47.Goymann W, Trappschuh M. 2011. Seasonal and diel variation of hormone metabolites in European stonechats: on the importance of high signal-to-noise ratios in noninvasive hormone studies. J. Biol. Rhythms 26, 44–54. ( 10.1177/0748730410388394) [DOI] [PubMed] [Google Scholar]
- 48.Apfelbeck B, Mortega K, Kiefer S, Kipper S, Vellema M, Villavicencio CP, Gahr M, Goymann W. 2013. Associated and disassociated patterns in hormones, song, behavior and brain receptor expression between life-cycle stages in male black redstarts, Phoenicurus ochruros. Gen. Comp. Endocrinol. 184, 93–102. ( 10.1016/j.ygcen.2012.11.027) [DOI] [PubMed] [Google Scholar]
- 49.Goymann W, Villavicencio CP, Apfelbeck B. 2015. Does a short-term increase in testosterone affect the intensity or persistence of territorial aggression? an approach using an individual's hormonal reactive scope to study hormonal effects on behavior. Physiol. Behav. 149, 310–316. ( 10.1016/j.physbeh.2015.06.029) [DOI] [PubMed] [Google Scholar]
- 50.Williams TD. 2008. Individual variation in endocrine systems: moving beyond the ‘tyranny of the Golden Mean’. Phil. Trans. R. Soc. B 363, 1687–1698. ( 10.1098/rstb.2007.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quispe R, Trappschuh M, Gahr M, Goymann W. 2015. Towards more physiological manipulations of hormones in field studies: comparing the release dynamics of three kinds of testosterone implants, silastic tubing, time-release pellets and beeswax. Gen. Comp. Endocrinol. 212, 100–105. ( 10.1016/j.ygcen.2015.01.007) [DOI] [PubMed] [Google Scholar]
- 52.Edler R, Goymann W, Schwabl I, Friedl TWP. 2011. Experimentally elevated testosterone levels enhance courtship behaviour and territoriality but depress acquired immune response in red bishops Euplectes orix. Ibis 153, 46–58. ( 10.1111/j.1474-919X.2010.01075.x) [DOI] [Google Scholar]
- 53.Hau M, Goymann W.. 2015. Endocrine mechanisms, behavioral phenotypes and plasticity: known relationships and open questions. Front. Zool. 12, S7 ( 10.1186/1742-9994-12-S1-S7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hews DK, Moore MC. 1997. Hormones and sex-specific traits: critical Questions. In Parasites and pathogens effects on host hormones and behavior (ed. Beckage NEE.), pp. 277–292. New York, NY: Chapman & Hall. [Google Scholar]
- 55.Harding CF. 1983. Hormonal influences on avian aggressive behavior. In Hormones and aggressive behavior (ed. Svare BB.), pp. 435–467. New York, NY: Plenum Press. [Google Scholar]
- 56.Ball GF, Balthazart J. 2009. Neuroendocrine regulation of reproductive behavior in birds. In Hormones, brain and behavior (second edition) (eds Arnold AP, Etgen AM, Fahrbach SE, Rubin RT), pp. 855–897. San Diego, CA: Academic Press. [Google Scholar]
- 57.Ball GF, Balthazart J. 2017. Neuroendocrine regulation of reproductive behavior in birds. In Hormones, brain and behavior (third edition) (ed. Joëls M.), pp. 217–254. Oxford, UK: Academic Press. [Google Scholar]
- 58.Millesi E, Hoffmann IE, Steurer S, Metwaly M, Dittami JP. 2002. Vernal changes in the behavioral and endocrine responses to GnRH application in male European ground squirrels. Horm. Behav. 41, 51–58. ( 10.1006/hbeh.2001.1735) [DOI] [PubMed] [Google Scholar]
- 59.Apfelbeck B, Mortega K, Kiefer S, Kipper S, Goymann W.. 2013. Life-history and hormonal control of aggression in black redstarts: blocking testosterone does not decrease territorial aggression, but changes the emphasis of vocal behaviours during simulated territorial intrusions. Front. Zool. 10, 8 ( 10.1186/1742-9994-10-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Apfelbeck B, Goymann W. 2011. Ignoring the challenge? Male black redstarts (Phoenicurus ochruros) do not increase testosterone levels during territorial conflicts but they do so in response to gonadotropin-releasing hormone. Proc. R. Soc. B 278, 3233–3242. ( 10.1098/rspb.2011.0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.R Development Core Team. 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 62.Bååth R. 2014. Bayesian First Aid: A package that implements Bayesian alternatives to the classical *.test functions in R In Proceedings of UseR! 2014 - the International R User Conference (University of California, Los Angeles, USA, 30 June–3 July 2014), p. 86 Vienna, Austria: R Foundation Conference Committee. [Google Scholar]
- 63.Gelman A, Su Y.-S., Yajima M, Hill J, Pittau MG, Kerman J, Zheng T, Dorie V. 2014. Data analysis using regression and multilevel/hierarchical models. (CRAN R repository, CRAN).
- 64.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 65.Korner-Nievergelt F, Roth T, von Felten S, Guélat J. 2015. Bayesian data analysis in ecology using linear models With R, BUGS, and Stan. Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 66.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 67.Villavicencio CP, Apfelbeck B, Goymann W. 2013. Experimental induction of social instability during early breeding does not alter testosterone levels in male black redstarts, a socially monogamous songbird. Horm. Behav. 64, 461–467. ( 10.1016/j.yhbeh.2013.08.005) [DOI] [PubMed] [Google Scholar]
- 68.Apfelbeck B, Kiefer S, Mortega KG, Goymann W, Kipper S. 2012. Testosterone affects song modulation during simulated territorial intrusions in male black redstarts (Phoenicurus ochruros). PLoS ONE 7, e52009 ( 10.1371/journal.pone.0052009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kempenaers B, Peters A, Foerster K. 2008. Sources of individual variation in plasma testosterone levels. Phil. Trans. R. Soc. B 363, 1711–1723. ( 10.1098/rstb.2007.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jawor JM, McGlothlin JW, Casto JM, Greives TJ, Snajdr EA, Bentley GE, Ketterson ED. 2006. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis). Gen. Comp. Endocrinol. 149, 182–189. ( 10.1016/j.ygcen.2006.05.013) [DOI] [PubMed] [Google Scholar]
- 71.Ambardar M, Grindstaff JL. 2017. Pre-GnRH and GnRH-induced testosterone levels do not vary across behavioral contexts: a role for individual variation. Gen. Comp. Endocrinol. 246, 51–62. ( 10.1016/j.ygcen.2017.03.009) [DOI] [PubMed] [Google Scholar]
- 72.Goymann W, Flores Dávila P. 2017. Data from: Acute peaks of testosterone suppress paternal care: evidence from individual hormonal reaction norms. Dryad Digital Repository. ( 10.5061/dryad.40kp5) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Goymann W, Flores Dávila P. 2017. Data from: Acute peaks of testosterone suppress paternal care: evidence from individual hormonal reaction norms. Dryad Digital Repository. ( 10.5061/dryad.40kp5) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data for this contribution are available at: http://dx.doi.org/10.5061/dryad.40kp5 [72].