Abstract
Numerous organisms integrate information from multiple sources and express adaptive behaviours, but how they do so at different developmental stages remains to be identified. Seeds, which are the embryonic stage of plants, need to make decisions about the timing of emergence in response to environmental cues related to survival. We investigated the timing of emergence of Plantago asiatica (Plantaginaceae) seed while manipulating the presence of Trifolium repens seed and the relatedness of neighbouring P. asiatica seed. The relatedness of neighbouring P. asiatica seed and the presence of seeds of T. repens did not on their own influence the timing of P. asiatica emergence. However, when encountering a T. repens seed, a P. asiatica seed emerged faster in the presence of a sibling seed than in the presence of a non-sibling seed. Water extracts of seeds gave the same result. We show that P. asiatica seeds integrate information about the relatedness of neighbouring P. asiatica seeds and the presence of seeds of a different species via water-soluble chemicals and adjust their emergence behaviour in response. These findings suggest the presence of kin-dependent interspecific interactions.
Keywords: germination behaviour, kin-discrimination, interspecific interaction, intraspecific interaction, information processing, water-soluble chemicals
1. Introduction
The integration of information from multiple sources to support decision-making is found in many organisms, including animals, plants and microbes [1–3], and is needed for adaptive behaviours under complex environmental conditions. For example, in the absence of competitors, plants adopt a broad foraging strategy that disregards the distribution of resources [1], but in the presence of competitors, they adopt a restricted strategy modified by resource distribution. In another example, acellular slime mould can grow to reach patches of different nutrient quality in the precise proportions necessary to derive an optimal diet [2].
Information integration has been studied in fully developed organisms, which are expected to need well developed information processing systems. Embryonic stages such as eggs or seeds may also need to make decisions about the timing of hatching or emergence in response to unpredictable variations in their environment, including pathogens, predation and competition [4–6]. By contrast to the intercellular neuronal networks seen in animals, plants possess both intracellular, intercellular and interorgan integration systems that allow such behaviours [7], which may thus enable information integration in seeds. However, it remains to be determined whether embryonic stage can adjust the timing of their emergence by integrating multiple sources of biological information.
Germinating seeds encounter neighbours of both the same species (kin and non-kin) and different species. In response to the presence of competitors, they accelerate their emergence to gain advantage [8–11]. The acceleration seems to be accomplished by increase of development speed of embryo in seeds. As increased intraspecific competition may limit interspecific competition [12], the intensity should depend on genetic relatedness of neighbour individuals. For example, the costs of competition should increase with the genetic relatedness of competitors, because genetic similarity should create a similarity in resource requirements [13]. On the other hand, some plants with close relatives often compete more weakly [9,14]. In such conditions, growing with siblings is advantageous for interspecific competition [12]. Therefore, seeds should discern both the presence of allospecific (different-species) competitors and the genetic similarity of conspecific (same-species) competitors and alter their emergence timing in response to competition intensity. We hypothesised that if competition intensity is high among kin, seeds would emerge faster when encountering other species in the presence of sibling seeds than in the presence of non-sibling seeds. Conversely, if competition intensity is high among non-kin, seeds would emerge faster when encountering other species in the presence of non-sibling seeds than in the presence of sibling seeds. In addition, if growing with siblings is advantageous for interspecific competition, the timing of emergence in the presence of both siblings and other species would be more synchronous than the timing in the presence of siblings alone.
To determine how seeds integrate information on the relatedness of conspecific seeds and the presence of allospecific seeds, we investigated the timing of emergence of Plantago asiatica (Plantaginaceae) seed while manipulating the presence of Trifolium repens (Fabaceae) and the number (or presence/absence) and relatedness of neighbour P. asiatica seed. Our goal was to determine whether the timing was change response to multiple biotic environmental information, or whether seeds use different timings under different combinations of conditions.
2. Material and methods
(a). Study organisms
Plantago asiatica is distributed throughout eastern Eurasia (Siberia to Java), including Japan [15]. It colonizes shady habitats, such as woodland tracts and wet grasslands [16]. It is a wind-pollinated, self-compatible, perennial rosette herb. Although the outcrossing rate in P. asiatica has not been determined, those of other wind-pollinated, self-compatible Plantago species are 3–14% [17]. Because their seeds disperse on the ground surface and germinate on the ground near the parent plant [18], seedlings often grow with kin, in addition to other species [15]. Native to Europe, the perennial Trifolium repens now grows worldwide, including with P. asiatica in Japan [16].
(b). Seed collection
In October 2013, seeds of P. asiatica were collected from 20 plants with aboveground diameters of greater than 15 cm in Saga city, western Japan (33° N, 130° E). Each plant grew greater than 100 m from the next. Each plant was randomly assigned a parent plant ID from 1 to 20. The parent plant ID was used to determine the combination of genotypes in all experiments. We refer to seeds collected from the same plant as siblings and seeds collected from different mother plants as ‘non-siblings’. Seeds of T. repens were collected from 10 plants at Saga University (33°24′ N, 130°29′ E) in September 2013. Each plant grew greater than 100 m from the next. Plantago seeds were kept in a refrigerator at 4°C until the experiments.
(c). Experimental design
In all experiments, seeds with no visible cracks were sown in each well (1.0 × 1.0 × 1.5 cm) of a multiwell dish (Thermo Scientific BioLite 48 Well Multidish) holding sterilized sand (7 mm deep) moistened with 100 µl of distilled water (DW). To reproduce the condition of seed-fall on the soil, all seeds were placed on the sand surface so that they were exposed in similar proportions to sand and air. Covers of dishes were fixed with adhesive tape. All dishes were kept in a growth chamber at 25°C under 12 h light which was typical to the germination condition of these plants. All experiments were conducted for 10 days, which was enough time for all seeds' germination. We used a target–neighbour design, in which each target seed was paired with a neighbour. Radicle emergence of target seeds was recorded daily. When multiple seeds were sown in a well, they were placed 3 mm apart. Dead seeds that turned black or on whose surface mould grew and healthy seeds neighbouring them were removed from all experiments.
(d). Effects of P. asiatica and T. repens seeds on germination timing
We investigated the effects of the presence of conspecific and allospecific seeds and their densities on the emergence of P. asiatica seeds in April 2014. To reveal the effects of T. repens and its density, we sowed single P. asiatica seeds with 0, 1 or 2 T. repens seeds (all n = 28; electronic supplementary material, figure S1a). Each genotype of P. asiatica was used once or twice under each condition. Each experimental condition was placed sequentially in the order of 0, 1 and 2 T. repens seeds on the multiwell dish.
To reveal the effects of the presence of another P. asiatica seed on the germination of P. asiatica seeds, including genotype and density, we provide five experiment conditions, target seeds of P. asiatica seeds were either alone (n = 22), with 1 or 2 seeds of the same family as the target seed (sibling condition) (n = 40), or with 1 or 2 seeds from a different family than the target seed (non-sibling condition (n = 40). (electronic supplementary material, figure S1b). Each genotype of P. asiatica was used once or twice when grown alone, and twice when grown with other seeds. Different neighbour genotypes were sown in the non-sibling combinations (electronic supplementary material, table S1). Each genotype combination was used once in the non-sibling combinations.
(e). Interactions between relatedness of P. asiatica seed and the presence of T. repens seed
To test the prediction that seeds integrate information on the relatedness of conspecific seed and the presence of allospecific seed, we provide four experimental conditions: sowing a target P. asiatica seed with either a sibling or a non-sibling seed, with or without a T. repens seed (four conditions, all n = 40). We sowed seeds in April 2014. Each genotype of P. asiatica was used twice in all conditions (electronic supplementary material, figure S2a). Different neighbour genotypes were assigned under the two non-sibling conditions; each genotype combination was used once in these two conditions (electronic supplementary material, table S1). The conditions were placed sequentially in the order of non-siblings, non-siblings + T. repens, siblings and siblings + T. repens on the multiwell dish. Each condition used 80 P. asiatica seeds.
(f). Extract experiments
To identify possible cues, we tested the following: (i) sibling seed + DW (control), (ii) non-sibling seed + 100 µl 5 d T. repens extract (NST5, n = 48), (iii) sibling seed + 100 µl 3 d T. repens extract (ST3, n = 48), (iv) sibling seed + 100 µl 5 d T. repens extract (ST5, n = 48), and (v) 100 µl 5 d sibling extract + 100 µl 5-d T. repens extract (S5T5, n = 20) (electronic supplementary material, figure S2b). Each 124 extract was derived from a single seed with no visible cracks (a commonly used approach to collect of seed extract [19,20]). If discrimination of the presence of seeds of another species depends on water-soluble chemicals, then seeds in ST5 will germinate faster than those in the sibling condition and faster than or in synchrony with those in the NST5 condition. If leaching of water-soluble chemicals from T. repens seed takes time, response of seeds in ST3 may be weaker than those in ST5. If the germination response to water-soluble chemicals depends on the detection of sibling seed, then P. asiatica seed in S5T5 will germinate later than seeds in the sibling and ST5 conditions. Different neighbour genotypes were assigned in non-sibling conditions (electronic supplementary material, table S1). Each genotype was used two or three times under sibling, NST5, ST3 and ST5 conditions, and once under the S5T5 condition. Each condition was placed sequentially, and 96 P. asiatica seeds were grown in all conditions except S5T5, which had 20 seeds. Experiments were conducted between September 2014 and January 2015.
(g). Statistical analyses
Statistical analyses were performed in R v. 2.15.1 software [21]. We use the generalized linear mixed models (GLMMs) for analysis, because our data did not satisfy the proportional hazard. In addition, we deal with over-dispersion by putting the random effects into the model. This method is major for dealing with against over-dispersion [22]. The effects of the presence of T. repens seed on the time to radicle emergence of P. asiatica seeds were tested with GLMMs with a Poisson distribution and a log-link function, including the parent plant ID and positions within the multiwell dish as a random effect, and with the likelihood-ratio test because the distribution of the residuals differed significantly from normality. The number of T. repens seeds was included as an explanatory variable. The effects of conspecific seed on the time to emergence were tested in the same way. Seed density, relatedness and their interactions were included as explanatory variables.
The effects of the presence of T. repens seed on the time to emergence of P. asiatica sibling and non-sibling seeds were tested using a generalized linear model with a Poisson distribution and a log-link function and the likelihood-ratio test because the distribution of the residuals differed significantly from normality. Relatedness, presence or absence of a T. repens seed and their interactions were included as explanatory variables. Multiple comparisons were performed using the Steel–Dwass test. To measure synchrony of P. asiatica sibling seeds with or without a T. repens seed, we calculated the difference in time to emergence between P. asiatica sibling seeds and tested it with a GLMM as above.
The effects of extracts on the time to emergence of P. asiatica seeds were tested by GLMM as above. Multiple comparisons were performed using the Steel–Dwass test. To measure synchrony of P. asiatica sibling seeds with either DW or 3- or 5-day extract of T. repens seed, we calculated the difference in time to emergence between P. asiatica sibling seeds. The differences were tested by GLMM as above. Multiple comparisons were performed using the Steel–Dwass test. In all statistical analysis, p < 0.05 was considered statistically significant.
3. Results and discussion
In all experiments, all healthy seeds were germinated. The timing of P. asiatica emergence was not influenced by the presence of T. repens seed alone (χ22 = 0.08, p = 0.96; table 1) or by the density or relatedness of conspecific seed alone (density, χ22 = 0.05, p = 0.98; relatedness, χ22 = 0.06, p = 0.97; table 1). Some studies found that the emergence of seeds was accelerated under competitive conditions, such as high seed density and the presence of the same or another species [8–11], but our results did not reveal such effects (table 1).
Table 1.
Mean (±SEM) days to emergence of P. asiatica seed. (Germination timing was not influenced by neighbour condition (GLMM, p > 0.05).)
| experimental conditions | n | Plantago asiatica | n | Trifolium repens |
|---|---|---|---|---|
| interspecific competition | ||||
| solitary | 22 | 5.32 ± 0.22 | — | — |
| with 1 T. repens seed | 28 | 5.39 ± 0.22 | 28 | 1.11 ± 0.08 |
| with 2 T. repens seeds | 28 | 5.21 ± 0.14 | 56 | 1.13 ± 0.05 |
| intraspecific competition | ||||
| solitary | 22 | 5.55 ± 0.28 | — | — |
| with one sibling seed | 40 | 5.35 ± 0.16 | — | — |
| with two sibling seeds | 33 | 5.48 ± 0.16 | — | — |
| with one non-sibling seed | 38 | 5.50 ± 0.20 | — | — |
| with two non-sibling seeds | 32 | 5.41 ± 0.21 | — | — |
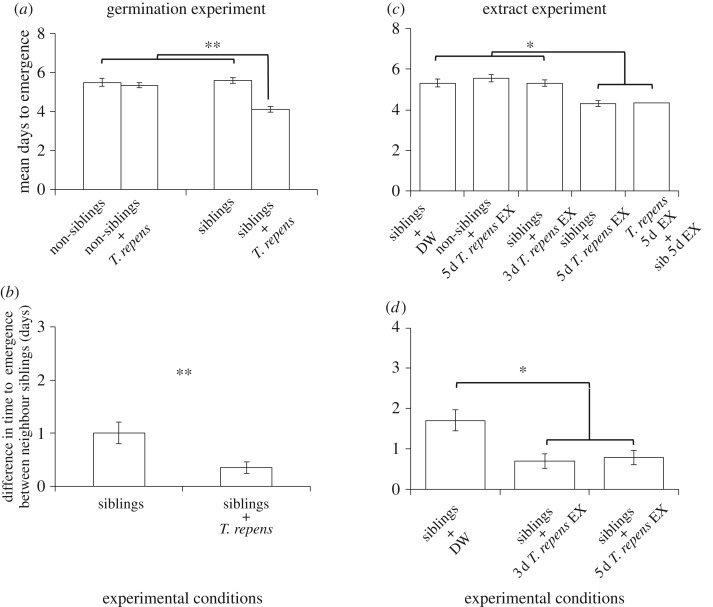
The timing of emergence of T. repens seeds was not influenced by the P. asiatica relatedness condition (sibling, 1.30 ± 0.56 days; non-sibling, 1.14 ± 0.36 days; GLMM, χ2 = 0.14, p > 0.05). The timing of emergence of P. asiatica seed pairs differed with the relatedness of P. asiatica neighbour seed and the presence or absence of T. repens seed (relatedness×competitor, χ2 = 3.88, p = 0.048): when encountering a T. repens seed, P. asiatica seeds emerged faster in the presence of a sibling seed than in the presence of a non-sibling seed (Steel–Dwass, p = 0.012; figure 1a), regardless of number of T. repens seed (electronic supplementary material, figure S3). This result was echoed in the extract experiment (χ2 = 10.67, p = 0.031; figure 1c). These results indicate that P. asiatica seeds integrate information about the relatedness of conspecific neighbour seeds and the presence of other species, probably by sensing water-soluble chemicals. Thus, our experiments provide the evidence that plants can integrate information not only at the seedling stage [1], but also at the embryonic stage. These results mean that developed organs, such as leaves, stems and roots, are not necessary for information integration.
Figure 1.
Emergence of P. asiatica seeds. (a) Time to emergence of P. asiatica seeds in the presence of different neighbours. (b) Differences in time to emergence among sibling seeds. (c) Time to emergence of P. asiatica seeds in the presence of various seed extracts. (d) Differences in time to emergence among sibling seeds in an extract experiment. DW, distilled water; EX, water extract of seed. Data are means ± s.e.m. (germination experiment: siblings, n = 40; non-siblings, n = 38; siblings versus T. repens, n = 40; non-siblings versus T. repens, n = 40; extract experiment: siblings, n = 48; non-siblings, n = 40; siblings versus 3 d T. repens extract, n = 48; siblings versus 5 d T. repens extract, n = 48; sibling versus 5 d T. repens extract + 5 d sibling extract, n = 20). Asterisks indicate statistically significant differences: (a,c,d) Steel–Dwass test (*p < 0.05, **p < 0.01); (b) GLMM (**p < 0.01).
The emergence of P. asiatica siblings was better synchronized in the presence of a T. repens seed than in its absence (χ12 = 7.67, p = 0.006; figure 1b). The timing of emergence of a neighbour sibling seed was better synchronized in the presence of 3- and 5-day extracts of T. repens seeds than in their absence (χ22 = 12.97, p = 0.002; figure 1d). Because the 3-day extract had no significant effect on emergence timing, but had a significant effect on synchronization, the results of the extract experiment suggest that synchronization is independent of the acceleration of emergence (figure 1c,d); Such synchrony suggests that the P. asiatica sibling seeds are able to sense the developmental stage of neighbouring conspecific seeds, thereby altering their development rate, through ‘embryonic communication’ [23,24].
Why do P. asiatica seeds accelerate their emergence when encountering other species only in the presence of sibling seed? One possible reason is (as we hypothesised) that intraspecific competition is more intense among siblings than among non-siblings. However, P. asiatica sibling seeds were better synchronised in the presence of T. repens seeds. Synchronous hatching of eggs can provide beneficial effects for each other such as obtaining food resources [25] and avoiding predators [26]. The co-emergence of sibling seeds suggests the presence of cooperative effects among siblings in the face of interspecific competition. As the early emergence of seeds can confer a competitive advantage [11], the acceleration of emergence of P. asiatica sibling seeds should provide an advantage in competition with T. repens. If P. asiatica siblings minimize competition as some plant species do [9,13], the acceleration of emergence and synchronous-emergence in the presence of T. repens may provide a competitive advantage. Additional competition experiments will be needed to reveal the adaptive significance of the acceleration of emergence and the co-emergence of P. asiatica sibling seeds.
In conclusion, we present experimental evidence that P. asiatica seeds integrate information about their biotic environment and alter their emergence timing accordingly. These results suggest that P. asiatica seeds have a strategy for competition with other species that depends on the relatedness of neighbouring P. asiatica seeds. Information integration is likely to occur in many other plant species, because both interspecific interaction and discrimination of competitors through water-soluble chemicals are common in plants [27]. Further studies in other species would be beneficial to understand of plant–plant interactions. If such integration of biotic information is widespread, it may form the basis for competitive interactions among seedlings, and examination of such interactions may improve our understanding of plant–plant interactions.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank E. Kasuya for his advice on statistical methods.
Data accessibility
The datasets supporting this article have been uploaded as part of the supplementary material.
Authors' contributions
A.Y. and H.M. developed the core idea and designed the experiments. A.Y. and H.M. performed all experiments, performed data analyses, and wrote the article.
Competing interests
We have no competing interests.
Funding
This work was supported by a JSPS Grant-in-Aid for Young Scientists (B) (grant no. 15K18611 to A.Y., grant no. 15K18618 to H.M.).
References
- 1.Cahill JF Jr, McNickle GG, Haag JJ, Lamb EG, Nyanumba SM, St Clair CC. 2010. Plants integrate information about nutrients and neighbors. Science 328, 1657 ( 10.1126/science.1189736) [DOI] [PubMed] [Google Scholar]
- 2.Dussutour A, Latty T, Beekman M, Simpson SJ. 2010. Amoeboid organism solves complex nutritional challenges. Proc. Natl Acad. Sci. USA 107, 4607–4611. ( 10.1073/pnas.0912198107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Heuvel MP, Sporns O. 2013. Network hubs in the human brain. Trends Cogn. Sci. 17, 683–696. ( 10.1016/j.tics.2013.09.012) [DOI] [PubMed] [Google Scholar]
- 4.Warkentin KM. 1995. Adaptive plasticity in hatching age: a response to predation risk trade-offs. Proc. Natl Acad. Sci. USA 92, 3507–3510. ( 10.1073/pnas.92.8.3507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyer AR, Fenech A, Rice KJ. 2000. Accelerated seedling emergence in interspecific competitive neighbourhoods. Ecol. Lett. 3, 523–529. ( 10.1111/j.1461-0248.2000.00187.x) [DOI] [Google Scholar]
- 6.Baskin CC, Baskin JM. 1998. Seeds: ecology, biogeography, and evolution of dormancy and germination. London, UK: Elsevier. [Google Scholar]
- 7.Chaiwanon J, Wang W, Zhu J-Y, Oh E, Wang Z-Y. 2016. Information integration and communication in plant growth regulation. Cell 164, 1257–1268. ( 10.1016/j.cell.2016.01.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergelson J, Perry R. 1989. Interspecific competition between seeds: relative planting date and density affect seedling emergence. Ecology 70, 1639–1644. ( 10.2307/1938097) [DOI] [Google Scholar]
- 9.Dudley SA, File AL. 2007. Kin recognition in an annual plant. Biol. Lett. 3, 435–438. ( 10.1098/rsbl.2007.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tielbörger K, Prasse R. 2009. Do seeds sense each other? Testing for density-dependent germination in desert perennial plants. Oikos 118, 792–800. ( 10.1111/j.1600-0706.2008.17175.x) [DOI] [Google Scholar]
- 11.Orrock JL, Christopher CC. 2010. Density of intraspecific competitors determines the occurrence and benefits of accelerated germination. Am. J. Bot. 97, 694–699. ( 10.3732/ajb.0900051) [DOI] [PubMed] [Google Scholar]
- 12.Ehlers BK, David P, Damgaard CF, Lenormand T. 2016. Competitor relatedness, indirect soil effects, and plant coexistence. J. Ecol. 140, 1126–1135. ( 10.1111/1365-2745.12568) [DOI] [Google Scholar]
- 13.File AL, Murphy GP, Dudley SA. 2012. Fitness consequences of plants growing with siblings: reconciling kin selection, niche partitioning and competitive ability. Proc. R. Soc. B 279, 209–218. ( 10.1098/rspb.2011.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crepy MA, Casal JJ. 2015. Photoreceptor-mediated kin recognition in plants. New Phytol. 205, 329–338. ( 10.1111/nph.13040) [DOI] [PubMed] [Google Scholar]
- 15.Tanaka S, Miura R, Tominaga T. 2010. Small-scale heterogeneity in the soil environment influences the distribution of lawn grass and weeds. Weed Biol. Manag. 10, 209–218. ( 10.1111/j.1445-6664.2010.00386.x) [DOI] [Google Scholar]
- 16.Matsuo K. 1999. Comparison of ecological distributions and life history characteristics between invasive and closely related native Plantago species in Japan. In Biological invasions of ecosystem by pests and beneficial organisms (eds Yano E, Matsuo K, Shiyomi M, Andow DA). Tsukuba, Japan: National Institute of Agro-Environmental Sciences. [Google Scholar]
- 17.Kuiper PJC, Bos M. 1992. Plantago: a multidisciplinary study. Berlin, Germany: Springer. [Google Scholar]
- 18.Kobayashi T, Okamoto K, Hori Y. 2001. Variations in size structure, growth and reproduction in Japanese plantain (Plantago asiatica L.) between exposed and shaded populations. Plant Species Biol. 16, 13–28. ( 10.1046/j.1442-1984.2001.00053.x) [DOI] [Google Scholar]
- 19.Kageyama K, Nelson EB. 2003. Differential inactivation of seed exudate stimulation of Pythium ultimum sporangium germination by Enterobacter cloacae influences biological control efficacy on different plant species. Appl. Environ. Microbiol. 69, 1114–1120. ( 10.1128/AEM.69.2.1114-1120.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tambalo DD, Vanderlinde EM, Robinson S, Halmillawewa A, Hynes MF, Yost CK. 2014. Legume seed exudates and Physcomitrella patens extracts influence swarming behavior in Rhizobium leguminosarum. Can. J. Microbiol. 60, 15–24. ( 10.1139/cjm-2013-0723) [DOI] [PubMed] [Google Scholar]
- 21.R Development Core Team. 2015. R: a language and environment for statistical computing. See: http://www.R-project.org/. [Google Scholar]
- 22.McCullagh P, Nelder JA. 1989. Generalized linear models. London, UK: Chapman & Hall. [Google Scholar]
- 23.McGlashan JK, Spencer R-J, Old JM. 2011. Embryonic communication in the nest: metabolic responses of reptilian embryos to developmental rates of siblings. Proc. R. Soc. B 279, 1709–1715. ( 10.1098/rspb.2011.2074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aubret F, Blanvillain G, Bignon F, Kok PJR. 2016. Heartbeat, embryo communication and hatching synchrony in snake eggs. Sci. Rep. 6, 23519 ( 10.1038/srep23519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colbert PL, Spencer R-J, Janzen FJ. 2010. Mechanism and cost of synchronous hatching. Funct. Ecol. 24, 112–121. ( 10.1111/j.1365-2435.2009.01602.x) [DOI] [Google Scholar]
- 26.Merkling T, Agdere L, Albert E, Durieux R, Hatch SA, Danchin E, Blanchard P. 2014. Is natural hatching asynchrony optimal? An experimental investigation of sibling competition patterns in a facultatively siblicidal seabird. Behav. Ecol. Sociobiol. 68, 309–319. ( 10.1007/s00265-013-1646-y) [DOI] [Google Scholar]
- 27.Renne IJ, Sinn BT, Shook GW, Sedlacko DM, Dull JR, Villarreal D, Hierro JL. 2014. Eavesdropping in plants: delayed germination via biochemical recognition. J. Ecol. 102, 86–94. ( 10.1111/1365-2745.12189) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the supplementary material.