Abstract
Many marine invertebrates provide their offspring with symbionts. Yet the consequences of maternally inherited symbionts on larval fitness remain largely unexplored. In the stony coral Favia fragum (Esper 1797), mothers produce larvae with highly variable amounts of endosymbiotic algae, and we examined the implications of this variation in symbiont density on the performance of F. fragum larvae under different environmental scenarios. High symbiont densities prolonged the period that larvae actively swam and searched for suitable settlement habitats. Thermal stress reduced survival and settlement success in F. fragum larvae, whereby larvae with high symbiont densities suffered more from non-lethal stress and were five times more likely to die compared with larvae with low symbiont densities. These results show that maternally inherited algal symbionts can be either beneficial or harmful to coral larvae depending on the environmental conditions at hand, and suggest that F. fragum mothers use a bet-hedging strategy to minimize risks associated with spatio-temporal variability in their offspring's environment.
Keywords: symbiont provisioning, vertical transmission, thermal stress, prolonged pelagic phase, bet-hedging theory
1. Introduction
Maternally inherited symbionts, such as bacteria, viruses, protists, fungi, algae and cyanobacteria, are widespread in multicellular organisms. They are important modulators of their hosts' phenotypes as they can provide energetic resources [1,2] and protection from diseases, predators and pathogens [3]. Within-host symbiont density can further influence the hosts' phenotypes by regulating physiological processes such as rates of energy acquisition [4], and susceptibility to thermal stress [5]. While symbiotic associations generally benefit both host and symbiont, they often represent a delicate balance that breaks down when environmental conditions change [6–8]. Yet, even in well-studied host–symbiont systems (e.g. in insects), the degree to which presumed benefits from maternally inherited symbionts (and variability therein) change in response to varying environmental conditions is not well known [9,10].
Endosymbiosis is common in marine phyla such as the Porifera [11], Mollusca [12], Cnidaria [13,14], Annelida, Nematoda and Tunicata [15], and is found in a wide variety of marine ecosystems, such as hydrothermal vents, cold seep habitats [15], coral reefs [14] and temperate waters [13,16]. Members of aforementioned taxa associate with various endosymbionts, such as methane- and sulfur-oxidizing bacteria [15], photosynthesizing single-celled algae [17], and nitrogen-fixing and photosynthesizing cyanobacteria [18]. These endosymbiotic partners provide their hosts with energetic resources that they are unable to obtain themselves, especially in nutrient-poor or extreme environments [2].
A subset of species that form obligate symbioses as adults transfer those symbionts vertically to their offspring (e.g. corals [19], sponges [20,21], ascidians [22], bivalves [23]), whereby the amount of symbionts that each offspring receives, and thus the potential benefit to larval fitness, can vary greatly [21,24,25]. Large variation in symbiont densities among individual offspring produced by the same mother [24–26] therefore suggests a ‘bet-hedging strategy’ typical of mothers facing uncertainty regarding the future environment their offspring will experience [27]. Furthermore, symbiotic relationships in marine invertebrates are often beneficial only within a narrow range of (a)biotic conditions. Changing environmental conditions can therefore, in addition to variable symbiont provisioning, affect the fitness benefits a host receives from their endosymbionts (e.g. Mollusca, Annelida and Nematoda [7], Cnidaria [8,17], Porifera [17,28,29]).
Most tropical stony coral species live in obligate symbiosis with endosymbiotic algae (Symbiodinium spp.). The corals provide shelter and CO2 produced during respiration, and in turn receive photosynthates that facilitate their survival in nutrient-poor, tropical waters [1]. In some species, algal symbionts are transferred directly from mothers to planula larvae during embryogenesis [19], and can fulfil up to 70% of a larva's energy demand, depending on symbiont density and the availability of other resources such as lipids [25,30,31]. Coral–algal symbiotic relationships are, however, extremely susceptible to temperature anomalies associated with climate change. Under high temperature and/or solar radiation, algal symbionts produce excessive amounts of reactive oxygen species, causing corals to expel their symbionts to prevent tissue damage, resulting in ‘coral bleaching’ [8]. During prolonged warmer periods, corals become deprived of the energetic resources provided by their now missing endosymbionts, and they die [32,33]. This illustrates that the presence of Symbiodinium can provide corals with necessary energetic resources, but that such benefit only occurs under particular environmental conditions. Yet most research has centred on algal symbionts in adult corals. How coral larvae provided with different densities of algal symbionts will perform in different environments is still a major open question.
Here, we used the stony coral Favia fragum (Esper 1797) to investigate the implications of variable maternal provisioning of algal symbionts on offspring performance under different environmental conditions. We first determined if trade-offs existed between a mother's symbiont investments and the size and number of offspring she produced. Second, we reared larvae provided with different symbiont densities under physiological (heat stress) and environmental (lack of positive settlement cues) stressors to determine under what conditions differential symbiont provisioning results in offspring fitness costs or benefits. We predicted that the benefits of endosymbiosis during the larval phase of this marine invertebrate are context-dependent, whereby larvae harbouring larger numbers of symbionts benefit from additional energetic resources, but are also more sensitive to higher temperatures relative to larvae provided with smaller numbers of symbionts.
2. Material and methods
(a). Study species
Favia fragum is a conspicuous coral species occurring in a wide range of shallow marine habitats [34] in the Western Atlantic. It releases symbiotic planula larvae between 6 and 16 days after new moon (ANM) with maximum release during day 11 ANM [35]. Favia fragum produces lecithotrophic larvae capable of settling immediately after release when positive settlement cues are present [36], and must settle before energetic resources required for settlement and metamorphosis are depleted.
(b). Collection and rearing of Favia fragum larvae
This study was carried out on the Caribbean island of Curaçao (12°N, 69°W), located 60 km north of Venezuela. Nineteen healthy (i.e. no signs of bleaching, disease or tissue discoloration) F. fragum colonies of similar sizes (13.9 cm2 ± 3.3, mean ± s.d.) were collected 5 days ANM in June 2013 between 3 and 6 m depth at the Curaçao Sea Aquarium reef (12°04′59″ N, 68°53′44″ W) and placed individually in 1 l beakers. Each beaker was constantly supplied with 100 µm filtered seawater to create an overflow into a semi-submerged larval collection cup with a 150 µm mesh filter bottom. Because F. fragum larvae are positively buoyant, the overflow caused larvae from each colony to concentrate in a separate collection cup. Both the beakers and the collection cups were partially submerged in a flow-through aquarium system at temperatures similar to natural seawater (approx. 28°C [37]) for the duration of the experiment. Larvae produced by each mother during the night were collected the following morning between 7.00 and 8.00 h and placed in a 500 ml polystyrene deli container (DART, MI, USA) filled with 300 ml of 0.7 µm filtered seawater (Whatman GF/F, GE Life Sciences, PA, USA), and kept at 28°C and exposed to approximately 12 h light natural light cycles until they were used in one of the following experiments.
(c). Variation in size and symbiont content in Favia fragum offspring
To determine the variability in size and symbiont content in F. fragum offspring, larvae produced by each colony were collected and counted daily throughout one reproductive cycle (6–18 ANM). From the total number of larvae released by each mother each day, 20 randomly selected larvae were stored in 0.5 ml of 3.7% formalin-filtered seawater in 1.5 ml tubes (Eppendorf, NY, USA). If a colony released fewer than 20 larvae in a day, the entire daily brood was sampled. To compare the size of all sampled larvae, the longitudinal and transversal axes were measured with a scale bar under a dissecting microscope and larval volume was calculated assuming the volume of a spheroid [38]. Each larva was subsequently homogenized in 3.7% formalin-filtered seawater and the number of symbionts was quantified using a haemocytometer (Bright-Line; Hausser Scientific, PA, USA). For more information on this method, refer to the electronic supplementary material, section S1a.
(d). Larval colour as proxy for symbiont density
Because larvae vary in size, symbiont density (i.e. the number of symbionts per mm3 of larva) appears more suitable than total symbiont number to study physiological processes [5]. Favia fragum larvae show large variation in coloration, reflecting differences in symbiont density [24,25]. Therefore, we investigated whether a distinction between beige, brown and dark-brown larvae could be used as a simple proxy to identify larvae with low, intermediate and high symbiont densities, respectively (electronic supplementary material, section S1b).
(e). Experiment 1: effects of an extended pelagic phase
To test if larvae can delay settlement (i.e. extend their pelagic phase) when they contain higher symbiont densities, larvae from each coloration group were reared in seawater with and without settlement cues to experimentally shorten or lengthen their pelagic phase, respectively. On the day they were released, eight larvae were placed in individual 350 ml polystyrene cups (n = 6; Darnel Inc., NC, USA) containing 250 ml of 0.7 µm filtered seawater and immediately provided with clay pottery tripods as settlement substrates (kiln stilts, 6 cm diameter; Carl Jaeger Tonindustriebedarf GmbH, Germany). These tripods were pre-conditioned in a flow-through aquarium for three months prior to the experiment to allow the colonization by crustose coralline algae (CCA) known to induce larval settlement in F. fragum [39]. In the second treatment, larvae of each coloration group were placed in identical cups as above (n = 6) but with 250 ml of 0.22 µm filtered seawater (EMD Millepore, MA, USA), which delays the development of microbial communities that trigger settlement, causing larvae to not settle and remain pelagic [40]. Seawater in both treatments was exchanged daily (approx. 75%) and kept at 28°C.
Larval survival and behaviour were assessed daily between 8.00 and 9.00 h for the duration of the experiment. For each surviving larva, we noted whether it lay motionless (i) at the surface or (ii) on the bottom, moved around (iii) in the water column, or (iv) on the bottom, or (v) had settled. After 5 days, 15–40% of the larvae forced to remain pelagic stopped swimming and had become inactive. Therefore, the experiment was ended to ensure that a sufficient number of larvae with an extended pelagic phase survived to assess their ability to settle once provided with positive cues (see below).
(f). Experiment 2: effects of heat stress
To investigate if F. fragum larvae provisioned with different symbiont densities have different survival rates and behaviour under elevated temperatures, larvae from each colour group were reared at the average monthly mean seawater temperature in June 2013 (28.5 ± 0.3°C, mean ± s.d.) and at elevated temperatures (31.1 ± 0.2°C) corresponding to the coral bleaching threshold on Curaçao [37]. Six 35 l plastic containers (Sterilite, MA, USA) were filled with 25 l of seawater (n = 3 containers per temperature treatment) and the water temperature inside each container was regulated using a 300 W submersible water heater (Aquatop, CA, USA). The water temperature was monitored every 5 min with a temperature logger (HOBO Pro V2; Onset Computer Corporation, MA, USA). Two water pumps placed at the opposite corners of each container ensured mixing of the water inside each container. Larvae (n = 8) were added to 15 ml conical polystyrene tubes (BD Biosciences, CA, USA) containing 14 ml of 0.7 µm filtered seawater. Nine tubes with larvae were assigned to each of six experimental treatments (symbiont density (3) × temperature (2)).
Larval behaviour was assessed daily between 8.00 and 9.00 h for the duration of the experiment by removing the tubes from the baths and examining the larvae with a dim light. Behavioural categories were scored as described above. Once a replicate was scored, the tube lid was removed for 5 s to allow gas exchange, closed, and randomly reassigned to a water bath of the same experimental treatment. In a similar set-up, Hartmann et al. [41] found that larval respiration did not lead to significant reductions in dissolved oxygen concentrations, and that larvae were therefore unlikely to suffer from low-oxygen stress [41]. On day 8, less than 20% of the larvae containing high symbiont densities reared under elevated temperature were still alive. We therefore ended the experiment to ensure enough larvae remained alive in all treatments to assess if their ability to subsequently settle depended on their prior exposure to heat stress (see below).
(g). Quantification of sublethal stress
Pulse amplitude-modulated (PAM) fluorometry was used to determine whether F. fragum larvae with different symbiont densities experienced different levels of sublethal stress when exposed to elevated temperatures or when forced to extend their pelagic phase. The effective quantum yield (ΔF/Fm′), which is a measure of the photochemical efficiency of photosystem II of the endosymbiotic algae under ambient light, was used as a proxy for ‘sublethal stress’ [42,43] and measured using a diving PAM (Walz, GmbH, Germany) fitted with a 1.5 mm diameter mini fibre-optic cable. Measurements were performed at 9.00 h in a dim room. Individual larvae were suspended in a small volume of seawater directly in front of the sensor and the effective quantum yield of four individual larvae was measured three times and averaged per larva. Following the methods of Putnam et al. [44], the measuring light of the PAM was set to a constant intensity of ‘8’ in ‘burst mode’ and the gain was set to ‘12’ to obtain Ft values between 4.00 and 8.00. Measurements were taken at days 0, 3 and 5 during experiment 1, and at days 0, 4 and 8 during experiment 2. After each time point, larvae were removed from the experiments to avoid potential stress effects caused by repetitive handling in later PAM measurements.
(h). Latent effects on settlement success
To test if stress experienced by a larva during its pelagic phase affected its ability to settle, larvae from experiments 1 and 2 were provided with settlement substrates and subsequent settlement rates were quantified. On day 8, surviving larvae from the heat stress experiment were transferred to individual 1 l polystyrene containers (DART, MI, USA) that contained 500 ml of 0.7 µm filtered seawater kept at 28°C, and one clay tripod as described above serving as a settlement surface. This could be replicated nine times for larvae reared under ambient temperatures (three to eight larvae per container), and seven to eight times (two to six larvae per container) for those previously exposed to elevated temperature. Settlement rates of larvae with high symbiont densities exposed to heat stress could not be assessed due to total mortality in six (out of nine) replicates during experiment 2. Survival and settlement rates were recorded daily thereafter. After day 12, larvae that had not settled no longer changed their behaviour nor settled, and the experiment was stopped at day 15. For larvae that were forced to extend their pelagic phase, one tripod was added to each replicate (n = 6) on day 5 similar to above after larval behaviour was scored, and survival and settlement rates were recorded daily until day 13, because no changes in settlement were observed after day 10.
(i). Statistical analysis
To test for differences in larval size and in symbiont provisions within and among individual broods, we used Welch's F tests for unequal variances followed by Bonferroni's post hoc multiple comparisons because data did not meet the assumption of homoscedasticity (Levene's test, p < 0.05). Differences in symbiont densities among the three larval colouration groups were assessed similarly. Regression analyses were used to test if brood size (i.e. number of larvae released by a mother throughout a planulation cycle) influenced (i) the average number and density of symbionts allocated to each larva, (ii) average larval size, and (iii) if larval symbiont density varied independently of larval size. Because fecundity in clonal organisms is partly determined by the number of gravid modules in a colony (polyps in corals) [45], we standardized fecundity among individual F. fragum colonies as the number of larvae released per polyp during one planulation cycle.
Differences in larval survival and settlement between treatments for experiments 1 and 2 were compared using the Kaplan–Meier (K–M) analysis followed by post hoc tests in the case of significant main effects. Survival curves were compared using the K–M log rank tests that for each group calculate the χ2 value for each ‘event time’, and subsequently sum the results to generate a single χ2 used to determine if survival curves of different experimental groups differ. Death and settlement were defined as ‘events’, whereas larvae for which event time could not be determined were included as ‘censored’—that is, they were alive at the end of the experiment and did not die (survival analyses) or died or did not settle (settlement analyses).
In experiment 1, a maximum-likelihood (ML) approach [46] (see the electronic supplementary material, section S1c for details) was used to detect differences in larval settlement and swimming activity among treatments at experimental time points (i.e. at day 5 when CCA were added, day 13 when the experiment was ended and at day 9 as an intermediate time point between aforementioned time points). We compared settlement rates at day 5 among larval coloration groups (symbiont density) reared with and without CCA. Differences in settlement were expressed as the proportion of larvae that settled relative to the initial number of larvae. Differences in larval settlement success were assessed similarly, but expressed as the proportion of larvae that settled relative to the total number of larvae that were alive on the day they were provided with settlement cues. The proportion of inactive larvae was calculated as the proportion of larvae that were motionless relative to the number of larvae that were still alive and had not settled, and compared among larval colour groups for each time point after delayed and immediate addition of settlement cues. Finally, differences in effective quantum yield (experiments 1 and 2) were assessed with a two-way ANOVA followed by Tukey's post hoc tests.
3. Results
(a). Variation in colour, size and symbiont content of offspring
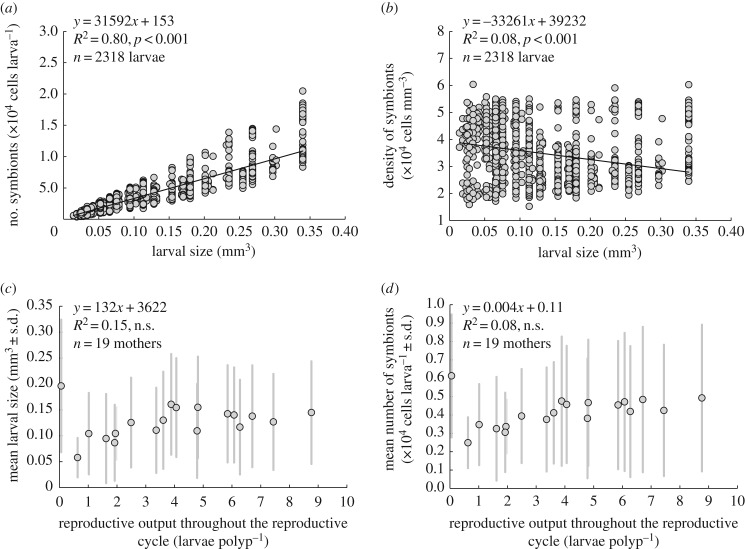
The total number of symbionts provided to individual larvae increased with larval size (R2 = 0.8, n = 2318, p < 0.001; figure 1a). However, larval size explained only 8% of the variation in symbiont density (R2 = 0.08, n = 2318, p < 0.001; figure 1b). Beige larvae had low symbiont densities (2.3 × 104 ± 0.3 cells mm−3; mean ± s.d.; n = 30), brown larvae had intermediate symbiont densities (3.6 × 104 ± 0.8 cells mm−3) and dark-brown larvae had high symbiont densities (4.7 × 104 ± 0.2 cells mm−3; electronic supplementary material, figure S1). The beige, brown and dark-brown larvae differed in symbiont density (Welch's F test, F50.3 = 798.7, p < 0.001; electronic supplementary material, figure S1a), but not in larval size (Welch's F test, F57.0 = 1.3, p = 0.29; electronic supplementary material, figure S1b and section S2a). Larval colour could therefore be used as a proxy for symbiont density, independently of larval size.
Figure 1.
Analysis of (a) number of algal symbionts and (b) symbiont density in coral larvae of different sizes, and investigation of possible trade-offs between the total number of larvae produced by each mother during a reproductive cycle and (c) mean larval size and (d) the mean number of symbionts per larva.
Larval size, symbiont number and symbiont density showed large variation among mothers and within broods of the same mother (electronic supplementary material, section 2b; figure S2). Throughout one planulation cycle, F. fragum mothers produced different amounts of larvae ranging from 0.3 to 53.3 larvae polyp−1. No trade-offs existed between the total number of larvae produced by each mother during a reproductive cycle and larval size (R2 = 0.15, n = 19, p = 0.23; figure 1c) or the number of symbionts per larva (R2 = 0.08, n = 19, p = 0.10; figure 1d).
(b). Effects of an extended pelagic phase
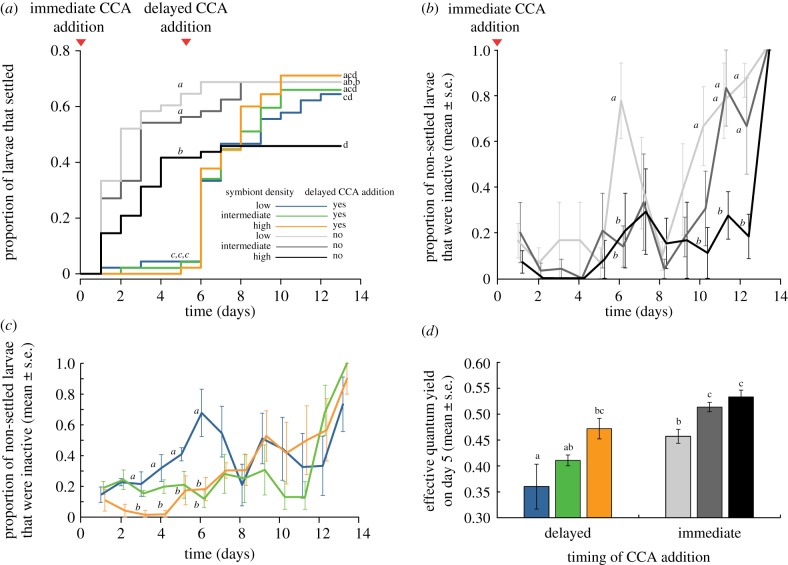
When settlement cues were provided to larvae immediately after release, those with low and intermediate symbiont densities settled faster and ultimately in higher numbers than larvae with high symbiont densities (K–M log-rank test, 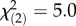 , p < 0.05; figure 2a; electronic supplementary material, table S1; day 13: ML, three-parameter model, p < 0.05; electronic supplementary material, table S2). Non-settled larvae with high symbiont densities remained more active (swimming) towards the end of our experiment compared with larvae with low symbiont densities (days 10–12: ML, two-parameter model, p < 0.05; electronic supplementary material, table S3), of which 67% had become inactive by day 10 (figure 2b).
, p < 0.05; figure 2a; electronic supplementary material, table S1; day 13: ML, three-parameter model, p < 0.05; electronic supplementary material, table S2). Non-settled larvae with high symbiont densities remained more active (swimming) towards the end of our experiment compared with larvae with low symbiont densities (days 10–12: ML, two-parameter model, p < 0.05; electronic supplementary material, table S3), of which 67% had become inactive by day 10 (figure 2b).
Figure 2.
Effects of symbiont density on activity and settlement of coral larvae. (a) Settlement rates of coral larvae with different symbiont densities when CCA were provided either immediately upon release or delayed to day 5 (n = 6 replicates). (b) Proportion of non-settled larvae that ceased to swim when provided with settlement cues immediately upon release. (c) Proportion of non-settled larvae that ceased to swim when forced to extend their pelagic phase. (d) Effective quantum yield of symbionts at day 5 in non-settled coral larvae that were provided with CCA either immediately upon release or at day 5 (n = 4 larvae per treatment). In (a) and (d), significantly different treatment groups are shown by non-italicized letters, as tested with a K–M analysis and a two-way ANOVA, respectively. Letters in (a), (b) and (c) indicate significant differences at individual time points, as tested with an ML approach, and are exclusively shown for treatment groups that differed.
The withholding of CCA as settlement cues forced F. fragum larvae to delay settlement and extend their pelagic phase. After 5 days, only 4% of all larvae without settlement cues had settled, whereas 54% of all larvae with settlement cues had settled by that time (day 5: ML, three-parameter model, p < 0.05; figure 2a; electronic supplementary material, table S2). Delaying settlement slightly reduced larval mortality (by 1.5%) over the entire experimental period (K–M log-rank test, 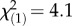 , p < 0.05). After 2 days without settlement cues, larvae containing low symbiont densities became 2–15-fold less active than those with high symbiont densities (days 3–5: ML, two-parameter model, p < 0.05; figure 2c; electronic supplementary material, table S3). When provided with settlement cues, low symbiont density larvae gradually resumed swimming and were equally active as those with intermediate and high symbiont densities by day 7 (days 7–13: ML, null model; figure 2c).
, p < 0.05). After 2 days without settlement cues, larvae containing low symbiont densities became 2–15-fold less active than those with high symbiont densities (days 3–5: ML, two-parameter model, p < 0.05; figure 2c; electronic supplementary material, table S3). When provided with settlement cues, low symbiont density larvae gradually resumed swimming and were equally active as those with intermediate and high symbiont densities by day 7 (days 7–13: ML, null model; figure 2c).
The algal symbionts of non-settled larvae forced to delay settlement were more stressed at day 5 than the symbionts of non-settled larvae provided with settlement substrates immediately after release (two-way ANOVA, F1,18 = 44.2, p < 0.001; figure 2d; electronic supplementary material, table S4). Furthermore, sublethal stress was higher in larvae with low symbiont densities than in larvae with high symbiont densities (two-way ANOVA, F2,18 = 17.6, p < 0.001; figure 2d; electronic supplementary material, table S4).
We did not detect latent effects in the form of reduced settlement success caused by an extended pelagic phase in F. fragum larvae, independent of differences in symbiont density. When larvae initially reared without CCA as settlement cues were provided with CCA on day 5, they initiated settlement within 24 h and, by day 9, had settled in equal numbers as larvae provided with settlement cues immediately after birth (day 9: ML, null model; figure 2a; electronic supplementary material, table S2).
(c). Effects of heat stress
Elevated temperature reduced survival of F. fragum larvae and larvae containing high symbiont densities were five times less likely to survive under elevated temperature compared with larvae with low symbiont densities (K–M log-rank test, 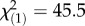 , p < 0.001; figure 3a; electronic supplementary material, table S5). Elevated temperature also inhibited settlement of coral larvae independent of symbiont density (K–M log-rank test,
, p < 0.001; figure 3a; electronic supplementary material, table S5). Elevated temperature also inhibited settlement of coral larvae independent of symbiont density (K–M log-rank test, 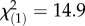 , p < 0.001; figure 3b; electronic supplementary material, table S6). In addition, physiological stress levels (indicated by lower effective quantum yield of the algal symbionts) was higher in larvae kept at elevated temperatures, particularly if the larvae contained high symbiont densities (two-way ANOVA, F2,11 = 4.2, p < 0.05; figure 3c, electronic supplementary material, table S7). Thus, while the direct negative effects of thermal stress on F. fragum larvae were more severe for larvae hosting high symbiont densities (figure 3a,c), latent effects caused by thermal stress resulted in low settlement rates for all F. fragum larvae, regardless of the density of symbionts they contained (figure 3b).
, p < 0.001; figure 3b; electronic supplementary material, table S6). In addition, physiological stress levels (indicated by lower effective quantum yield of the algal symbionts) was higher in larvae kept at elevated temperatures, particularly if the larvae contained high symbiont densities (two-way ANOVA, F2,11 = 4.2, p < 0.05; figure 3c, electronic supplementary material, table S7). Thus, while the direct negative effects of thermal stress on F. fragum larvae were more severe for larvae hosting high symbiont densities (figure 3a,c), latent effects caused by thermal stress resulted in low settlement rates for all F. fragum larvae, regardless of the density of symbionts they contained (figure 3b).
Figure 3.
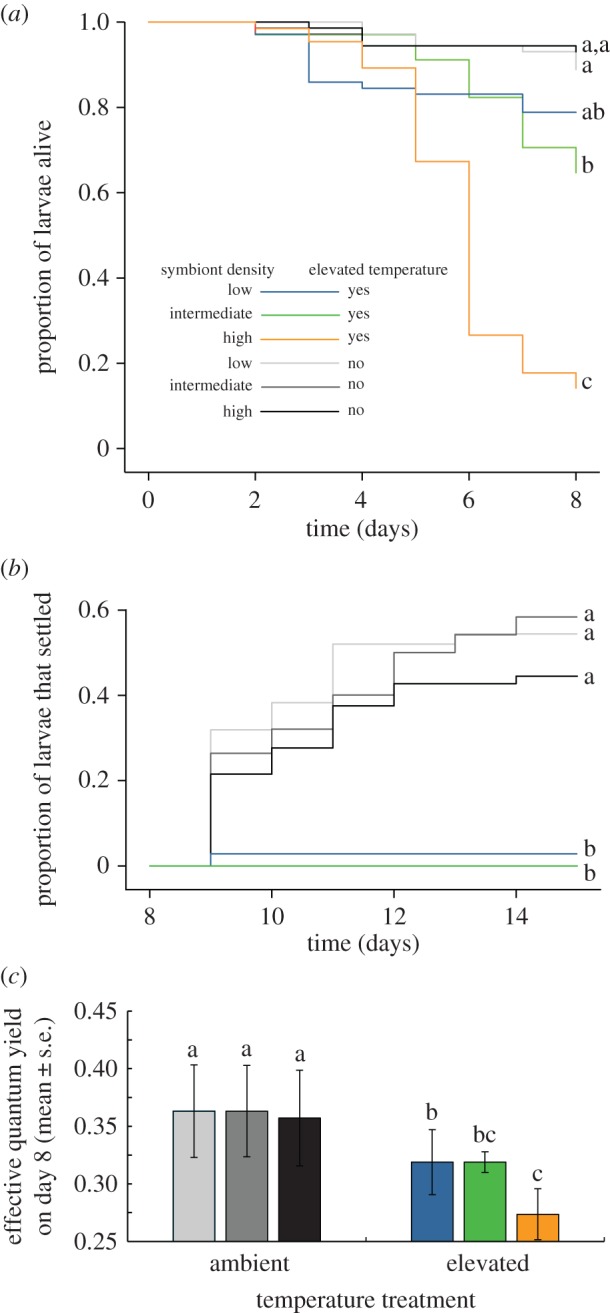
Effects of symbiont density on coral larvae exposed to heat stress. (a) Survival rates of larvae with different symbiont densities at ambient versus elevated temperature, plotted throughout their pelagic phase (n = 9 replicates). (b) Settlement rates of larvae after they were relieved from heat stress and provided with CCA as settlement cues at day 8 (ambient: n = 9 replicates with three to eight larvae; elevated: n = 7 to 8 replicates with two to six larvae). Note that the treatment combining elevated temperature and high symbiont densities is excluded because too many larvae had died in this treatment during the preceding experiment. (c) Effective quantum yield of symbionts at day 8 in non-settled coral larvae exposed to ambient versus elevated temperature (n = 4 larvae per treatment). Lines and bars that do not share the same letter are significantly different, as tested with (a,b) K–M analyses and (c) a two-way ANOVA.
4. Discussion
Differential provisioning of symbionts by mothers could be of fundamental importance for their offspring's fitness because symbionts can provide their larval host with energy resources. We used the brooding coral F. fragum as a model species to examine the ecological consequences of potential trade-offs in larval characteristics that result from variable symbiont provisioning. We found that larger per-offspring symbiont investments by coral mothers incurred both costs and benefits, depending on the environmental context experienced by the offspring.
(a). Absence of trade-offs between brood size and per-offspring investment
Studies on maternal provisioning often focus on a possible trade-off between the number of offspring a mother produces and her per-offspring resource allocation, whereby a larger reproductive output results in smaller per-offspring investments [47]. Negative relationships between offspring number and size are indeed widespread in both plants and animals (e.g. reptiles [48], insects [49], plants [50] and fish [51]), but there is increasing evidence that this trade-off is not as ubiquitous as previously assumed. In many taxa, no correlation between offspring number and size exists, and in some cases, positive correlations were even observed [52,53]. In our study, we also found no correlation between the number of larvae produced by F. fragum mothers and their per-offspring allocation of resources in terms of either larval size or symbiont number (figure 1c,d). Hence, our results provide additional evidence supporting that mothers are not always bound to a compromise between total reproductive output and per-offspring investments.
(b). Larger symbiont provisions allow for prolonged larval activity
Forcing F. fragum offspring to extend their pelagic phase did not reduce their survival or ability to settle (figure 2a). Within a week, however, it led to physiological stress and inactivity in larvae with low symbiont densities (figure 2c,d). By contrast, larvae with intermediate and high symbiont densities maintained a higher photosynthetic efficiency, possibly resulting from benefits associated with self-shading among algal symbionts [54], and swam continuously during the first week (figure 2c,d). Active swimming represents the largest energetic cost during a marine invertebrate's larval stage [55], and in symbiotic coral larvae, this energy may be derived from lipid catabolism [56], photosynthetic products supplied by their endosymbionts [31] or both. Large lipid reserves, for example, allow ascidian larvae to search for high-quality settlement habitats for a longer period of time compared with smaller conspecifics [57]. By ceasing to swim, F. fragum larvae with low symbiont densities, and therefore probably lower energetic resources, could reduce their energy demand to avoid exhaustion and mortality at the cost of encountering favourable habitats for settlement. In environments where positive settlement cues are scarce, coral larvae with small symbiont provisions are therefore expected to be less successful in finding optimal habitats for settlement and post-settlement survival.
When provided with settlement cues immediately upon release, larvae with high symbiont densities swam longer (figure 2b) and settled at lower rates than those with lower symbiont densities (figure 2a). Lower immediate rates of settlement coupled with active swimming and general lower physiological stress in larvae with high symbiont densities (figure 2d) suggest that larger symbiont provisions enable larger dispersal distances and promote population connectivity [58], but only when settlement cues were present. When settlement substrates were initially withheld, such ‘dispersal behaviour’ disappeared (figure 2a). By delaying settlement, larvae risk passing their competency period and, while alive, can no longer settle and metamorphose [59]. Larvae in other species have often been observed to separate out in a group that settles relatively quickly after release and another group of larvae that settles much later [60]. Such phenomenon would be in agreement with our aforementioned suggestion that these larvae actively explore habitats outside their native range, though it is not possible, within the context of this study, to definitively confirm this scenario as we did not test if non-settled larvae had retained their ability to settle and metamorphose at the end of our experiment.
(c). High symbiont densities can become harmful to the host
Heat stress exerted direct negative effects on F. fragum larvae (figure 3), in particular in larvae that hosted higher densities of symbionts, as they had a lower photosynthetic efficiency and survival rate (figure 3a,c). When larvae were relieved of thermal stress, latent effects were widespread. Nearly all F. fragum larvae that had been exposed to heat stress failed to settle, regardless of symbiont density (figure 3b). Hartmann et al. [41] observed similar patterns in Agaricia humilis, another Caribbean brooding coral that produces symbiotic larvae. In this species, large larval size (indicative of larger lipid reserves) did not counteract the negative latent effects of heat stress, resulting in low settlement success equivalent to that of smaller larvae. By contrast, aposymbiotic larvae produced by broadcast spawning coral species suffered no or only small direct and latent effects from exposure to heat stress compared with symbiotic larvae [41,61]. Heat stress is largely mediated via the algal symbionts, which produce excessive amounts of reactive oxygen species at elevated temperatures [8]. Thus, despite benefits in terms of energy provisioning, harbouring symbiotic algae lowers the ability of coral larvae to tolerate higher temperatures, whereby larval susceptibility to heat stress increases with symbiont density.
(d). Mixed benefits of symbiont provisioning in different environments
Our finding that maternally inherited symbionts can be both beneficial and harmful to larvae contrasts with the common, almost invariably positive effects of larger maternal lipid provisioning (i.e. larger sizes) on the performance of marine invertebrate offspring [62]. Larger-sized, non-feeding marine invertebrate larvae typically can search for suitable settlement surfaces for longer periods of time, show increased post-metamorphic survival and growth, are stronger competitors for space, attain sexual maturity faster and are more fecund than smaller conspecifics (e.g. marine snails [63], bryozoans [62,64], ascidians [65]). While the aforementioned benefits appear straightforward when considered in terms of lipid provisions alone, marine invertebrate larvae that also contain symbionts could experience physiological stress, as found in this study, due to altered symbiont performance under certain environmental conditions (e.g. Mollusca, Annelida and Nematoda [7], Cnidaria [8], Porifera [28,29]). For example, we hypothesize that in an environment where F. fragum larvae risk depleting their energy reserves before they settle due to, for example, a scarcity of suitable settlement habitats, smaller larvae are at a disadvantage due to limited energetic reserves that can be derived from lipid catabolism. If they contain high symbiont densities, they can compensate for this lack of resources by using energy derived from algal photosynthates resulting in equal settlement success as larger larvae that host low symbiont densities. However, during warmer periods, larvae harbouring high symbiont densities, regardless of size, are prone to oxidative damage so that large larvae with high symbiont densities can be expected to perform worse than smaller larvae provisioned with low symbiont densities. In this scenario, the benefits of larger maternal lipid contributions could be enhanced by large symbiont provisions, but also offset by the disadvantages associated with high symbiont densities under certain environmental contexts. Future studies in which larvae are not only separated according to symbiont density (this study), but also according to size, are needed to conclusively confirm this hypothesis.
(e). Bet-hedging as a strategy in variable environments
In marine invertebrate larvae, size variability is highest for lecithotrophic species with internal fertilization [62]. Offspring size and symbiont content in F. fragum was remarkably variable (electronic supplementary material, figure S2a,b), greatly exceeding the variability found in many other invertebrates, including many coral species [25,62]. As a result, F. fragum larvae of all sizes could contain all ranges of symbiont densities (figure 1b), thus generating a diversified pool of phenotypes mixing these two traits. The dynamic bet-hedging theory predicts that such variability in offspring phenotypes benefits populations or species growing in fluctuating and unpredictable environments [27]. Favia fragum occurs in tidal pools, shallow reefs and seagrass beds, which are known for high spatial and temporal fluctuations in (a)biotic parameters (e.g. light, habitat availability, temperature, distance between conspecifics, water movement) [34]. Therefore, we hypothesize that the extreme variability in maternal provisioning in F. fragum is indeed a response to the high level of unpredictability of this species's environment. Because large symbiont provisions can come with both costs and benefits depending on the environmental context, producing larvae with a wide range of symbiont densities prevents the complete loss of one reproductive cycle investment in the advent of harsh environmental conditions. Thus, F. fragum mothers appear to minimize risks associated with spatio-temporal uncertainty in their offspring's environment by producing larvae of all possible combinations of sizes and symbiont densities, whereby each combination can benefit or negatively impact larval performance depending on the environmental conditions at hand. In addition to gradual adaptation over multiple generations, variation in maternal symbiont provisions could act as another pathway by which corals prove capable of responding to environmental stressors such as environmental degradation and global warming.
Supplementary Material
Supplementary Material
Acknowledgements
We are thankful to two anonymous reviewers for providing us with insightful suggestions to improve earlier versions of this manuscript.
Data accessibility
Data supporting these findings are fully available as the electronic supplementary material.
Authors' contributions
V.F.C., K.R.W.L., A.C.H. and M.J.A.V. conceptualized the project; V.F.C., K.R.W.L., A.C.H., J.H. and M.J.A.V. wrote the manuscript; V.F.C., K.R.W.L. and M.J.A.V. performed sample collection, processing, experiments and analyses. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This research was made possible through funding and/or support received by the CARMABI Foundation, the University of Amsterdam, SECORE International, the Curaçao Sea Aquarium and the Fonds de Recherche du Québec-Nature et Technologies.
References
- 1.Muscatine L, Porter JW. 1977. Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27, 454–460. ( 10.2307/1297526) [DOI] [Google Scholar]
- 2.Cavanaugh CM. 1994. Microbial symbiosis: patterns of diversity in the marine environment. Am. Zool. 34, 79–89. ( 10.1093/icb/34.1.79) [DOI] [Google Scholar]
- 3.Haine ER. 2008. Symbiont-mediated protection. Proc. R. Soc. B 275, 353–361. ( 10.1098/rspb.2007.1211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoogenboom M, Beraud E, Ferrier-Pagès C. 2010. Relationship between symbiont density and photosynthetic carbon acquisition in the temperate coral Cladocora caespitosa. Coral Reefs 29, 21–29. ( 10.1007/s00338-009-0558-9) [DOI] [Google Scholar]
- 5.Cunning R, Baker AC. 2012. Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 3, 259–262. ( 10.1038/nclimate1711) [DOI] [Google Scholar]
- 6.Weldon SR, Strand MR, Oliver KM.. 2013. Phage loss and the breakdown of a defensive symbiosis in aphids. Proc. R. Soc. B 280, 20122103 ( 10.1098/rspb.2012.2103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Childress J, Fisher C. 1992. The biology of hydrothermal vent animals: physiology, biochemistry, and autotrophic symbioses. Oceanogr. Mar. Biol. 30, 337–447. [Google Scholar]
- 8.Downs CA, Fauth JE, Halas JC, Dustan P, Bemiss J, Woodley CM. 2002. Oxidative stress and seasonal coral bleaching. Free Radic. Biol. Med. 33, 533–543. ( 10.1016/S0891-5849(02)00907-3) [DOI] [PubMed] [Google Scholar]
- 9.Corbin C, Heyworth ER, Ferrari J, Hurst GDD. 2017. Heritable symbionts in a world of varying temperature. Heredity (Edinb). 118, 10–20. ( 10.1038/hdy.2016.71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosokawa T, Kikuchi Y, Fukatsu T. 2007. How many symbionts are provided by mothers, acquired by offspring, and needed for successful vertical transmission in an obligate insect–bacterium mutualism? Mol. Ecol. 16, 5316–5325. ( 10.1111/j.1365-294X.2007.03592.x) [DOI] [PubMed] [Google Scholar]
- 11.Lee YK, Lee JH, Lee HK. 2001. Microbial symbiosis in marine sponges. J. Microbiol. 39, 254–264. [Google Scholar]
- 12.Cheng TC. 1967. Marine molluscs as hosts for symbiosis with a review of known parasites of commercially important species. Adv. Mar. Biol. 5, 1–300. ( 10.1016/s0065-2881(08)60321-1) [DOI] [Google Scholar]
- 13.Muller-Parker G, Davy SK. 2001. Temperate and tropical algal–sea anemone symbioses. Invertebr. Biol. 120, 104–123. ( 10.1111/j.1744-7410.2001.tb00115.x) [DOI] [Google Scholar]
- 14.Baker AC. 2003. Flexibility and specificity in coral–algal symbiosis: diversity, ecology, and biogeography of symbiodinium. Annu. Rev. Ecol. Evol. Syst. 34, 661–689. ( 10.1146/132417) [DOI] [Google Scholar]
- 15.Dubilier N, Bergin C, Lott C. 2008. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nat. Rev. Microbiol. 6, 725–740. ( 10.1038/nrmicro1992) [DOI] [PubMed] [Google Scholar]
- 16.Sutton DC, Hoegh-Guldberg O. 1990. Host-zooxanthella interactions in four temperate marine invertebrate symbioses: assessment of effect of host extracts on symbionts. Biol. Bull. 178, 175–186. ( 10.2307/1541975) [DOI] [PubMed] [Google Scholar]
- 17.Venn AA, Loram JE, Douglas AE. 2008. Photosynthetic symbioses in animals. J. Exp. Bot. 59, 1069–1080. ( 10.1093/jxb/erm328) [DOI] [PubMed] [Google Scholar]
- 18.Carpenter EJ. 2002. Marine cyanobacterial symbioses. Biol. Environ. Proc. R. Irish Acad. 102B, 15–18. ( 10.3318/BIOE.2002.102.1.15) [DOI] [Google Scholar]
- 19.Hirose M, Kinzie RA III, Hidaka M. 2001. Timing and process of entry of zooxanthellae into oocytes of hermatypic corals. Coral Reefs 20, 273–280. ( 10.1007/s003380100171) [DOI] [Google Scholar]
- 20.Ereskovsky AV, Gonobobleva E, Vishnyakov A. 2005. Morphological evidence for vertical transmission of symbiotic bacteria in the viviparous sponge Halisarca dujardini Johnston (Porifera, Demospongiae, Halisarcida). Mar. Biol. 146, 869–875. ( 10.1007/s00227-004-1489-1) [DOI] [Google Scholar]
- 21.Usher KM, Kuo J, Fromont J, Sutton DC. 2001. Vertical transmission of cyanobacterial symbionts in the marine sponge Chondrilla australiensis (Demospongiae). Hydrobiologia 461, 15–23. ( 10.1071/mf04304) [DOI] [Google Scholar]
- 22.Kojima A, Hirose E. 2012. Transmission of cyanobacterial symbionts during embryogenesis in the coral reef ascidians Trididemnum nubilum and T. clinides (Didemnidae, Ascidiacea, Chordata). Biol. Bull. 222, 63–73. ( 10.1086/BBLv222n1p63) [DOI] [PubMed] [Google Scholar]
- 23.Krueger DM, Gustafson RG, Cavanaugh CM. 1996. Vertical transmission of chemoautotrophic symbionts in the bivalve Solemya velum (Bivalvia: Protobranchia). Biol. Bull. 190, 195–202. ( 10.2307/1542539) [DOI] [PubMed] [Google Scholar]
- 24.Roth MS, Fan T-Y, Deheyn DD. 2013. Life history changes in coral fluorescence and the effects of light intensity on larval physiology and settlement in Seriatopora hystrix. PLoS ONE 8, e59476 ( 10.1371/journal.pone.0059476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaither MR, Rowan R. 2010. Zooxanthellar symbiosis in planula larvae of the coral Pocillopora damicornis. J. Exp. Mar. Biol. Ecol. 386, 45–53. ( 10.1016/j.jembe.2010.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isomura N, Nishihira M. 2001. Size variation of planulae and its effect on the lifetime of planulae in three pocilloporid corals. Coral Reefs 20, 309–315. ( 10.1007/s003380100180) [DOI] [Google Scholar]
- 27.Crean AJ, Marshall DJ. 2009. Coping with environmental uncertainty: dynamic bet hedging as a maternal effect. Phil. Trans. R. Soc. B 364, 1087–1096. ( 10.1098/rstb.2008.0237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster NS, Cobb RE, Negri AP. 2008. Temperature thresholds for bacterial symbiosis with a sponge. ISME J. 2, 830–842. ( 10.1038/ismej.2008.42) [DOI] [PubMed] [Google Scholar]
- 29.Cebrian E, Uriz MJ, Garrabou J, Ballesteros E.. 2011. Sponge mass mortalities in a warming Mediterranean Sea: are cyanobacteria-harboring species worse off? PLoS ONE 6, e20211 ( 10.1371/journal.pone.0020211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp C, Domart-Coulon I, Barthelemy D, Meibom A.. 2016. Nutritional input from dinoflagellate symbionts in reef-building corals is minimal during planula larval life stage. Sci. Adv. 2, e1500681 ( 10.1126/sciadv.1500681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harii S, Yamamoto M, Hoegh-Guldberg O. 2010. The relative contribution of dinoflagellate photosynthesis and stored lipids to the survivorship of symbiotic larvae of the reef-building corals. Mar. Biol. 157, 1215–1224. ( 10.1007/s00227-010-1401-0) [DOI] [Google Scholar]
- 32.Baker AC, Glynn PW, Riegl B. 2008. Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471. ( 10.1016/j.ecss.2008.09.003) [DOI] [Google Scholar]
- 33.Hoegh-Guldberg O., et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 34.Carlon DB, Budd AF. 2002. Incipient speciation across a depth gradient in a scleractinian coral? Evolution 56, 2227–2242. ( 10.1111/j.0014-3820.2002.tb00147.x) [DOI] [PubMed] [Google Scholar]
- 35.Szmant-Froelich A, Reutter M, Riggs L. 1985. Sexual reproduction of Favia fragum (Esper): lunar patterns of gametogenesis, embryogenesis and planulation in Puerto Rico. Coral Reefs 37, 880–892. [Google Scholar]
- 36.Carlon DB, Olson RR. 1993. Larval dispersal distance as an explanation for adult spatial pattern in two Caribbean reef corals. J. Exp. Mar. Biol. Ecol. 173, 247–263. ( 10.1016/0022-0981(93)90056-T) [DOI] [Google Scholar]
- 37.NOAA Coral Reef Watch. 2013. Daily global 5-km satellite virtual station time series data for Curaçao and Aruba, 1 January–31 December 2013. College Park, MD: NOAA Coral Reef Watch. [Google Scholar]
- 38.Van Moorsel G. 1983. Reproductive strategies in two closely related stony corals (Agaricia, Scleractinia). Mar. Ecol. Prog. Ser. 13, 273–283. ( 10.3354/meps013273) [DOI] [Google Scholar]
- 39.Randall CJ, Szmant AM. 2009. Elevated temperature reduces survivorship and settlement of the larvae of the Caribbean scleractinian coral, Favia fragum (Esper). Coral Reefs 28, 537–545. ( 10.1007/s00338-009-0482-z) [DOI] [Google Scholar]
- 40.Vermeij MJA, Smith JE, Smith CM, Vega Thurber R, Sandin SA. 2009. Survival and settlement success of coral planulae: independent and synergistic effects of macroalgae and microbes. Oecologia 159, 325–336. ( 10.1007/s00442-008-1223-7) [DOI] [PubMed] [Google Scholar]
- 41.Hartmann AC, Marhaver KL, Chamberland VF, Sandin SA, Vermeij MJA. 2013. Large birth size does not reduce negative latent effects of harsh environments across life stages in two coral species. Ecology 94, 1966–1976. ( 10.1890/13-0161.1) [DOI] [PubMed] [Google Scholar]
- 42.Phillip E, Fabricius KE. 2003. Photophysiological stress in scleractinian corals in response to short-term sedimentation. J. Exp. Mar. Biol. Ecol. 287, 57–78. ( 10.1016/s0022-0981(02)00495-1) [DOI] [Google Scholar]
- 43.Fai BP, Grant A, Reid B. 2007. Chlorophyll a fluorescence as a biomarker for rapid toxicity assessment. Environ. Toxicol. Chem. 26, 1520–1531. ( 10.1897/06-394r1.1) [DOI] [PubMed] [Google Scholar]
- 44.Putnam HM, Edmunds PJ, Fan T. 2008. Effect of temperature on the settlement choice and photophysiology of larvae from the reef coral Stylophora pistillata. Biol. Bull. 215, 135–142. ( 10.2307/25470694) [DOI] [PubMed] [Google Scholar]
- 45.Hall V, Hughes T. 1996. Reproductive strategies of modular organisms: comparative studies of reef-building corals. Ecology 77, 950–963. ( 10.2307/2265514) [DOI] [Google Scholar]
- 46.Hilborn R, Mangel M. 1997. The ecological detective: confronting models with data. Princeton, NJ: Princeton University Press. [Google Scholar]
- 47.Smith CC, Fretwell SD. 1974. The optimal balance between size and number of offspring. Am. Nat. 108, 499–506. ( 10.1086/282929) [DOI] [Google Scholar]
- 48.Ford NB, Seigel RA. 1989. Relationships among body size, clutch size, and egg size in three species of oviparous snakes. Herpetologica 45, 75–83. [Google Scholar]
- 49.Fox CW, Thakar MS, Mousseau TA.. 1997. Egg size plasticity in a seed beetle: an adaptive maternal effect. Am. Nat. 149, 149–163. ( 10.1086/285983) [DOI] [PubMed] [Google Scholar]
- 50.Aarssen LW, Jordan CY. 2001. Between-species patterns of covariation in plant size, seed size, and fecundity in monocarpic herbs. Ecoscience 8, 471–477. ( 10.1080/11956860.2001.11682677) [DOI] [Google Scholar]
- 51.Williams TD. 2001. Experimental manipulation of female reproduction reveals an intraspecific egg size–clutch size trade-off. Proc. R. Soc. Lond. B 268, 423–428. ( 10.1098/rspb.2000.1374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernardo J. 1996. The particular maternal effect of propagule size, especially egg size: patterns, models, quality of evidence and interpretations. Integr. Comp. Biol. 36, 216–236. ( 10.1093/icb/36.2.216) [DOI] [Google Scholar]
- 53.Venable DL. 1992. Size–number trade-offs and the variation of seed size with plant resource status. Am. Nat. 140, 287–304. ( 10.1086/285413) [DOI] [Google Scholar]
- 54.Roth MS. 2014. The engine of the reef: photobiology of the coral–algal symbiosis. Front. Microbiol. 5, 1–22. ( 10.3389/fmicb.2014.00422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marshall D, Pechenik J, Keough M. 2003. Larval activity levels and delayed metamorphosis affect post-larval performance in the colonial ascidian Diplosoma listerianum. Mar. Ecol. Prog. Ser. 246, 153–162. ( 10.3354/meps246153) [DOI] [Google Scholar]
- 56.Harii S, Nadaoka K, Yamamoto M, Iwao K. 2007. Temporal changes in settlement, lipid content and lipid composition of larvae of the spawning hermatypic coral Acropora tenuis. Mar. Ecol. Prog. Ser. 346, 89–96. ( 10.3354/meps07114) [DOI] [Google Scholar]
- 57.Marshall DJ, Keough MJ. 2003. Variation in the dispersal potential of non-feeding invertebrate larvae: the desperate larva hypothesis and larval size. Mar. Ecol. Prog. Ser. 255, 145–153. ( 10.3354/meps255145) [DOI] [Google Scholar]
- 58.Goodbody-Gringley G, Vollmer SV, Woollacott RM, Giribet G. 2010. Limited gene flow in the brooding coral Favia fragum. Mar. Biol. 157, 2591–2602. ( 10.1007/s00227-010-1521-6) [DOI] [Google Scholar]
- 59.Nishikawa A, Sakai K. 2005. Settlement-competency period of planulae and genetic differentiation of the scleractinian coral Acropora digitifera. Zool. Sci. 22, 391–399. ( 10.2108/zsj.22.391) [DOI] [PubMed] [Google Scholar]
- 60.Wilson JR, Harrison PL. 1998. Settlement-competency periods of larvae of three species of scleractinian corals. Mar. Biol. 131, 339–345. ( 10.1007/s002270050327) [DOI] [Google Scholar]
- 61.Yakovleva I, Baird A, Yamamoto H, Bhagooli R, Nonaka M, Hidaka M. 2009. Algal symbionts increase oxidative damage and death in coral larvae at high temperatures. Mar. Ecol. Prog. Ser. 378, 105–112. ( 10.3354/meps07857) [DOI] [Google Scholar]
- 62.Marshall DJ, Keough MJ. 2008. The evolutionary ecology of offspring size in marine invertebrates. Adv. Mar. Biol. 53, 1–60. ( 10.1016/S0065-2881(07)53001-4) [DOI] [PubMed] [Google Scholar]
- 63.Moran AL, Emlet RB. 2001. Offspring size and performance in variable environments: field studies on a marine snail. Ecology 82, 1597–1612. ( 10.2307/2679803) [DOI] [Google Scholar]
- 64.Marshall DJ, Bolton TF, Keough MJ. 2003. Offspring size affects the post-metamorphic performance of a colonial marine invertebrate. Ecology 84, 3131–3137. ( 10.1890/02-0311) [DOI] [Google Scholar]
- 65.Marshall DJ, Cook CN, Emlet RB. 2006. Offspring size effects mediate competitive interactions in a colonial marine invertebrate. Ecology 87, 214–225. ( 10.1890/05-0350) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting these findings are fully available as the electronic supplementary material.