Abstract
Fossil remains provide useful insights into the long-term impact of anthropogenic phenomena on faunas and are often used to reveal the local (extirpations) or global (extinctions) losses of populations or species. However, other phenomena such as minor morphological changes can remain inconspicuous in the fossil record depending on the methodology used. In this study, we used the anole of Marie-Galante Island (Anolis ferreus) in Guadeloupe (French, West Indies) as a model to demonstrate how the morphological evolution of an insular lizard can be tracked through the Pleistocene/Holocene climatic transition and the recent anthropization of the island. We used a fossil assemblage of nearly 30 000 remains and a combination of anatomical description, traditional morphometry and geometric morphometrics. These fossils are attributed to a single taxon, most likely to be A. ferreus on the basis of morphological and morphometric arguments. Our results show the disappearance of a distinct (sub)population of large specimens that were about 25% larger than the modern representatives of A. ferreus. We also demonstrate an apparent size stability of the main fossil population of this species since the Late Pleistocene but with the possible occurrence of a reduction in morphological diversity during the Late Holocene. These results highlight the impact of anthropic disturbances on a lizard whose morphology otherwise remained stable since the Late Pleistocene.
Keywords: Anolis, extinction, geometric morphometrics, osteology, palaeontology, West Indies
1. Introduction
It is now generally accepted that our planet is undergoing global changes including climatic modifications and environmental perturbations due to anthropogenic activities. Global phenomena impact living organisms worldwide [1] but often have strong impacts on islands that are, depending on their size, isolation and environmental specificity, more vulnerable to environmental perturbations [2–4]. The impact on islands is often faster than on the continent and thus islands are excellent model systems to document the influence of environmental modifications. Whereas in some cases, global change can lead to the total extinction of species on islands [5,6], other less spectacular impacts are also possible, such as a reduction in the morphological disparity of a species. However, the smaller the change, the harder it is to detect. Moreover, some of these changes may take dozens, hundreds or even thousands of years and consequently are impossible to document without fossil remains. In addition, even when fossil remains exist, their investigation can be limited by the lack of available analytical tools able to detect more subtle variations in disparity or form. The aim of this study is to test whether climate change and recent environmental disturbance induced by modern human societies impact insular faunas. To do so, we use the Marie-Galante (Guadeloupe archipelago, French West Indies) solitary anole, Anolis ferreus (Cope, 1864), as a model. Anoles are small arboreal lizards presenting a high phenotypic plasticity and high population density on most of the Lesser Antillean islands where they are represented by several endemic species. Generally, two species occur on a single island but on some islands, including Marie-Galante, only a single species is present [7–9]. These characteristics potentially make them resistant to extinction phenomena and anthropogenic pressures. They are consequently an excellent study case to investigate how anthropization can impact insular taxa without immediately leading to their extinction.
In order to provide data concerning the morphology of past Marie-Galante anole communities and to investigate the impact of climate change and anthropogenic disturbance, we here perform a detailed morphological analysis of a large assemblage of fossil anole remains dated from the Late Pleistocene to the Late Holocene. These remains were recovered in the three Marie-Galante Island main fossil deposits: Blanchard cave [10], Cadet 2 cave [11] and Cadet 3 rock shelter [12]. To study this material, we applied traditional descriptive palaeontology and recently published methods [13] used to investigate the size of fossil lizards. We also conducted a geometric morphometric (GMM) analysis on isolated Anolis fossil bones to describe the phenotypic diversity present on the island through time.
2. Material and methods
(a). Modern and fossil samples
The modern comparative sample used in the GMM and morphological studies is composed of 214 skeletons representing eight of the 18 lesser Antillean species of Anolis (see electronic supplementary material, S1 and S2). The fossil sample is composed of 29 183 anole remains coming from Blanchard cave (N = 5207), Cadet 2 cave (N = 8638) and Cadet 3 rock shelter (N = 15 338). For the GMM analysis, we selected 137 fossil Anolis dentaries collected in these deposits (see electronic supplementary material, S3).
(b). Past snout–vent length size study
Only the bones that were previously recognized as good predictors of the snout–vent length (SVL) of fossil Anolis (dentary, maxilla, ilium and humerus; see [14]) were used to calculate the SVL of fossils following the protocol of Bochaton & Kemp [14]. The equations and measurements used are given in electronic supplementary material, S4. In addition, we used the minimal theoretical maximal size (MTMS) as defined by Bochaton [13]. This last value is obtained by combining the estimated size and skeletal maturity of the specimens using a relation established by Maisano [15] between these two variables.
(c). Geometric morphometrics study
We chose to perform our analysis on the dentary bone because it was the most frequent fossil anatomical part in the deposits. Given that the dentary is a rather flat bone, we chose to perform our study in two dimensions using landmarks and sliding semi-landmarks placed on pictures of the lingual side of the bone. A mirror function was used in order to study both right and left dentaries in the same analysis. Details concerning this approach and subsequent analysis can be found in electronic supplementary material, S5. Statistical tests were performed using the basic library stats v. 3.1.2 (Kruskal–Walis and Manovas) and Geomorph [16] (Procrustes ANOVA with permutation procedures) R libraries; the mixture analysis was performed using the package mixtools v. 1.0.4 in R [17]. All p-values were considered significant if below 0.05.
3. Results
(a). Size estimation
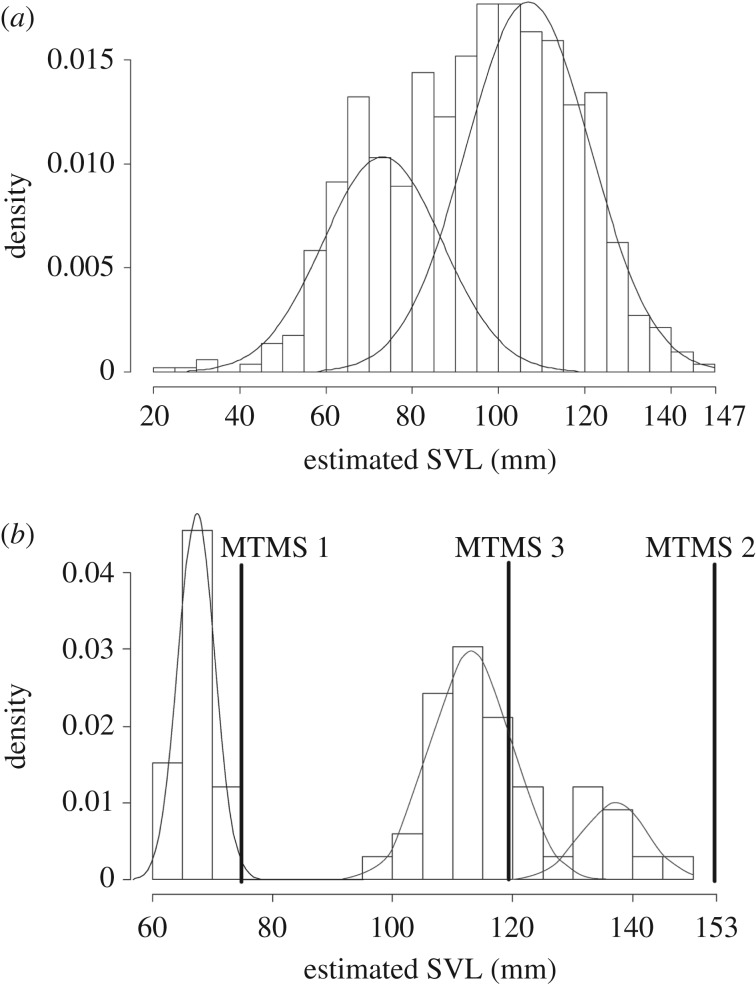
A total of 1028 bones (424 ilium bones, 302 dentaries, 191 humeri and 111 maxillary bones) from the three investigated fossil deposits were measured to obtain a set of estimated SVLs ranging from 23 to 147 mm. The global distribution of the estimated sizes tends to be bimodal as demonstrated by a mixture analysis (figure 1a). We observed differences between the sizes in the three sites (Kruskal–Wallis's test, p < 0.05) that could reflect differences in conservation or accumulation processes including accumulator agent choices. However, no differences exist between the sizes observed in Pleistocene and Holocene layers (Kruskal–Wallis test, p > 0.05) of the three deposits. The identification of the sizes associated with fully mature humeri (humeri for which the epiphysis were fused) allowed the removal of non-mature individuals from the analysis (figure 1b). This process reveals the occurrence of a clearly distinct population of smaller adult specimens with an SVL between 61 and 75 mm. The larger specimens constitute one or more likely two populations with SVLs of 95–125 mm and 120–145 mm according to the results of a mixture analysis. Following Maisano [15], the diaphysis of the humerus fuses with its epiphysis when the specimen attains around 80% of its maximal size. Thus, the differences between the smallest and the largest individuals of each group should not exceed 20%. This condition would be fulfilled for each group only if the large specimens above 99 mm SVL are considered as belonging to two groups, as suggested by the mixture analysis. The MTMS obtained with the smallest fully mature humerus provides a value of 75 mm (MTMS 1) and the largest immature humerus (with unfused epiphysis) a value of 153 mm (MTMS 2). These values could, respectively, correspond to the maximal sizes of the smallest and largest groups (figure 1b). Another value can be estimated using the smallest mature humerus included in the intermediately sized group (99 mm SVL), which results in an MTMS of 119 mm (MTMS 3 in figure 1b). We consider these estimations as reliable because they match with sizes directly observed in the fossil assemblage with the exception of the highest one (MTMS 2). However, considering that the largest group of individuals is only represented by a small number of specimens, it is likely that the full range of sizes is not observed in the fossil remains.
Figure 1.
(a) All reconstructed SVLs from the three sites (N = 1028) with the results of a mixture analysis indicating a bimodal distribution. (b) SVL reconstructed from the three sites on the basis of fully mature humeri (N = 66) with the results of a mixture analysis indicating a trimodal distribution. MTMS1, minimal theoretical maximal size obtained from the smallest fully mature humerus; MTMS 2, minimal theoretical maximal size obtained from the largest immature humerus; MTMS 3, minimal theoretical maximal size obtained from the smallest mature humerus included in the intermediately sized group.
(b). Morphology
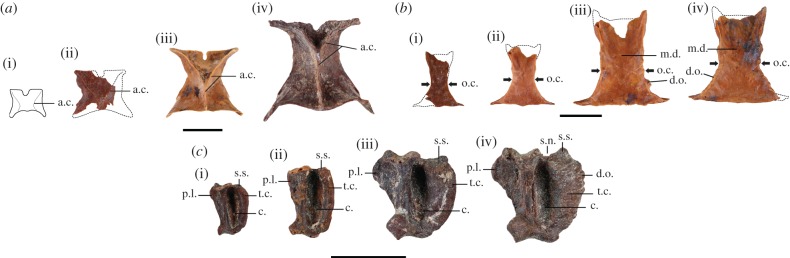
The morphology of the studied remains allows their identification as Anolis of the bimaculatus series (sensu [18]), likely to be A. ferreus. This attribution is mainly based on the occurrence of Y-shaped adductor crests on the parietal and of a pineal foramina on the frontal margin of this bone (all morphological characters used and anatomical descriptions are included in electronic supplementary material, S6). In addition, large fossil specimens bear a high caudal crest that only occurs in A. ferreus among Lesser Antillean anoles (Poe [19] indicates the occurrence of a caudal crest in Anolis oculatus, but this crest is far more developed in A. ferreus). However, the anole remains collected in the three investigated sites present an important morphological variability, especially between small and large bones. We here describe the three bones we found to be morphologically the most variable (parietal, frontal and quadrate bones). Parietal (N = 215; figure 2a): the smallest bones are short and nearly square in dorsal view with no contact between the adductor crests. In the larger bones, the parietal is posteriorly extended and has a more rectangular shape in dorsal view. The adductor crests are higher and are posteriorly fused to form a Y shape. The largest parietals are even more posteriorly extended and present higher adductor crests. This pattern of variability was previously described as ontogenetic by Etheridge [20] in Anolis carolinensis, but the earliest stage (figure 2a(i)) did not occur in our material, probably because the parietals, and especially the smaller ones, are fragile bones that are rarely found in fossil assemblages. Frontal (N = 456; figure 2b): in the smallest specimens, the bone is thin, nearly flat and presents an important orbital constriction. In the larger specimens, the bone is wider and thicker. Moreover, the orbital constriction is weaker, a tuberculous dermal ornamentation is observed on the orbital margins and the dorsal surface is medially depressed. This medial depression becomes stronger in the largest specimens. Quadrate bone (N = 589; figure 2c): the small quadrate bones have a rather rectangular shape in posterior view, but the larger quadrates are wider and have a more squared shape in posterior view. This difference in shape is mainly the effect of the development of the tympanic crest and the dorsal part of the pterygoid lamina that are more extensive in the large specimens, whereas the size of the conch maintains its proportions. The squamosal articular surface is also more developed in large specimens. In the largest specimens, the tympanic crest also presents a well-marked dermal ornamentation consisting of a ‘vermiculation’ and a deep squamosal notch. For the three bones described here, the same morphological variability was also observed in modern A. ferreus, although at the exception of the most extreme large morphologies. This is unsurprising considering that our largest modern specimen was only 94 mm SVL compared with 130 mm SVL calculated for the largest fossils. Additional fossil material is described in electronic supplementary material, S6.
Figure 2.
(a) Morphological variability of fossil parietal bones from the smallest (ii) to the largest (iv) and an earlier ontogenetic stage observed by Etheridge [20] (i) (dorsal view). (b) Morphological variability of fossil frontal bones from the smallest (i) to the largest (iv) (dorsal view). (c) Morphological variability of fossil left quadrate bones from the smallest (i) to the largest (iv) (posterior view). a.c., adductor crest; c., conch; d.o., dermal ornamentation; m.d., medial depression, o.c., orbital constriction; p.l., pterygoid lamina; t.c., tympanic crest; s.n., squamosal notch; s.s., squamosal articular surface. (Scale bar, 5 mm.) (Online version in colour.)
(c). Geometric morphometrics on the dentaries
(i). Modern Lesser Antillean anoles
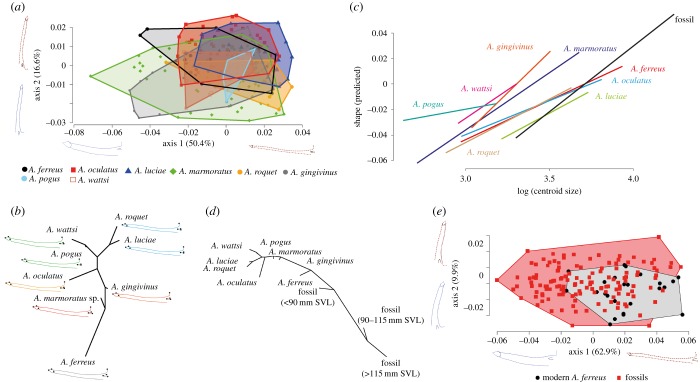
A principal component analysis (PCA) conducted on the shape data obtained from the dentaries of the modern Lesser Antillean anoles shows an important intraspecific variability and morphological overlap between the different taxa (figure 3a). This analysis shows that most of the variability (50.4%) is explained by the first axis of the PCA, representing the overall robustness of the bone. This axis is partly positively correlated with the centroid sizes of the bones (R2 = 0.25; p < 0.05), indicating significant allometry associated with the development of a more robust bone. The second axis (16% of the total variability) represents the relative length of the dental row, which is weakly correlated with the centroid size (R2 = 0.09; p < 0.05). A linear discriminant analysis confirms the strong morphological proximity between the taxa and revealed a weak proportion of correct identification (60% globally and between 80 and 0% depending of the taxon). However, the neighbour-joining tree constructed on the Mahalanobis distances between the modern species shows results that are partly in accordance with the anole molecular phylogeny [21] (figure 3b). The two members of the Dactyloa group [18], Anolis luciae and Anolis roquet, are closer to each other than to any other species. All the other taxa are part of the Ctenonotus group [18], but the morphology of their dentary only partly reflects the phylogeny with a strong morphological affinity between Anolis pogus and Anolis wattsi, two closely related species forming a distinct clade in the group. Moreover, as in the phylogeny, A. ferreus is, although distant from all other species, morphologically closer to Anolis marmoratus than to other species. However, the position of A. oculatus does not match the phylogeny in which this species is considered closer to A. marmoratus than to Anolis gingivinus [18]. A Kmult test [22] calculated on shape data of the samples also indicates the presence of phylogenetic signal in the data (K = 0.89, p < 0.05). Procrustes ANOVA with permutation procedures demonstrated a strong interaction between centroid size and shape (p < 0.05) but also shows a significant interaction with species (p < 0.05), although all taxa do not significantly differ in this regard (see electronic supplementary material, S7). This result can be visualized by the regression lines between species-specific shape and size data (figure 3c). The Kmult test is no longer significant (K = 0.63, p = 0.12) if conducted on allometry-free shape data. This indicates that differences in allometry between taxa yield significant phylogenetic information.
Figure 3.
(a) Two first axes of the PCA conducted on the shape data of the modern species dentaries showing important morphological overlaps between taxa. (b) Neighbour-joining tree using the Mahalanobis distances based on shape data of modern species. (c) Predicted values of dentary shape from species-specific regressions versus log of centroid size showing differences of allometry in modern and fossil specimens. (d) Neighbour-joining tree constructed on the Mahalanobis distances between the shape data of fossil and modern species showing morphological distances between fossils and modern groups. (e) Two first axes of the PCA conducted on shape data collected for fossil and modern A. ferreus dentaries showing a diminution of morphological variability between fossil and modern anoles. (Online version in colour.)
(ii). Fossil specimens
In order to test the specific homogeneity of the fossil assemblage of dentaries in regard to the different size groups observed in the size estimation study, fossil dentaries were divided into three groups on the basis of their size. A first group corresponding to specimens below 90 mm SVL is similar to the size observed in modern A. ferreus, a second group likely to correspond to large A. ferreus with specimens between 90 and 115 mm SVL and a third group corresponding to the large morphotype above 115 mm SVL. These three groups are found to be morphologically different (Manova, p < 0.05) but all share a similar allometric relation between shape and centroid size data (Procrustes ANOVA with permutation procedures, p > 0.05). These fossil groups were added to the neighbour-joining tree constructed based on modern taxa (figure 3d). The two larger fossil groups appear more similar to each other than to any other groups and also to be closer to the smaller fossils than to modern taxa. Mahalanobis distances also indicate the predominance of allometry in these data, considering that the largest fossil groups are the most distant from modern ones. These data indicate the strong morphological affinities between all fossil size groups, despite their strong morphological variability linked by the same allometric growth pattern. Fossil groups are distant from all other groups in the Mahalanobis distance tree but are positioned closer to A. ferreus than to any other species in that tree (figure 3d). However, these modern A. ferreus and our fossil groups are still significantly different in regard to their shape (Manova, p < 0.05) and also present significantly different allometries (Procrustes ANOVA with permutation procedures, p < 0.05) (figure 3c). Most of the difference between modern A. ferreus and the fossil groups is observed on the first axis of the PCA (62% of the variance), which is strongly correlated with size (R2 = 0.7; p < 0.05) (figure 3e). This axis is likely to represent an allometric growth component comparable to the one observed for modern specimens (figure 3a) but attaining far greater sizes and more robust morphology in fossils as shown by the shapes associated (figure 3e). Indeed, fossils are larger (mean centroid size = 51.4) than modern A. ferreus (mean centroid size = 39.5) and all modern specimens in general (mean centroid size = 29.9). However, all the differences between fossil dentaries and modern A. ferreus are not explained by size. Indeed, the second axis of the PCA (9.9% of the variance) (figure 3e) is not correlated with centroid size (R2 = 0.005; p > 0.05). Yet, this axis shows that many fossil specimens have longer dental rows than modern A. ferreus (Welch t-test; p < 0.05). As for the analysis on SVL, no significant differences in shape between fossil dentaries from the Pleistocene and the Holocene layers were observed (Manova; p > 0.05).
4. Discussion
The occurrence of three adult morphotypes of different sizes led to question concerning the specific uniformity of the Anolis bone assemblage. A first subset of small sized adult specimens between 60 and 75 mm SVL matches with the size of modern female A. ferreus, the species currently inhabiting Marie-Galante Island. Two subsets of larger specimens also exist. The larger specimens of first subset (max. 119 mm SVL) match with the maximal size of modern male A. ferreus (119 mm SVL), while the larger specimen of the second subset (153 mm SVL) exceeds this size by far. These data disprove the possibility of the occurrence of fossil species smaller than modern A. ferreus females and indicate size similarity between modern and fossil Marie-Galante anoles. Yet, the largest specimens observed do not match the size of any modern known A. ferreus, which raises the question of the possible occurrence of a distinct large species in the fossil record. This hypothesis is, however, not supported by the GMM analysis, which does not provide evidence of taxonomic heterogeneity in the fossil assemblages regarding the shape of the dentary. In addition, the morphological characters observed in fossil assemblages indicate the attribution of the whole assemblage to anoles of the bimaculatus series and at least large specimens to A. ferreus. However, the shape data collected on dentaries indicate that fossil specimens—regardless of their size—are significantly different from modern A. ferreus both in shape and allometry. This implies that fossils cannot be unambiguously referred to A. ferreus on the basis of morphological data. However, considering the molecular divergence time of A. ferreus relative to its most closely related species (more than 10 Ma [23]), it is very likely that this species colonized Marie-Galante at least hundreds of thousands of years ago. From a more general point of view, the size stability of our fossil morphotype from the Late Pleistocene to the present is a strong argument against the past occurrence of a species other than A. ferreus on Marie-Galante. Indeed, intraspecific competition is known to have a strong impact on the size of Lesser Antillean Anolis and the extinction of a large taxon would likely have impacted the size evolution of other species, as is hypothesized by several models [24–26]. Moreover, the situation with three morphotypes of the same species as observed in the fossil record is also observed in some modern taxa [27]. The occurrence of a distinct large species is also in contradiction to the important sexual size dimorphism of fossil and modern A. ferreus. Indeed, an important sexual size dimorphism can be explained by the fact that males and females of a solitary species occupy the ecological niches that are otherwise occupied by two species. This pattern is general in Lesser Antillean Islands that are occupied by either one or two species of anole lizards [25]. The most parsimonious hypothesis is thus that fossil Marie-Galante anoles correspond to A. ferreus that underwent morphological changes during the past centuries, making their strict comparison with modern specimens partly inconsistent.
The anatomical description of the fossil anole remains shows that, although highly variable, the morphology of the bones seems to represent an ontogenetic variability reflecting an allometric growth also observed in modern A. ferreus and other anoles of the bimaculatus series [20]. The largest specimens bear features that change the shape of the skull (dermal ornamentation on the quadrate and the frontal, depression of the frontal and very high adductor crests). Several osteological structures that are especially well developed in fossil and modern large specimens are associated with muscles known to play a role in bite force generation [28,29]. The height of the adductor crest may be an indicator of the important development of the M. adductor mandibulae externus medialis, whereas the development of the tympanic crest and pterygoid lamina of the quadrate bones could be related to the development of the M. adductor mandibulae externis superficialis anterior, M. adductor mandibulae externus posterior and M. adductor mandibulae posterior [29,30]. In addition, the important width of the quadrate bone might be related to the size of the adductor muscle chamber, which has been demonstrated to be linked with bite force [28]. These elements indicate that the large specimens probably had a stronger bite than the small ones, a characteristic important in Anolis male–male combat (review in [31]). These characters are likely to reflect the strong sexual dimorphism occurring in modern A. ferreus and fossil anoles. The fact that these characters are more accentuated in some fossils than in modern A. ferreus suggests that the sexual dimorphism was stronger in extinct populations, although we may not have characterized the full morphological variability of extant populations.
The GMM approach on the dentaries provided promising results, although it also revealed the strong morphological variability of Lesser Antillean anoles and the important morphological overlap between taxa. Thus, the identification of anole species based on a single dentary is impossible. However, when enough specimens of each species are taken into account, morphological trends were revealed. Thus, the identification of fossil remains of Lesser Antillean anoles might be possible to some degree. The main information provided by the modern reference sample is that the fossil is morphologically closer to A. ferreus than to other Lesser Antillean taxa. Yet, the fossil was also different, mainly because of the allometric growth that impacts the shape of the fossils. The comparison between modern A. ferreus and fossil specimens shows that the morphological variability was greater in the fossils and that differences between modern and fossil specimens were statistically significant. If the differences observed are not considered methodological artefacts, fossil specimens appear to be morphologically more variable than modern A. ferreus, especially with respect to length of their dental row.
A first possibility to explain the occurrence of a large morphotype of A. ferreus in fossil deposits is that this subset of larger size was collected by the accumulator agent in another region of the island were A. ferreus was larger than in the direct vicinity of the deposit. Considering the costal location of the deposits, it is likely that the population sampled by the accumulator agent was a coastal population and the larger specimens may, for example, have originated from the plateau (altitude 200 m) that was densely forested in the past [32] and still presents more mesic vegetation than the surrounding areas [33,34]. An increase in body size related to altitude was observed in A. oculatus on the neighbouring island of Dominica [35] and could also be hypothesized on Marie-Galante. Another argument would be that the maximum size of modern A. ferreus (MCZ R-70767; 119 mm SVL) was observed in specimens collected near the coast. This would suggest that the size of these coastal anoles has not changed since the Pleistocene. This hypothesis implies, however, a size decrease in the anoles occurring on the plateau because specimens as large as the largest fossils have never been observed. This would not seem surprising considering that the primary forest of the plateau, the putative habitat of these anoles, was entirely cut during past centuries for agricultural purposes [36]. On the contrary, the size stability of the coastal anoles could be explained by the existence of several refuges in areas unfit for agriculture, including slopes or gullies. Testing this hypothesis would require more data about the size distribution of extant A. ferreus on Marie-Galante. A second explanation, not mutually exclusive of the former, could be that a now-extinct morphotype of larger, possibly dominant, males occurred on Marie-Galante in the past. Extant anoles such as A. carolinensis show a small subgroup of very large dominant males that could also have occurred in fossil A. ferreus [27]. The disappearance of this dominant male phenotype might be explained by a switch in sexual strategy or simply a lack of available habitat of mature forest trees where these large dominant morphs would typically perch and display with relative protection from predators provided by the trees.
Both the SVL reconstruction and the GMM analysis show no significant differences between specimens contained in Pleistocene and Holocene layers (see also [11]). However, fossil A. ferreus differ from modern forms, for the following reasons. (i) The largest morph of A. ferreus occurring in the fossil record is no longer observed on the island. (ii) The fossils were possibly morphologically more variable and presented a longer dental row than modern A. ferreus. (iii) The allometric relation between shape and size of the dentaries differs between fossil and modern specimens. This difference may reflect a change in feeding habits [37] or maybe sexual competition, but ecological data needed to test these ideas are lacking. Thus, after at least 30 000 years of morphological stability, A. ferreus may have very recently undergone a loss of its morphological diversity with the disappearance of a large morph. Based on the raw SVL data, a reduction in size of A. ferreus between the Late Holocene and the modern period is evident, as observed for several other lizards [38,39]. Yet, our data suggest a more complex scenario, given that populations whose sizes are similar to modern female and male A. ferreus exist in the fossil record. Thus, A. ferreus may have been differently impacted rather than having undergone a strict overall size reduction. The disappearance of the large morph would not be surprising considering the size bias extinction of Quaternary lizards in the Lesser Antilles [40], which is also observed on Marie-Galante Island [10,11]. These modifications that seem to have occurred after 30 000 years of morphological stability cannot be related to climatic and environmental modifications [41,42], which occurred during the period documented by the fossiliferous deposits. The large fossil morph of A. ferreus appears for the last time in the most recent layer of Cadet 2 cave dated to the fourteenth century [11]. There is a void in fossil data from historical periods and specimens of such size are not mentioned either in the first description of A. ferreus in the nineteenth century [43] or in the subsequent studies of this lizard [44,45]. A similar observation can be made for several squamates (Pholidoscelis sp., Leiocephalus sp. and Antillotyphlops) that disappeared around the same period [10,11] during which the island was colonized by Europeans and intensively deforested for agricultural purposes [33]. The lack of fossil data for these recent periods precludes linking these extinction phenomena to a precise event. However, the quick diminution in only a few centuries of the morphological variability of A. ferreus after thousands of years of stability throughout the Pleistocene/Holocene climatic transition [41,42,46] and the pre-Columbian anthropic periods seems indisputably connected to the environmental impact of historical European societies. Deforestation occurring in this period on Marie-Galante [33] could explain the lack of large trees suitable for a morph of large A. ferreus males. Concomitant extinction events explained by the same phenomenon were also demonstrated on the nearby Island of La Désirade [47]. These events are parts of a global extinction phenomenon linked to anthropogenic disturbances impacting West Indian [6,40,48] and global [1,49] biodiversity.
Our study highlights the potential role of Late Holocene palaeontological data to observe the evolution of extant taxa under anthropogenic impacts. Such approaches using both palaeontological and archaeological material are of critical importance for the quantification of human impacts on fauna, especially in fragile and vulnerable insular environments. In addition, we demonstrate that even species with large modern populations and considered weakly impacted by anthropic disturbance respond to these phenomena and can be affected in more complex ways.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank two anonymous reviewers for helpful and constructive comments on an earlier version of the manuscript. We are also grateful to the excavation directors of the studied sites: P. Courtaud, S. Grouard, A. Lenoble and C. Stouvenot. We thank the Muséum national d'Histoire naturelle morphometry platform and the institutions that provided comparative specimens: the National Museum of Natural History (Washington/USA) (K. De Queiroz), the Museum of Comparative Zoology (Harvard/USA) (J. Rosado), the Muséum National d'Histoire Naturelle (Paris/France), the PACEA laboratory (Bordeaux/France) (A. Lenoble) and the Edgar Clerc Museum (Moule/Guadeloupe) (S. Guimaraez). We are very grateful to M. E. Kemp who assisted us during our stay at Boston and also to people who provided comparative specimens: V. Bels, C. Martin, A. Lenoble and M. Breuil.
Data accessibility
Additional data can be found in the electronic supplementary material.
Authors' contributions
C.B. conceived and designed the study, performed all the analysis and drafted the manuscript; S.B. took part in the description and identification of the fossils and helped draft the manuscript; A.H. took part in the interpretation of the results and helped draft the manuscript; S.G. took part in the conception of the study, collection of fossils on the field and helped to obtain comparative specimens; I.I. helped to obtain comparative specimens and helped draft the manuscript; A.T. helped draft the manuscript; R.C. took part in the conception of the study, the geometric morphometric analysis and helped draft the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the Collective Research Program ‘Cavités naturelles de Guadeloupe:aspects géologiques, fauniques et archéologiques’ and CNRS BIVAAG project: ‘Biodiversité Insulaire Vertébrée, floristique et malacologique Ancienne de l'Archipel de Guadeloupe’, with support from a European PO-FEDER grant 2007-2013 n°2/2.4/-33456, the Guadeloupe Regional Council, Regional Service of Archaeology, the DEAL of Guadeloupe and the DAC of Guadeloupe.
References
- 1.Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM. 2015. Accelerated modern human-induced species losses: entering the sixth mass extinction. Sci. Adv. 1, e1400253 ( 10.1126/sciadv.1400253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulay G. 1994. Biodiversity on oceanic islands: its origin and extinction. Am. Zool. 34, 134–144. ( 10.1093/icb/34.1.134) [DOI] [Google Scholar]
- 3.Loope LL, Giambelluca TW. 1998. Vulnerability of island tropical montane cloud forests to climate change, with special reference to East Maui, Hawaii. Clim. Change 39, 503–517. ( 10.1023/A:1005372118420) [DOI] [Google Scholar]
- 4.Slavenko A, Tallowin OJS, Itescu Y, Raia P, Meiri S. 2016. Late Quaternary reptile extinctions: size matters, insularity dominates. Glob. Ecol. Biogeogr. 25, 1308–1320. ( 10.1111/geb.12491) [DOI] [Google Scholar]
- 5.Case TJ, Bolger DT, Richman AD. 1992. Reptilian extinctions: the last ten thousand years. In Conservation biology (eds Fiedler PL, Jain SK), pp. 91–125. New York, NY: Springer. [Google Scholar]
- 6.Steadman DW, Albury NA, Kakuk B, Mead JI, Soto-Centeno JA, Singleton HM, Franklin J. 2015. Vertebrate community on an ice-age Caribbean island. Proc. Natl Acad. Sci. USA 112, E5963–E5971. ( 10.1073/pnas.1516490112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson R, Powell R. 2009. Natural history of West Indian reptiles and amphibians. Gainesville, FL: University Press of Florida. [Google Scholar]
- 8.Schwartz A, Henderson RW. 1991. Amphibians and reptiles of the West Indies: descriptions, distributions and natural history. Gainesville, FL: University of Florida Press. [Google Scholar]
- 9.Lazell JD. 1972. The anoles (Sauria, Iguanidae) of the Lesser Antilles. Bull. Mus. Comp. Zool. 143, 1–115. [Google Scholar]
- 10.Bailon S, Bochaton C, Lenoble A. 2015. New data on Pleistocene and Holocene herpetofauna of Marie-Galante (Blanchard Cave, Guadeloupe Islands, French West Indies): insular faunal turnover and human impact. Quat. Sci. Rev. 128, 127–137. ( 10.1016/j.quascirev.2015.09.023) [DOI] [Google Scholar]
- 11.Bochaton C, Grouard S, Cornette R, Ineich I, Tresset A, Bailon S. 2015. Fossil and subfossil herpetofauna from Cadet 2 Cave (Marie-Galante, Guadeloupe Islands, F. W. I.): evolution of an insular herpetofauna since the Late Pleistocene. C. R. Palévol. 14, 101–110. ( 10.1016/j.crpv.2014.10.005) [DOI] [Google Scholar]
- 12.Stouvenot C, Grouard S, Bailon S, Bonnissent D, Lenoble A, Serrand N, Sierpe V. 2014. L'abri sous roche Cadet 3 (Marie-Galante): un gisement à accumulations de faune et à vestiges archéologiques. In Un demi-siècle d'archéologie caribéenne, pp. 126–140. Martinique, France: AIAC. [Google Scholar]
- 13.Bochaton C. 2016. Describing archaeological Iguana Laurenti, 1768 (Squamata: Iguanidae) populations: size and skeletal maturity. Int. J. Osteoarchaeol. 26, 716–724. ( 10.1002/oa.2463) [DOI] [Google Scholar]
- 14.Bochaton C, Kemp ME. 2017. Reconstructing the body sizes of Quaternary lizards using Pholidoscelis Fitzinger, 1843, and Anolis Daudin, 1802, as case studies. J. Vertebr. Paleontol. 37, e1239626 ( 10.1080/02724634.2017.1239626) [DOI] [Google Scholar]
- 15.Maisano JA. 2002. Terminal fusions of skeletal elements as indicators of maturity in squamates. J. Vertebr. Paleontol. 22, 268–275. ( 10.1671/0272-4634(2002)022%5B0268:TFOSEA%5D2.0.CO;2) [DOI] [Google Scholar]
- 16.Adams D, Collyer M, Sherratt E. 2016. geomorph: Geometric morphometric analyses of 2D/3D landmark data. See https://cran.r-project.org/web/packages/geomorph/index.html.
- 17.Young D, Benaglia T, Chauveau D, Hunter D, Elmore R, Hettmansperger T, Thomas H, Xuan F. 2016. mixtools: Tools for analyzing finite mixture models. See https://cran.r-project.org/web/packages/mixtools/index.html.
- 18.Nicholson K, Crother BI, Guyer G, Savage JM. 2012. It is time for a new classification of anoles (Squamata: Dactyloidae). Zootaxa 3477, 1–108. [Google Scholar]
- 19.Poe S. 2004. Phylogeny of Anoles. Herpetol. Monogr. 18, 37–89. ( 10.1655/0733-1347(2004)018%5B0037:POA%5D2.0.CO;2) [DOI] [Google Scholar]
- 20.Etheridge R. 1959. The relationships of the anoles (Reptilia: Sauria: Iguanidae): an interpretation based on skeletal morphology. Unpublished PhD thesis, University of Michigan.
- 21.Pyron RA, Burbrink FT, Wiens JJ. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 13, 1–53. ( 10.1186/1471-2148-13-93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams DC. 2014. A generalized K statistic for estimating phylogenetic signal from shape and other high-dimensional multivariate data. Syst. Biol. 63, 685–697. ( 10.1093/sysbio/syu030) [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Wiens JJ. 2016. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol. Phylogenet. Evol. 94, 537–547. ( 10.1016/j.ympev.2015.10.009) [DOI] [PubMed] [Google Scholar]
- 24.Roughgarden J, Pacala S. 1989. Taxon cycle among Anolis lizard populations: review of evidence. In Speciation and its consequences (eds Otte D, Jendler JA), pp. 403–432. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 25.Williams EE. 1972. The origin of faunas. Evolution of lizard congeners in a complex island fauna: a trial analysis. In Evolutionary biology, Volume 6 (eds Dobzhansky T, Hecht MK, Steere WC), pp. 47–89. New York, NY: Springer. [Google Scholar]
- 26.Losos JB. 1992. A critical comparison of the taxon-cycle and character-displacement models for size evolution of Anolis lizards in the Lesser Antilles. Copeia 1992, 279–288. ( 10.2307/1446189) [DOI] [Google Scholar]
- 27.Lailvaux SP, Herrel A, Vanhooydonck B, Meyers JJ, Irschick DJ. 2004. Performance capacity, fighting tactics and the evolution of life-stage male morphs in the green anole lizard (Anolis carolinensis). Proc. R. Soc. Lond. B 271, 2501–2508. ( 10.1098/rspb.2004.2891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrel A, Mcbrayer LD, Larson PM. 2007. Functional basis for sexual differences in bite force in the lizard Anolis carolinensis. Biol. J. Linn. Soc. 91, 111–119. ( 10.1111/j.1095-8312.2007.00772.x) [DOI] [Google Scholar]
- 29.Wittorski A, Losos JB, Herrel A. 2016. Proximate determinants of bite force in Anolis lizards. J. Anat. 228, 85–95. ( 10.1111/joa.12394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas G. 1973. Muscles of the jaws and associated structures in the Rhynchocephalia and Squamata. In Biology of the reptilia. Volume 4, morphology D (eds Gans C, Parsons TS), pp. 285–490. New York, NY: Academic. [Google Scholar]
- 31.Losos JB. 2009. Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. Berkeley, CA: University of California Press. [Google Scholar]
- 32.Rochefort CD. 1658. Histoire naturelle et morale des Antilles de l'Amérique. Histoire générale des Antilles habitées par les Français. Rotterdam, The Netherlands: Arnould Liers. [Google Scholar]
- 33.Lasserre G. 1961. La Guadeloupe: étude géographique Tome 1: Le milieu naturel et l'héritage du passé. Bordeaux, France: Union française d'impression. [Google Scholar]
- 34.Rousteau A. 1996. Carte écologique de la Guadeloupe. (Maps with bibligraphic references.) Guadeloupe, France: ONF, UAG, Parc National de la Guadeloupe.
- 35.Malhotra A, Thorpe R. 1997. Size and shape variation in a Lesser Antillean anole, Anolis oculatus (Sauria: Iguanidae) in relation to habitat. Biol. J. Linn. Soc. 60, 53–72. ( 10.1006/bijl.1996.0088) [DOI] [Google Scholar]
- 36.Lasserre G. 1950. Marie-Galante. Cah. O.-m. 3, 123–152. [Google Scholar]
- 37.Metzger KA, Herrel A. 2005. Correlations between lizard cranial shape and diet: a quantitative, phylogenetically informed analysis. Biol. J. Linn. Soc. 86, 433–466. ( 10.1111/j.1095-8312.2005.00546.x) [DOI] [Google Scholar]
- 38.Pregill GK. 1986. Body size of insular lizards: a pattern of Holocene dwarfism. Evolution 40, 997–1008. ( 10.1111/j.1558-5646.1986.tb00567.x) [DOI] [PubMed] [Google Scholar]
- 39.Bochaton C, Bailon S, Ineich I, Breuil M, Tresset A, Grouard S. 2016. From a thriving past to an uncertain future: zooarchaeological evidence of two millennia of human impact on a large emblematic lizard (Iguana delicatissima) on the Guadeloupe Islands (French West Indies). Quat. Sci. Rev. 150, 172–183. ( 10.1016/j.quascirev.2016.08.017) [DOI] [Google Scholar]
- 40.Kemp ME, Hadly EA. 2015. Extinction biases in Quaternary Caribbean lizards. Glob. Ecol. Biogeogr. 24, 1281–1289. ( 10.1111/geb.12366) [DOI] [Google Scholar]
- 41.Hodell DA, Curtis JH, Jones GA, Higuera-Gundy A, Brenner M, Binford MW, Dorsey KT. 1991. Reconstruction of Caribbean climate change over the past 10,500 years. Nature 352, 790–793. ( 10.1038/352790a0) [DOI] [Google Scholar]
- 42.Curtis JH, Brenner M, Hodell DA. 2001. Climate change in the circum-caribbean (Late Pleistocene to present) and implications for regional biogeography. In Biogeography of the West Indies (ed. Woods CA.), pp. 35–54. Boca Raton, FL: CRC Press. [Google Scholar]
- 43.Cope ED. 1864. Contributions to the herpetology of tropical America. Proc. Acad. Nat. Sci. Phila. 16, 166–176. [Google Scholar]
- 44.Lazell JD. 1964. The anoles (Sauria, Iguanidae) of the Guadeloupéen archipelago. Bull. Mus. Comp. Zool. 131, 359–401. [Google Scholar]
- 45.Underwood G. 1959. Revisionary notes. The anoles of the eastern Caribbean (Sauria, Iguanidae). Part III. Bull. Mus. Comp. Zool. 121, 191–226. [Google Scholar]
- 46.Royer A, Malaizé B, Lécuyer C, Queffelec A, Charlier K, Caley T, Lenoble A. 2017. A high-resolution temporal record of environmental changes in the Eastern Caribbean (Guadeloupe) from 40 to 10 ka BP. Quat. Sci. Rev. 155, 198–212. ( 10.1016/j.quascirev.2016.11.010) [DOI] [Google Scholar]
- 47.Boudadi-Maligne M, Bailon S, Bochaton C, Cassagrande F, Grouard S, Serrand N, Lenoble A. 2016. Evidence for historical human-induced extinctions of vertebrate species on La Désirade (French West Indies). Quat. Res. 85, 54–65. ( 10.1016/j.yqres.2015.11.001) [DOI] [Google Scholar]
- 48.Bochaton C, Boistel R, Cassagrande F, Grouard S, Bailon S. 2016. A fossil Diploglossus (Squamata, Anguidae) lizard from Basse-Terre and Grande-Terre islands (Guadeloupe, French West-Indies). Sci. Rep. 28475, 1–12. ( 10.1038/srep28475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnosky AD, et al. 2011. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57. ( 10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data can be found in the electronic supplementary material.