Abstract
Objective
To evaluate microbiologic effectiveness, i.e. culture negativity of a non-blinded eradication protocol (Rx) compared to observation (Obs) in clinically stable cystic fibrosis participants with newly positive MRSA cultures.
Design
This non-blinded trial randomized participants ages 4-45 years with first or early (≤2 positive cultures within 3 years) MRSA positive culture without MRSA-active antibiotics within 4 week 1:1 to Rx or Obs. The Rx protocol was: oral trimethoprim-sulfamethoxazole or if sulfa-allergic, minocycline plus oral rifampin; chlorhexidine mouthwash for two weeks; nasal mupirocin and chlorhexidine body wipes for five days, and environmental decontamination for 21 days. The primary endpoint was MRSA culture status at day 28.
Results
Between April 1, 2011 to September 2014, forty-five participants (44% female, mean age 11.5 years) were randomized (24 Rx, 21 Obs). At Day 28, 82% (n=18/22) of participants in the Rx-arm compared to 26% (n=5/19) in Obs-arm were MRSA negative. Adjusted for interim monitoring, this difference was 52% (95% CI: 23%, 80%; p<0.001). Limiting analyses to participants who were MRSA positive at the screening visit, 67% (8/12) in the Rx and 13% (2/15) in the Obs-arm were MRSA negative at Day 28, adjusted difference: 49% (95% CI: 22%,71%, p<0.001). Fifty-four percent in the Rx compared to 10% participants in the Obs-arm remained MRSA negative through Day 84. Mild gastrointestinal side effects were higher in the Rx-arm.
Conculsions
This MRSA eradication protocol for newly acquired MRSA demonstrated microbiologic efficacy with a large treatment effect.
Trial Registration
Keywords: Methicillin resistant Staphylococcus aureus, eradication, cystic fibrosis, lung infection
Introduction
Infection with methicillin resistant S. aureus (MRSA) continues to have a significant impact in hospital and community acquired infections1,2. In subjects with cystic fibrosis (CF) the prevalence of MRSA positive respiratory cultures increased from 11.9% in 2003 to 25.6% in 2013 in US CF-centers and contributes to adverse outcomes in CF.3,4 Cross-sectional, epidemiologic studies demonstrated that MRSA was associated with lower lung function in CF5,6 and greater use of medical therapies.7 Longitudinal outcomes of MRSA in CF have noted differing results; one study found no difference in lung function decline whereas another study showed greater lung function decline in those acquiring persistent MRSA infection.8,9 Similarly, CF patients with persistent MRSA infection may have increased mortality.10
Treatment approaches highlighted by case series have varied widely from observation to long term or intravenous antibiotics with the goal of eradicating MRSA.11-13 Despite the evolving concern of this organism in the CF community, to date there are no randomized studies demonstrating if treating MRSA at initial detection can eradicate MRSA and prevent chronic respiratory infection. Despite MRSA rates being significantly lower than in the U.S., many European countries treat any positive respiratory cultures of MRSA and MSSA; alternatively, U.S. guidelines do not recommend treatment of incident MRSA or MSSA in CF5. The risks of indiscriminate and prolonged treatment of MRSA acquisition include the emergence of new/increasingly resistant organisms and treatment related toxicity.
The current study aimed to evaluate the 28 day safety and microbiologic efficacy, i.e. microbiologic treatment effect, of a MRSA eradication protocol in CF patients with newly acquired MRSA as compared to observation alone. We hypothesized that patients with CF who are clinically stable at time of first detection of MRSA in a respiratory culture are more likely to be MRSA culture negative following an intense multi-faceted eradication protocol compared to the current U.S. standard of care of not treating MRSA when patients are stable.
Materials and Methods
Study Centers
The trial was conducted from April 1, 2011 to September 2014 at 14 CF Foundation accredited care centers in the United States. The trial was coordinated by the CF Foundation Therapeutics Development Network Coordinating Center (TDNCC; Seattle, WA) and registered on clinicaltrials.gov (NCT01349192). Institutional review boards at each participating center approved the study and each participant and/or their parent voluntarily provided written consent to participate in the trial. Age appropriate assent was obtained as indicated.
Study Participants
Eligibility criteria included a confirmed diagnosis of CF, age 4-45 years at time of consent and a new onset MRSA positive culture from sputum, or oro-pharyngeal (OP) swab or bronchoscopy. New onset was defined as a MRSA positive culture within 6 months that was either the first lifetime MRSA positive culture or new emergence of MRSA after at least 1 year of documented negative cultures (minimum of two cultures/year while off MRSA active antibiotics) for MRSA in participants with ≤ 2 MRSA positive cultures in the past 3.5 years. After the planned interim review the DMC recommended that the number of study sites be increased and that subjects MRSA negative at screening visit be included if the initial clinical MRSA isolate was available. This was to enhance enrolment and to reflect clinical practice. Participants had to be clinically stable within the 14 days prior to screening. Exclusion criteria included having received antibiotics with activity against MRSA or use of an investigational drug within 28 days of screening, and FEV1 <30% of predicted based on reference equations.14,15 Participants with contraindications for study medications i.e. allergy or renal or hepatic dysfunction were not eligible. Microbiologic contraindications were resistance of the available MRSA isolate to TMP-SMX and minocycline or to rifampin.
Randomization and Blinding
Participants were randomized (1:1) to a MRSA eradication protocol as outlined below or to no treatment within strata defined by site, age (4 to 12 years, 13 to 45 years) and presence of P. aeruginosa at screening. Randomization assignments were generated via a centralized, secure web based randomization system for each enrolled subject. Study personnel and participants were not blinded to the treatment regimen.
Treatment Regimen (Rx)
The treatment protocol for those randomized to the MRSA eradication protocol consisted of two oral antibiotics for two weeks combined with nasal, skin and oral decontamination as well as a three week enhanced household cleaning. Study medications were oral trimethoprim-sulfamethoxazole (TMP-SMX) dosed per CF guidelines at 8 mg/kg trimethoprim/40 mg/kg sulfamethoxazole for children <40 kg and 320mg/1600 mg for adults given twice daily for 14 days. In participants intolerant to TMP-SMX, minocycline, if >8 years, at a dose of 100 mg BID was substituted. All participants randomized to the treatment protocol received combination therapy with rifampin (15 mg/kg/day up to 40 kg or 300 mg BID). Nasal mupirocin and whole body cleansing with chlorhexidine wipes was used for 5 days in addition to twice daily gurgling with 0.12% chlorhexidine gluconate oral rinse for 14 days. Enhanced household cleaning included weekly washing of linens and towels, wiping down high contact surfaces e.g. toys and computers with chlorhexidine and extra cleaning of airway clearance devices. Selection of the Rx regimen was based on decolonization strategies in non-CF populations and TMP-SMX was selected based on an U.S. observational trial in CF which had shown that most MRSA isolates were susceptible to TMP-SMX and rifampin, with very low resistance rates to mupirocin.16,17
For participants randomized to the observation arm (Obs), treatment with anti-MRSA therapy prior to Day 28 was only allowed for a protocol defined exacerbation with choice of antibiotics per the participant's treating physician. Use of anti-MRSA antibiotics after Day 28 was allowed for both arms.
Clinical Evaluations
Medical history, physical examination, specimen sampling for microbiology (OP, nasal, groin and axilla swabs on all participants, additional sputum in those expectorating) and spirometry were obtained at the screening visit (Day -14). Clinical evaluations, physical examination and spirometry were performed at Day 1 (randomization), 15, 28, 84, and 168. Follow-up after day 28 was used to assess durability of treatment effect. Pulmonary function testing was performed in accordance with American Thoracic Society standards.18 Adverse events and concomitant medications were recorded during each visit and by phone calls conducted at Day 7. Protocol defined pulmonary exacerbations were defined using a combination of spirometry, X-ray and clinical symptoms as in prior studies.19
Primary and Secondary Outcomes
The primary outcome of the study was difference in the proportion of MRSA-negative subjects based on OP-swab or sputum at day 28 between the two study arms. Secondary endpoints included safety, tolerability of the treatment regimen, protocol adherence, duration of microbiologic effect, number of pulmonary exacerbations, use of antibiotics, change in spirometry (as measured by FEV1), respiratory symptoms as measured by the CF specific patient outcomes: Cystic Fibrosis Questionnaire Revised (CFQ-R) respiratory domain scores and Cystic Fibrosis Respiratory Symptom Diary Chronic Respiratory Infection Symptom Scale (CFRSD-CRISS), and weight.
Statistical Analysis
The study design specified randomization of 90 participants providing 80% power to detect a difference of 30% or greater in the proportion of respiratory cultures negative for MRSA at Day 28 (Rx minus Obs). The sample size calculations assumed a dropout rate of 10%, ensuring 40 randomized participants to Rx and 40 to Obs-arms.
Safety outcomes were monitored throughout the trial by a Data Monitoring Committee (DMC) appointed by the CF Foundation Safety Monitoring Board. One interim analysis with early stopping for futility was scheduled to take place after approximately half of the participants had been randomized into the study and had completed the Day 28 visit. Because of slow accrual, an early interim review and futility analysis of the primary endpoint was initiated when 24 participants had completed the first 28 days of the study. Upon review of the interim results, the DMC recommended continuance of the study with a second interim analysis for early efficacy. The study team remained blinded to findings presented to the DMC. Statistical ramifications of this unplanned efficacy analysis are addressed in the Online Supplement.
All of the safety and secondary efficacy analyses were conducted on the Intent-to-Treat population (ITT). The primary efficacy analyses were performed on the Intent-to-Treat-Efficacy (ITT-E) population. The Per-Protocol Efficacy (PPE) population was used in sensitivity analyses of the primary endpoint. The detailed definitions of analyses populations are provided in Figure 1. T-tests were used to compare continuous variables by study arm. The comparisons of proportions were performed using Fisher exact test, with corresponding 95% confidence interval (CI) derived using the Newcombe-Wilson method. Event rate comparisons were performed using Poisson regression. In the analyses of the primary endpoint, the difference in the proportion of respiratory cultures negative for MRSA at Day 28 (Rx minus Obs), the treatment effect, 95% CI's and p-value estimates were adjusted for the interim reviews. The use of group sequential stopping rules alters the sampling distribution of the usual fixed sample statistics and so adjustments need to be made to compute the point estimates, CI's and p-values. The results of final analyses had to be adjusted for bias to account for multiple looks at the data during the two interim reviews (referred to “adj. for interim monitoring”).
Figure 1. CONSORT diagram of participant disposition.
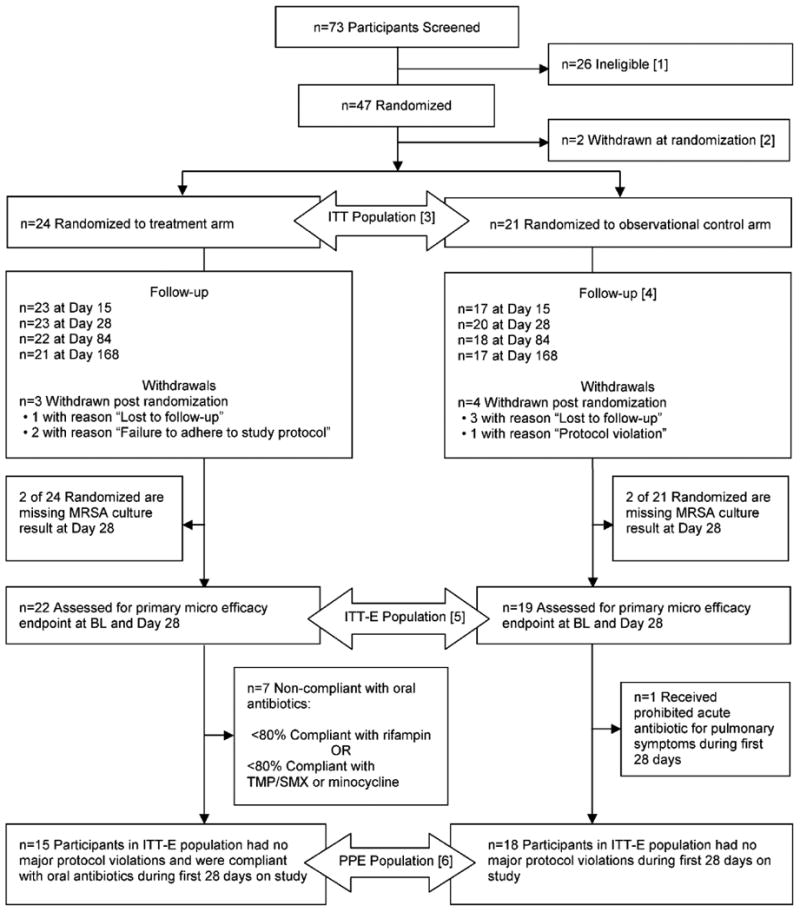
Flow diagram of participants through each stage of the randomized trial.
Footnote: Nine participants did not meet the inclusion criteria of being clinically stable. One of these participants was subsequently re-screened but failed the inclusion criteria of MRSA positive culture at screening or within 6 months prior to screening.
Eleven participants failed the inclusion criteria of MRSA positive culture at screening or within 6 months prior to screening.
One participant did not meet the inclusion criteria by withdrawing their consent.
One participant did not meet the inclusion criteria of having a documented CF diagnosis.
Two participants met the exclusion criteria of receiving anti-MRSA antibiotics within 28 days prior to screening.
One participant met the exclusion criteria of abnormal renal function at screening.
One participant met the exclusion criteria that warranted a screen failure due to investigator's opinion.
[2] Two participants randomized to the observational control arm withdrew with reason “Subject decision”.
[3] The intent-to-treat (ITT) population is defined as participants who are randomized to a study arm and followed post randomization. The ITT population is used for all safety and secondary efficacy analyses.
[4] One site, which enrolled a total of four participants (two in the treatment arm, two in the observational control arm), experienced numerous study conduct issues and protocol violations resulting in missing primary endpoint and other endpoint data. One of these four participants had withdrawn immediately following randomization to the observational control arm. The remaining three participants' data is summarized as available post-randomization.
[5] The intent-to-treat efficacy (ITT-E) population consists of all the participants in the ITT population who were assessed for the primary microbiologic efficacy endpoint at baseline and Day 28. The ITT-E population is used for the primary efficacy analyses.
[6] The per-protocol efficacy population (PPE) is comprised of all participants in the ITT-E population excluding the participants with major protocol violation or those non-compliant with oral antibiotic use during the first 28 days of the study. The PP population is used in sensitivity analyses of primary efficacy endpoint.
P-values and confidence intervals are two-sided, 0.05 significance level; analyses were performed using SAS (version 9.4, SAS Institute Inc., Cary, NC, USA, 2013) and R (version 3.2.1, The R Foundation for Statistical Computing, Vienna, Austria, 2015).
Results
Although the planned sample size was 90 patients, the DMC recommended that the enrollment of new participants be stopped early with ongoing follow-up of enrolled subjects, after interim review by the DMC showed a statistically significant microbiological treatment effect. At the time the trial was stopped, 73 participants had been screened at 14 participating centers; 45/73 participants were randomized and followed post-randomization (ITT population), 24 in the Rx and 21 in the Obs-arm (Figure 1).
Clinical characteristics of the ITT-participants (n=45) showed a mean age of 11.5 years with well-preserved lung function (Table 1). The two arms were comparable at baseline (Table 1) and distribution of participants who had a sputum sample in addition to OP swab did not differ by study arm (no participant included based on bronchoscopy results). Of the 45 randomized participants, 41 (22 Rx and 19 Obs) were included in the ITT-E analyses. Although the protocol allowed changing from TMP-SMX to minocycline in case of side effects, none changed treatment during the study; however, two subjects in the Rx arm were started on minocycline due to previously known intolerances to TMP-SMX. A total of seven participants withdrew post randomization (3 [13%] in Rx and 4 [19%] in Obs); 4 withdrew before Day 28 visit and were not assessed for the primary microbiologic endpoint (Figure 1).
Table 1. Demographics and Baseline Characteristics by Study Arm.
This table summarizes demographic and baseline characteristics by study arm in the ITT population. All measures were recorded at Screening.
| No. (%) | |||
|---|---|---|---|
|
| |||
| Treatment (N = 24) | Observational Control (N = 21) | Total (N = 45) | |
| Sex – Female | 10 (42) | 10 (48) | 20 (44) |
| Race | |||
| Caucasian | 19 (79) | 17 (81) | 36 (80) |
| Hispanic | 3 (13) | 2 (10) | 5 (11) |
| African-American | 1 (4) | 1 (5) | 2 (4) |
| Other | 1 (4) | 1 (5) | 2 (4) |
| Genotype | |||
| F508 del Homozygous | 6 (25) | 12 (57) | 18 (40) |
| F508 del Heterozygous | 14 (58) | 7 (33) | 21 (47) |
| Other [1] | 4 (17) | 2 (10) | 6 (13) |
| Age Group | |||
| 4 - 12 years | 13 (54) | 15 (71) | 28 (62) |
| >12 - 18 years | 6 (25) | 5 (24) | 11 (24) |
| >18 years | 5 (21) | 1 (5) | 6 (13) |
| P. aeruginosa Positive | 4 (17) | 4 (19) | 8 (18) |
| FEV1 % Predicted Group [2] | |||
| 30% - 50% predicted | 1 (5) | 0 (0) | 1 (3) |
| >50% - 75% predicted | 1 (5) | 0 (0) | 1 (3) |
| >75% - 100% predicted | 7 (35) | 5 (29) | 12 (32) |
| >100% predicted | 11 (55) | 12 (71) | 23 (62) |
|
| |||
| Mean (SD) | |||
|
| |||
| Age (years) | 12.3 (6.6) | 10.5 (5.5) | 11.5 (6.1) |
| FEV1 % Predicted [2] | 98.5 (21.6) | 101.2 (11.8) | 99.8 (17.6) |
| Weight (kg) | 40.5 (17.0) | 38.2 (19.8) | 39.4 (18.2) |
| Weight (%)[3] | 50.5 (27.4) | 53.7 (23.9) | 52.0 (25.5) |
| Body Mass Index (%)[3] | 60.8 (25.4) | 64.6 (20.5) | 62.7 (23.0) |
Other refers to participants with either two known, non-Delta F508 CF mutations, or one known, non-F508 del CF mutation and one unidentified allele which has not been classified as a CF mutation.
For participants 6 years or older, FEV1 % predicted is calculated based on the Wang (males < 18 years, females < 16 years) or Hankinson (males ≥ 18 years, females ≥ 16 years) reference equations. Percentages are based on number of participants with FEV1 measurements available (20 in the treatment arm and 17 in the observational control arm).
The percentiles are derived using CDC standards for participants ≤ 20 years old.
Table 2 summarizes the primary endpoint, i.e. changes in MRSA culture status from screening through Day 28. Those who were not positive at screening had a confirmed MRSA isolate at a clinic visit within a median of 46 days (range 14 to 154 days) prior to screening. The proportion of participants in the ITT-E population who were MRSA negative at Day 28 was 82% (n=18/22) in the Rx compared to 26% (n=5/19) in the Obs-arm. Adjusted for the interim reviews, the difference in the proportion being MRSA negative at Day 28 (Rx minus Obs) was 52% (95% CI: 23%, 80%; p<0.001). In a sensitivity analysis limited to participants who were MRSA positive at the screening visit, 67% in the Rx compared to 13% in the Obs-arm were MRSA negative at Day 28, with an adjusted difference of 49% (95% CI: 22%,71%, p<0.001). These results prompted the DMC to recommend early study closure (see Online Supplement labelled Statistical Monitoring Guidelines and Interim Primary Endpoint Analyses).
Table 2. Microbiologic Effect at Day 28.
This table summarizes analysis of primary endpoint, i.e. proportion of participants in the ITT-E population with a MRSA-negative culture at Day 28, adjusted for interim review. Also summarized is the proportion of MRSA-negative cultures at Day 28 among participants with a MRSA-positive culture at screening. For participants that have both an OP and expectorated sputum sample available at a given visit, a positive respiratory culture result is based on MRSA being present in either the OP or expectorated sputum sample; a negative result is based on MRSA being absent from both the OP and expectorated sputum samples.
| Treatment (N=24) | Observational Control (N=21) | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| Screening | ||||
| Number screened | 24 | 21 | ||
| MRSA Positive at screen, n (%) | 14 (58%) | 17 (81%) | -23% (-45%, 4%)[1] | 0.12[2] |
|
| ||||
| Day 28 | ||||
| Number completed | 22 | 19 | ||
| MRSA Negative at Day 28, n (%) | 18 (82%) | 5 (26%) | 52% (23%, 80%)[3] | <0.001 [3] |
|
| ||||
| Change from Screening to Day 28 | ||||
| Number Cultures MRSA Positive at Screening [4] | 12 | 15 | ||
| Changed to MRSA Negative from Screening to Day 28, n (%) [5] | 8 (67%) | 2 (13%) | 49% (22%, 71%)[3] | <0.001 [3] |
95% confidence interval calculated using the Newcombe -Wilson method without continuity correction.
The p-value is obtained from the Fisher's exact test.
Adjusted for the interim reviews.
Number of participants with both a MRSA positive respiratory culture result available at Screening, and a non-missing MRSA culture result at Day 28.
Percent value is based on the number of participants with a MRSA positive respiratory culture result available at Screening.
Table S1 shows results of sensitivity analyses within the first 28 days for the primary efficacy endpoint. These sensitivity analyses addressed the use of anti-MRSA antibiotics within the first 28 days. For all of the sensitivity analyses, the inference was consistent with the primary analysis and in particular, the observed treatment effect was stronger in the PPE population.
In the ITT population, fifteen participants (65%) were compliant in their use of both oral antibiotics by taking at least 80% doses of rifampin and TMP-SMX, or minocycline (only two subjects in this group were treated with minocycline). Overall, the protocol was acceptable to patients and families with a relatively low treatment burden (See Online Supplement). Adherence with environmental decontamination was very good with 2 participants (9%) reporting missing ≥5 days of wipes and one participant missing ≥1 time of washing the linens. There were three instances of oral antibiotic discontinuation due to adverse events “probably related” to study drug: two were temporary discontinuation of rifampin due to GI complaints, whereas one participant had to discontinue all antibiotics due to urticaria. None of the adverse events were considered serious or required hospitalization. Unrelated to adverse events one participant discontinued rifampin early and one participant reported taking TMP/SMX only once daily.
Two serious adverse events (SAEs) occurred during the first 28 days on study, one in the Rx-arm (increased cough) and one in the Obs-arm (cellulitis of the eyelid). Types of adverse events were more likely related to gastrointestinal and skin/subcutaneous tissues disorders in the treatment arm. No significant laboratory related adverse events were identified (see Online Supplement Table S2 and Safety Laboratory Assessment). There were no microbiologic adverse events (i.e. no emergent MRSA resistances to antibiotics used or appearance of small colony variants).
Use of anti-MRSA antibiotics is shown by individual participants in Figure 2 from screening through Day 168 by study arm together with MRSA culture status. After Day 28, nine (38%) participants in the Rx and nine (43%) in the Obs-arm were treated with anti-MRSA antibiotics. The usage of non-MRSA active acute oral, inhaled, or IV antibiotic was comparable between treatment arms during the first 28 days as well as throughout the study. Participant-specific MRSA culture results show that 13 of 24 participants (54%) in the Rx-arm were MRSA negative at Day 28 and remained negative through Day 84 (Figure 2A) as compared to two of 21 (10%) participants in the Obs-arm (Figure 2B). The impact of acute antibiotic administration in the Obs-arm on the treatment effect is examined in Online Supplement Tables S3.1 and S3.2.
Figure 2. (A Rx and B Obs-arm) Participant-specific MRSA culture results through Day 168 by treatment arm.
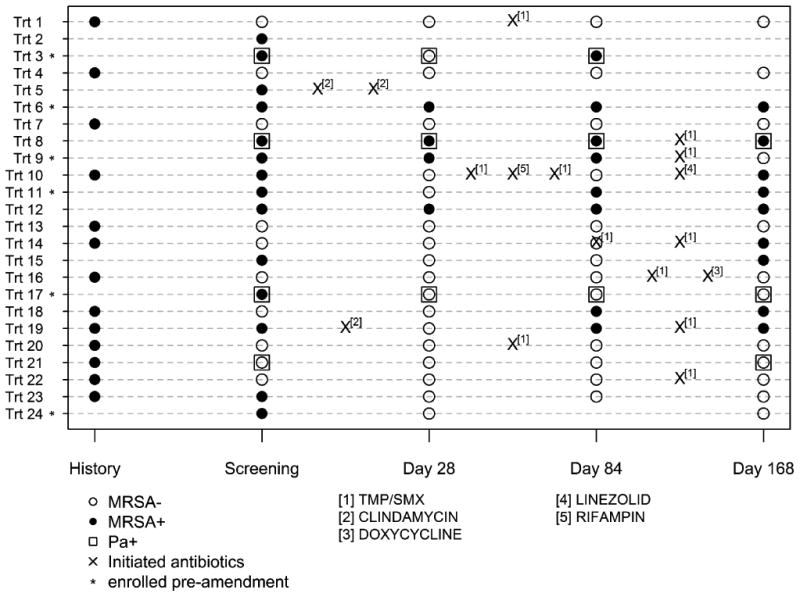
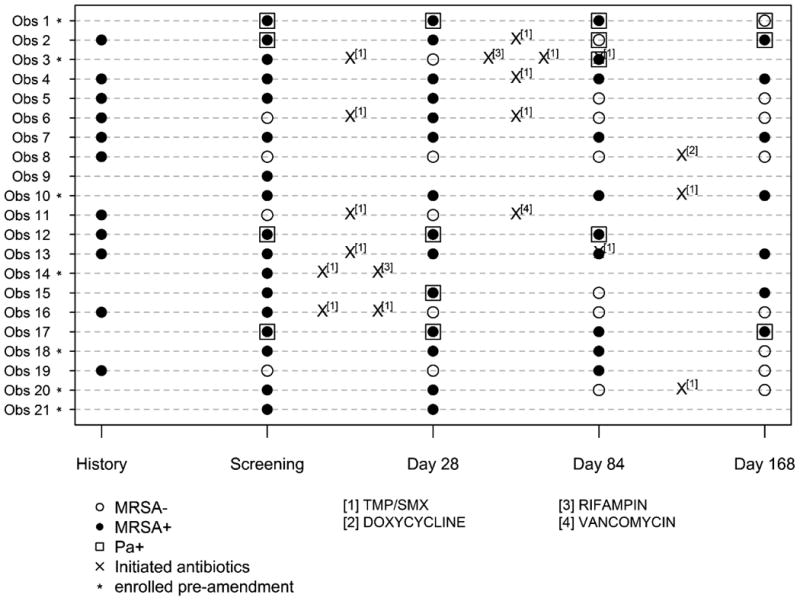
Individual participant MRSA culture status across time is shown. History of MRSA positive isolate is also shown. Participants with who are P. aeruginosa positive (Pa +) are indicated by □. Acute events treated with anti-MRSA active antibiotics are marked with an ‘X’. The locations of the ‘X’s indicate the timing of the antibiotic course in relationship to the study visits but do not represent an actual day as measured from screening. Participants enrolled prior to protocol amendment are marked with an asterisk (see Discussion).
Two participants, one in each arm, were hospitalized during the first 28 days of the study. Over the entire course of the study, two (8%) participants in the Rx-arm were hospitalized three times and five (24%) were hospitalized in the Obs-arm 11 times (difference = -15%, 95% CI: -38%, 6%, p=0.22). The rate of hospitalization from screening through Day 168 was significantly lower in the Rx vs. the Obs-arm (RR = 0.22, 95% CI: 0.05, 0.72, p=0.01).
The proportion of participants experiencing at least one pulmonary exacerbation between screening and Day 28 (calculated as the proportion of patients experiencing an event per 28 days of follow-up) was 13% in the Rx-arm as compared to 33% in the Obs-arm (95% CI: -44%, 4%, p=0.15). Similarly, the rate of exacerbation from screening through Day 28 was lower in the Rx-arm vs. Obs-arm, (RR = 0.36, 95% CI: 0.08, 1.29, p=0.12), although not statistically significant.
At screening 14 of 45 participants had nasal MRSA colonization. The proportion colonized was similar in the two treatment arms: six of 24 (25%) in the Rx and eight of 21 (38%) in the Obs-arm (p=0.52). No treatment related differences emerged. Only one patient was MRSA colonized at the skin (Supplement Tables S4.1, S4.2). Participants with persistent or re-emergent MRSA infection kept the same SCCmec type. There were no differences in proportion colonized with P. aeruginosa at screening, with four of 24 (17%) in Rx and 4 of 21 (19%) in the Obs-arm (p>0.999). No differences emerged during the course of the trial.
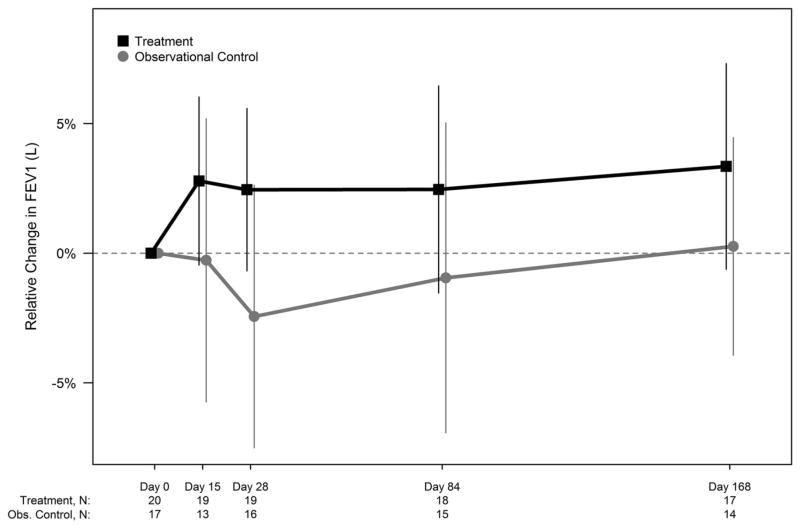
At Day 28, the mean relative change in FEV1 (L) from screening was 2.5% in the Rx-arm (n=19) and -2.4% in the Obs-arm (n=16) (difference = 4.9%, 95% CI: -0.6%,10.4%, p=0·08); the mean absolute change in FEV1 (% predicted) was 0.7% in the Rx-arm and -4.1% in the Obs-arm with a difference of 4.8%, (95% CI: -0.9%,10.5%, p=0.10). At Day 168, the differences (Rx-Obs) in mean relative change in FEV1 (L) was 3·1% (95% CI: -2.5%, 8.6%, p=0.27) and the mean absolute change in FEV1 (% predicted), 4·7% (95% CI: -0.4%, 9.8%, p=0.07) (See Figure 3). Figure S1.1 and S1.2 show the changes in FEV1 (Liters) and FEV1 (% predicted)).
Figure 3. Relative change from screening in FEV1 (Liters) over time by study arm (ITT Population).
Relative change in FEV1 (Liters) from baseline to each post-baseline visit for both study arms in ITT population is shown. 95% confidence intervals (using t-distribution approximation) are included at each time point. The number of participants at each time point is included in a legend below the figure. Per protocol, participants younger than 6 years of age from both the treatment arm (n=3) and observational control arm (n=4) were not assessed for pulmonary function.
There were no significant differences between study arms with respect to changes in weight or patient reported outcomes (Online Supplement Figures S2.1, S2.2, S2.3, S3.1, and S3.2).
Discussion
Prevalence of MRSA in CF is ∼26% in the U.S. and chronic infection has been associated with higher rates of lung function decline and mortality.9,10 The current randomized controlled trial evaluated a comprehensive protocol with two oral antibiotics for two weeks combined with nasal, skin, and oral decontamination as well as a three week environmental decontamination in clinically stable CF patients. This treatment led to a marked reduction in culture positivity (OP swab or sputum) at 28 days compared to the observational arm. The treatment effect was large enough to recommend early study termination by the DMC due to evidence of microbiologic efficacy, i.e. the high rate or MRSA negative cultures in the treatment compared to the observation arm. Positive trends were also seen for secondary outcomes with reduction in the rate of pulmonary exacerbation and a trend towards improved lung function despite the study population having preserved lung function at baseline. As expected for these antibiotics, the rate of GI side effects was higher in the Rx arm, however the only permanent discontinuation of antibiotics was for urticaria. Thus, the treatment regimen appeared overall safe and, despite some drug related side effects, well tolerated. Despite most participants reporting that the regimen was acceptable, compliance with all aspects of this regimen was not ideal, which may be related to the multi-faceted approach.
The possibility of treating MRSA successfully and achieving eradication even in settings of chronic infection has been reported.11-13,20-22 None of these studies included a control arm and all had smaller sample sizes than the current study. Despite these limitations, they demonstrated the potential benefit of eradication. Of note, these reports included countries and CF centers with much lower MRSA prevalence than the U.S., which would be especially relevant in examining rates of MRSA recurrence. A systemic review for the early treatment of MRSA in CF concluded that there were no randomized trials available to assess early eradication.23
The protocol specified antibiotics were based on drug availability, cost, synergy and antibiotic susceptibility in CF MRSA isolates in the U.S.16,24,17 Although fusidic acid showed low rates of resistance for U.S. CF MRSA isolates17 and that MRSA eradiation protocols outside the U.S. reported combination therapy with fusidic acid and rifampin, this drug is not approved in the U.S. We included nasal or throat decontamination procedures based on the use of mupirocin in non-CF eradication/decontamination25 and the high rate of positive throat cultures in CF.26 Skin decontamination was included based on possible high skin colonization with SCCmec IV isolates and approaches in non-CF decolonization.27 Interestingly, despite high rates of SCCmec IV MRSA, skin positivity was rare and the topical skin treatment may not be essential in CF. Further trials comparing our intense protocol to less elaborate treatment approaches are required to demonstrate whether skin decontamination is required.
This study was primarily designed as a controlled trial of MRSA treatment with a short controlled microbiology outcome. Thus, longer term clinical outcomes after day 28 in our trial are harder to interpret given the very high rate of oral, intravenous and inhaled antibiotics (many with anti-MRSA activity) in both study arms, even in subjects not culturing MRSA (Fig. 2). The participant and treatment heterogeneity after the intervention period limited interpretation of long term clinical outcomes and we are not able to definitively comment regarding long term lower airway eradication. At day 84, a higher proportion of participants in the Rx-arm than in the Obs-arm were still MRSA negative. It is difficult to evaluate if recurrence of MRSA is due to persistence not detected by culture or due to reinfection and, although repeat isolates were of the same SCCmec type, this method has insufficient sensitivity to address high degree genetic relatedness. Further studies will need to address these questions. Prior literature has demonstrated heavy antibiotics use in U.S. MRSA-positive CF populations.28,29,30
Two aspects of the study warrant special attention and should be considered in regards to generalisability of results. First, because of poor enrollment rates, the number of study sites was expanded and participants were allowed to be enrolled if they had MRSA isolated from the respiratory tract (sputum or OP culture) at the most recent clinical care visit if this MRSA isolate was available for susceptibility testing. This amendment mirrored common clinical practice and was similar to the approach taken in the Early Pseudomonas Infection Control trial.19 Importantly, when the primary endpoint was analyzed based only on participants who were culture positive for MRSA at screening (27/41 in ITT-E population), the results were similar.
Second, the study was stopped based on an unplanned efficacy interim analysis. Interestingly, early stopping for microbiologic efficacy was also initiated in the early inhaled tobramycin eradication trial.31 At the first, planned review, the DMC recommended an unplanned interim efficacy analysis. In response to this unplanned review, additional statistical analyses evaluated the sensitivity of the results to variations in stopping rules (see Online Supplement Table S1).32-36 Because the stopping rule for efficacy was not specified in advance, this sensitivity analysis assessed how sensitive the inference (related to the difference in the proportion MRSA negative between study arms) was to a myriad of stopping rules that the DMC could have considered. These estimates of treatment differences adjusted for the corresponding group sequential stopping rule are shown in detail in Table S1.
This trial was not blinded to either staff or participants because several of the interventions e.g. rifampin could not be blinded. However, the primary endpoint was an objective measure i.e. culture positivity. This design may have led to higher use of antibiotics in the Obs arm after day 28.
There are a number of important limitations to interpreting the results of this clinical trial. First, only participants ages 4-45 years with early MRSA infection were included. Thus, one cannot infer that this protocol would have a similar treatment effect in patient groups not fulfilling these inclusion criteria. Consistent with the highest incidence of MRSA occurring in mid-childhood in the U,S, CF population, the majority of participants were children with FEV1 >75% predicted (Table 1) and the majority could not expectorate sputum. Further, patients presenting in the setting of an acute exacerbation who were culture positive for MRSA or who had received antibiotics effective against MRSA in the prior 28 days were excluded. These participant characteristics and other exclusion criteria limit the extension of these results to the broader CF population infected with MRSA. Last, the study was not designed to assess the long term impact of MRSA eradication or effect of repeated treatment MRSA eradication courses. Data gathered in the observational extension portion of the study noted that both Rx and Obs-arms received multiple courses of antibiotics with potential MRSA activity.
Our study did demonstrate that spontaneous clearance of MRSA at day 28 does occur at a rate of approximately 13% in those that were culture positive at screening and 26% in the entire observation arm. Data from the U.S. CF Registry database collected from 1996-2006 indicates that among patients ages ≥6 years up to 50% of subjects have only one time positive MRSA cultures or intermittent MRSA infection, however treatment history is missing on these subjects.10 Such data however do highlight the value to repeat cultures prior to eradication attempts if one was to employ such a protocol.
Conclusion
This trial is the first randomized clinical trial to study the microbiologic impact of an eradication protocol. While there was a significant difference in microbiological success and evidence towards fewer exacerbations in the Rx-arm in this short trial, more questions remain prior to recommending universal early anti-MRSA therapy. These include optimization of the treatment regimen i.e. are all measures necessary and assessing individuals who either have mild exacerbations or are outside the currently selected age range. The high rate of antibiotic use after the primary endpoint begs the question if a repeat course is indicated in those who remain culture positive at the end of the treatment. These questions will need further clinical trials and close observation of clinical practice in all countries. However, we did demonstrate that currently available antibiotics with a well-established safety profile are effective in early MRSA infection.
Supplementary Material
What is the key question?
Is aggressive treatment of incident MRSA positive respiratory culture in cystic fibrosis (CF) effective at reducing MRSA culture positivity and is it clinically safe?
What is the bottom line?
This multi-faceted oral, topical and environmental treatment protocol demonstrated a strong microbiologic treatment effect in children and adults with few treatment related side effects, which were mostly gastrointestinal and skin-related.
Why read on?
To learn about the duration of MRSA negative cultures and secondary outcomes, which showed favorable trends despite a small sample size.
Acknowledgments
The research for this article was supported by the Cystic Fibrosis Foundation grant number STAR10K, and CTSA at individual sites: UL1TR001111, UL1 TR000433, UL1 TR000077, UL1 TR000423, NCT02249182, UL1 TR001417, UL1 TR000448, UL1 TR001082, UL1 RR025780.
CHG receives funding from the Cystic Fibrosis Foundation, the NIH (R01HL103965, R01HL113382, R01AI101307, U M1HL119073, P30DK089507) and the FDA (R01FD003704).
Special thanks to CF participants, families of CF children who participated in the study, and whose dedication to research made the trial possible. We would also like to thank Bob Beall, PhD and the Cystic Fibrosis Foundation for supporting this clinical trial.
Study Acronym STAR-too: STaph Aureus Resistance – treat or observe
Participating Sites: Site (Investigator and lead Research Coordinator): University of Washington (Christopher H. Goss, Debbie Ng); Minneapolis Children's Affiliate (John McNamara, Mahrya Johnson); Texas Children's Hospital (Silby Moonnumakal, Nicoline Schaap); University of Colorado (Edith Zemanick, Meg Anthony); University of Cincinnati (John P Clancy); Seattle Children's Hospital (Ronald Gibson, Sharon McNamara); Washington University, St. Louis Children's (Peter Michelson, Tina Hicks); University of Texas Southwestern (Preeti Sharma, Andrew Hebert); University of Alabama at Birmingham (Wynton Hoover, Katie Brand); University of Florida at Gainesville (Pamela Schuler, Dawn Baker); University of Michigan (Amy Filbrun, Marisa Linn); University of Nebraska (Paul Sammut, Raquel Telfer); University of North Carolina at Chapel Hill (Marianne Muhlebach, Kelly Moormann); Ft. Worth Cook Children's Hospital (Karen Schultz, Heather Urbanek).
Data and Safety Monitoring Board / DMC: Margaret Guill, MD, DMC Chair; Dartmouth-Hitchcock Medical Center, Pediatric Pulmonary Medicine
John J. LiPuma, MD, Pediatric Infectious Disease Medicine, University of Michigan
Marci Sontag, PhD, Epidemiology/Community Health, Preventive Medicine/Biometrics, The Children's Hospital, Aurora, CO
Susan Murray, ScD, University of Michigan, School of Public Health, Department of Biostatistics (CFFT DMC Ad-hoc Specialist, Sequential Monitoring)
Miriam Hunt, CFFT DMC, Prog. Coord., Sr.
CFF Therapeutics Development Coordinating Center: Dianne L. Howe, Jasna Hocevar-Trnka, Rose Mitchell, Lynette Browne
Footnotes
The names and affiliations of the STAR-too study team Investigators are listed in the Acknowledgments.
Twitter feed: A randomized trial in cystic fibrosis patients with early MRSA infection shows a strong microbiologic treatment effect at 28 days compared to observation.
Author Contributions: Marianne S. Muhlebach: Inception of study, study design, supervising conduction of study, discussion of data and writing, revising manuscript. Communication with all authors and Thorax.
Elena Popowitch: Contributed to protocol development. Performed all laboratory analyses on bacterial samples.
Melissa B. Miller: Contributed to design of the microbiology endpoints and provided oversight of all MRSA related processing and interpretation of MRSA typing results.
Preston J. Campbell contributed to the study inception and ongoing advice during the trial.
Wynton C. Hoover contributed to patient recruitment and enrollment at his study site, provided input for study modification and ongoing feed-back on the protocol. Participated in review and modifications of the final manuscript.
Edith T. Zemanick contributed to patient recruitment and enrollment at her study site, provided input for study modification and ongoing feed-back on the protocol. Participated in review and modifications of the final manuscript.
Christopher H. Goss, Nicole Mayer-Hamblett, and Jill M. Van Dalfsen contributed to study conception and study design. Nicole Mayer-Hamblett, Valeria V Thompson, and Arthur Baines participated in data management and statistical analyses.
All authors participated in data analysis/interpretation, drafting and/or revising the manuscript for intellectual content, and editing the manuscript for final approval.
Competing Interests: None declared.
References
- 1.CDC. Active Bacterial Core Surveillance Report, Emerging INfections Program Network, MRSA. Secondary Active Bacterial Core Surveillance Report, Emerging INfections Program Network, MRSA. 2012 http://www.cdc.gov/abcs/reports-findings/survreports/mrsa12.pdf.
- 2.Lee BY, Singh A, David MZ, et al. The economic burden of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013;19(6):528–36. doi: 10.1111/j.1469-0691.2012.03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knapp EA, Salsgiver E, Fink A, et al. Trends in respiratory microbiology of people with cystic fibrosis in the United States, 2006-2012. Ped Pulm. 2014;S38:282. [Google Scholar]
- 4.CFF Registry CFF. Patient Registry Annual Report 2013. 2013 https://wwwcfforg/2013_CFF_Patient_Registry_Annual_Data_Reportpdf.
- 5.Goss CH, Muhlebach MS. Review: Staphylococcus aureus and MRSA in cystic fibrosis. J Cyst Fibros. 2011;10(5):298–306. doi: 10.1016/j.jcf.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Ren CL, Morgan WJ, Konstan MW, et al. Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol. 2007;42(6):513–8. doi: 10.1002/ppul.20604. [DOI] [PubMed] [Google Scholar]
- 7.Muhlebach M, Miller M, Goodrich J, et al. Impact of Methicillin Resistant S. aureus on Clinical outcomes in CF. Ped Pulmonology. 2007;42(30):331. [Google Scholar]
- 8.Sawicki GS, Rasouliyan L, Ren CL. The impact of MRSA on lung function in patients with cystic fibrosis. Am J Respir Crit Care Med. 2009;179(8):734–5. doi: 10.1164/ajrccm.179.8.734a. author reply 35. [DOI] [PubMed] [Google Scholar]
- 9.Dasenbrook EC, Merlo CA, Diener-West M, et al. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med. 2008;178(8):814–21. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 10.Dasenbrook EC, Checkley W, Merlo CA, et al. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303(23):2386–92. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 11.Garske LA, Kidd TJ, Gan R, et al. Rifampicin and sodium fusidate reduces the frequency of methicillin-resistant Staphylococcus aureus (MRSA) isolation in adults with cystic fibrosis and chronic MRSA infection. J Hosp Infect. 2004;56(3):208–14. doi: 10.1016/j.jhin.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Macfarlane M, Leavy A, McCaughan J, et al. Successful decolonization of methicillin-resistant Staphylococcus aureus in paediatric patients with cystic fibrosis (CF) using a three-step protocol. J Hosp Infect. 2007;65(3):231–6. doi: 10.1016/j.jhin.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Solis A, Brown D, Hughes J, et al. Methicillin-resistant Staphylococcus aureus in children with cystic fibrosis: An eradication protocol. Pediatric pulmonology. 2003;36(3):189–95. doi: 10.1002/ppul.10231. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Dockery DW, Wypij D, et al. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 16.Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2008;29(6):510–6. doi: 10.1086/588201. [DOI] [PubMed] [Google Scholar]
- 17.Champion EA, Miller MB, Popowitch EB, et al. Antimicrobial susceptibility and molecular typing of MRSA in cystic fibrosis. Pediatric pulmonology. 2013;49(3):230–37. doi: 10.1002/ppul.22815. [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165(9):847–56. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doe SJ, McSorley A, Isalska B, et al. Patient segregation and aggressive antibiotic eradication therapy can control methicillin-resistant Staphylococcus aureus at large cystic fibrosis centres. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2010;9(2):104–9. doi: 10.1016/j.jcf.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Halton K, Zobell J, M M, et al. Evaluation of the effectiveness of a MRSA eradication protocol in pediatric CF patients. Ped Pulm. 2009;S32:366. [Google Scholar]
- 22.Vanderhelst E, Wachter E, Willekens J, et al. Increase in ventilated air spaces after eradication of chronic methicillin-resistant Staphylococcus aureus infection in cystic fibrosis patients. Acta Clin Belg. 2015;70(1):30–33. doi: 10.1179/2295333714Y.0000000079. [DOI] [PubMed] [Google Scholar]
- 23.Lo DK, Hurley MN, Muhlebach MS, et al. Interventions for the eradication of meticillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database Syst Rev. 2015;2 doi: 10.1002/14651858.CD009650.pub3. CD009650. [DOI] [PubMed] [Google Scholar]
- 24.Simor AE, Daneman N. Staphylococcus aureus decolonization as a prevention strategy. Infect Dis Clin North Am. 2009;23(1):133–51. doi: 10.1016/j.idc.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Buehlmann M, Frei R, Fenner L, et al. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2008;29(6):510–6. doi: 10.1086/588201. [DOI] [PubMed] [Google Scholar]
- 26.Ridder-Schaphorn S, Ratjen F, Dübbers A, H J, Falk S, Küster P, Schuster A, Mellies U, Löwe B, Reintjes R, Peters G, Kahl BC. Nasal Staphylococcus aureus carriage is not a risk factor for lower-airway infection in young cystic fibrosis patients. Journal of clinical microbiology. 2007;45(9):2979–84. doi: 10.1128/JCM.00855-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West SK, Plantenga MS, Strausbaugh LJ, et al. Use of decolonization to prevent staphylococcal infections in various healthcare settings: results of an Emerging Infections Network survey. Infect Control Hosp Epidemiol. 2007;28(9):1111–3. doi: 10.1086/519930. [DOI] [PubMed] [Google Scholar]
- 28.Muhlebach MS, Miller M, LaVange LM, et al. Treatment intensity and characteristics of MRSA infection in CF. J Cyst Fibrosis. 2011;10(3):201–06. doi: 10.1016/j.jcf.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Heltshe SL, Saiman L, Popowitch EB, et al. Outcomes and Treatment of Chronic Methicillin-Resistant Staphylococcus aureus Differs by Staphylococcal Cassette Chromosome mec (SCCmec) Type in Children With Cystic Fibrosis. J Pediatric Infect Dis Soc. 2015;4(3):225–31. doi: 10.1093/jpids/piu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heltshe SL, Goss CH, Thompson V, et al. Short-term and long-term response to pulmonary exacerbation treatment in cystic fibrosis. Thorax. 2016;71(3):223–9. doi: 10.1136/thoraxjnl-2014-206750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson RL, Emerson J, McNamara S, et al. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med. 2003;167(6):841–9. doi: 10.1164/rccm.200208-855OC. [DOI] [PubMed] [Google Scholar]
- 32.Emerson SS. Stopping a clinical trial very early based on unplanned interim analyses: a group sequential approach. Biometrics. 1995;51(3):1152–62. [PubMed] [Google Scholar]
- 33.Emerson SS, Banks PLC. Interpretation of a leukemia trial stopped early. In: Lange N, Ryan L, Billiard L, et al., editors. Case Studies in Biometry. New York City: John Wiley & Sons; 1994. [Google Scholar]
- 34.Emerson SS, Fleming TR. Symmetric group sequential test designs. Biometrics. 1989;45(3):905–23. [PubMed] [Google Scholar]
- 35.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–56. [PubMed] [Google Scholar]
- 36.Pocock SJ. Group sequential methods in the design and analysis of clinical trials. Biometrika. 1977;64:191–99. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.